Abstract
Aim
Prostate cancer (PCa) is the second most common nonskin malignancy and the second most common cause of cancer-related deaths in men. The most common site of metastasis in PCa is the axial skeleton which may lead to back pain or pathological fractures. Hematogenous spread to the brain and involvement of the central nervous system (CNS) are a rare occurrence. However, failed androgen deprivation therapy (ADT) may facilitate such a spread resulting in an advanced metastatic stage of PCa, which carries a poor prognosis.
Methods
In this systematic review, we searched the PubMed, Scopus, and Web of Science online databases based on the PRISMA guideline and used all the medical subject headings (MeSH) in terms of the following search line: (“Brain Neoplasms” OR “Central Nervous System Neoplasms”) and (“Prostatic Neoplasms” OR “Prostate”). Related studies were identified and reviewed.
Results
A total of 59 eligible studies (902 patients) were included in this systematic review. In order to gain a deeper understanding, we extracted and presented the data from included articles based on clinical manifestations, diagnostic methods, therapeutic approaches, and prognostic status of PCa patients having BMs.
Conclusion
We have demonstrated the current knowledge regarding the mechanism, clinical manifestations, diagnostic methods, therapeutic approaches, and prognosis of BMs in PCa. These data shed more light on the way to help clinicians and physicians to understand, diagnose, and manage BMs in PCa patients better.
1. Introduction
Prostate cancer (PCa) is the second most common nonskin malignancy and the second most common cause of cancer-related deaths in men [1–3]. PCa is a clinically heterogeneous cancer that develops and progresses through various stages. These can range from initial prostatic intraepithelial neoplasia to metastatic disease, as well as hormone-refractory disease [1, 3]. It is demonstrated that several environmental and genetic risk factors such as age, genetic mutations, race/ethnicity, family history, lifestyle, and diet can strongly impact the progression of PCa [1, 4, 5]. PCa can often be asymptomatic, however, the most common signs and symptoms are difficulty in micturition, straining to start, increased frequency, and nocturia [2]. Surgery and radiotherapy are the established standard treatments of PCa. However, patients in whom such treatments prove unsuccessful are mainly treated with androgen deprivation therapy (ADT), which works by shrinking androgen-dependent tumors. A possible consequence of failed ADT is the subsequent development of recurrent androgen-independent PCa, with brain metastases (BMs) and reduced cognitive functions [3, 6, 7].
The most common site of metastasis in PCa is the axial skeleton which may lead to back pain or pathological fractures [2]. Hematogenous spread to the brain and involvement of the central nervous system (CNS) are a rare occurrence. However, failed ADT may facilitate such a spread resulting in an advanced metastatic stage of PCa, which carries a poor prognosis. This occurs mostly in intracranial sites such as the leptomeninges, cerebrum, and cerebellum, with many of the nonfocal neurologic symptoms being attributed to intracranial hypertension [6, 8–13]. It has been demonstrated that patients with nonadenocarcinoma PCa have a higher chance of BMs [10]. Recently, there has been a trend showing an increase in the number of PCa cases with a reported metastatic brain lesion. This brings to light the numerous challenges we face in terms of properly understanding, diagnosing, and managing PCa patients with BMs [14–33]. Given the lack of knowledge regarding the issue of metastatic brain lesions in PCa, we decided to comprehensively and systematically review the latest evidence regarding the clinical manifestations, diagnosis, treatment, and prognosis of the BMs in PCa.
2. Methods
2.1. Search Strategy
This systematic review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [34]. Two researchers (S. M and A. AJ) independently searched the PubMed, Scopus, and Web of Science online databases using all the medical subject headings (MeSH) in terms of the following search line: (“Brain Neoplasms” OR “Central Nervous System Neoplasms”) and (“Prostatic Neoplasms” OR “Prostate”) until November 7th, 2021, in the title and abstract and without any date or language restrictions. The intention of this systematic search was to search and find studies reporting clinical manifestations, diagnostic approaches, treatments, and prognosis of BMs in PCa patients. Moreover, to find any studies which are not resulted through the online searches, we performed a manual hand-searching process to identify and include any further relevant studies.
2.2. Study Selection
As described in Figure 1, all the records resulting from the systematic search underwent a screening assessment separately by two independent researchers (S. M and A. AJ). Subsequent to removing the duplicate records, the researchers assessed the articles through screening by title and abstract, and sequentially in the final stage, they reassessed the studies using full-text screening. At this stage, we excluded studies meeting our exclusion criteria such as clinical trials, letters, reviews, animal studies, in vitro studies, articles without any useful data, studies reporting other metastases from PCa, and studies reporting BMs originated from other cancers. In case of any discrepancies and conflicts about studies, they were resolved by discussion with the third researcher (A. S). In the end, 59 eligible articles met the inclusion criteria, and therefore, they were included in this systematic review. The PRISMA process of this study is presented in detail in Figure 1.
Figure 1.
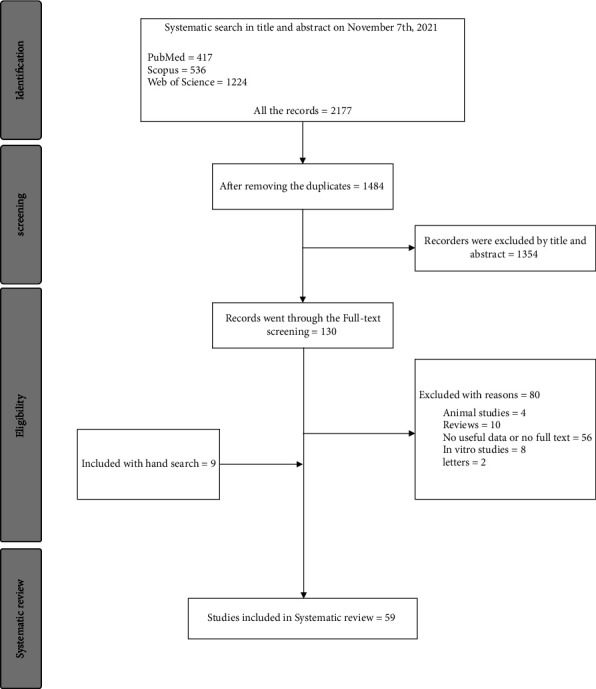
The PRISMA flow diagram of study selection.
2.3. Exclusion and Inclusion Criteria
Our main inclusion criteria included observational studies such as cohort studies, cross-sectional studies, and case series, and case report studies that reported diagnostic approaches, clinical manifestations, available treatments, and prognosis of BMs in PCa patients.
Our exclusion criteria included (1) articles reporting BMs originated from other cancers, (2) articles reporting other organ metastases from PCa instead of the brain, and (3) clinical trials, letters, reviews, animal studies, in vitro studies, and articles without any useful data or without any available full text.
2.4. Quality Assessment
As shown in Table 1, we assessed the methodological quality of all included studies independently by two researchers (S. M and A. AJ) using the NIH quality assessment tool for observational studies [35].
Table 1.
The demographic information of all included studies reporting BMs in PCa.
| Author | Year of publication | Type of studies | Total sample size | Quality assessment (good, fair, or poor) | Reference |
|---|---|---|---|---|---|
| Sena et al. | 2021 | Case report | 1 | Good | [36] |
| Pikis et al. | 2021 | Case series | 46 | Good | [37] |
| Parihar et al. | 2021 | Case report | 1 | Good | [38] |
| Hung et al. | 2021 | Retrospective study | 204 | Good | [39] |
| Boxley et al. | 2021 | Retrospective study | 29 | Fair | [40] |
| Son et al. | 2020 | Case report | 1 | Fair | [30] |
| Shida et al. | 2020 | Case report | 3 | Good | [29] |
| Saadatpour et al. | 2020 | Case report | 1 | Fair | [41] |
| Ross et al. | 2020 | Case report | 1 | Good | [28] |
| Nguyen et al. | 2020 | Retrospective cohort | 21 | Fair | [42] |
| Marchand Crety et al. | 2020 | Case report | 1 | Good | [27] |
| Liu et al. | 2020 | Case report | 1 | Good | [26] |
| Janda et al. | 2020 | Case report | 1 | Good | [24] |
| Bhambhvani et al. | 2020 | Retrospective cohort | 31 | Good | [13] |
| Aljarba et al. | 2020 | Case report | 1 | Good | [32] |
| Ahmad et al. | 2020 | Case report | 1 | Good | [43] |
| Kosaka et al. | 2019 | Case report | 1 | Fair | [23] |
| Kanyilmaz et al. | 2019 | Retrospective cohort | 339 | Good | [44] |
| Ishizaki et al. | 2019 | Case report | 1 | Good | [22] |
| Hogan et al. | 2019 | Case report | 1 | Good | [45] |
| Zanatta et al. | 2018 | Case report | 1 | Good | [46] |
| Reinas et al. | 2018 | Case report | 1 | Fair | [47] |
| Nunno et al. | 2018 | Case report | 1 | Fair | [48] |
| Jack et al. | 2018 | Case report | 1 | Good | [49] |
| Campagna et al. | 2018 | Case report | 1 | Fair | [21] |
| Watanabe et al. | 2017 | Case report | 1 | Good | [20] |
| Lam et al. | 2017 | Case report | 2 | Fair | [19] |
| Chang et al. | 2017 | Case report | 1 | Good | [18] |
| Guraya et al. | 2016 | Case report | 1 | Good | [50] |
| Barakat et al. | 2016 | Case report | 1 | Good | [17] |
| Mandaliya et al. | 2015 | Case report | 2 | Good | [51] |
| Hutton et al. | 2015 | Case report | 1 | Fair | [16] |
| Gajewska et al. | 2015 | Case report | 1 | Fair | [15] |
| Craig et al. | 2015 | Case report | 1 | Good | [14] |
| Hatzoglou et al. | 2014 | Retrospective cohort | 21 | Good | [10] |
| Placido et al. | 2014 | Case report | 3 | Fair | [52] |
| Hazell et al. | 2013 | Case report | 4 | Good | [53] |
| Caffo et al. | 2013 | Cohort | 9 | Fair | [54] |
| Caffo et al. | 2012 | Case series | 22 | Good | [55] |
| Flannery et al. | 2010 | Case series | 10 | Good | [56] |
| Yamada et al. | 2009 | Case report | 1 | Good | [57] |
| Sweets et al. | 2009 | Case report | 1 | Good | [58] |
| Kim et al. | 2008 | Case series | 5 | Good | [59] |
| Grenader et al. | 2007 | Case report | 1 | Good | [60] |
| Lyons et al. | 2006 | Case report | 1 | Good | [61] |
| Wullich et al. | 2004 | Case report | 2 | Fair | [62] |
| Schoenwaelder et al. | 2004 | Case report | 1 | Good | [63] |
| Tremont-Lukats et al. | 2003 | Case series | 103 | Good | [9] |
| Erasmus et al. | 2002 | Case report | 1 | Good | [64] |
| Minami, H. | 2001 | Case report | 1 | Fair | [65] |
| Behrens et al. | 2001 | Case report | 1 | Good | [66] |
| Garcia-Morales et al. | 2000 | Case report | 1 | Good | [67] |
| Fervenza et al. | 2000 | Case report | 1 | Good | [68] |
| Hayashi et al. | 1998 | Case report | 1 | Fair | [69] |
| Zhang et al. | 1997 | Case report | 1 | Fair | [70] |
| Leibman et al. | 1995 | Case report | 2 | Good | [71] |
| Bland et al. | 1992 | Case report | 1 | Good | [72] |
| Lynes et al. | 1986 | Case report | 2 | Good | [73] |
| Sarma et al. | 1983 | Case report | 4 | Fair | [74] |
2.5. Data Extraction
Two independent researchers (S. M and A. AJ) extracted all the intended data from the final eligible articles, and in terms of any disagreement, they consulted with the third researcher (A. S). For each included study, the authors' names, publication year, type of study, total sample size, type of PCa, all the reported clinical manifestations, diagnostic methods, treatments, and prognosis status.
