Summary
Objectives : To review current studies about designing and implementing clinician-facing clinical decision support (CDS) integrated or interoperable with an electronic health record (EHR) to improve health care for populations facing disparities.
Methods : We searched PubMed to identify studies published between January 1, 2011 and October 22, 2021 about clinician-facing CDS integrated or interoperable with an EHR. We screened abstracts and titles and extracted study data from articles using a protocol developed by team consensus. Extracted data included patient population characteristics, clinical specialty, setting, EHR, clinical problem, CDS type, reported user-centered design, implementation strategies, and outcomes.
Results : There were 28 studies (36 articles) included. Most studies were performed at safety net institutions (14 studies) or Indian Health Service sites (6 studies). CDS tools were implemented in primary care outpatient settings in 24 studies (86%) for screening or treatment. CDS included point-of-care alerts (93%), order facilitators (46%), workflow support (39%), relevant information display (36%), expert systems (11%), and medication dosing support (7%). Successful outcomes were reported in 19 of 26 studies that reported outcomes (73%). User-centered design was reported during CDS planning (39%), development (32%), and implementation phase (25%). Most frequent implementation strategies were education (89%) and consensus facilitation (50%).
Conclusions : CDS tools may improve health equity and outcomes for patients who face disparities. The present review underscores the need for high-quality analyses of CDS-associated health outcomes, reporting of user-centered design and implementation strategies used in low-resource settings, and methods to disseminate CDS created to improve health equity.
Keywords: Equity, screening, vulnerable populations, medically underserved, safety net
1 Introduction
Health disparities are differences in health that are unnecessary, avoidable, unfair, and unjust, whereas health equity may be defined as the absence of systemic disparities in health, including underlying social determinants of health [ 1 2 3 ]. Achieving equity is an imperative for clinical practice and public health. Identifying health disparities and pathways to equity have important consequences such as allotment of resources by institutions and governments to improve health outcomes.
Electronic health record (EHR)-integrated clinical decision support (CDS) may improve quality of care including guideline adherence [ 4 , 5 ]. However, CDS may be based on research or algorithms that may perpetuate disparities through exclusion of risk factors for specific subgroups [ 6 ], inappropriate application of subgroup risk [ 7 , 8 ], or the lack of representation of subgroups [ 9 10 11 ]. In the United States, the health care safety net is defined as “providers that organize and deliver a significant level of health care and other health-related services to uninsured, Medicaid, and other vulnerable patients” in varied settings such as public hospitals, community health centers, or local health departments [ 12 ]. Safety net organizations typically have limited EHR and health information technology capabilities compared to non-safety net organizations [ 13 ]. Disparities in quality of care may be widened further because developing and integrating custom CDS into an EHR may incur major human resource costs [ 14 ] that may be prohibitive to systems that care for underserved populations.
User-centered design and effective implementation strategies are vital to the success of CDS systems [ 15 ]. In a recent study, when a CDS tool was customized using recommended design practices and an implementation science framework, the tool was used more frequently and was more effective than a commercial alert designed for general use in varied populations and settings [ 16 ]. However, user-centered design may be challenging in settings with limited resources to support provider engagement [ 17 , 18 ]. There is limited research evaluating implementation strategies in safety net settings to define practical and high-value methods [ 17 , 19 ].
Providers in low-resource settings serve populations that are affected disproportionately by chronic conditions and need specific tools to treat illness that is complicated by the social determinants of health [ 20 ]. Although several reviews have described patient-facing CDS for populations experiencing health care disparities [ 21 22 23 ], there is limited information available summarizing the design, implementation, and use of clinician-facing EHR-integrated or interoperable CDS in low-resource settings. The purpose of this study was to address this information gap by reviewing current studies about designing and implementing EHR-integrated or interoperable CDS that was created for clinicians to decrease health disparities or improve the quality of health care for populations that may face disparities.
2 Methods
2.1 Inclusion Criteria
This review included research studies about health care interventions that (1) had a clinician-facing, EHR-integrated or interoperable CDS component [ 21 ], and (2) were designed to decrease a known health disparity or serve a vulnerable or underserved population that may face health disparities [ 25 ]. In this study, health care workers were defined as any clinician or support staff involved in clinical workflow including providers (defined as health care workers who make medical diagnoses and prescribe medical treatment including physicians, physician associates or assistants, and nurse practitioners), nurses, medical assistants, care coordinators, and other staff including pharmacists and social workers.
2.2 Exclusion Criteria
We excluded reviews, viewpoint articles, and non-English language articles. We also excluded patient-facing applications, online or mobile clinician-facing applications that were not interoperable with an EHR, tools to collect data to assess disparities without CDS to reduce the disparities, and CDS for universal screening of all patients, even if a positive screen defined a population that may face disparities.
2.3 Literature Search
The PubMed database (National Library of Medicine, Bethesda, MD) was searched for relevant citations in English that were published between January 1, 2011 and October 22, 2021 using terms related to populations that may face health care disparities, including minorities of sexual identity or gender orientation, racial and ethnic minorities, and people with disabilities or who were homeless, incarcerated, medically underserved, rural, vulnerable, uninsured, or underinsured. Terms for CDS were added to the population terms with exclusion terms to limit citations about mobile health and smartphone applications ( Appendix ).
