Abstract
Purpose:
To identify biomarkers for predicting response to anti-VEGF therapy in diabetic macular edema (DME) and evaluate any links between cytokine expression and OCT phenotype.
Design:
IMAGINE DME is a post-hoc image analysis and cytokine expression assessment of the DAVE randomized clinical trial.
Methods:
Subjects were categorized as anatomical Responders or Nonresponders,and within the Responder group as Rebounders and Nonrebounders based on quantitative, longitudinal optical coherence tomography (OCT) criteria. Retinal layer and fluid features were extracted using an OCT machine-learning augmented segmentation platform. Responders were further sub-classified by rapidity of response. Aqueous concentrations of 54 cytokines at multiple timepoints. Expression was compared between Responder groups and correlated with OCT imaging biomarkers.
Results:
Of the 24 eyes studied, 79% were anatomical Responders with 38% Super Responders, 17% Early Responders, 25% Slow Responders. Twenty-one percent were Nonresponders. Super Responders had increased baseline VEGF (880.0 vs 245.4 pg/mL, p=0.012) and decreased MCP-1 (513.3 vs 809.5 pg/mL, 0.042) concentrations compared to Nonresponders. IL-6 (−24.9 vs 442.8 pg/mL, p=0.032) concentrations increased among Nonresponders during therapy. VEGF concentrations correlated with central subfield thickness (r=0.49, p=0.01). Panmacular retinal volume correlated with increased IL-6 (r=0.47, p=0.02) and decreased MCP-1 (r=−0.45, p=0.03). MMP-1 correlated with SRF volume (r=0.50, p=0.01).
Conclusions:
OCT imaging biomarkers correlated with both intraocular cytokines and responsiveness to anti-VEGF therapy, indicating a possible link to underlying pathways and their relevance to DME prognosis. Baseline concentrations of VEGF and MCP-1 are associated with anatomic response to anti-VEGF therapy.
Table of Contents Statement
This study evaluated the prognostic potential of aqueous humor cytokines for predicting anatomic response to intravitreal ranibizumab in patients with diabetic macular edema and additionally evaluated the anatomic-biologic bridge through correlation of OCT imaging biomarkers with cytokine concentrations. The results indicate that VEGF and MCP-1 pre-treatment concentrations differ between eyes likely to experience anatomic response to ranibizumab. Further investigations into intraocular cytokine dynamics may engender effective personalized treatment regimens and prognoses for eyes with DME.
INTRODUCTION
Diabetic macular edema (DME) is the leading cause of visual loss associated with diabetes ahead of proliferative diabetic retinopathy as fluid accumulates in the macula as either intraretinal (IRF) or subretinal fluid (SRF).1 The pathogenesis of DME has been tied to multiple factors including increased oxidative stress, perturbation of the blood-retinal barrier, and subsequent vascular permeability dysfunction.1,2
Vascular endothelial growth factor (VEGF) is a prominent mediator of DME pathophysiology.3 Inhibitors of VEGF have become first-line treatment in DME management after multiple clinical trials including the RISE/RIDE and VISTA/VIVID phase III studies demonstrated significant clinical efficacy compared to prior standard therapies including photocoagulation.4–7 However, importantly these clinical trials revealed that only 31–46% of patients receiving anti-VEGF therapy gained 3 or more lines of vision, while significant proportions of patients have an incomplete response to anti-VEGF therapy anatomically, functionally or both.4–7 Moreover, these data suggest delays in therapy may be associated with irrecoverable vision loss. An improved understanding of the biologic underpinnings of an specific individual patient’s DME may enable personalized management, optimizing visual and anatomic outcomes, which may not always be congruent. Specifically, as intraocular cytokines represent secretion of proteins from the retina, a thorough exploration could distinguish between a phenotype driven predominately by VEGF vs multifactorial inflammatory mediators.1,2 This may provide a unique opportunity for correlation with specific imaging or clinical phenotypes that could facilitate more precise therapeutic decision-making.
Prior analyses of aqueous humor cytokines have provided some insights into which patients are likely to respond to anti-VEGF therapy, but there is substantial heterogeneity in their results.8–10 For VEGF levels alone, previous reports have been conflicting with some identifying baseline differences between eyes classified as responsive or unresponsive to anti-VEGF therapy, while others did not.9,11 Moreover, prior studies have reported conflicting results on whether increased or decreased aqueous humor VEGF is associated with response to anti-VEGF therapy. Interestingly, increased levels of other cytokines including ICAM-1, MCP-1, and IL-6 have been associated with responsiveness to intravitreal ranibizumab.8 To engender effective, timely personalized treatment regimens for DME patients, additional cohort analyses on aqueous humor cytokine profiles are required to clarify literature discrepancies and expand knowledge on the role of cytokines other than VEGF in disease pathophysiology and responsiveness.