3. Results
3.1. Demographic Information
Following the completion of the study selection process, as shown in Figure 1, a total of 59 eligible studies [9, 10, 13–24, 26–30, 32, 36–74] (902 PCa patients with brain BMs) were included in this systematic review. Details regarding the included studies such as the year of publication, type of the study (case report or case series), sample size, and the quality assessment are presented in Table 1.
In order to gain a deeper understanding, we extracted and presented the data from included articles based on the clinical manifestations, diagnostic methods, therapeutic approaches, and prognostic status of PCa patients having BMs.
3.2. Clinical Manifestations and Diagnosis Methods
Fifty-seven [9, 10, 13–24, 26–30, 32, 36–39, 41, 43–74] out of the 59 studies including 852 patients had reported BMs in PCa patients presenting with several clinical manifestations ranging from general symptoms such as hematuria, increased urinary frequency, and weakness to neurologic signs and symptoms such as aphasia, dysphasia, dysarthria, hemiplegia, headache, dizziness, confusion, double vision, visual field cut, ataxia, seizures, delirium, dementia, loss of appetite, and even behavioral changes. Moreover, different diagnostic methods including prostate-specific antigen (PSA), brain computed tomography (CT), brain magnetic resonance imaging (MRI), bone scan, multispectral immunofluorescence, immunohistochemistry (IHC), DNA sequencing, positron emission tomography (PET), prostate biopsy, and CSF analysis were used to diagnose BM in patients with PCa. More information regarding the clinical manifestations and diagnostic methods used for these patients is presented in Table 2.
Table 2.
Clinical manifestations and diagnosis methods in PCa patients presenting with BMs.
| Author | Year of publication | Type of studies | Total sample size | All reported clinical manifestations from all the patients | Diagnostic methods | Reference |
|---|---|---|---|---|---|---|
| Sena et al. | 2021 | Case report | 1 | Confusion, expressive aphasia, impaired coordination of his right hand | PSA, MRI, multispectral immunofluorescence, IHC, and DNA sequencing | [36] |
| Pikis et al. | 2021 | Case series | 46 | Neurological symptoms | PSA | [37] |
| Parihar et al. | 2021 | Case report | 1 | Any neurologic or visual symptoms | PSA and PET/CT | [38] |
| Hung et al. | 2021 | Retrospective study | 204 | — | PSA | [39] |
| Son et al. | 2020 | Case report | 1 | Headache, weakness, and unintentional weight loss | PSA, brain CT, brain MRI, and bone scan | [30] |
| Shida et al. | 2020 | Case report | 3 | Headache, nausea, double vision, and aphasia | PSA, brain CT, and brain MRI | [29] |
| Saadatpour et al. | 2020 | Case report | 1 | Afebrile and unresponsive | Brain CT | [41] |
| Ross et al. | 2020 | Case report | 1 | No neurological symptoms | PSA, brain CT, brain MRI, and bone scan | [28] |
| Marchand Crety et al. | 2020 | Case report | 1 | No neurological symptoms | PSA, prostate biopsy, and brain MRI | [27] |
| Liu et al. | 2020 | Case report | 1 | Weakness, loss of appetite, and chronic multiple bone and joint pain | PSA, brain CT scan, brain MRI, and CSF analysis | [26] |
| Janda et al. | 2020 | Case report | 1 | Headache, vomiting, and decreased visual acuity | Brain CT scan, brain MRI, and PSA | [24] |
| Bhambhvani et al. | 2020 | Retrospective cohort | 31 | Headache, delirium, weakness, cranial nerve palsy, visual field cut, ataxia, and seizures | — | [13] |
| Aljarba et al. | 2020 | Case report | 1 | Headache, dizziness, and upper and lower limb weakness | Brain CT scan, brain MRI, PSA, IHC, and CSF analysis | [32] |
| Ahmad et al. | 2020 | Case report | 1 | Headache, right-sided facial pain, intermittent diplopia in the right eye, and partial right third nerve palsy | Brain CT, blood test, MRI, CT thorax, abdomen and pelvis, PSA, prostate biopsy, and IHC | [43] |
| Kosaka et al. | 2019 | Case report | 1 | Reduced motivation | PSA, brain CT scan, brain MRI, and biopsy | [23] |
| Kanyilmaz et al. | 2019 | Retrospective cohort | 339 | Headache and hemiparesis | PSA, brain MRI, brain CT, and histopathology | [44] |
| Ishizaki et al. | 2019 | Case report | 1 | Dizziness and gross hematuria | PSA, brain MRI, brain CT, and DRE | [22] |
| Hogan et al. | 2019 | Case report | 1 | Confusion, visual disturbances, | Brain CT scan, ETV, biopsy, and PSA | [45] |
| Zanatta et al. | 2018 | Case report | 1 | double vision, holocranial headache, 6th cranial nerve paresis, and 5th cranial nerve paresthesia | PSA, MRI, multispectral immunofluorescence and IHC, and DNA sequencing, | [46] |
| Reinas et al. | 2018 | Case report | 1 | Headache and confusion | PSA, bone scintigraphy, CT scan of the thorax, abdomen and pelvis, and MRI | [47] |
| Nunno et al. | 2018 | Case report | 1 | Mental status, restlessness, headache, nausea, and vomiting, gait unsteadiness, unresponsive, and mumbling incomprehensible words | Brain CT | [48] |
| Jack et al. | 2018 | Case report | 1 | Dizziness, nausea, vomiting, headache, diaphoresis, and hemianopsia, | PSA, MRI, and biopsy | [49] |
| Campagna and Feia | 2018 | Case report | 1 | Falls, memory deficits, and weight loss | PSA, brain MRI, and brain CT | [21] |
| Watanabe et al. | 2017 | Case report | 1 | Back pain, headache, and nausea | CT, PSA, biopsy, bone scintigraphy, contrast-enhanced CT of the chest, abdomen, and pelvis | [20] |
| Lam et al. | 2017 | Case report | 2 | Dysphasia | PSA, brain MRI, brain CT, and biopsy | [19] |
| Chang et al. | 2017 | Case report | 1 | Memory loss, delusions, and dementia | PSA, brain MRI, brain CT, IHC, and biopsy | [18] |
| Guraya et al. | 2016 | Case report | 1 | Confusion, altered mental status, urinary frequency, occasional incontinence, and difficulty urinating | CT, PSA, and brain MRI | [50] |
| Barakat et al. | 2016 | Case report | 1 | Dizziness, mild dysphagia, and imbalance | PSA, brain MRI, and brain CT | [17] |
| Mandaliya et al. | 2015 | Case report | 2 | Right-sided facial palsy, dysarthria, and hemiparesis, | CT scan, MRI, blood tests, and PSA | [51] |
| Hutton et al. | 2015 | Case report | 1 | Bony pain, lower limb weakness, collapse, increased tone, and hematuria | PSA, bone scan, MRI, and brain CT | [16] |
| Gajewska et al. | 2015 | Case report | 1 | — | PET/CT scan, PSA, MRI, and bone scintigraphy | [15] |
| Craig et al. | 2015 | Case report | 1 | Increased urinary frequency, headaches, unsteady gait, and memory loss | DRE, PSA, transrectal ultrasonography, CT, chest radiography, MRI, IHC | [14] |
| Hatzoglou et al. | 2014 | Retrospective cohort | 21 | — | and MRI | [10] |
| Placido et al. | 2014 | Case report | 3 | — | PSA and MRI | [52] |
| Hazell et al. | 2013 | Case report | 4 | Weakness, seizure, ataxia, back pain, confusion, headache, dysarthria, and expressive dysphasia | MRI, PSA, bone scan, and brain CT | [53] |
| Caffo et al. | 2013 | Cohort | 490/9 | — | Brain CT and MRI | [54] |
| Caffo et al. | 2012 | Case series | 22 | Headache, confusion, coma, and hyposthenia | Brain CT and MRI | [55] |
| Flannery et al. | 2010 | Case series | 10 | Headaches, visual deterioration, seizures, diplopia, facial weakness, and confusion | PSA and MRI | [56] |
| Yamada et al. | 2009 | Case report | 1 | Back pain, headache, frequent vomiting, a decreased level of consciousness, his condition deteriorated quickly | Biopsy, PSA, progastrin-releasing peptide, brain MRI, and CSF | [57] |
| Sweets et al. | 2009 | Case report | 1 | Palpable prostate, and grand mal seizure | PSA, abdomen and pelvis CT, prostaScint scan, brain MRI, and PAP | [58] |
| Kim, S. H. | 2008 | Case series?? Pros? | 5 | Headache, memory deficit, hemiparesis, visual field deficits, ataxia, dysphasia, and seizure | PSA, volumetric axial, and contrast-enhanced T1 scan | [59] |
| Grenader et al. | 2007 | Case report | 1 | Confusion and behavioral changes | Brain CT and MRI, PSA, and histology | [60] |
| Lyons et al. | 2006 | Case report | 1 | Left-sided headache, right hemiparesis, and partial epilepsy | Brain CT, cerebral angiography, and biopsy | [61] |
| Wullich et al. | 2004 | Case report | 2 | Neurologic alterations | PSA, brain MRI, axial T1-weighted scan, and histopathology | [62] |
| Schoenwaelder et al. | 2004 | Case report | 1 | Worsening headaches, mild nausea and vomiting, mild gait ataxia, brisk reflexes, and upgoing plantar reflex on the right | PSA, brain CT, MRI, and whole body bone scan | [63] |
| Tremont-Lukats et al. | 2003 | Case series | 103 | Headaches, delirium, memory deficits, diplopia, with III, IV, or VI CN palsies, VII and other lower CN neuropathies, hemiparesis, visual field cuts, aphasia, seizures, ataxia, and asymptomatic | Cytology, MRI, and CT myelography | [9] |
| Erasmus et al. | 2002 | Case report | 1 | Homonymous hemianopsia, | PSA, prostate biopsy, brain CT and MRI, and histological examination | [64] |
| Minami et al. | 2001 | Case report | 1 | Dysarthria and hemiplegia | PSA, intravenous pyelography and urethrocystography, brain CT, and MRI | [65] |