2.4 Literature Screening
Abstracts and titles of citations were screened by three reviewers who worked independently and graded the citations for inclusion or exclusion (CHS, PVK, QTN). The full articles of all included citations were reviewed by two coauthors separately (CHS, ET) to confirm eligibility, and selected full articles were reviewed by three other coauthors separately (PVK, QTN, LV) to unify taxonomy and data extraction. Uncertainties or disagreements about inclusion of articles were resolved by discussion to achieve agreement between reviewers. Additional nonredundant articles about included studies and one other study discovered during article review were added and reviewed.
2.5 Data Extraction
Evaluation criteria were defined by consensus, and characteristics of study patients, clinical setting, type of health care worker supported by CDS, EHR type, and CDS management type were extracted from included articles by the full-text reviewers. Study patient characteristics included population description, patient age range, and sex distribution. Clinical settings were emergency department, inpatient, mobile clinic, and outpatient settings. The EHRs used were extracted and categorized as commercial (sold commercially) or noncommercial (not sold commercially) EHRs.
Study characteristics were extracted using the problem-intervention-comparison-outcome (PICO) framework. Study design was categorized (observational, quasi-experimental, or cluster randomized) as described previously [ 26 , 27 ]. The phase of CDS life cycle for each study was categorized (planning, development, implementation, or operation) as described previously [ 28 ]. The CDS tools planned or implemented in the included studies were categorized (medication dosing support, order facilitator, point-of-care alert, relevant information display, expert systems, workflow support) as described previously [ 24 ].
Outcomes were extracted including pertinent clinical and informatics findings. Study outcomes were rated as successful (improvement in a primary clinical study outcome confirmed with statistical testing or reported achievement of an informatics objective), indeterminate (neither statistical testing nor comparison group included), or no change (insignificant change). As study outcomes may have been affected by design or implementation procedures, characteristics of CDS user-centered design during the planning, development, and implementation phases and implementation strategies were extracted based on terms described previously ( Table 1 ) [ 28 , 29 ].
Table 1.
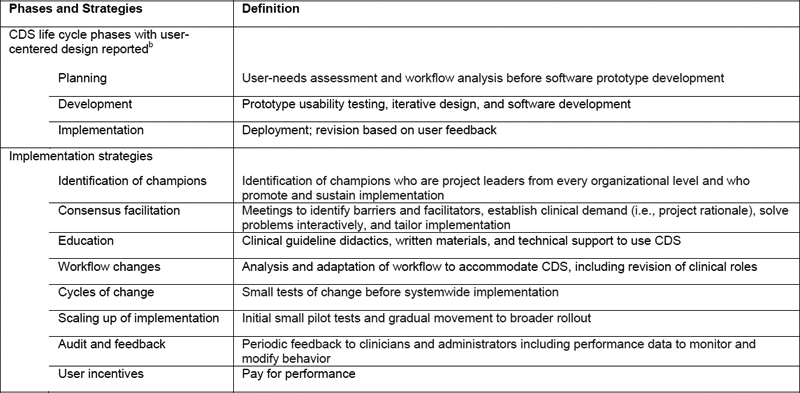
Taxonomy for assessment of user-centered design and implementation strategies for clinical decision support toward reducing health care disparities a
Abbreviation : CDS, clinical decision support.
a CDS life cycle phases and user-centered design strategies modified from Kukhareva [ 28 ]. Implementation strategies modified from Powell [ 29 ].
b Operation phase that included maintenance and dissemination to other health care systems was not included because operation phase occurred after user-centered design reported.
During the review, we identified two broad categories of CDS that were developed for clinicians who provided health care services to underserved populations: (1) CDS that was developed within a study health system and implemented only at clinical sites affiliated with the study (defined as internally-used CDS ), and (2) CDS that was designed, implemented, and maintained within an umbrella nonprofit or government health care organization and was available for use at clinical sites external to the sites reported in the study (defined as externally-available CDS ). Therefore, the CDS for each study was categorized as internally-used or externally-available CDS.
2.6 Data Analysis
Data analysis was performed with statistical software (Stata, StataCorp, College Station, TX). To determine whether externally-available CDS had advantages due to an economy of scale, the association between externally-available CDS vs number of implementation sites was evaluated with linear regression and reported as beta coefficient ± standard deviation (SD) and 95% confidence interval (CI). For studies that did not include statistical analysis of pre- vs postimplementation numeric results, reported results were evaluated with chi-square test. Statistical significance was defined by P < .05.
3 Results
The PubMed search identified 189 articles, and one redundant article that reported the planning stage of an intervention was removed. After titles and abstracts for the remaining 188 articles were reviewed, 158 articles were excluded, mostly for not having CDS ( Figure 1 ). After full-text review of the remaining 30 studies, three studies not pertaining to underserved patients were excluded. An additional study (one article) and eight non-redundant additional articles about remaining studies that were not identified by the PubMed search were added, resulting in 28 studies (36 articles) included in the review [ 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 ].
Fig. 1.

Flow diagram of search and screening of articles about clinical decision support for populations facing health care disparities
There were 19 studies (68%) that were performed at safety net institutions or Indian Health Service (IHS) sites serving American Indian/Alaskan Native people and two studies (7%) that were performed with vulnerable or underserved populations outside the United States ( Table 2 ). There were 24 studies (86%) that reported CDS implementation in primary care outpatient settings ranging from 1 to 60 sites per study, and the CDS supported providers in all except one study and nurses in seven studies (25%). There were four EHRs reported including two commercial and two non-commercial EHRs, but 13 studies (46%) did not report the EHR used ( Table 2 ).
Table 2.