Emerging technology now enables higher-order optical coherence tomography (OCT) analysis, including targeted feature extraction through multi-layer segmentation and pathologic feature characterization, including panmacular fluid feature volumetric assessment.12–14 This sophisticated platform generates higher-order parameters such as the Retinal Fluid Index (RFI) which has been correlated with visual outcomes in DME in the VISTA study.13 The use of this technology in tandem with a thorough correlation of aqueous humor cytokines may expand knowledge regarding specific imaging phenotypes, facilitating more precise therapeutic decision-making.
The goals of the current study was to (1) characterize the longitudinal cytokine profile in DME patients receiving intravitreal anti-VEGF therapy and delineate baseline cytokines which may be predictive of anatomic resolution of macular edema and (2) evaluate the association of higher-order OCT features with underlying intraocular cytokine expression and the link to DME treatment response to anti-VEGF therapy.
METHODS
Study Design
The IMAGINE study is a post-hoc study evaluating aqueous cytokine expression with in-depth assessment of the imaging studies obtained throughout the phase I/II DAVE study performed by Brown and colleagues.15 The endpoint of this study was evaluating the baseline cytokine profiles in DME patients for their association with treatment response. The IMAGINE study was determined to be exempt by the Cleveland Clinic’s Institutional Review Board and adhered to the tenants of the Declaration of Helsinki.
Briefly, the DAVE study was a 3-year prospective randomized trial evaluating ranibizumab alone compared to combination therapy with targeted retinal photocoagulation (TRP) to areas of nonperfusion in treatment-naïve eyes with DME. All eyes received 4 doses of monthly 0.3mg ranibizumab injections before starting monthly visits with as needed retreatment based on disease activity (pro re nata (PRN)) for the remainder of the study. Reinjection criteria during the PRN phase was the presence of DME within the foveal depression based on SD-OCT.15 TRP was performed at week 1 in the eyes randomized to that treatment arm to areas of retinal capillary nonperfusion outside the macula with possible retreatment at months 6, 18, and 25. Inclusion criteria for the original study included treatment naïve visually affected DME patients >18 years of age with severe non-proliferative diabetic retinopathy (NPDR) or early proliferative diabetic retinopathy (PDR). Spectral domain optical coherence tomography (SD-OCT) were obtained at baseline and months 1, 2, 3, 5, 6, and 12. Demographic and clinical information including gender, age, years with diabetes mellitus, hemoglobin A1c, and diabetic retinopathy severity were collected at baseline. A detailed description of the study protocol is outlined, as previously described.15
Cytokine Analysis
All study eyes with available concurrent aqueous humor samples from baseline were included in the analysis. Aqueous humor samples were obtained when able at baseline, month 3, and month 12 through paracentesis and frozen and stored at −80 degrees Celsius. Samples underwent commercial multiplex ELISA based assays (RayBioTech) targeting angiogenesis and inflammatory pathways, including measurement of Activin A, Agouti Related Neuropeptide (AgRP), Angiogenin, Angiopoietin-2 (ANG-2), Angiopoietin like 4 (ANGPTL4), Basic Fibroblast Growth Factor (bFGF), Epithelial-Neutrophil Activating Peptide (ENA-78), Growth Related Alpha Protein (GRO), Heparin-binding EGF-like Growth Factor (HB-EGF), Hepatocyte Growth Factor (HGF), Interferon Gamma (IFNg), Insulin-like Growth Factor (IGF-1), Interleukin-1a (IL-1a), Interleukin-2 (IL-2), Interleukin-6 (IL-6), Interleukin-8 (IL-8), Interleukin-17 (IL-17), Interferon Gamma-Induced Protein 10 (IP-10), Leptin, Leukemia Inhibitory Factor (LIF), Monocyte Chemotactic Protein-1 (MCP-1), Platelet Derived Growth Factor Subunit B (PDGF-BB), Phosphatidylinositol Glycan Anchor Biosynthesis Class F (PIGF), C-C Motif Chemokine Ligand 5 (RANTES), Transforming Growth Factor Beta 1 (TGFb1), Tissue Inhibitor Of Metalloproteinases 1 (TIMP-1), Tissue Inhibitor Of Metalloproteinases 2 (TIMP-2), Angiopoietin-1 (ANG-1), Angiostatin, C-X-C Motif Chemokine Ligand 16 (CXCL16), Epidermal Growth Factor (EGF), Fibroblast Growth Factor-4 (FGF-4), Follistatin, Granulocyte Colony Stimulating Factor (G-CSF), Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), C-C Motif Chemokine Ligand 1 (I-309), Interleukin-1b (IL-1b), Interleukin-4 (IL-4), Interleukin-10 (IL-10), Interleukin-12, p40(IL-12p40), Interleukin-12, p70 (IL-12p70), Iterferon-inducible T cell a-Chemoattractant (I-TAC), Monocyte Chemotactic Protein-2 (MCP-2), Monocyte Chemotactic Protein-3 (MCP-3), Monocyte Chemotactic Protein-4 (MCP-4), Matrix Metallopeptidase 1 (MMP-1), Matrix Metallopeptidase 9 (MMP-9), Platelet And Endothelial Cell Adhesion Molecule 1 (PECAM-1), Transforming Growth Factor Alpha (TGFa), Transforming Growth Factor Beta 3 (TGFb3), Tyrosine Kinase With Immunoglobulin Like And EGF Like Domains 1 (Tie-1), Tyrosine Kinase With Immunoglobulin Like And EGF Like Domains 2 (Tie-2), Plasminogen Activator, Urokinase Receptor (uPAR), VEGF. Concentrations of the cytokines were measured at each time-point in quadruplicate, averaged, normalized, and values below the limit of detection (LOD) for each cytokine were forced to 0. For this report, only cytokines with a detectable level in at least 20% or more samples were included in analysis.
Categorization of Eyes by Anatomical Response and Rebound Profiles
SD-OCT macular cube scans (Spectralis, Heidelberg) were uploaded into a previously described software platform that enables linear, area, and volumetric features of multiple imaging biomarkers.12–14 Using automated analysis, scans underwent fluid feature extraction and multi-layer segmentation that evaluated IRF, subretinal fluid (SRF), and various retinal layer thickness parameters such as internal limiting membrane (ILM)-retinal pigment epithelium (RPE) and ellipsoid zone (EZ)-RPE with manual correction as needed. An image analyst reviewed each B-scan for segmentation accuracy in retinal layers and fluid segmentation. A secondary quality control pass was performed to evaluate imaging and segmentation consistency.
Several quantitative metrics were exported for cytokine correlation including retinal thickness parameters (ILM-RPE) and outer retina parameters (EZ-RPE) that evaluated panmacular and central subfield regions. In addition, intraretinal and subretinal volumes were extracted with similar regional stratification. Previously described Retinal Fluid Indices (RFI) that calculate the ratio of fluid to tissue in either the entire macular cube or subfield were also exported.13
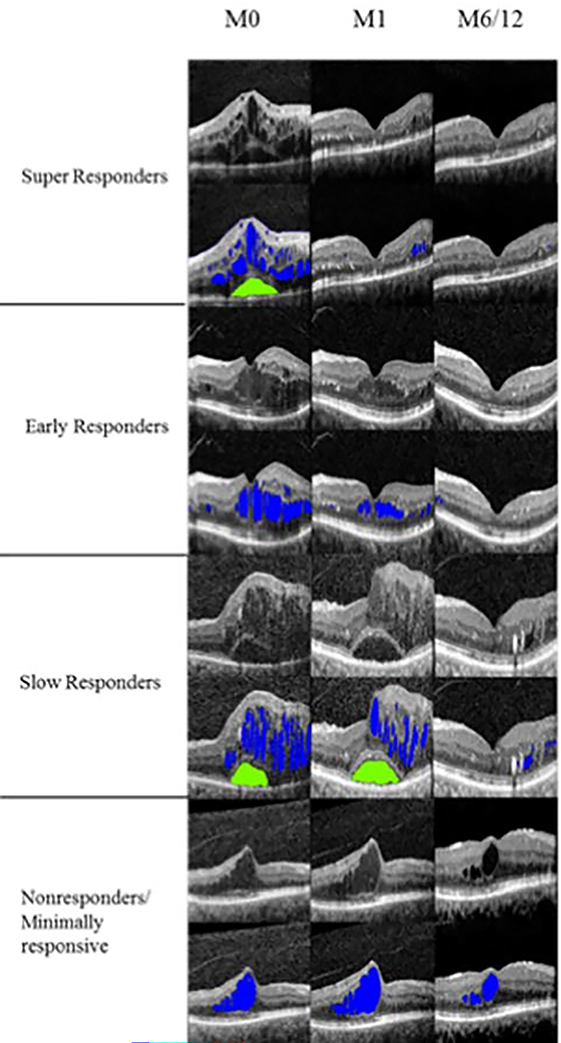
Eyes were categorized into anatomical Responder groups by the following criteria: 1) Super Responders were those eyes in which IRF volume reduced by over 80% or to <0.001mm3 after 1 injection and/or an 80% reduction of excess thickening of the central subfield thickness (CST). Excess thickening was defined as CST greater than 300μm, 2) Early Responders were those eyes who met these criteria after the 3rd injection at month 3, 3) Slow Responders were those eyes who met this criteria between months 3 and 12, 4) Non-Responders/Minimal Responders were those eyes that maintained >50% of CST excess and/or IRF >50% of initial volume by 12 months of treatment. 5) Indeterminate were those eyes that met none of the above criteria (Figure 1).