| Behrens et al. | 2001 | Case report | 1 | Personality change, forgetfulness and episodic confusion, and slow gait and inability to tandem-walk | PSA, brain MRI and CT, and histopathology | [66] |
| Garcia-Morales et al. | 2000 | Case report | 1 | Slight memory deficit | Bone scan, brain MRI and CT, 111In-ProstaScint scan, tomography images, and histopathology | [67] |
| Fervenza et al. | 2000 | Case report | 1 | Confusion, nausea, unresponsiveness, headache, unsteadiness, and mild muscle weakness | PSA and brain MRI | [68] |
| Hayashi et al. | 1998 | Case report | 1 | Asymptomatic | PSA, histology, and brain CT | [69] |
| Zhang et al. | 1997 | Case report | 1 | Transient attack of unconsciousness | PSA, biopsy, CT, and histopathology | [70] |
| Leibman et al. | 1995 | Case report | 2 | Rapid deterioration, dementia, rigidity and disorientation, and difficulty with walking | PSA, PAP, biopsy, brain CT and MRI, and bone scan | [71] |
| Bland et al. | 1992 | Case report | 1 | Seizures, left cheek pain, left orbital pain, periorbital swelling, diplopia, proptosis of the left eye, chemosis and lateral periorbital edema, poor short-term memory, diplopia, limitation of all extraocular movements of the left eye, a diminished left corneal reflex, and mild right hemiparesis. | Brain CT, histopathology, PAP, PSA, and bone scan | [72] |
| Lynes et al. | 1986 | Case report | 2 | Headaches, gait disturbances, incoordination, memory loss, and lethargy, lateral gaze nystagmus, papilledema, hyperactive deep tendon reflexes, and motor incoordination, and rapidly progressive dementia | Brain CT, biopsy, diethylstilbestrol/lumbar puncture, and EEG | [73] |
| Sarma et al. | 1983 | Case report | 4 | Left hemiplegia, hemiparesis, and continued deterioration | Brain CT | [74] |
Prostate specific antigen (PSA); brain computed tomography (CT); magnetic resonance imaging (MRI); positron emission tomography (PET); cerebrospinal fluid (CSF); digital rectal examination (DRE); immunohistochemistry (IHC); endoscopic third ventriculostomy (ETV); prostatic acid phosphatase (PAP); electroencephalogram (EEG).
3.3. Therapeutic Approaches and Prognosis Status
As shown in Table 3, 901 patients from fifty-eight [9, 10, 13–24, 26–30, 32, 36–40, 42–74] out of the 59 studies had demonstrated varied therapeutic approaches such as immunotherapy, ADT, whole-brain radiation therapy (WBRT), craniotomy, prostatectomy, adjuvant radiation therapy, docetaxel chemotherapy, and medications such as abiraterone, prednisone, pembrolizumab, dexamethasone, bicalutamide, and leuprorelin. Additionally, the prognostic overview of the PCa patients developing BMs was not promising. Most of the cases passed away despite the fact that they received different kinds of treatments ranging from radiotherapy, chemotherapy, surgery, and even immunotherapy.
Table 3.
Therapeutic approaches and prognosis status of PCa patients having BMs.
| Author | Year of publication | Type of studies | Total sample size | Treatments | Prognosis | Overall survival time after the diagnosis of BM | Reference |
|---|---|---|---|---|---|---|---|
| Sena et al. | 2021 | Case report | 1 | Sipuleucel-T immunotherapy, abiraterone, prednisone, pembrolizumab, dexamethasone, craniotomy, brain-directed radiotherapy, and docetaxel chemotherapy | Passed away | — | [36] |
| Pikis et al. | 2021 | Case series | 46 | Prostatectomy, adjuvant radiation therapy, SRS, craniotomy, WBRT, and ADTs | Passed away | 1 year and 8 months | [37] |
| Parihar et al. | 2021 | Case report | 1 | Docetaxel, cabazitaxel, enzalutamide, LuPSMA radioligand therapy, alpha therapy, and Ac-PSMA, | Survived | — | [38] |
| Hung et al. | 2021 | Retrospective study | 204 | Docetaxel, AA, enzalutamid, and cabazitaxel | — | — | [39] |
| Boxley et al. | 2021 | Retrospective study | 29 | ADTs, enzalutamide, abiraterone acetate, docetaxel, and cabazitaxel | — | — | [40] |
| Son et al. | 2020 | Case report | 1 | Bicalutamide, degarelix, leuprorelin, WBRT, chemotherapy, and craniotomy | — | — | [30] |
| Shida et al. | 2020 | Case report | 3 | Bicalutamide, degarelix, brachytherapy, and WBRT | 3 passed away | 1/3/5 months | [29] |
| Ross et al. | 2020 | Case report | 1 | craniotomy | Survived | — | [28] |
| Nguyen et al. | 2020 | Retrospective cohort | 21 | WBRT | Passed away | 2/3/6 months | [42] |
| Marchand Crety et al. | 2020 | Case report | 1 | Hormone therapy, prostatic radiotherapy, docetaxel, and chemotherapy | Survived | — | [27] |
| Liu et al. | 2020 | Case report | 1 | Dexamethasone and palliative care | Passed away | 5 months | [26] |
| Janda et al. | 2020 | Case report | 1 | Complete androgen blocking, GnRH analog, and nonsteroidal antiandrogen, chemotherapy with analgesic radiotherapy | Passed away | 1 year and 8 months | [24] |
| Bhambhvani et al. | 2020 | Retrospective cohort | 31 | Stereotactic radiosurgery and surgical resection | Passed away | 4.6/13 months | [13] |
| Aljarba et al. | 2020 | Case report | 1 | ADTs, surgery, and palliative care | Passed away | 43 days | [32] |
| Ahmad et al. | 2020 | Case report | 1 | Thyroxine (steroid replacement), exposure therapy, testosterone replacement, and bicalutamide, radiotherapy | Survived | — | [43] |
| Kosaka et al. | 2019 | Case report | 1 | Craniotomy and ADTs, chemotherapy | — | — | [23] |
| Kanyilmaz et al. | 2019 | Retrospective cohort | 339 | WBRT and radiotherapy | Passed away | 4.5 months | [44] |
| Ishizaki et al. | 2019 | Case report | 1 | ADTs and WBRT | — | — | [22] |
| Hogan et al. | 2019 | Case report | 1 | SRS | Survived | — | [45] |
| Zanatta et al. | 2018 | Case report | 1 | Cerebellopontine angle microsurgery | Survived | — | [46] |
| Reinas et al. | 2018 | Case report | 1 | External beam radiotherapy, bicalutamide, right parietal craniotomy, and luteinizing | — | — | [47] |
| Nunno et al. | 2018 | Case report | 1 | Craniotomy | — | — | [48] |
| Jack et al. | 2018 | Case report | 1 | Zoladex, casodex, docetaxel, lupron, ketoconazole, lupron therapy, and WBRT | Passed away | 3 months | [49] |
| Campagna and Feia | 2018 | Case report | 1 | Craniotomy with biopsy and SRS | — | — | [21] |
| Watanabe et al. | 2017 | Case report | 1 | Androgen blockade therapy, denosumab, docetaxel, enzalutamide, abiraterone, and cabazitaxel | Survived | — | [20] |
| Lam et al. | 2017 | Case report | 2 | Near-total excision surgery | Survived | — | [19] |
| Chang et al. | 2017 | Case report | 1 | Dexamethasone, SRS, excision and orchiectomy, and left subcapsular orchiectomy | — | — | [18] |
| Guraya et al. | 2016 | Case report | 1 | Dexamethasone, steroid injection, levetiracetam, craniotomy, and radiation therapy | Passed away | 12 months | [50] |
| Barakat et al. | 2016 | Case report | 1 | Tumor resection, SRS, craniotomy, and ADTs | Survived | — | [17] |
| Mandaliya et al. | 2015 | Case report | 2 | Stereotactic craniotomy, WBRT, and ADTs | Survived | — | [51] |
| Hutton et al. | 2015 | Case report | 1 | Dexamethasone | Passed away | — | [16] |
| Gajewska et al. | 2015 | Case report | 1 | Cabazitaxel | — | — | [15] |
| Craig et al. | 2015 | Case report | 1 | Radiotherapy, ADTs, craniotomy, dexamethasone, WBRT, bicalutamide, abiraterone, and prednisone | Passed away | — | [14] |
| Hatzoglou et al. | 2014 | Retrospective cohort | 21 | — | Passed away | 2.8 months | [10] |
| Placido et al. | 2014 | Case report | 3 | Luteinizing, complete androgen blockage, bicalutamide, prostatectomy and radiation therapy, ADTs, docetaxel, abiraterone, and cabazitaxel | 2 passed away, 1 survived | — | [52] |
| Hazell et al. | 2013 | Case report | 4 | ADTs, radiation therapy, craniotomy, WBRT, docetaxel, prednisone, and zoledronic acid | Passed away | — | [53] |
| Caffo et al. | 2013 | Cohort | 9 | Castration-resistantdocetaxel-based chemotherapy, and surgery | — | — | [54] |
| Caffo et al. | 2012 | Case series | 22 | Undergone docetaxel-based treatment, surgery, WBRT, gamma knife, chemotherapy with docetaxel and mitoxantrone, and radiotherapy | 20 passed away | 4 months | [55] |
| Flannery et al. | 2010 | Case series | 10 | ADTs, WBRT, SRS, and gamma knife | 9 passed away | 2–23 months | [56] |
| Yamada et al. | 2009 | Case report | 1 | GnRH, cisplatin plus etoposide, and combined therapy with carboplatin plus irinotecan | Survived | — | [57] |
| Sweets et al. | 2009 | Case report | 1 | Chemotherapy with doxorubicin, vinblastine, nilutamide, leuprolide, prostatectomy, salvage three-dimensional conformal radiotherapy, and craniotomy | Survived | — | [58] |
| Kim et al. | 2008 | Case series | 5 | Gamma knife SRS and WBRT | 4 passed away | 10 months | [59] |
| Grenader et al. | 2007 | Case report | 1 | Dexamethasone, phenytoin, palliative radiotherapy, antiandrogen agent, bicalutamide, subcutaneous luteinizing hormone releasing hormone agonist goserelin, zoledronic acid, and craniotomy | Survived | — | [60] |