Characteristics of patients and clinical settings for studies included in the review about clinical decision support and health care disparities
Abbreviations : AI/AN, American Indian/Alaska Native; CC, care coordinator; CKD, chronic kidney disease; ED, emergency department; EHR, electronic health record; F, female; Fam, family medicine; FQHC, Federally Qualified Health Center; Hosp, hospital staff; IM, internal medicine; Inpt, inpatient; M, male; MA, medical assistant; Mobile, mobile clinics; NR, not reported; OBGYN, obstetrics and gynecology; Outpt, outpatient; Peds, pediatrics; Prim, primary care; Prov, provider; Pt, patient; Urg, urgent care.
a N = 36 articles for 28 studies; 1 article per study except for 3 studies (Peiris; Price-Haywood; Shah) that had 2 articles each, 1 study (Tuot) with 3 articles, and 1 study (Gold) with 4 articles. For studies with > 1 article, year listed was the earliest year of the articles. Review limited to clinician-facing CDS tools.
b Safety net (population treated regardless of insurance status including uninsured and underinsured), 14 studies (50%), including FQHCs (6 studies). AI/AN-Indian Health Service, 6 studies (21%). Studies performed outside the United States, 2 studies (Peiris; Patel).
c Joseph: other including minorities, poverty, and uninsured.
d Shaibi: age reported for groups: 44 ± 14 and 49 ± 11 y.
e Rudd: inclusion or exclusion of males, NR.
f Clinical specialty: primary care, 24 studies (86%).
g Hughes: 6 clinical specialties: ED, Fam, IM, Peds, OBGYN, Urg.
h Clinical setting: outpt, 25 studies (89%); inpt, 6 studies (21%); ED, 2 studies (7%); mobile clinics, 1 study (4%).
i Gold, clinical setting: pilot, 3 sites [ 46 ]; protocols, 30 and 36 sites [ 47 , 48 ].
j Shaibi: program development, 2 institutions (1 academic center and 1 FQHC); implementation, 1 site (FQHC).
k Orenstein: working group, 19 institutions; implementation, 3 sites.
l EHRs: Epic (commercial; Epic Systems Corp., Verona, WI), 8 studies including 1 study with OCHIN (formerly Oregon Community Health Information Network Inc., Portland, OR); IHS-RPMS (noncommercial; Indian Health Service Resource and Patient Management System, Rockville, MD), 5 studies; Centricity (commercial; GE HealthCare, Chicago, IL), 1 study; eCRF (noncommercial; Electronic Case Record Form, Government of India), 1 study; NR, 13 studies (46%) including Shapiro that had EHR changed during study, Peiris that used 2 EHRs but identity NR, and Rosenman that had internal EHR that was changed to a vendor. There were 8 studies (29%) that included noncommercial (nonprofit or government) organizations (eCRF, IHS-RPMS, OCHIN, Peiris).
The CDS tools were used for screening or treatment of diverse clinical problems including chronic and infectious diseases and mostly included point-of-care alerts (93% of studies), order facilitators (46%), workflow support (39%), and relevant information display (36%), with some studies including several CDS intervention types ( Table 3 ). There were eight studies (29%) that reported externally-available CDS implemented in seven non-commercial EHRs and one commercial EHR ( Table 2 and Table 3 ). The use of externally-available CDS was associated with a larger number of implementation sites (coefficient, 16 ± 6; 95% CI, 2.6–29; P = .021).
Table 3.
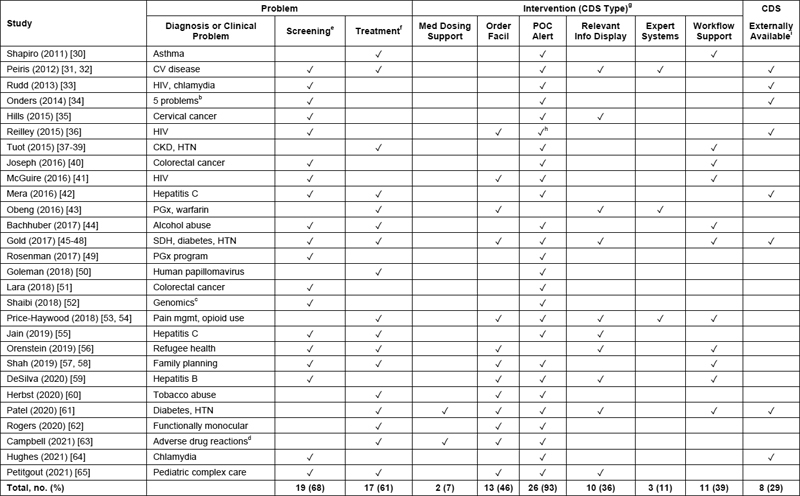
Characteristics of studies included in the review about clinical decision support and health care disparities: problem and intervention
Abbreviations : CDS, clinical decision support; CKD, chronic kidney disease; CV, cardiovascular; Facil, facilitator; HTN, hypertension; Info, information; Med, medication; Mgmt, management; PGx, pharmacogenomics; POC, point of care; SDH, social determinants of health.
a N = 36 articles for 28 studies; 1 article per study except for 3 studies (Peiris; Price-Haywood; Shah) that had 2 articles each, 1 study (Tuot) with 3 articles, and 1 study (Gold) with 4 articles. For studies with > 1 article, year listed was the earliest year of the articles. Totals reported as no. of studies (%). Review limited to clinician-facing CDS tools. Outcomes not reported for 2 studies: Obeng, Patel.
b Onders, primary care screening for 5 problems: CV disease, tobacco abuse, alcohol abuse, intimate partner violence, and depression.
c Shaibi: genomics for dyslipidemia and colon polyps.
d Campbell: adverse drug reactions to anticholinergics in geriatric patients; intervention limited to tricyclic antidepressants and urinary antispasmodics.
e Screening included documentation and genetic testing.
f Treatment included documentation, referral for treatment, and vaccination.
g CDS type was classified according to published taxonomy [ 24 ].
h Reilley: In 51 federal sites surveyed, 47 sites (92%) used POC alert of some type, and 32 sites (63%) used a national Indian Health Service POC alert.
i Externally-available CDS was defined as CDS that was designed, implemented, and maintained within an umbrella nonprofit or government health care organization and was available for use at clinical sites external to the sites reported in the study.