Figure 1.
Selection of representative foveal B-scans from Responder categories with volumetric fluid segmentation off (first row) and on (second row) for each classification taken at months 0, 1, and 6 or 12. Color distinguishes intraretinal (blue) and subretinal (green) fluid.
Eyes that were within the anatomical Super Responder, Early Responder, or Slow Responder groups were treated as Rebounders if they experienced worsening of 50% of resolved IRF volume or 50% of maximum CST reduction over the treatment course.
Statistical Analysis
Due to several cytokines having non-normal distributions assessed by Shapiro-Wilk tests and small group sample sizes, non-parametric testing was used throughout to calculate p-values. Longitudinal changes in aqueous humor cytokine concentrations from baseline to month 3 or 12 were compared using Wilcoxon Signed Rank tests. Comparisons between mean cytokine levels within responder and rebounder groups were performed using Mann Whitney U tests. Spearman’s Correlation Coefficients were used to measure the association between exported imaging features and cytokine concentrations at baseline. The effect of ranibizumab monotherapy versus combination therapy ranibizumab with TRP and baseline diabetic profiling (HA1c) were evaluated for their impact on aqueous cytokine dynamics using Mann Whitney U testing and Spearman’s correlation respectively. A p-value below 0.05 was considered significant. All statistical analyses were conducted using R.
RESULTS
Baseline Clinical Characteristics and Demographics
In total of the 40 eyes studied in the DAVE trial, 24 eyes from 20 patients (17 men, 3 women) who had sufficient baseline aqueous humor samples for analysis were included in the study (Table 1). The mean age at presentation was 54.6 ± 9.1 years (range: 31 to 75). Eleven eyes (46%) received anti-VEGF monotherapy with ranibizumab, while thirteen eyes (54%) received ranibizumab and TRP. The average HA1c pre-treatment was 8.2% ± 2.2%, while 13.0 ± 8.1 was the mean years with diabetes mellitus. Twelve of the patients had severe NPDR (50%), 4 had mild-moderate NPDR (17%), and 8 had PDR (33%). The mean baseline best-corrected visual acuity was 58 ± 13 measured by ETDRS letters. In the first 12 months of treatment that this study evaluated, patients received ranibizumab injections in 244/261 visits (93%).
Table 1.
Demographic characteristics of All eyes, Responders, including subtypes, and Nonresponders.
| Parameter | All (n=24) | Responders (n = 19) | Super Responders (n=9) | Early Responders (n=4) | Slow Responders (n=6) | Nonresponders (n = 5) |
|---|---|---|---|---|---|---|
|
| ||||||
| Age (y), mean (SD) | 54.6 (9.1) | 51.5 (8.5) | 47.2 (7.8) | 10.5 (55.5) | 55.2 (5.8) | 66.6 (5.7) |