| Lyons et al. | 2006 | Case report | 1 | Prostatectomy, dexamethasone, phenytoin, craniotomy and resection of tumor, and external beam radiotherapy to the brain, casodex and lupron therapy | Survived | — | [61] |
| Wullich et al. | 2004 | Case report | 2 | Bilateral orchiectomy, fosfestrol, transurethral resection, craniotomy, and WBRT | Passed away | 1-2 month | [62] |
| Schoenwaelder et al. | 2004 | Case report | 1 | Dexamethasone, phenytoin, posterior fossa craniectomy, insertion of a ventriculoperitoneal shunt for hydrocephalus, oral antiandrogen therapy, and palliative radiotherapy | Passed away | 4 months | [63] |
| Tremont-Lukats et al. | 2003 | Case series | 103 | Supportive care and SRS | Passed away | 1 month | [9] |
| Erasmus et al. | 2002 | Case report | 1 | Hormonal therapy(cyproterone acetate), craniotomy, and radiation therapy | Passed away | — | [64] |
| Minami et al. | 2001 | Case report | 1 | Transurethral resection of prostate, hormone therapy with goserelin acetate and flutamide, and intermittent arterial infusion chemotherapy with cisplatin and pirarubicin | Passed away | Less than 1 month | [65] |
| Behrens et al. | 2001 | Case report | 1 | Dexamethasone, phenytoin, craniotomy | — | — | [66] |
| Garcia-Morales et al. | 2000 | Case report | 1 | chemotherapy, radiotherapy, and craniotomy | — | — | [67] |
| Fervenza et al. | 2000 | Case report | 1 | WBRT, SRS, craniotomy, and ADTs | Survived | — | [68] |
| Hayashi et al. | 1998 | Case report | 1 | Prostatectomy and craniotomy | Survived | — | [69] |
| Zhang et al. | 1997 | Case report | 1 | Prostatectomy, ifosfamide, local radiation therapy, and craniotomy | Passed away | 19 months | [70] |
| Leibman et al. | 1995 | Case report | 2 | Hormonal therapy (leuprolide and flutamide) and chemotherapy (cyclophosphamide and methotrexate), and WBRT | Passed away | — | [71] |
| Bland et al. | 1992 | Case report | 1 | Radiation therapy and bilateral orchiectomy | Survived | — | [72] |
| Lynes et al. | 1986 | Case report | 2 | WBRT, suboccipital craniotomy, bilateral orchiectomy, doxorubicin hydrochloride (adriamycin), and frontal craniotomy | 1 survived, 1 passed away | 9 months | [73] |
| Sarma et al. | 1983 | Case report | 4 | Transurethral resection and bilateral orchiectomy | Passed away | — | [74] |
Stereotactic radiosurgery (SRS); whole brain radiation therapy (WBRT); antiandrogen deprivation therapies (ADTs); abiraterone acetate (AA); gonadotropin-releasing hormone (GnRH).
4. Discussion
Metastatic lesions in the brain arising from lung cancer (∼30% of patients), breast cancer (∼30%), and melanoma (∼45%) are relatively common and well-studied. There is, however, scarceness of information regarding BMs arising from PCa, due to its rarity. Reports in the literature estimate the incidence to be between 0.16% and 0.63%, with a median survival time after BMs detection between 2.8 and 4.5 months [13].
In a manner similar to other neoplasia's, there is a progression from initial normal prostatic epithelium to intraepithelial neoplasia, which can lead to either localized adenocarcinoma, squamous carcinoma, neuroendocrine carcinoma, or a combination of the above. Continued neoplastic change leads to eventual metastatic spread, once the basal layer/basement membrane has been breached [75]. It has been mentioned that prostate small cell carcinoma seems to have a greater tendency to produce BMs compared to prostate adenocarcinoma [64].]
Various theoretical mechanisms have been proposed for the CNS metastases of PCa. As shown in Figure 2, these can be broadly classified as the hematogenous and lymphatic spread and severe impairment of the immune system leading to a breakdown of the blood barrier and the soil-and-seed and epithelial-to-mesenchymal transition theory, as well as the multistep or cascade process theory [76–78]. After a wide and thorough review of the available evidence, it is evident that the true nature of PCa varies from case to case. Thus, it is a complex interaction among the numerous multiple mechanisms listed above.
Figure 2.
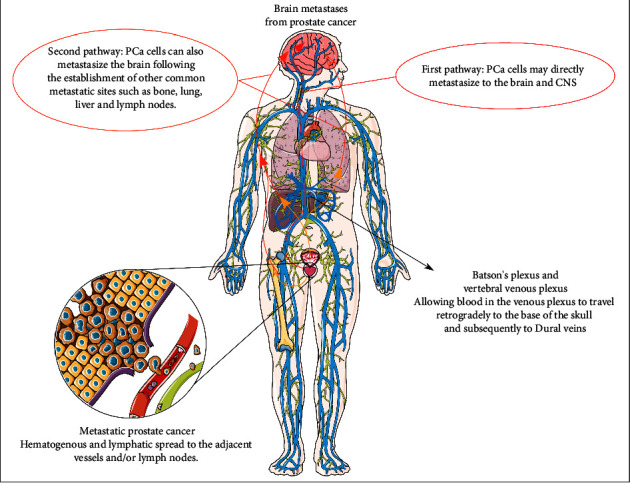
The pathways and mechanisms of brain metastases in prostate cancer (this figure was drawn using images from Servier medical art (https://smart.servier.com) licensed by a Creative Commons Attribution 3.0 unported license.)
The broad concept consists of tumor cells detaching from the primary tumor mass to invade the basement membrane and the surrounding microenvironment. They intravasate into either the surrounding blood vessels or lymphatics. They then synthesize proangiogenic factors that will initiate neoangiogenesis. This is followed by extravasation at the secondary sites, the formation of micrometastases, and the subsequent process of metastatic colonization [79].
4.1. Clinical Manifestations
The neurological signs and symptoms of BMs in PCa lesions are usually related to the consequent intracranial hypertension [76]. Additionally, issues also arise based on the specific area of localization.
As described in Table 2, these include symptoms such as headache, nausea, vomiting, seizures, confusion, weakness, aphasia, visual disturbances, ataxia, motor dysfunction, mono/hemiparesis, mental status or behavioral changes, cranial nerve dysfunction, and delirium. It was also demonstrated that patients can be asymptomatic, and the diagnosis of BMs in PCa may be an incidental finding during other investigations [9, 10, 13–24, 26–30, 32, 36–39, 41, 43–74, 76, 77].
Prostate neoplastic cells rarely involve the brain stem and cerebellum, therefore, focal neurologic presentations including seizures and ataxia are uncommon. As we showed in the results, most patients show unspecific neurologic symptoms including headaches and papilledema, which can be explained as being caused by elevated intracranial pressure and frontal lobe syndrome. However, in some rare cases, PCa BMs are asymptomatic. PCa patients with BMs are often in the end stages of the disease, or they may also have other accompanying chronic illnesses such as atherosclerosis, which may hide their CNS involvement and make the diagnosis even more challenging. Due to these multiple factors, cranial nerve and pituitary tumor spread, tumor mass effect, and meningeal involvement with hemorrhage of the contiguous brain tissue are frequently missed in these patients [9, 10, 13–24, 26–30, 32, 36–39, 41, 43–74, 76, 77, 80–83].
4.2. Diagnostic Approaches to the BMs of PCa
Considering the findings, the brain involvement acts as an uncommon yet serious presentation of PCa notably in patients with widespread metastases and multiorgan tumor spread. It has significant implications on patient prognosis and overall survival. Hence, the necessity is for early diagnosis and management, especially in patients with high clinical suspicion for metastases [36–40, 82, 84]. As shown in Table 2, several diagnostic approaches were used in BMs of PCa [9, 10, 13–24, 26–30, 32, 36–39, 41, 43–74]. Herein, we categorized and discussed them separately based on the nature of the diagnostic approach.
4.2.1. Imaging Modalities
Due to the impacts of CNS involvement on PCa, staging imaging is indicated in specific clinical situations. MRI and CT are the modalities which are chosen to detect brain involvement in systemic malignancies with or without neurologic symptoms. Moreover, imaging is also indicated in patients with neurological symptoms without signs of malignancy [37, 39, 44, 85–88].
In metastatic PCa, routine radiologic screening is performed to detect asymptomatic brain invasion, since tumors spreading to the lymph nodes and bone are more likely to have intracranial metastases [89]. In regards to cost-effectiveness, Hatzoglou et al. mentioned that screening asymptomatic PCa patients with BMs for early detection may warrant further studies before it can become a routine practice [10]. Due to the low incidence of BMs in PCa, MRI is not indicated in asymptomatic patients or those with mild neurological symptoms [76]. However, increased incidence of metastatic PCa, especially in those with neurological presentations, requires screening guidelines to reduce mortality, morbidity, and treatment costs, as well as to improve overall patient survival [9, 10, 90].
As illustrated in the results, the first line imaging modality to detect BMs in PCa is a noncontrast CT scan which identifies lethal neurological conditions including hemorrhage, hydrocephaly, or massive compressive lesions [9, 29, 30, 38, 41, 44, 87]. Before using MRI as a diagnostic tool for detecting CNS metastases, imaging modalities lack the sensitivity to identify multiple intraparenchymal metastatic lesions. Therefore, most BMs were reported as unifocal [9, 73, 91].
Some PCa patients present brain hemorrhages as a result of tumor invasion which appears as hyperdense lesions on CT scans. However, nonhemorrhagic spots demonstrate variable densities compared with the surrounding brain tissue [10]. Calcification can make the diagnosis of BMs in PCa unlikely, as well as other malignancies [86, 92].