In the 26 studies that reported an evaluation, most CDS tools were in the operation phase (88%), and most study designs were observational (85%) with pre- vs postimplementation comparisons (62%) ( Table 4 ). There were 19 of the 26 studies (73%) that reported success in clinical screening or treatment associated with CDS use or achievement of an informatics objective ( Table 4 ).
Table 4.
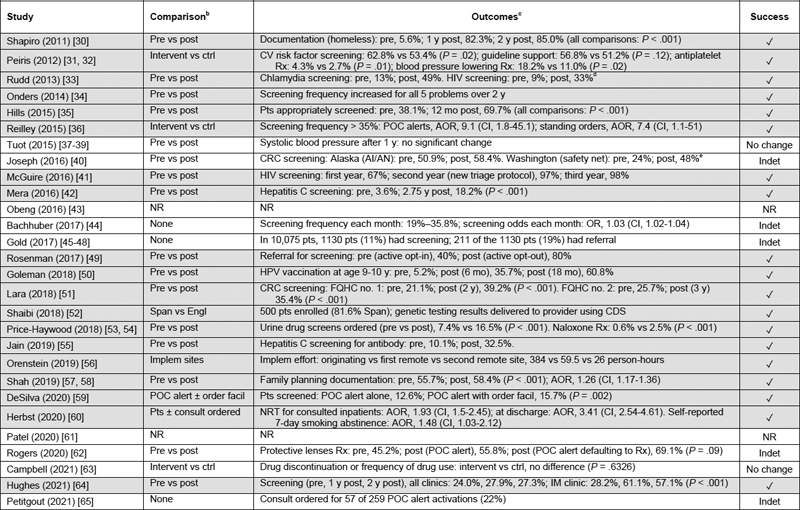
Characteristics of studies about clinical decision support and health care disparities: comparison and outcomes
Abbreviations : AI/AN, American Indian/Alaska Native; AOR, adjusted odds ratio; CI, 95% confidence interval; CDS, clinical decision support; CRC, colorectal cancer; CV, cardiovascular; Engl, English-speaking patients; Facil, facilitator; FQHC, Federally Qualified Health Center; HPV, human papillomavirus; IM, internal medicine; Implem, implementation; Indet, indeterminate; Intervent vs ctrl, intervention vs control clinics; NR, not reported; NRT, nicotine-replacement therapy; OR, odds ratio; POC, point of care; Post, after implementation; Pre, before implementation; Pt(s), patient(s); RR, risk ratio; Rx, prescription; Span, Spanish-speaking patients.
a N = 36 articles for 28 studies; 1 article per study except for 3 studies (Peiris; Price-Haywood; Shah) that had 2 articles each, 1 study (Tuot) with 3 articles, and 1 study (Gold) with 4 articles. For studies with > 1 article, year listed was the earliest year of the articles. Review limited to clinician-facing CDS tools. Outcomes not reported for 2 studies (Obeng; Patel). Rows with gray highlights indicate studies that had success reported.
b In the 26 studies that reported outcomes, study design: observational, 22 studies (85%) including 1 study (Herbst) that was observational and quasi-experimental; cluster randomized controlled trial, 4 studies (15%) (Peiris, Tuot, DeSilva, Campbell). Comparison was pre vs post in 16 studies (62%). Software lifecycle phase according to reported classification [ 28 ]: implementation, 3 studies (12%) (Shaibi, Orenstein, Petitgout); operation, 23 studies (88%).
c P values and CIs were included when reported.
d Rudd: percentages estimated from graphs in article.
e Joseph: significance could not be verified because reporting of population size was incomplete.
The use of user-centered design was reported in 18 of the 28 studies (64%), including 11 studies during the planning phase (39%), nine studies during the development phase (32%), and seven studies during the implementation phase (25%), with seven studies that reported the use of user-centered design in more than one study phase ( Table 5 ). The most frequently reported implementation strategies included education in 25 studies (89%), consensus facilitation in 14 studies (50%), and identification of champions, workflow changes, and audit and feedback each in 13 studies (46%) ( Table 5 ).
Table 5.
