Abstract
Objectives:
Neoadjuvant therapy prior to surgical resection for locally advanced lung cancer has evolved to incorporate systemic cytotoxic chemotherapy +/− immunotherapy +/− radiotherapy. The role of neoadjuvant precision therapies remains understudied.
Materials and Methods:
We report cases with major and complete pathologic responses to off-label neoadjuvant alectinib.
Results:
A case with stage IIIA (cT1b cN2 cM0) EML4-ALK variant 3a/b lung adenocarcinoma received 6 weeks of alectinib followed by R0 left upper lobectomy with complete pathological response (ypT0 ypN0). Another case with stage IIIA (cT3 cN2 cM0) EML4-ALK variant 2 received 12 weeks of alectinib followed by R0 right middle lobectomy with a major pathologic response (ypT1a ypN0) but systemic recurrence 12 months post-operatively.
Conclusion:
Ongoing clinical trials are evaluating the role of both neoadjuvant and adjuvant ALK-directed therapy. Our cases support the completion of ongoing trials (ALINA: NCT03456076 and ALNEO: NCT05015010), and highlight the ability of second generation ALK inhibitors to induce major and complete pathologic responses in the neoadjuvant setting plus the likely role of long-term adjuvant kinase inhibitor therapy to prevent radiographic/clinical recurrence.
Keywords: Lung cancer, ALK, Neoadjuvant, Alectinib, Complete pathologic response
1. Introduction:
Neoadjuvant therapy prior to microscopically margin-negative (R0) resection for locally advanced lung cancer has evolved to incorporate systemic cytotoxic chemotherapy +/− immunotherapy +/− radiotherapy. With those anti-neoplastic interventions, both mediastinal node clearance and complete pathologic response have emerged as potent prognostic determinants of long-term clinical benefit plus cure rates [1,2]. However, the role of neoadjuvant precision therapies and the value of tumor regression when a kinase inhibitor is used remain understudied.
2. Material and methods
We report cases with major and complete pathologic response to off-label neoadjuvant alectinib. Data on clinical, radiographic, and survival outcomes used to report these cases were obtained from an ongoing institutional review board-approved protocol at our institution.
3. Results/case presentations
A 61-year-old with a prior 5 pack-year history of smoking presented with asymptomatic lingular lung adenocarcinoma and biopsy-proven ipsilateral (4L) nodal invasion without additional sites of active disease (clinical-radiographic-bronchoscopic stage IIIA; 8th American Joint Committee on Cancer [AJCC]: cT1b cN2 cM0). Tumor genotype (FoundationOne CDx) disclosed ALK rearrangement with EML4-ALK fusion (variant 3a/b), NF1-R1534* and ARID1A-Q1346*mutations. Alectinib 600 mg twice daily was provided for 6 weeks before a R0 robotic-assisted thoracoscopic left upper lobectomy and mediastinal lymph node dissection. Alectinib was held 4 days before surgery. The specimen showed a complete pathological response (ypT0 ypN0, Fig. 1). The patient was recommended additional 2 years of alectinib post-operatively and is currently receiving therapy without recurrence (3-month mark).