Before the development of advanced MRI techniques, contrast-enhanced CT scan was the second diagnostic option to confirm BMs in challenging PCa cases. PCa metastases may appear as solid, cystic, nodular, mixed, or ring-like enhancements in this modality [93–95].
Nowadays, MRI is the gold standard diagnostic option to identify malignant CNS lesions in PCa, and it can also be used to follow-up the patients during the course of treatment. Advanced techniques/sequences such as magnetic resonance spectroscopy (MRS), magnetic resonance perfusion (MRP), diffusion-weighted imaging (DWI), and diffusion tensor imaging (DTI) are advantageous due to them having a higher sensitivity in order to better differentiate BMs in PCa from other CNS malignant or nonmalignant lesions [86]. MRI is indicated to find any possible tumoral lesion which may have been missed/undiagnosed on the CT scan. It is used before surgery, radiosurgery, or when an underlying malignancy is suspected. In spite of a CT scan, MRI can differentiate a malignant metastatic lesion from a noncancerous pathology such as an infection [88]. In T1, signal nonhemorrhagic BMs appears isointense or hypointense, and metastatic hemorrhagic lesions are hyperintense. In T2 weighted MRI, tumoral masses show hyperintense signals [86, 96, 97]. Nevertheless, BMs in PCa show unspecific and variable signs in MRI, making it difficult to differentiate it from other brain lesions, including metastases from other cancers, and primary brain tumors such as gliomas, or even infectious masses [44].
Most metastatic brain tumors including those from PCa are unifocal, however, multifocal lesions are reported in some cases. Malignant cells often invade the brain tissue at the junction between gray and white matter, particularly at the areas which border the main arteries supplying the brain with blood [98, 99]. Besides involving the cerebellum, PCa cells tend to involve the posterior fossa [100, 101]. It is suggested that the adjacent structures of brain parenchyma such as the skull and meninges might also become involved [102]. 18 fluorodeoxyglucose positron emission tomography (FDG-PET) and other advanced molecular imaging techniques may also help in diagnosing more challenging cases [86].
4.2.2. Histopathology
Based on our findings showing that biopsy and tissue assessment was used in most cases, histopathology is the most accurate test for diagnosing CNS metastases in PCa, although, its invasive nature and side effects outweigh the benefits, especially for end-stage or critically ill PCa patients. Therefore, the tissue assessment of other distant metastases of PCa or brain imaging may act as substitutes to diagnose neurologic involvement in most patients [9, 10, 14, 18–20, 36, 43–46, 49, 57, 60–62, 64, 66, 69, 70, 72, 73].
Most brain metastatic lesions originate from prostate adenocarcinoma since it is the most common type of PCa. However, other less frequent histological subtypes including small cell carcinoma and neuroendocrine carcinoma of the prostate have the highest tendency to cause brain involvement in a shorter period. Besides tumor staging, histology plays a notable role in the spread of PCa to the CNS [10, 103]. Hematoxylin and eosin staining of BMs in prostate adenocarcinoma demonstrate glandular patterns that are PSA-positive in immunohistochemistry [10].
Memorial Sloan Kettering Cancer Center evaluated the gross pathology of BMs in deceased PCa patients and found hemorrhagic metastases in some autopsies. Like primary CNS neoplasms, the metastatic masses of malignant prostate cells exhibited distinct borders surrounded by vasogenic edema [10].
4.2.3. Molecular and Genetic Biomarkers
Of particular interest is one of our results which demonstrated that progressively higher reelin levels have been found in 39% of PCa patients. Reelin is an extracellular glycoprotein that is involved in regulating neuronal migration during brain development and also in cancer biology. Thus, it can be suggested that reelin levels could be used as a marker to predict the aggressiveness of prostatic cells [77]. Another finding of interest showed that the brain involvement of PCa should be assessed in high-risk patients; also understanding molecular characteristics of malignant cells might help us in the diagnosis and management process [9]. In this regard, a study found that forty percent of patients with prostate tumors demonstrated mild ADAM metallopeptidase with thrombospondin type 1 motif 13 (ADAMTS-13) deficiency. Low ADAMTS-13 activity could result in elevated levels of highly polymeric von Willebrand factor (vWF), which might facilitate adhesive interactions with both circulating tumor cells and platelets, resulting in thrombus formation and the presumptive development of a metastatic colony [104].
4.3. Treatments of BMs in PCa
Due to more efficient treatment facilities and earlier diagnosis, the prognosis of patients with PCa has become more favorable. Therefore, the rate of rare complications such as BMs in PCa has become more common [90, 105, 106]. Hence, it becomes necessary to study and find more effective therapeutic approaches for cases of BMs in patients with PCa. As shown in Table 3, many diverse classes of treatments were administrated in BMs of PCa. Selected therapies for BMs can be divided into two general categories, main therapies and supportive therapies [9, 10, 13–24, 26–30, 32, 36–40, 42–74, 107].
We demonstrated that supportive therapies may reduce and alleviate the symptoms of cerebral metastases which typically include anticoagulants, antiepileptic drugs (AEDs), and corticosteroids. The main treatments that are considered directly for tumor management usually include chemotherapy, radiotherapy, and surgery [9, 107]. Corticosteroids, especially dexamethasone, are used to treat cerebral edema and any symptoms in patients with BMs, reporting a 75% reduction in neurological symptoms. Dexamethasone is usually given in two separate doses of 4 to 8 mg daily, according to the instructions, and higher doses are used only in more severe cases or if there is no effective response within 48 hours after administration [60, 73, 88, 108, 109]. However, corticosteroids have no place in asymptomatic patients. In some studies, it is recommended that dexamethasone can be prescribed to reduce the incidence of acute radiation toxicity if patients were asymptomatic and showed signs of cerebral edema on radiographic imaging before the radiotherapy [110]. In one study, mannitol, furosemide, and dexamethasone as supportive therapy were used to reduce intracerebral pressure, and have since started as major therapies for the treatment of BMs such as WBRT [29].
The most effective main treatments in the management of BMs are considered based on the number and type of BMs, the type of primary tumor, the location of the BMs, the rate of spread and control of the disease, and how the patient responds to treatment [107].
Our findings suggest that radiotherapy is currently used as a common treatment in most patients with BMs [9, 14, 17, 18, 21, 27, 29, 30, 36, 37, 43, 44, 47, 51, 55, 58, 60, 61, 63, 67, 71, 111]. In this regard, a study by Sita et al. focusing on radiotherapy in BMs following PCa provides a significant breakthrough for radiotherapy-prioritized treatment patterns. It suggests that five treatment approaches could be offered for comorbid radiotherapy treatments, which include WBRT, stereotactic radiosurgery (SRS), the base of skull radiotherapy, concurrent cabazitaxel plus WBRT, and surgery followed by adjuvant WBRT. This study has demonstrated that the survival rate of patients undergoing WBRT increased from 4 to 9 months, and for SRS, it increased from 9 to 13 months [112].
Additionally, we showed the investigation of 31 PCa patients who experienced BMs and underwent the treatment with radiosurgery, and there was a reported increased life expectancy from 1.2 to 4.6 months. Moreover, patients who received surgical resection plus radiotherapy had an elevated survival rate from 1.2 to 13 months [13]. Also, during the follow-up of three PCa patients with brain involvement, surgical treatment was considered as the initial treatment, but the systemic status of the cancer was out of control and the number of metastases was more than 5, and SRS has not been considered because it cannot affect the whole disease, so all three patients were treated with WBRT only. The WBRT reduced the symptoms of BMs in all three patients, however, its effect on the prognosis of patients was not promising [29, 113]. Because elderly patients do not often tolerate standard radiotherapy treatments, personalized treatments can be a good option in managing elderly patients [29, 113].
Our findings showed that solitary BMs can be also treated with resection surgery followed by radiotherapy, specially WBRT [17]. In confirmation of that, another study demonstrated that a solitary BMs was reported in a 63-year-old patient with PCa, craniotomy with gross total resection of the tumor was performed and was followed by adjuvant WBRT. In this patient, during the 23-monthfollow-up after surgery, they saw an unrecognizable decrease in PSA levels with no evidence of recurrence [22]. Another patient was diagnosed with metastatic prostate adenocarcinoma who underwent craniotomy with biopsy and resection of the mass lesion simultaneously and then was subsequently treated with SRS due to the limited volume of involvement. After 7 months of follow-up, no recurrence of the lesion was reported [21]. Another elderly patient with a history of prostate adenocarcinoma highlighted the possibility of intracranial metastases of the prostate based on neurological symptoms. Due to his disability and poor prognosis, the patient preferred palliative treatment rather than radiotherapy. Although acute neurological symptoms improved after 3 days of dexamethasone use, the patient died 5 months after being diagnosed with BMs [26].
4.4. Prognosis and Mortality
We found that despite being devoted to PCa, BMs is rare and uncommon [9, 10, 13, 27, 112, 114–116], and the main survival rate estimations suggest that BMs in PCa has a poor prognosis [10, 112, 116, 117]. As presented in Table 3, many studies have been conducted to measure the survival rate and the mortality rate of PCa with BMs, for instance, it is reported in one study that the mean survival rate for only BMs in PCa is less than 28 months [9, 10, 13–24, 26–30, 32, 36–74, 117]. Sita et al. [112] has also demonstrated that the median survival for intracranial metastases in PC is 4 to 13 months. Moreover, it is established that the prognosis of parenchymal BMs is poor, with a mean survival rate of 1 to 7.6 months [116]. Tremont-Lukats IW and colleagues have reported that the overall median survival rate of PCa patients with BMs was 1 month from the time of diagnosis of CNS involvement; however, they suggested that undergoing the radiotherapy treatment can increase their median survival rate up to 3.5 months [9].
On the other hand, Cagney et al. [118] in a cohort study indicated that the median survival of BMs just in PCa patients was 12 months which was more than other primary cancers such as breast, small cell lung, and melanoma. One study found the Karnofsky performance score (KPS) to be effective in estimating the prognosis of elderly patients with BMs in PCa treated with WBRT. This study reported that patients with more than 70% of KPS are good candidates for treatment with long-term courses with WBRT [42].
Thus, in this review, we cannot compare the prognosis and the survival rate of BMs following PCa with other types of cancers. As diagnostic factors can play a major role in discovering the BMs in PCa, prognostic agents can also be considered crucial and even necessary in the prediction of disease prognosis. Malignant brain tumor domain-containing protein 1 (MBTD1) can play a critical role as a novel prognostic or diagnostic factor. As its overexpression is associated with poor prognosis and consequently, short survival time [119].