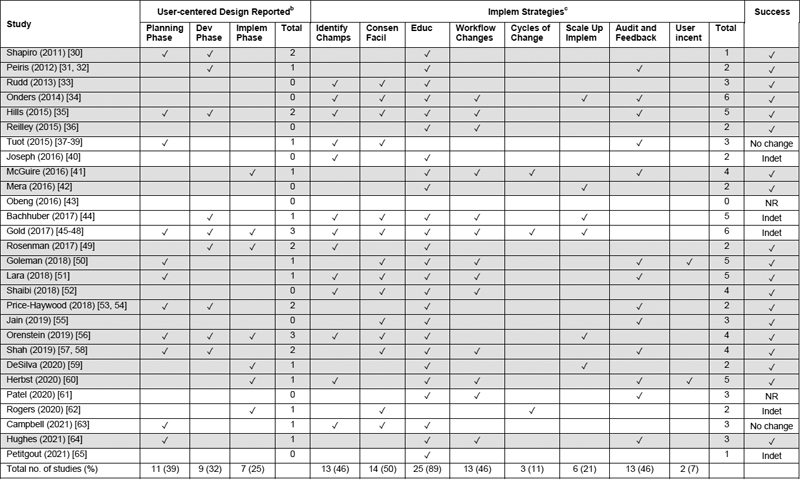
Clinical decision support life cycle phases with user-centered design and implementation strategies reported
Abbreviations : Abbreviations: Champs, champions; Consen, consensus; CDS, clinical decision support; Deploy, deployment; Dev, development; Educ, education; Facil, facilitation; Identify champs, identification of champions; Implem, implementation; Incent, incentive; Indet, indeterminate; NR, not reported, Scale up implem, scaling up of implementation.
a N = 36 articles for 28 studies; 1 article per study except for 3 studies (Peiris; Price-Haywood; Shah) that had 2 articles each, 1 study (Tuot) with 3 articles, and 1 study (Gold) with 4 articles. For studies with > 1 article, year listed was the earliest year of the articles. Review limited to clinician-facing CDS tools. Taxonomy for user-centered design phases and implementation strategies defined in Table 1 . Columns of totals are the total number of user-centered design phases or implementation strategies for each study. Rows with gray highlights indicate studies that had success reported ( Table 4 ).
b User-centered design: user-centered design was reported in any of the 3 CDS life cycle phases in 18 of the 28 studies (64%).
c Implementation strategies: any of the 8 implementation strategies were reported in 27 of the 28 studies (96%).
4 Discussion
The present review showed diverse interventions in the included studies, with varied complexity of CDS ranging from a single point-of-care alert to CDS having up to five distinct functions according to a standardized CDS taxonomy ( Table 3 ) [ 24 ]. Most studies reported success with CDS use in clinical screening, treatment, or achieving an informatics objective toward improving health equity ( Table 4 ). Despite the diversity of CDS complexity reported in the studies, the importance of previously described recommendations for successful CDS design and implementation was evident [ 15 , 28 , 66 , 67 ]. In a study that evaluated an HIV testing alert, the order facilitator and workflow support were ignored by emergency department providers despite education and audit and feedback until the CDS target was changed to the triage nurse, in support of the strategy of targeting the CDS to the “right person” and “right point in the workflow” [ 41 , 68 ]. In another study of CDS for HIV screening, the highest odds of HIV testing orders resulted when nurses were allowed to sign standing orders [ 36 ]. There were three studies using CDS for order facilitation that showed low use of CDS by providers and few reported user-centered design elements or implementation strategies [ 59 , 62 , 63 ].
Rigorous study design is needed to understand the value of CDS [ 15 ]. Only four studies reported cluster randomized designs [ 31 , 32 , 37 38 39 , 59 , 63 ], and there were no patient-randomized studies, underscoring the challenges of implementing controlled CDS trials in safety net settings associated with frequent leadership and staff turnover, limited EHR support, and added time burden on providers and staff with low staff-to-provider ratios [ 69 , 70 ]. The challenges of performing research in resource-limited settings were highlighted in a genomics study using CDS in which 12 months and extensive staff resources were required to enroll 500 study participants in a Federally Qualified Health Center; in contrast, in a non-safety net population, 2,500 participants were enrolled by eight months using mailings only [ 52 ].
The six IHS studies showed the capability of implementing CDS to promote change over large geographic areas with externally-available population-specific CDS and quality improvement methods [ 33 , 34 , 36 , 40 , 42 , 64 ]. Beginning in 2004, federally operated IHS facilities began implementing a single EHR, Resource and Patient Management System (RPMS), that was developed for IHS with end-user input and major contributions for shared core applications from the Department of Veterans Affairs legacy EHR, Veterans Health Information Systems and Technology Architecture (VistA) [ 71 , 72 ]. RPMS has been used across the United States by all facilities operated directly by IHS and most tribal and urban American Indian/Alaska Native health care programs [ 73 , 74 ]. IHS maintains a national library of electronic clinical reminders (ECR) that can be modified for local taxonomy and code [ 36 ]. With support from the Institute for Healthcare Improvement, IHS has sponsored a national improvement collaborative that has designed a 5-step implementation of the ECRs using quality improvement methods, including (1) establish clinical demand, (2) pilot test, (3) expand to all providers, (4) measure outcomes and share results, and (5) delegate clinical reminders to other staff [ 34 ]. The ECRs are all visible in a single front-end location to reduce end-user alert fatigue, and clinics have integrated clinical applications coordinators who are informaticists responsible for EHR function and end-user training. The improved outcomes reported in most of the IHS studies in this review, that were based on the RPMS EHR and ECR library toward improving public health and equity, underscore the potential benefits of externally-available CDS targeting traditionally underserved populations in helping to improve care for these vulnerable populations.
Another example of the success that can be achieved through externally-available CDS tailored to underserved populations was evident from the study by a nonprofit health center-controlled organization (OCHIN Inc., formerly known as Oregon Community Health Information Network Inc., Portland, OR) that receives funding from the Health Resources and Services Administration and develops externally-available CDS for use with an instance of a commercial EHR (Epic, Epic Systems Corp., Verona, WI) for more than 500 clinic sites across 20 states [ 48 , 75 76 77 ]. OCHIN creates custom CDS with user-centered design, including support for diverse population needs such as those for correctional facilities, school-based clinics, HIV management, or refugee health [ 78 , 79 ], and an implementation protocol for social-risk screening of more than 400,000 patients has been described with functionality to identify and facilitate referrals for at-risk patients [ 48 ]. Another study with externally-available CDS reported implementation in 51 clinical sites with a single build [ 36 ], and this model may represent an opportunity for efficiencies and cost savings that are important in safety net settings.