| Male sex, n (%) | 17 (85.0) | 17 (89.5) | 9 (100) | 3 (75) | 5 (83) | 2 (40.0) |
| Right eye, n (%) | 10 (50) | 7 (36.8) | 3 (33) | 1 (25%) | 3 (50) | 3 (60.0) |
| Hemoglobin A1c (%), mean (SD) | 8.2 (2.2) | 8.4 (2.1) | 8.5 (2.3) | 8.7 (3.0) | 8.2 (2.4) | 7.3 (2.5) |
| ETDRS BCVA, mean (SD) | 58 (13.0) | 60 (11.5) | 53 (12.6) | 65 (17.1) | 61 (7.0) | 50 (16.3) |
| Severity of retinopathy, n (%) | ||||||
| Mild NPDR | 1 (4.2) | 0 (0) | 0 | 0 (0) | 0 (0) | 1 (20) |
| Moderate NPDR | 3 (12.5) | 3 (15.8) | 2 (22.2) | 0 (0) | 1 (16.7) | 0 (0) |
| Severe NPDR | 12 (50.0) | 10 (52.6) | 2 (22.2) | 4 (100) | 4 (66.7) | 2 (40) |
| PDR | 8 (3.3) | 6 (31.5) | 5 (55.6) | 0 (0) | 1 (16.7) | 2 (40) |
Baseline and Longitudinal Cytokine Dynamics
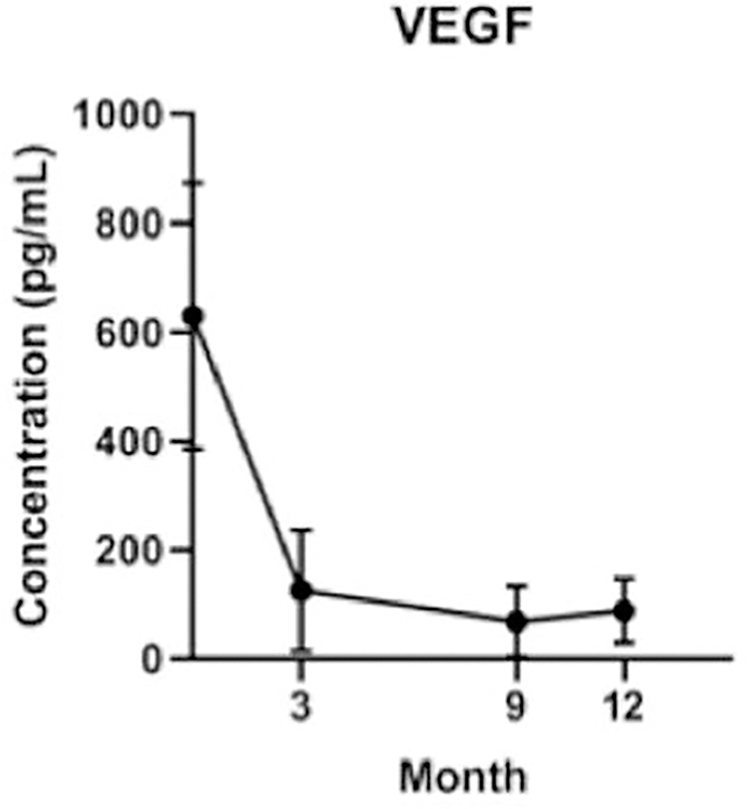
Of the 54 cytokines evaluated, 27 had levels above detection threshold across all visits. Across all eyes, mean VEGF concentrations were significantly lower at month 3 (n=16, p<0.001) and month 12 (n=15, p<0.001) following initiation of treatment (Figure 2). Mean MMP-1 increased at 12 months following treatment (p=0.036), while IP-10 trended toward a significant elevation at month 12 (p=0.065) and mean MCP-1 trended towards decreased levels (p=0.073). There were no significant differences or trends observed among the concentrations of the other detected cytokines (Table 2).
Figure 2.
Longitudinal mean VEGF concentrations across all eyes from baseline to month 12 of treatment showing a significant drop in VEGF concentrations after treatment initiation. Error bars represent 95% confidence intervals.
Table 2.
Longitudinal cytokine concentrations among all patients.
| Cytokine: mean pg/mL (SD)1 | Baseline | Month 3 | Month 9 | Month 12 |
|---|---|---|---|---|
|
| ||||
| AgRP | 3.64 (4.0) | 3.32 (2.94) | 4.79 (3.91) | 4.32 (4.54) |
| ANG-1 | 36.67 (56.76) | 26.82 (43.43) | 29.43 (46.17) | 58.78 (90.58) |
| Angiogenin | 6249.07 (991.63) | 6514.71 (1359.58) | 6499.98 (845.77) | 6629.58 (1057.5) |
| ANGPTL4 | 1198.25 (1637.5) | 1136.1 (2836.2) | 478.6 (574.31) | 1686.06 (3677.1) |
| bFGF | 1.4 (2.53) | 1.82 (3.54) | 0.78 (2.41) | 1.55 (2.11) |
| CXCL16 | 642.4 (316.77) | 557.18 (304.51) | 654.13 (368.06) | 712.51 (348.36) |
| EGF | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) | 0.03 (0.09) |
| FGF-4 | 49.59 (169.01) | 78.58 (229.75) | 44.19 (101.79) | 0 (0) |
| G-CSF | 20.03 (20.99) | 13.36 (16.59) | 32.48 (92.76) | 29.26 (65.21) |
| HGF | 245.71 (176.41) | 232.47 (255.51) | 204.79 (135.58) | 309.55 (355.96) |
| I-309 | 142.28 (188.53) | 79.8 (144.52) | 117.51 (166.88) | 118.59 (179.54) |
| IL-12p40 | 6.07 (5.18) | 6.23 (6.09) | 6.3 (5.5) | 7.1 (5.21) |
| IL-12p70 | 1.04 (1.59) | 0.72 (1.36) | 0.82 (1.75) | 0.82 (1.9) |