5. Conclusion
The intended purpose of conducting this systematic review was to gather and highlight all the current knowledge of BMs in PCa patients. We emphasized the importance of all the basic and clinical aspects of BMs in PCa. We have discussed the possible risk factors and mechanisms causing the BMs in PCa and then, we have described the available clinical approaches and diagnostic methods that can be used by physicians to identify the BMs in PCa patients and differentiate it from other neurological diseases. We have also demonstrated the therapeutic guidelines and treatments in BMs in PCa and along with the prognostic status of BMs in PCa. These data shed more light on the way to help clinicians and physicians to understand, diagnose, and manage BMs in PCa patients better.
Data Availability
The data will be provided by the corresponding author on request.
Ethical Approval
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All authors verify their participation in preparing this manuscript. SM conceptualized the study and reviewed edited the manuscript. AAJ reviewed and edited the manuscript. MS, SZH, and FS wrote the original draft. AS wrote the original draft and supervis.
References
- 1.Karan D., Thrasher J. B., Lubaroff D. Prostate cancer: genes, environment, immunity and the use of immunotherapy. Prostate Cancer and Prostatic Diseases . 2008;11(3):230–236. doi: 10.1038/pcan.2008.3. [DOI] [PubMed] [Google Scholar]
- 2.Daniyal M., Siddiqui Z. A., Akram M., Asif H., Sultana S., Khan A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pacific Journal of Cancer Prevention . 2014;15(22):9575–9578. doi: 10.7314/apjcp.2014.15.22.9575. [DOI] [PubMed] [Google Scholar]
- 3.Testa U., Castelli G., Pelosi E. Cellular and molecular mechanisms underlying prostate cancer development: therapeutic implications. Medicine (Baltimore) . 2019;6(3):p. 82. doi: 10.3390/medicines6030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell T., Neal D. E. The genomic evolution of human prostate cancer. British Journal of Cancer . 2015;113(2):193–198. doi: 10.1038/bjc.2015.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colloca G., Venturino A. The evolving role of familial history for prostate cancer. Acta Oncologica . 2011;50(1):14–24. doi: 10.3109/0284186x.2010.521191. [DOI] [PubMed] [Google Scholar]
- 6.Kluger J., Roy A., Chao H. H. Androgen deprivation therapy and cognitive function in prostate cancer. Current Oncology Reports . 2020;22(3):p. 24. doi: 10.1007/s11912-020-0884-1. [DOI] [PubMed] [Google Scholar]
- 7.Holtfrerich S. K. C., Knipper S., Purwins J., et al. The impact of long-term androgen deprivation therapy on cognitive function and socioeconomic decision making in prostate cancer patients. Psycho-Oncology . 2020;29(8):1338–1346. doi: 10.1002/pon.5442. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin R. Neurologic complications of prostate cancer. American Family Physician . 2002;65(9):1834–1840. [PubMed] [Google Scholar]
- 9.Tremont-Lukats I. W., Bobustuc G., Lagos G. K., Lolas K., Kyritsis A. P., Puduvalli V. K. Brain metastasis from prostate carcinoma - the M. D. Anderson cancer center experience. Cancer . 2003;98(2):363–368. doi: 10.1002/cncr.11522. [DOI] [PubMed] [Google Scholar]
- 10.Hatzoglou V., Patel G. V., Morris M. J., et al. Brain metastases from prostate cancer: an 11-year analysis in the MRI era with emphasis on imaging characteristics, incidence, and prognosis. Journal of Neuroimaging . 2014;24(2):161–166. doi: 10.1111/j.1552-6569.2012.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolzenbach L. F., Rosiello G., Deuker M., et al. The impact of race and age on distribution of metastases in patients with prostate cancer. The Journal of Urology . 2020;204(5):962–968. doi: 10.1097/ju.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 12.Nafissi N. N., Kosiorek H. E., Butterfield R. J., et al. Evolving natural history of metastatic prostate cancer. Cureus . 2020;12(11) doi: 10.7759/cureus.11484.e11484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhambhvani H. P., Greenberg D. R., Srinivas S., Hayden Gephart M. Prostate cancer brain metastases: a single -institution experience. World Neurosurgery . 2020;138:E445–E449. doi: 10.1016/j.wneu.2020.02.152. [DOI] [PubMed] [Google Scholar]
- 14.Craig J., Woulfe J., Sinclair J., Malone S. Isolated brain metastases as first site of recurrence in prostate cancer: case report and review of the literature. Current Oncology . 2015;22(6):e493–e497. doi: 10.3747/co.22.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gizewska A., Witkowska-Patena E., Stembrowicz-Nowakowska Z., Buraczewska A., Dziuk M. Brain metastases in patient with prostate cancer found in 18F-choline PET/CT. Nuclear Medicine Review . 2015;18(1):39–41. doi: 10.5603/nmr.2015.0010. [DOI] [PubMed] [Google Scholar]
- 16.Hutton R., Maguire J., Amer T., et al. Intracranial metastasis of adenocarcinoma of the prostate presenting with symptoms of spinal cord compression. Indian Journal of Surgery . 2015;77(S1):75–76. doi: 10.1007/s12262-014-1143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barakat T., Agarwal A., McDonald R., et al. Solitary brain metastasis from prostate cancer: a case report. Annals of Palliative Medicine . 2016;5(3):227–232. doi: 10.21037/apm.2016.04.02. [DOI] [PubMed] [Google Scholar]
- 18.Chang J., Kwan B., Panjwani N., et al. Prostate adenocarcinoma metastases to the testis and brain: case report and review of the literature. Oxford Medical Case Reports . 2017;2017(8) doi: 10.1093/omcr/omx042.omx042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam A., Gan P. Y. Metastatic prostate adenocarcinoma to the brain: case reports and literature review. Journal of Neurological Surgery Reports . 2017;78(1):e55–e58. doi: 10.1055/s-0037-1601304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe K., Kosaka T., Hongo H., Tamaki S., Oya M. Headache caused by brain metastases of castration-resistant prostate cancer during cabazitaxel therapy. Keio Journal of Medicine . 2017;66(4):65–71. doi: 10.2302/kjm.2016-0014-cr. [DOI] [PubMed] [Google Scholar]
- 21.Campagna J. P., Feia K. Isolated brain metastasis of prostate carcinoma in the setting of normal prostate specific antigen. Urology Case Reports . 2018;21:67–69. doi: 10.1016/j.eucr.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishizaki F., Maruyama R., Yamana K., Kasahara T., Nishiyama T., Tomita Y. Solitary brain metastasis from prostate cancer after multi modality treatment: a case report. Urology Case Reports . 2019;24 doi: 10.1016/j.eucr.2019.100879.100879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosaka T., Hongo H., Aimono E., et al. A first Japanese case of neuroendocrine prostate cancer accompanied by lung and brain metastasis with somatic and germline BRCA2 mutation. Pathology International . 2019;69(12):715–720. doi: 10.1111/pin.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jandou I., Moataz A., Larrache Y., Mohammed D., Debbagh A., Aboutaieb R. Fronto-orbital metastasis of a prostatic adenocarcinoma. Urology Case Reports . 2020;33 doi: 10.1016/j.eucr.2020.101335.101335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korkmaz U., Ustun F. <sup>18</sup>F-NaF PET/CT and extraordinary involvement: non-calcific brain involvement in a prostate cancer case. Molecular Imaging and Radionuclide Therapy . 2020;29(1):41–44. doi: 10.4274/mirt.galenos.2019.85547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S. H., Qavi A. H., Kim Y., Basak P., Jesmajian S. Intracranial metastasis from prostate adenocarcinoma: a case report and literature review. Journal of Community Hospital Internal Medicine Perspectives . 2020;10(6):583–586. doi: 10.1080/20009666.2020.1811069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchand Crety C., Ettalhaoui L., Servagi Vernat S. Cystic brain metastasis from prostate cancer: a case report and literature review. Urology Case Reports . 2020;32 doi: 10.1016/j.eucr.2020.101219.101219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross M. I., Bird N., Mendichovszky I. A., Rimmer Y. L. Neurologically asymptomatic cerebral oligometastatic prostate carcinoma metastasis identified on [Ga]Ga-THP-PSMA PET/CT. EJNMMI Research . 2020;10(1):p. 108. doi: 10.1186/s13550-020-00696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shida Y., Hakariya T., Miyata Y., Sakai H. Three cases of brain metastasis from castration-resistant prostate cancer. Clin Case Rep . 2020;8(1):96–99. doi: 10.1002/ccr3.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son Y., Chialastri P., Scali J. T., Mueller T. J. Metastatic adenocarcinoma of the prostate to the brain initially suspected as meningioma by magnetic resonance imaging. Cureus . 2020;12(12) doi: 10.7759/cureus.12285.e12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livings C., Uemura M., Patel R., Afshar M. Nocardia farcinica masquerading as intracerebral metastases in advanced metastatic prostatic cancer. BMJ Case Reports . 2020;13(9) doi: 10.1136/bcr-2019-233678.e233678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aljarba S. I., Murad M., Bafaquh M., Alshakweer W. Brain metastasis from large cell neuroendocrine carcinoma of the prostate: a case report and literature review. International Journal of Surgery Case Reports . 2020;67:245–249. doi: 10.1016/j.ijscr.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aleksić V. Dural metastasis of prostate carcinoma misdiagnosed as a bilateral subdural hematoma: a case report. Neurocirugia . 2020;20 doi: 10.1016/j.neucir.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine . 2009;6(7) doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quality N. I. H. Assessment tool for observational studies. 2013. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Available from:
- 36.Sena L. A., Salles D. C., Engle E. L., et al. Mismatch repair-deficient prostate cancer with parenchymal brain metastases treated with immune checkpoint blockade. Cold Spring Harb Mol Case Stud . 2021;7(4) doi: 10.1101/mcs.a006094.a006094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pikis S., Bunevicius A., Lee C. C., et al. Stereotactic radiosurgery for prostate cancer cerebral metastases: an international multicenter study. Journal of Neurosurgery . 2021;136:1–7. doi: 10.3171/2021.4.JNS21246. [DOI] [PubMed] [Google Scholar]