Fig. 1.
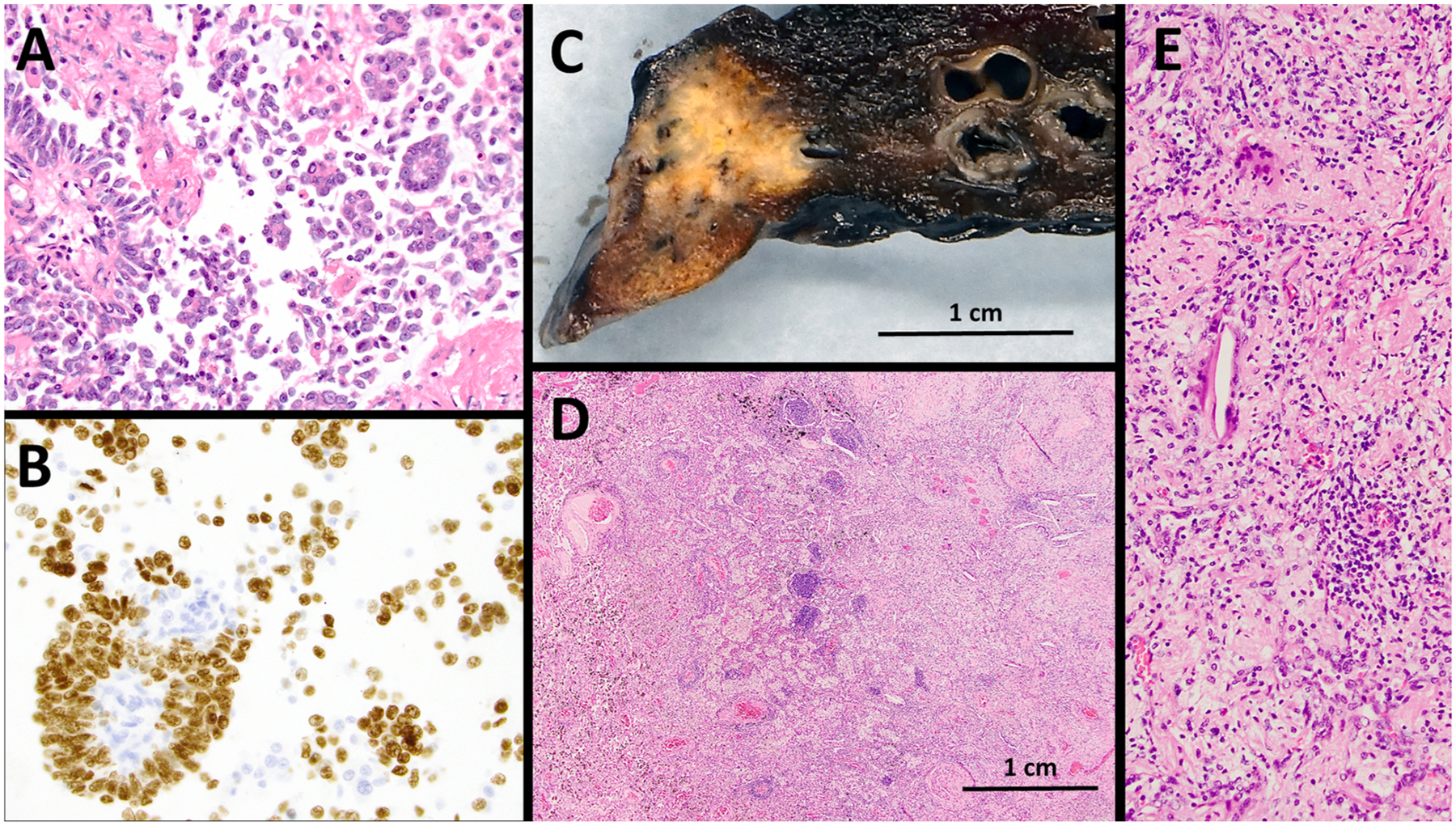
Complete pathologic response to neoadjuvant alectinib. A. Trans-bronchial needle aspirate (TBNA) from left upper lobe lung mass showing lung adenocarcinoma with papillary and micropapillary architecture. B. Tumor cells positive by immunohistochemistry for TTF-1 and Napsin (not shown) (cell block, 400x). C. Surgical resection showing 1.1 cm partially necrotic nodule. D and E. Histologic examination demonstrating extensive stromal fibrosis with associated chronic inflammation and histiocytic reaction with no residual viable adenocarcinoma (40x and 200x, respectively).
In a separate case, a 65-year-old with a prior 20 pack-year smoking history and an asymptomatic stage IIIA right middle lobe lung adenocarcinoma (cT3 cN2 c M0) harboring EML4-ALK fusion (variant 2, FoundationOne CDx) was treated with alectinib (initially at 600 mg twice daily but dose reduced to 450 mg due to elevated transaminases) for 12 weeks before a R0 right middle lobectomy. Alectinib was held 6 days before surgery. The specimen showed a major pathologic response (ypT1a ypN0, Fig. 2) and the patient did not receive adjuvant therapy. One year after surgical resection, imaging studies disclosed metastatic adenocarcinoma recurrence (intra-thoracic and extra-thoracic lymph nodes) with confirmation of the EML4-ALK rearrangement without ALK kinase domain mutations; and re-initiation of next generation ALK inhibitor (such as alectinib) was recommended at time of report.
Fig. 2.

Major pathologic response to neoadjuvant alectinib. A. TBNA from level 4R lymph node demonstrating metastatic poorly differentiated lung adenocarcinoma. B. Tumor cells positive by immunohistochemistry for both TTF-1 and Napsin (not shown) (cell block, 400x). C. Surgical resection showing a solid 2.2 cm fibrotic mass. D. Histologic evaluation demonstrated extensive fibroelastotic scaring with minimal chronic inflammation but only scant nests (comprising less than 1 % of the tumor volume) of viable adenocarcinoma (20x with 400x callout).
Pathologic response of ALK rearranged lung cancer to other neoadjuvant therapies (chemotherapy, immunotherapy, radiotherapy) is seldom reported and rates of major pathologic response are uncertain. In addition, cases with ALK rearrangement have been excluded from the registration clinical trials of chemo-immunotherapy [2]. In our own institution, neoadjuvant chemoradiotherapy has anecdotally produced disappointing responses in ALK rearranged lung cancer without pathologic response (data not shown). A case with stage IIIA (cT1b cN2 cM0) ALK FISH positive non-small-cell lung cancer received neoadjuvant cisplatin/etoposide and 5400 cGy radiotherapy but, at time of surgery, was found to have M1a (pleural and pericardial) disease. Another patient with stage IIIA (cT2a cN2b cM0) EML4-ALK fusion (variant 5) positive lung adenocarcinoma received neoadjuvant carboplatin/pemetrexed and 5400 cGy radiotherapy but the R0 robotic-assisted right upper lobectomy showed lack of significant response (ypT1a ypN2b).