Safety net clinics that are not using externally-available CDS and a population optimized EHR may face barriers to customizing and maintaining CDS designed to decrease disparities. There were two studies that reported a change of EHR used during study implementation [ 30 , 49 ], and other authors noted that IHS tribal sites migrating to commercial EHR platforms may face increased expenses associated with customization compared with IHS sites using RPMS [ 36 ]. A feasible approach to enabling desired CDS regardless of EHR used could include the sharing of CDS resources designed for safety net health care systems or specific to improving equity in an interoperable manner. Interoperable CDS resources could be shared through a repository such as the CDS Connect repository of the Agency for Healthcare Research and Quality that was launched in 2016 [ 80 , 81 ]. In a study that reported dissemination of instructions about building a non-interoperable, multifaceted refugee health CDS to an external site via CDS Connect download, implementation required only 26 person-hours vs 384 person-hours for the original build, even though the information shared on CDS Connect was primarily a build guide [ 56 , 82 ].
Most of the articles reported interventions in outpatient primary care. Primary care is defined as first contact care for all health care needs and patient-focused, longitudinal, comprehensive, and coordinated care across settings [ 83 ]. Access to primary care decreases health disparities across racial and socioeconomic groups [ 84 ]. The burden of information required to provide this breadth of care is increased by layers of social complexity and higher frequency of chronic disease in safety net settings. To understand how to reduce this burden of information and complexity using CDS within safety net organizational capacities, reporting may be strengthened in several areas. In the 26 studies reporting outcomes, five studies (19%) were rated indeterminate because they did not include sufficient comparisons or reporting to confirm success. Resources to support data curation and analysis and guidelines to perform randomization in resource-limited settings are needed [ 69 ]. Although we extracted the user-centered design and implementation strategies reported in the included studies, it is unknown how many strategies were used but not reported. As user time typically is dedicated to the complex needs of patients in safety net settings, structured reporting of how user-centered design was achieved in resource-limited settings is needed. In addition, structured reporting of implementation strategies that are effective for local populations may provide guidance needed for generalization between safety net organizations [ 19 ].
Limitations of the present study included the use of only one database (PubMed) for the search to identify pertinent articles. Relevant articles may have been missed because of limitations in the selected search terms or overlooked because the review of titles and abstracts was divided between three coauthors. We did not consider studies before 2011 because of our purpose of evaluating the current state of EHR-interoperable CDS in decreasing health disparities. Furthermore, user-centered design and implementation science techniques may have been used but not reported.
5 Conclusion
CDS tools may be effective in improving health equity and outcomes for patients who face disparities. The present review underscores the need for high-quality analyses of CDS-associated health outcomes, reporting of user-centered design and implementation strategies used in low-resource settings, and methods to disseminate CDS created to improve health equity.
Acknowledgments
The authors thank Jonathan Iralu, MD for information about the EHR used in the Gallup Indian Medical Center study, Jorge Mera, MD for information about the EHR used in the Cherokee Nation Health Services study, and Shawn Steidinger (University of Utah Eccles Health Sciences Library) for assistance with the database query. Outside of the submitted work, KK reports honoraria, consulting, sponsored research, licensing, or co-development in the past year with Hitachi, Pfizer, RTI International, NORC at the University of Chicago, the University of California at San Francisco, Indiana University, MD Aware, the Korean Society of Medical Informatics, and the U.S. Office of the National Coordinator for Health IT (via Security Risk Solutions) in the area of health information technology. KK was also an unpaid board member of the non-profit Health Level Seven International health IT standard development organization, he is an unpaid member of the U.S. Health Information Technology Advisory Committee, and he has helped develop a number of health IT tools which have been or may be commercialized to enable wider impact. None of these relationships have direct relevance to the manuscript but are reported in the interest of full disclosure.
Supplementary Material
References
- 1.Whitehead M, Dahlgren G. Concepts and Principles for Tackling Social Inequities in Health: Levelling up Part 1. Copenhagen: World Health Organization; 2006.
- 2.Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health 2006;27:167-94. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed]
- 3.Braveman P. What are health disparities and health equity? We need to be clear. Public Health Rep 2014 Jan-Feb;129(Suppl 2):5-8. [DOI] [PMC free article] [PubMed]
- 4.
- 5.
- 6.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA 2016 Jun 7;315(21):2300-11. [DOI] [PMC free article] [PubMed]
- 7.Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019 Oct 25;366(6464):447-53. [DOI] [PubMed]
- 8.Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight - Reconsidering the Use of Race Correction in Clinical Algorithms. N Engl J Med 2020 Aug 27;383(9):874-82. [DOI] [PubMed]
- 9.Feldman S, Ammar W, Lo K, Trepman E, van Zuylen M, Etzioni O. Quantifying Sex Bias in Clinical Studies at Scale With Automated Data Extraction. JAMA Netw Open 2019 Jul 3;2(7):e196700. [DOI] [PMC free article] [PubMed]
- 10.
- 11.