- 38.Parihar A. S., Chandekar K. R., Singh H., Sood A., Mittal B. R. Orbital and brain metastases on (68)Ga-PSMA PET/CT in a patient with prostate carcinoma refractory to (177)Lu-PSMA and (225)Ac-PSMA therapy. Asia Ocean J Nucl Med Biol . 2021;9(1):67–70. doi: 10.22038/AOJNMB.2020.50820.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung S. C., Chang L. W., Li J. R., et al. Docetaxel rechallenge improves survival in patients with metastatic castration-resistant prostate cancer: a retrospective study. In Vivo . 2021;35(6):3509–3519. doi: 10.21873/invivo.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boxley P. J., Smith D. E., Gao D., et al. Prostate cancer central nervous system metastasis in a contemporary cohort. Clinical Genitourinary Cancer . 2021;19(3):217–222.e1. doi: 10.1016/j.clgc.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Saadatpour Z., Rezaei A., Singhal A., Sotoudeh H., Tavakol K. A solitary hypothalamic metastasis from prostatic cancer mimicking a giant thrombotic aneurysm and presenting with intraventricular hemorrhage and acute hydrocephalus: a case report. Egyptian Journal of Radiology and Nuclear Medicine . 2020;51(1):p. 257. doi: 10.1186/s43055-020-00367-z. [DOI] [Google Scholar]
- 42.Nguyen T., Bartscht T., Schild S. E., Rades D. Performance status is associated with survival in elderly patients irradiated for cerebral metastases from prostate cancer. Anticancer Research . 2020;40(3):1665–1668. doi: 10.21873/anticanres.14117. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad S., Smeeton F., Hayhurst C., Lansdown A. Pituitary metastasis of prostate cancer presenting as a unilateral third nerve palsy. BMJ Case Reports . 2020;13(6) doi: 10.1136/bcr-2020-234550.e234550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanyilmaz G., Aktan M., Benli Yavuz B., Koc M. Brain metastases from prostate cancer: a single-center experience. Turkish Journal of Urology . 2019;45(4):279–283. doi: 10.5152/tud.2018.74555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogan E., Almira-Suarez I., Li S., Collins S. P., Jean W. C. Clinical management of prostate cancer metastasis to pineal gland: case report and review of literature. World Neurosurgery . 2019;122:464–468. doi: 10.1016/j.wneu.2018.11.111. [DOI] [PubMed] [Google Scholar]
- 46.Zanatta J. P., Zanella L., Kurtz G., Gabardo B., Roman A., Pastorello J. Trigeminal cave brain metastasis from prostate adenocarcinoma: case report and review of the literature. Arquivos Brasileiros de Neurocirurgia: Brazilian Neurosurgery . 2018;37(04):330–333. doi: 10.1055/s-0038-1676525. [DOI] [Google Scholar]
- 47.Reinas R., Costa P., Baggen Santos R., et al. Cerebral metastasis as first systemic event in a patient with prostate adenocarcinoma. Arquivos Brasileiros de Neurocirurgia: Brazilian Neurosurgery . 2018;37(01):38–41. doi: 10.1055/s-0038-1623515. [DOI] [Google Scholar]
- 48.Nunno A., Johnson M. D., Wu G., Li Y. M. Metastatic prostate cancer mimicking a subdural hematoma: a case report and literature review. Journal of Clinical Neuroscience . 2018;55:109–112. doi: 10.1016/j.jocn.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 49.Jack M. M., Gattozzi D. A., Arnold P. M. Multiple intraparenchymal cystic brain metastases - a rare manifestation of metastatic adenocarcinoma of the prostate. Interdisciplinary Neurosurgery . 2018;14:53–55. doi: 10.1016/j.inat.2018.04.008. [DOI] [Google Scholar]
- 50.Guraya S. S., Prayson R. A. Metastatic prostatic adenocarcinoma with neuroendocrine differentiation to meningioma. Journal of Clinical Neuroscience . 2016;34:30–32. doi: 10.1016/j.jocn.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Mandaliya H., Sung J., Hill J., Samali R., George M. Prostate cancer: cases of rare presentation and rare metastasis. Case Reports in Oncology . 2015;8(3):526–529. doi: 10.1159/000442045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Placido S. D., Rescigno P., Federico P., et al. Cabazitaxel in castration resistant prostate cancer with brain metastases: 3 case reports. World Journal of Clinical Cases . 2014;2(6):228–231. doi: 10.12998/wjcc.v2.i6.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gzell C. E., Kench J. G., Stockler M. R., Hruby G. Biopsy-proven brain metastases from prostate cancer: a series of four cases with review of the literature. International Urology and Nephrology . 2013;45(3):735–742. doi: 10.1007/s11255-013-0462-7. [DOI] [PubMed] [Google Scholar]
- 54.Caffo O., Veccia A., Fellin G., et al. Frequency of brain metastases from prostate cancer: an 18-yearsingle-institution experience. Journal of Neuro-Oncology . 2013;111(2):163–167. doi: 10.1007/s11060-012-0994-1. [DOI] [PubMed] [Google Scholar]
- 55.Caffo O., Gernone A., Ortega C., et al. Central nervous system metastases from castration-resistant prostate cancer in the docetaxel era. Journal of Neuro-Oncology . 2012;107(1):191–196. doi: 10.1007/s11060-011-0734-y. [DOI] [PubMed] [Google Scholar]
- 56.Flannery T., Kano H., Niranjan A., et al. Stereotactic radiosurgery as a therapeutic strategy for intracranial metastatic prostate carcinoma. Journal of Neuro-Oncology . 2010;96(3):369–374. doi: 10.1007/s11060-009-9966-5. [DOI] [PubMed] [Google Scholar]
- 57.Yamada T., Ohtsubo K., Mouri H., et al. Combined chemotherapy with carboplatin plus irinotecan showed favorable efficacy in a patient with relapsed small cell carcinoma of the prostate complicated with meningeal carcinomatosis. International Journal of Clinical Oncology . 2009;14(5):468–472. doi: 10.1007/s10147-008-0869-9. [DOI] [PubMed] [Google Scholar]
- 58.Sweets T., Bracken R. B., Geisler E. J., Warnick R. Intracranial treatment for solitary prostatic adenocarcinoma brain metastasis is curative. Urology . 2009;73(3):681.e7–681.e9. doi: 10.1016/j.urology.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 59.Kim S. H., Chao S. T., Toms S. A., et al. Stereotactic radiosurgical treatment of parenchymal brain metastases from prostate adenocarcinoma. Surgical Neurology . 2008;69(6):641–646. doi: 10.1016/j.surneu.2007.05.035. ; discussion 646. [DOI] [PubMed] [Google Scholar]
- 60.Grenader T., Shavit L., Lossos A., Pizov G., Wygoda M. Brain metastases: a rare initial presentation of prostate cancer. International Urology and Nephrology . 2007;39(2):537–539. doi: 10.1007/s11255-006-9065-x. [DOI] [PubMed] [Google Scholar]
- 61.Lyons M. K., Drazkowski J. F., Wong W. W., Fitch T. R., Nelson K. D. Metastatic prostate carcinoma mimicking meningioma - case report and review of the literature. The Neurologist . 2006;12(1):48–52. doi: 10.1097/01.nrl.0000186809.04283.17. [DOI] [PubMed] [Google Scholar]
- 62.Wullich B., Riedinger S., Brinck U., et al. Evidence for gains at 15q and 20q in brain metastases of prostate cancer. Cancer Genetics and Cytogenetics . 2004;154(2):119–123. doi: 10.1016/j.cancergencyto.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Schoenwaelder M., Waugh J., Russell P. Cerebellar metastases from prostatic carcinoma. Australasian Radiology . 2004;48(3):430–433. doi: 10.1111/j.0004-8461.2004.01335.x. [DOI] [PubMed] [Google Scholar]
- 64.Erasmus C. E., Verhagen W. I., Wauters C. A., Lindert E. Jv. Brain metastasis from prostate small cell carcinoma: not to be neglected. The Canadian Journal of Neurological Sciences . 2002;29(4):375–377. doi: 10.1017/s0317167100002250. [DOI] [PubMed] [Google Scholar]
- 65.Minami H., Kanagawa K., Watanabe Y., et al. Complete remission of brain metastases from prostate cancer by gamma knife radiosurgery: a case report. Hinyokika kiyo. Acta urologica Japonica . 2001;47(5):333–336. [PubMed] [Google Scholar]
- 66.Behrens B., Husain M. M., Schmidley J. W. Cystic solitary intracerebral metastasis from prostate adenocarcinoma. Neuroradiology . 2001;43(2):162–164. doi: 10.1007/s002340000509. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Morales F., Chengazi V. U., O’Mara R. E. Detection of brain metastasis with indium-111-capromab pendetide (ProstaScint) due to prostatic carcinoma. Urology . 2000;55(2):286xiii–286xv. doi: 10.1016/s0090-4295(99)00426-4. [DOI] [Google Scholar]
- 68.Fervenza F. C., Wolanskyj A. P., Eklund H. E., Richardson R. L. Brain metastasis: an unusual complication from prostatic adenocarcinoma. Mayo Clinic Proceedings . 2000;75(1):79–82. doi: 10.4065/75.1.79. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi T., Igarashi K., Tanizawa A., Terada Y., Sekine H. Brain metastasis as a sole recurrence of prostate cancer after total prostatectomy. Urologia Internationalis . 1998;60(2):121–123. doi: 10.1159/000030225. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X., Tsukuda F., Yamamoto N., Takenaka I. Brain metastasis from prostate cancer: a case report. International Journal of Urology . 1997;4(5):519–521. doi: 10.1111/j.1442-2042.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 71.Leibman B. D., Dillioglugil O., Wheeler T. M., Scardino P. T. Distant metastasis after radical prostatectomy in patients without an elevated serum prostate specific antigen level. Cancer . 1995;76(12):2530–2534. doi: 10.1002/1097-0142(19951215)76:12<2530::aid-cncr2820761219>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 72.Bland L. I., Welch W. C., Okawara S. H. Large cystic intraparenchymal brain metastasis from prostate cancer. Neuroradiology . 1992;34(1):70–72. doi: 10.1007/bf00588437. [DOI] [PubMed] [Google Scholar]
- 73.Lynes W. L., Bostwick D. G., Freiha F. S., Stamey T. A. Parenchymal brain metastases from adenocarcinoma of prostate. Urology . 1986;28(4):280–287. doi: 10.1016/0090-4295(86)90005-1. [DOI] [PubMed] [Google Scholar]
- 74.Sarma D. P., Godeau L. Brain metastasis from prostatic cancer. Journal of Surgical Oncology . 1983;23(3):173–174. doi: 10.1002/jso.2930230310. [DOI] [PubMed] [Google Scholar]
- 75.Wang G., Zhao D., Spring D. J., DePinho R. A. Genetics and biology of prostate cancer. Genes & Development . 2018;32(17-18):1105–1140. doi: 10.1101/gad.315739.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salvati M., Frati A., Russo N., et al. Brain metastasis from prostate cancer. Report of 13 cases and critical analysis of the literature. Journal of Experimental & Clinical Cancer Research . 2005;24(2):203–207. [PubMed] [Google Scholar]