| IL-6 | 18.86 (44.09) | 32.14 (110.23) | 54.01 (83.71) | 125.15 (447.91) |
| IP-10 | 9.74 (11.83) | 15.81 (32.11) | 14.5 (19.43) | 41.39 (65.38) |
| Leptin | 35.94 (89.92) | 41.9 (93.19) | 174.02 (464.2) | 123.04 (338.02) |
| LIF | 24.87 (32.34) | 29.68 (34.18) | 18.82 (32.87) | 30.07 (39.13) |
| MCP-1 | 490.63 (562.15) | 382.72 (433.31) | 366.18 (426.89) | 400.68 (428.24) |
| MCP-4 | 2.4 (2.97) | 3.44 (8.03) | 3.54 (5.12) | 1.51 (2.6) |
| MMP-1 * | 25.82 (91.91) | 57.13 (129.03) | 56.88 (139.53) | 166.13 (318.19) |
| MMP-9 | 95.78 (160.13) | 100 (264.61) | 88.05 (153.67) | 109.05 (198.23) |
| PECAM-1 | 2443.11 (2015.2) | 1702.11 (2090.6) | 2166.63 (2477.4) | 1902.03 (2290.5) |
| TGFa | 0.04 (0.05) | 0.05 (0.06) | 0.04 (0.04) | 0.06 (0.08) |
| TIMP-1 | 15571.16 (3122.9) | 15241.85 (4259.78) | 15432.98 (2852.99) | 16573.56 (2142.5) |
| TIMP-2 | 10864.71 (3914.5) | 9262.07 (4341.2) | 10537.22 (4007.2) | 11527 (4083.5) |
| uPAR | 355.8 (200.2) | 361.65 (263.06) | 396.66 (389.01) | 485.65 (478.72) |
| VEGF * | 631.09 (578.52) | 126.44 (218.03) | 68.67 (135.22) | 88.69 (131.15) |
represents a significant change in concentration in Wilcoxon signed rank testing from baseline at p < 0.05.
Standard deviation (SD), Agouti Related Neuropeptide (AgRP), Angiogenin, Angiopoietin like 4 (ANGPTL4), Basic Fibroblast Growth Factor (bFGF), Hepatocyte Growth Factor (HGF), Interleukin-6 (IL-6), Interferon Gamma-Induced Protein 10 (IP-10), Leukemia Inhibitory Factor (LIF), Monocyte Chemotactic Protein-1 (MCP-1), Tissue Inhibitor Of Metalloproteinases 1 (TIMP-1), Tissue Inhibitor Of Metalloproteinases 2 (TIMP-2), Angiopoietin-1 (ANG-1), C-X-C Motif Chemokine Ligand 16 (CXCL16), Epidermal Growth Factor (EGF), Fibroblast Growth Factor-4 (FGF-4), Follistatin, Granulocyte Colony Stimulating Factor (G-CSF), C-C Motif Chemokine Ligand 1 (I-309), Interleukin-12, p40(IL-12p40), Interleukin-12, p70 (IL-12p70), Monocyte Chemotactic Protein-4 (MCP-4), Matrix Metallopeptidase 1 (MMP-1), Matrix Metallopeptidase 9 (MMP-9), Platelet And Endothelial Cell Adhesion Molecule 1 (PECAM-1), Transforming Growth Factor Alpha (TGFa), Plasminogen Activator, Urokinase Receptor (uPAR), Vascular Endothelial Growth Factor (VEGF).
At month 12, subjects receiving combination therapy (n=13) with TRP had increased mean uPAR (p=0.05) and TGFa (p=0.026) compared to ranibizumab alone (n=11). There was no significant difference in reduction in mean VEGF between the groups at month 12 (p=0.10). HA1c at baseline correlated with increased levels of I-309 (p=0.044), increased MCP-4 (p=0.013), and decreased TIMP-1 (p=0.013). There were no differences between HA1c at baseline between anatomical Responders and Nonresponders.