- 12.Lewin ME, Altman S, editors. America’s Health Care Safety Net: Intact but Endangered. Washington DC: National Academy Press; 2000. [PubMed]
- 13.Lewis VA, Spivack S, Murray GF, Rodriguez HP. FQHC Designation and Safety Net Patient Revenue Associated with Primary Care Practice Capabilities for Access and Quality. J Gen Intern Med 2021 Oct;36(10):2922-8. [DOI] [PMC free article] [PubMed]
- 14.Panattoni L, Stults CD, Chan AS, Tai-Seale M. The human resource costs of implementing autopend clinical decision support to improve health maintenance. Am J Manag Care 2020 Jul 1;26(7):e232-e236. [DOI] [PubMed]
- 15.Kawamoto K, McDonald CJ. Designing, Conducting, and Reporting Clinical Decision Support Studies: Recommendations and Call to Action. Ann Intern Med 2020 Jun 2;172(11 Suppl):S101-S109. [DOI] [PubMed]
- 16.
- 17.Yapa HM, Bärnighausen T. Implementation science in resource-poor countries and communities. Implement Sci 2018 Dec 27;13(1):154. [DOI] [PMC free article] [PubMed]
- 18.Corsini L, Aranda-Jan CB, Henderson C, Moultrie J. Recommendations for participatory design in low-resource settings: a case study of Simprints. Academy for Design Innovation Management Conference, London, UK, June 19-21, 2019. Conference Proceedings of the Academy for Design Innovation Management 2019;951-61.
- 19.
- 20.NORC. Understanding the impact of health IT in underserved communities and those with health disparities. HealthIT.gov [Internet]. October 29, 2010. Available from: https://www.healthit.gov/sites/default/files/pdf/hit-underserved-communities-health-disparities.pdf [cited 2021 November 15].
- 21.
- 22.Choi W, Wang S, Lee Y, Oh H, Zheng Z. A systematic review of mobile health technologies to support self-management of concurrent diabetes and hypertension. J Am Med Inform Assoc 2020 Jun 1;27(6):939-45. [DOI] [PMC free article] [PubMed]
- 23.Chattopadhyay D, Ma T, Sharifi H, Martyn-Nemeth P. Computer-Controlled Virtual Humans in Patient-Facing Systems: Systematic Review and Meta-Analysis. J Med Internet Res 2020 Jul 30;22(7):e18839. [DOI] [PMC free article] [PubMed]
- 24.
- 25.Serving Vulnerable and Underserved Populations. Centers for Medicare & Medicaid Services Web site. Available from: https://marketplace.cms.gov/technical-assistance-resources/training-materials/vulnerable-and-underserved-populations.pdf [cited 2022 March 28].
- 26.Thiese MS. Observational and interventional study design types; an overview. Biochem Med (Zagreb) 2014;24(2):199-210. [DOI] [PMC free article] [PubMed]
- 27.Bland JM. Cluster randomised trials in the medical literature: two bibliometric surveys. BMC Med Res Methodol 2004 Aug 13;4:21. [DOI] [PMC free article] [PubMed]
- 28.
- 29.
- 30.Shapiro A, Gracy D, Quinones W, Applebaum J, Sarmiento A. Putting guidelines into practice: improving documentation of pediatric asthma management using a decision-making tool. Arch Pediatr Adolesc Med 2011 May;165(5):412-8. [DOI] [PubMed]
- 31.
- 32.
- 33.Rudd S, Gemelas J, Reilley B, Leston J, Tulloch S. Integrating clinical decision support to increase HIV and chlamydia screening. Prev Med 2013 Dec;57(6):908-9. [DOI] [PubMed]
- 34.Onders R, Spillane J, Reilley B, Leston J. Use of electronic clinical reminders to increase preventive screenings in a primary care setting: blueprint from a successful process in Kodiak, Alaska. J Prim Care Community Health 2014 Jan 1;5(1):50-4. [DOI] [PubMed]
- 35.Hills RL, Kulbok PA, Clark M. Evaluating a Quality Improvement Program for Cervical Cancer Screening at an Urban Safety Net Clinic. Health Promot Pract 2015 Sep;16(5):631-41. [DOI] [PubMed]
- 36.Reilley B, Leston J, Tulloch S, Neel L, Galope M, Taylor M. Implementation of National HIV Screening Recommendations in the Indian Health Service. J Int Assoc Provid AIDS Care 2015 Jul-Aug;14(4):291-4. [DOI] [PMC free article] [PubMed]
- 37.
- 38.
- 39.
- 40.Joseph DA, Redwood D, DeGroff A, Butler EL. Use of Evidence-Based Interventions to Address Disparities in Colorectal Cancer Screening. MMWR Suppl 2016 Feb 12;65(1):21-8. [DOI] [PubMed]
- 41.McGuire R, Moore E. Using a configurable EMR and decision support tools to promote process integration for routine HIV screening in the emergency department. J Am Med Inform Assoc 2016 Mar;23(2):396-401. [DOI] [PubMed]
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- 48.
- 49.Rosenman MB, Decker B, Levy KD, Holmes AM, Pratt VM, Eadon MT. Lessons Learned When Introducing Pharmacogenomic Panel Testing into Clinical Practice. Value Health 2017 Jan;20(1):54-9. [DOI] [PMC free article] [PubMed]
- 50.Goleman MJ, Dolce M, Morack J. Quality Improvement Initiative to Improve Human Papillomavirus Vaccine Initiation at 9 Years of Age. Acad Pediatr 2018 Sep-Oct;18(7):769-75. [DOI] [PubMed]
- 51.
- 52.