- 77.Caffo O., Veccia A., Russo L., Galligioni E. Brain metastases from prostate cancer: an emerging clinical problem with implications for the future therapeutic scenario. Future Oncology . 2012;8(12):1585–1595. doi: 10.2217/fon.12.156. [DOI] [PubMed] [Google Scholar]
- 78.Catane R., Kaufman J., West C., Merrin C., Tsukada Y., Murphy G. P. Brain metastasis from prostatic carcinoma. Cancer . 1976;38(6):2583–2587. doi: 10.1002/1097-0142(197612)38:6<2583::aid-cncr2820380652>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 79.de Oliveira Barros E. G., Meireles Da Costa N., Palmero C. Y., Ribeiro Pinto L. F., Nasciutti L. E., Palumbo A. Malignant invasion of the central nervous system: the hidden face of a poorly understood outcome of prostate cancer. World Journal of Urology . 2018;36(12):2009–2019. doi: 10.1007/s00345-018-2392-6. [DOI] [PubMed] [Google Scholar]
- 80.Posner J. B., Chernik N. L. Intracranial metastases from systemic cancer. Advances in Neurology . 1978;19:579–592. [PubMed] [Google Scholar]
- 81.Posner J. B. Brain metastases: 1995. A brief review. Journal of neuro-oncology . 1996;27(3):287–293. doi: 10.1007/bf00165486. [DOI] [PubMed] [Google Scholar]
- 82.Taylor H. G., Lefkowitz M., Coggin J. T., Skoog S. J., Miles B. J., McLeod D. G. Intracranial metastases in prostate cancer. Cancer . 1984;53(12):2728–2730. doi: 10.1002/1097-0142(19840615)53:12<2728::aid-cncr2820531231>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 83.Wilson G., Rupp C. Metastatic tumors of the central nervous system. American Practitioner and Digest of Treatment . 1949;3(6):350–352. [PubMed] [Google Scholar]
- 84.Varkarakis M., Winterberger A., Gaeta J., Moore R., Murphy G. Lung metastases in prostatic carcinoma: clinical significance. Urology . 1974;3(4):447–452. doi: 10.1016/s0090-4295(74)80160-3. [DOI] [PubMed] [Google Scholar]
- 85.Silvestri G. A., Gould M. K., Margolis M. L., et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines. Chest . 2007;132(3):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 86.Fink K. R., Fink J. R. Imaging of brain metastases. Surgical Neurology International . 2013;4(5):p. S209. doi: 10.4103/2152-7806.111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barajas R. F., Jr, Cha S. Imaging diagnosis of brain metastasis. Progress in Neurological Surgery . 2012;25:55–73. doi: 10.1159/000331174. [DOI] [PubMed] [Google Scholar]
- 88.Soffietti R., Cornu P., Delattre J. Y., et al. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. European Journal of Neurology . 2006;13(7):674–681. doi: 10.1111/j.1468-1331.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- 89.Gandaglia G., Abdollah F., Schiffmann J., et al. Distribution of metastatic sites in patients with prostate cancer: a population‐based analysis. The Prostate . 2014;74(2):210–216. doi: 10.1002/pros.22742. [DOI] [PubMed] [Google Scholar]
- 90.Nevedomskaya E., Baumgart S. J., Haendler B. Recent advances in prostate cancer treatment and drug discovery. International Journal of Molecular Sciences . 2018;19(5):p. 1359. doi: 10.3390/ijms19051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grossman Z. D. Cost-effective Diagnostic Imaging . Amsterdam, The Netherlands: Elsevier; 2006. [Google Scholar]
- 92.Nakase H., Sakaki T., Fujita T., et al. Multiple calcified metastatic brain tumor. Neurologia Medico-Chirurgica . 1991;31(12):787–791. doi: 10.2176/nmc.31.787. [DOI] [PubMed] [Google Scholar]
- 93.Blatt D. R., Friedman W. A., Agee O. F. Delayed computed tomography contrast enhancement patterns in biopsy proven cases. Neurosurgery . 1993;32(4):560–569. doi: 10.1097/00006123-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 94.Shalen P. R., Hayman L. A., Wallace S., Handel S. F. Protocol for delayed contrast enhancement in computed tomography of cerebral neoplasia. Radiology . 1981;139(2):397–402. doi: 10.1148/radiology.139.2.7220885. [DOI] [PubMed] [Google Scholar]
- 95.Sidhu K., Cooper P., Ramani R., Schwartz M., Franssen E., Davey P. Delineation of brain metastases on CT images for planning radiosurgery: concerns regarding accuracy. British Journal of Radiology . 2004;77(913):39–42. doi: 10.1259/bjr/68080920. [DOI] [PubMed] [Google Scholar]
- 96.Hakyemez B., Erdogan C., Gokalp G., Dusak A., Parlak M. Solitary metastases and high-grade gliomas: radiological differentiation by morphometric analysis and perfusion-weighted MRI. Clinical Radiology . 2010;65(1):15–20. doi: 10.1016/j.crad.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Nagai A., Shibamoto Y., Mori Y., Hashizume C., Hagiwara M., Kobayashi T. Increases in the number of brain metastases detected at frame-fixed, thin-slice MRI for gamma knife surgery planning. Neuro-Oncology . 2010;12(11):1187–1192. doi: 10.1093/neuonc/noq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Delattre J. Y., Krol G., Thaler H. T., Posner J. B. Distribution of brain metastases. Archives of Neurology . 1988;45(7):741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 99.Hwang T. L., Close T. P., Grego J. M., Brannon W. L., Gonzales F. Predilection of brain metastasis in gray and white matter junction and vascular border zones. Cancer . 1996;77(8):1551–1555. doi: 10.1002/(sici)1097-0142(19960415)77:8<1551::aid-cncr19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 100.Osborn A. G. Diagnostic Imaging: Brain E-Book . Amsterdam, The Netherlands: Elsevier Health Sciences; 2015. [Google Scholar]
- 101.Potts D. G., Abbott G. F., von Sneidern J. V. National Cancer Institute study: evaluation of computed tomography in the diagnosis of intracranial neoplasms. III. Metastatic tumors. Radiology . 1980;136(3):657–664. doi: 10.1148/radiology.136.3.7403544. [DOI] [PubMed] [Google Scholar]
- 102.Meyer P. C., Reah T. G. Secondary neoplasms of the central nervous system and meninges. British Journal of Cancer . 1953;7(4):438–448. doi: 10.1038/bjc.1953.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nadal R., Schweizer M., Kryvenko O. N., Epstein J. I., Eisenberger M. A. Small cell carcinoma of the prostate. Nature Reviews Urology . 2014;11(4):213–219. doi: 10.1038/nrurol.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Böhm M., Gerlach R., Beecken W. D., Scheuer T., Stier-Bruck I., Scharrer I. ADAMTS-13 activity in patients with brain and prostate tumors is mildly reduced, but not correlated to stage of malignancy and metastasis. Thrombosis Research . 2003;111(1-2):33–37. doi: 10.1016/j.thromres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 105.Berg K. D., Thomsen F. B., Mikkelsen M. K., et al. Improved survival for patients with de novo metastatic prostate cancer in the last 20 years. European Journal of Cancer . 2017;72:20–27. doi: 10.1016/j.ejca.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 106.Tannock I. F., de Wit R., Berry W. R., et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New England Journal of Medicine . 2004;351(15):1502–1512. doi: 10.1056/nejmoa040720. [DOI] [PubMed] [Google Scholar]
- 107.Lin X., DeAngelis L. M. Treatment of brain metastases. Journal of Clinical Oncology . 2015;33(30):3475–3484. doi: 10.1200/jco.2015.60.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ryken T. C., McDermott M., Robinson P. D., et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. Journal of Neuro-Oncology . 2010;96(1):103–114. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vecht C. J., Hovestadt A., Verbiest H., van Vliet J. J., van Putten W. L. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology . 1994;44(4):675–680. doi: 10.1212/wnl.44.4.675. [DOI] [PubMed] [Google Scholar]
- 110.Hempen C., Weiss E., Hess C. F. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects? Supportive Care in Cancer . 2002;10(4):322–328. doi: 10.1007/s00520-001-0333-0. [DOI] [PubMed] [Google Scholar]
- 111.Khuntia D., Brown P., Li J., Mehta M. P. Whole-brain radiotherapy in the management of brain metastasis. Journal of Clinical Oncology . 2006;24(8):1295–1304. doi: 10.1200/jco.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 112.Sita T. L., Petras K. G., Wafford Q. E., Berendsen M. A., Kruser T. J. Radiotherapy for cranial and brain metastases from prostate cancer: a systematic review. Journal of neuro-oncology . 2017;133(3):531–538. doi: 10.1007/s11060-017-2460-6. [DOI] [PubMed] [Google Scholar]
- 113.Fecci P. E., Champion C. D., Hoj J., et al. The evolving modern management of brain metastasis. Clinical Cancer Research . 2019;25(22):6570–6580. doi: 10.1158/1078-0432.ccr-18-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ganau M., Gallinaro P., Cebula H., et al. Intracranial metastases from prostate carcinoma: classification, management, and prognostication. World Neurosurgery . 2020;134:e559–e565. doi: 10.1016/j.wneu.2019.10.125. [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez-Homs M., Wiesen B., Rizeq M., Randau C., Lloyd G. L. Solitary brain metastasis after recurrent adenocarcinoma of the prostate. The Canadian Journal of Urology . 2021;28(1):10565–10567. [PubMed] [Google Scholar]
- 116.Macedo J., Carneiro E., Ferreira D., et al. Neuroimaging analysis of rare brain metastases from prostate cancer: PS171. Porto Biomedical Journal . 2017;2(5):p. 220. doi: 10.1016/j.pbj.2017.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shou J., Zhang Q., Wang S., Zhang D. The prognosis of different distant metastases pattern in prostate cancer: a population based retrospective study. The Prostate . 2018;78(7):491–497. doi: 10.1002/pros.23492. [DOI] [PubMed] [Google Scholar]
- 118.Cagney D. N., Martin A. M., Catalano P. J., et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro-Oncology . 2017;19(11):1511–1521. doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu W., Bai S., Zhu D., et al. Overexpression of malignant brain tumor domain containing protein 1 predicts a poor prognosis of prostate cancer. Oncology Letters . 2019;17(5):4640–4646. doi: 10.3892/ol.2019.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be provided by the corresponding author on request.