4. Discussion
Few prior reports have described neoadjuvant targeted therapies for ALK rearranged lung cancer. In single cases, major pathologic responses to neoadjuvant alectinib have been described but our literature review did not identify a prior case with complete pathologic response [3,4]. Ongoing clinical trials are evaluating the role of both neoadjuvant and adjuvant ALK-directed therapy for ALK rearranged lung cancer.
The ALINA trial (n = 255, ClinicalTrials.gov Identifier: NCT03456076) is comparing adjuvant alectinib (600 mg twice daily for 24 months) to standard platinum-doublet chemotherapy in stage IB (tumors ≥ 4 cm, 7th AJCC) to IIIA resected ALK rearranged lung cancer; with primary outcome of disease-free survival and secondary endpoint of overall survival.
The ALNEO trial (n = 33, NCT05015010) is evaluating alectinib 600 mg twice daily for 8 weeks in the neoadjuvant phase of potentially resectable stage III (any cT cN2 or cT4 cN0–1, 8th AJCC) tumor burden, followed by surgical resection and 96 weeks of adjuvant alectinib at same doses with primary outcome of major pathologic response (≤10 % residual viable tumor cells) and secondary endpoints of event-free and overall survival [5].
These trials will likely provide more robust evidence-based data to define the role of neoadjuvant and adjuvant alectinib, as well as determine the prognostic value of major and complete pathologic responses to ALK-directed therapy.
5. Conclusion
Our cases support the aforementioned phase II and III studies, and highlight the ability of precision therapies to induce major and complete pathologic responses in the neoadjuvant setting plus the likely role of long-term adjuvant suppressive kinase inhibitor therapy to prevent radiographic/clinical recurrence.
Declaration of Competing Interest
Dr. Costa reports receiving consulting fees and honoraria from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, Blueprint Medicines, and Janssen, institutional research support from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, Merck Sharp and Dohme, Merrimack Pharmaceuticals, Bristol Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals, Tesaro and Daiichi Sankyo, and consulting fees from Teladoc and Grand Rounds by Included Health. Dr. Rangachari reports receiving consulting fees and honoraria from AstraZeneca, institutional research support from Bristol Myers Squibb, Novocure, and AbbVie/Stemcentrx, and consulting fees from Teladoc and DynaMed. Dr. VanderLaan reports receiving consulting fees from Gala Therapeutics, Galvanize Therapeutics, and Ruby Robotics. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations:
- ALK, ALK
receptor tyrosine kinase or anaplastic lymphoma kinase
- EML4
Echinoderm microtubule associated protein like 4
- c
Clinical assessment data
- p
Pathological data
- yp
Pathological data following systemic or radiation therapy be it prior to surgery or as a primary treatment
- TBNA
Transbronchial needle aspiration
References
- [1].Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara DR, Fry WA, Darling G, Johnson DH, Green MR, Miller RC, Ley J, Sause WT, Cox JD, Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009. Aug 1;374(9687):379–86. doi: 10.1016/S0140-6736(09)60737-6. Epub 2009 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, Kerr K, Wang C, Ciuleanu TE, Saylors GB, Tanaka F, Ito H, Chen KN, Liberman M, Vokes EE, Taube JM, Dorange C, Cai J, Fiore J, Jarkowski A, Balli D, Sausen M, Pandya D, Calvet CY, Girard N, CheckMate 816 Investigators., Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer, N Engl J Med. 386 (21) (2022. May 26) 1973–1985, 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gu R, Shi Z, Duan T, Song M, Feasibility and Safety of Neoadjuvant Alectinib in Pulmonary Invasive Mucinous Adenocarcinoma with ALK Rearrangement: Case Report and Literature Review, Onco Targets Ther. 21 (14) (2021. Oct) 5107–5113, 10.2147/OTT.S334213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yue P, Zhang S, Zhou L, Xiang J, Zhao S, Chen X, Dong L, Yang W, Xiang Y, Perioperative alectinib in a patient with locally advanced anaplastic lymphoma kinase positive non-small cell lung cancer (NSCLC): a case report, Transl Cancer Res. 10 (8) (2021. Aug) 3856–3863, 10.21037/tcr-21-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Leonetti A, Minari R, Boni L, Gnetti L, Verzè M, Ventura L, Musini L, Tognetto M, Tiseo M, Phase II, Open-label, Single-arm, Multicenter Study to Assess the Activity and Safety of Alectinib as Neoadjuvant Treatment in Surgically Resectable Stage III ALK-positive NSCLC: ALNEO Trial, Clin. Lung Cancer 22 (5) (2021. Sep) 473–477, 10.1016/j.cllc.2021.02.014. Epub 2021 Feb 24. [DOI] [PubMed] [Google Scholar]