Baseline OCT Imaging Biomarkers and Cytokine Expression
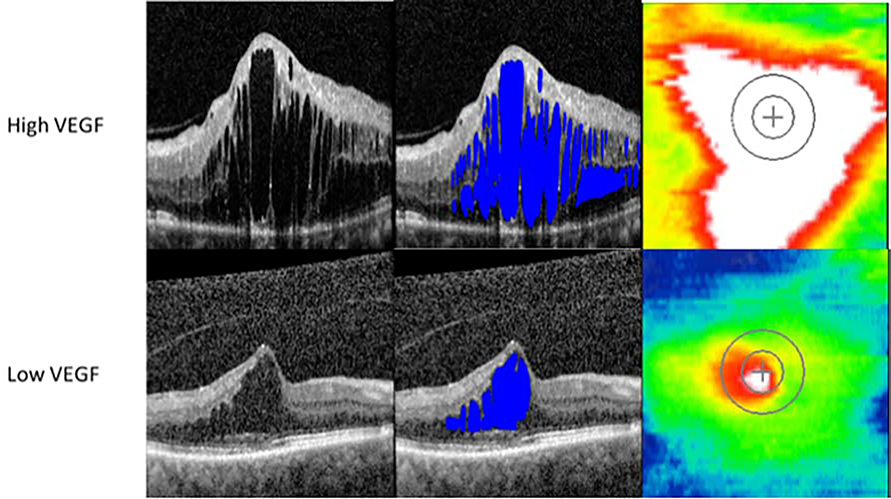
VEGF correlated with CST (r=0.49, p=0.01) and trended toward significance with multiple fluid parameters including Macular IRF Index and Central Macular IRF Index (p<0.1) (Figure 3). Panmacular retinal volume correlated with increased IL-6 (r=0.47, p=0.02) and decreased MCP-1 (r=−0.45, p=0.03). Increased PECAM-1 was associated with decreased IRF Central Subfield Volume (r=−0.42, p=0.04), decreased Central Macular IRF Index (r=−0.42, p=0.04), and decreased Central Subfield Total RFI (r=−0.44, p=0.03). There was additionally a trend towards an association between IRF Central Subfield Volume with IL-6 (p=0.13). Multiple SRF parameters correlated with MMP-1 including Total Macular SRF Index (r=0.50, p=0.01), Central Macular SRF Index (r=0.48, p=0.02), Central Subfield SRF Index (r=0.45, p=0.03) and SRF Central Subfield Volume (r=0.45, p=0.03).
Figure 3.
Representative optical coherence tomography foveal B-scans from high and low VEGF eyes without volumetric fluid segmentation (first column), with fluid segmentation (second column), and retinal thickness maps (third column) taken at baseline.
Multiple cytokines correlated with panmacular EZ attenuation, including ANGPTL4 (r=0.57, p=0.003), bFGF (r=0.48, p=0.02), FGF-4 (r=−0.48, p=0.02), LIF (r=0.46, p=0.02), and HGF (r=0.45, p=0.03). In addition, increased central subfield EZ-RPE volume correlated with decreased CXCL16 (r=−0.51, p=0.01), HGF (r=−0.44, p=0.03), and VEGF-A (r=−0.44, p=0.04). Panmacular EZ-RPE volume similarly correlated with decreased ANGPTL4 (r=−0.48, p=0.02), uPAR (r=−0.41, p=0.05), and CXCL16 (r=−0.41, p=0.05).
Anatomical Responders and Non-Responders
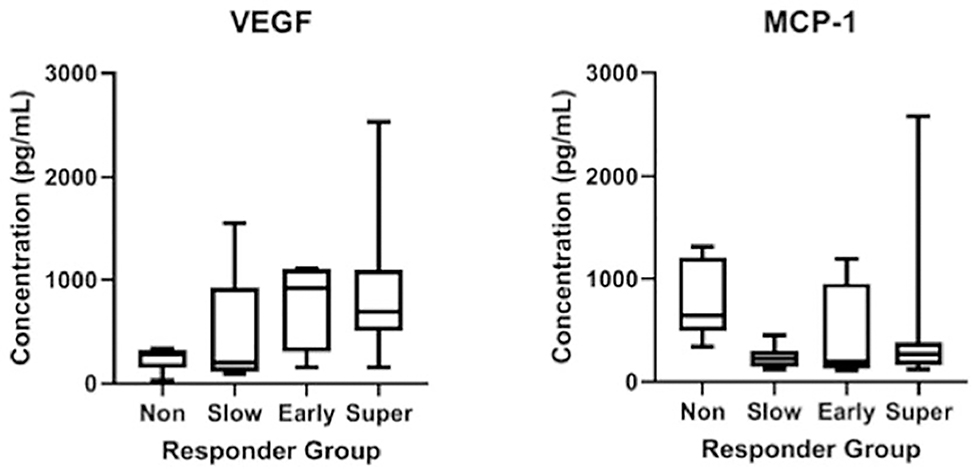
Of the 24 eyes studied, 79% (19/24) of eyes were anatomical Responders split between 38% Super Responders (9/24), 17% Early Responders (4/24), and 25% Slow Responders (6/24), while 21% (5/24) of eyes were Nonresponders. There were no eyes in the indeterminate group. At baseline, anatomical Super Responders had a significantly greater average VEGF (880.0 vs 245.4 pg/mL, p=0.012) and lower average MCP-1 (513.3 vs 809.5 pg/mL, 0.042) concentrations compared to Nonresponders (Figure 4). Aggregating anatomical Super Responders with Early Responders and comparing these to Nonresponders revealed a similar greater baseline mean VEGF (848.2 vs 245.4 pg/mL, p=0.014) in addition to lower mean MCP-1 (486.4 vs 809.5 pg/mL, 0.035) concentrations. When comparing all anatomical Responders to Nonresponders, average VEGF concentration was higher among Responders though this difference did not reach statistical significance (732.6 vs 245.4 pg/mL, p=0.10) while baseline average MCP-1 remained significantly higher in the Nonresponders (406.7 vs 809.5 pg/mL, p=0.0093). Evaluating anatomical Super and Early Responders against Slow Responders and Nonresponders showed increased mean VEGF (848.2 vs 374.5 pg/mL, p=0.018) and increased mean ANGPTL4 (1677.4 vs 632.0 pg/mL, p=0.023) in the former groups. Multiple other cytokines had sizeable effect size differences in this analysis including Leptin, and AgRP that did not reach statistical significance (Supplemental Table 1 at AJO.com).