- 53.Price-Haywood EG, Robinson W, Harden-Barrios J, Burton J, Burstain T. Intelligent Clinical Decision Support to Improve Safe Opioid Management of Chronic Noncancer Pain in Primary Care. Ochsner J 2018 Spring;18(1):30-5. [PMC free article] [PubMed]
- 54.
- 55.
- 56.
- 57.Shah SD, Prine L, Waltermaurer E, Rubin SE. Feasibility study of family planning services screening as clinical decision support at an urban Federally Qualified Health Center network. Contraception 2019 Jan;99(1):27-31. [DOI] [PubMed]
- 58.Srinivasulu S, Shah SD, Schechter CB, Prine L, Rubin SE. Effectiveness of clinical decision support to enhance delivery of family planning services in primary care settings. Contraception 2020 Mar;101(3):199-204. [DOI] [PubMed]
- 59.DeSilva MB, Kodet A, Walker PF. A Best Practice Alert for Identifying Hepatitis B-Infected Patients. Am J Trop Med Hyg 2020 Aug;103(2):884-6. [DOI] [PMC free article] [PubMed]
- 60.
- 61.
- 62.Rogers KA, Wilson AA, Wyckoff MA, Siatkowski RM. Computer-Based Quality Improvement for Management of Functionally Monocular Patients. Ophthalmology 2020 Jan;127(1):138-9. [DOI] [PubMed]
- 63.
- 64.Hughes MS, Apostolou A, Reilley B, Leston J, McCollum J, Iralu J. Electronic Health Record Reminders for Chlamydia Screening in an American Indian Population. Public Health Rep 2021 May;136(3):320-6. [DOI] [PMC free article] [PubMed]
- 65.Petitgout JM, Werner J, Stewart S. Pediatric Complexity Tool Best Practice Alert: Early Identification of Care Coordination for Children with Special Health Care Needs. J Pediatr Health Care 2021 Sep-Oct;35(5):485-90. [DOI] [PubMed]
- 66.
- 67.
- 68.Sirajuddin AM, Osheroff JA, Sittig DF, Chuo J, Velasco F, Collins DA. Implementation pearls from a new guidebook on improving medication use and outcomes with clinical decision support. Effective CDS is essential for addressing healthcare performance improvement imperatives. J Healthc Inf Manag 2009 Fall;23(4):38-45. [PMC free article] [PubMed]
- 69.Coronado GD, Schneider JL, Petrik A, Rivelli J, Taplin S, Green BB. Implementation successes and challenges in participating in a pragmatic study to improve colon cancer screening: perspectives of health center leaders. Transl Behav Med 2017 Sep;7(3):557-66. [DOI] [PMC free article] [PubMed]
- 70.Coronado GD, Retecki S, Schneider J, Taplin SH, Burdick T, Green BB. Recruiting community health centers into pragmatic research: Findings from STOP CRC. Clin Trials 2016 Apr;13(2):214-22. [DOI] [PMC free article] [PubMed]
- 71.About VistA. WorldVistA.org [Internet]. Available from: https://worldvista.org/AboutVistA [cited 2021 November 19].
- 72.Other VistA Adopters. WorldVistA.org [Internet]. Available from: https://worldvista.org/AboutVistA/copy_of_index_html [cited 2021 November 19].
- 73.Kruse GR, Hays H, Orav EJ, Palan M, Sequist TD. Meaningful Use of the Indian Health Service Electronic Health Record. Health Serv Res 2017 Aug;52(4):1349-63. [DOI] [PMC free article] [PubMed]
- 74.Indian Health Service Health IT Modernization Project. Strategic Options for the Modernization of the Indian Health Service Electronic Health Record. U.S. Department of Health & Human Services [Internet]. June 6, 2019. Available from: https://www.hhs.gov/sites/default/files/ihs-health-it-mod-analysis-of-alternatives.pdf [cited 2021 November 19].
- 75.OCHIN. National impact: nationwide reach. OCHIN [Internet]. Available from: https://ochin.org/national-impact. [cited 2021 November 19].
- 76.
- 77.Raths D. New funding makes OCHIN largest health center controlled network. Healthcare Innovation [Internet]. July 29, 2019. Available from: https://www.hcinnovationgroup.com/clinical-it/electronic-health-record-electronic-medical-record-ehr-emr/news/21090392/new-funding-makes-ochin-largest-health-center-controlled-network [cited 2021 November 19].
- 78.Devoe JE, Sears A. The OCHIN community information network: bringing together community health centers, information technology, and data to support a patient-centered medical village. J Am Board Fam Med 2013 May-Jun;26(3):271-8. [DOI] [PMC free article] [PubMed]
- 79.OCHIN. OCHIN hosted Epic electronic health record. OCHIN [Internet]. 2019. Available from: https://static1.squarespace.com/static/5ade0eb85cfd79247926399a/t/5d9b939522e5867cf037471d/1570476949935/Hosted+Epic+EHR+Two-Pager+190531.pdf [cited 2021 November 19].
- 80.Agency for Healthcare Research and Quality. Welcome to CDS Connect. AHRQ Web site. Available from: https://cds.ahrq.gov/cdsconnect [cited 2021 November 19].
- 81.Lomotan EA, Meadows G, Michaels M, Michel JJ, Miller K. To Share is Human! Advancing Evidence into Practice through a National Repository of Interoperable Clinical Decision Support. Appl Clin Inform 2020 Jan;11(1):112-21. [DOI] [PMC free article] [PubMed]
- 82.
- 83.Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary Care: America’s Health in a New Era. Washington DC: Institute of Medicine, National Academy Press; 1996. [PubMed]
- 84.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83(3):457-502. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