Figure 4.
Box and whisker plots of baseline VEGF and MCP-1 concentrations by anatomical Responder category visualizing increased VEGF and decreased MCP-1 among anatomical Responders compared to Nonresponders.
Following 3 months of treatment, no significant differences in cytokine changes comparing anatomical Super Responders vs Nonresponders and all anatomical Responders vs Nonresponders in paired analyses were detected. However, at 12 months, Super Responders had a greater reduction in mean VEGF (−737.8 vs −103.9 pg/mL, p=0.009) and mean LIF (−37.5 vs 30.1 pg/mL, p=0.028) concentrations compared to Nonresponders. Comparing all anatomical Responders to Nonresponders at 12 months, the change in mean concentration of VEGF was significantly greater (−683.9 vs −103.9 pg/mL, p=0.005) among the Responders, while mean LIF (−17.7 vs 30.1 pg/mL, p=0.028) and IL-6 (−24.9 vs 442.755 pg/mL, p=0.032) concentrations increased among Nonresponders (Supplemental Table 2 at AJO.com).
OCT characteristics of Anatomical Response Group
When compared against Nonresponders, anatomical Super Responders at baseline had increased panmacular retinal volume (16 mm3 vs 11 mm3, p=0.004), increased Macular Total RFI (12% vs 4%, p=0.02), increased mean retinal CST (614μm vs 360μm, p= 0.03), increased IRF volume (2.0mm3 vs 0.44mm3, p=0.04), and increased Macular IRF Index (11% vs 4%, p=0.04). Comparing all anatomical Responders against Nonresponders, Responder eyes were associated with increased panmacular retinal volume (15 mm3 vs 11 mm3, p=0.003), mean retinal CST (557um vs 360um, p=0.009), and several fluid parameters including IRF volume (1.6 mm3 vs 0.44 mm3, p=0.02), Central Subfield Total RFI (43% vs 12%, p=0.01), and Macular Total RFI (11% vs 4%, p=0.01) (Supplemental Table 3 at AJO.com).
Rebounders and Non-Rebounders
Among the 19 responders, 5 eyes exhibited rebounder behavior as defined in this study. At baseline multiple cytokines differentiated the rebounders from the non-rebounders including increased mean concentrations of TGFa (0.11 vs 0.029 pg/mL, p=0.008), LIF (58.6 vs 13.5 pg/mL, p=0.015), and uPAR (562.2 vs 289.1, p=0.034). Of the rebounder eyes, 4 had cytokines available at follow-up timepoint associated with disease rebounding. There were no statistically significant differences detectable between the cytokines at baseline and at the rebound timepoint. Mean VEGF levels were elevated compared to a follow-up timepoint associated with anatomic response (37.5 pg/mL vs 918.1pg/mL p=0.125).
Baseline SD-OCT parameters were similar between Rebounders and Nonrebounders. However, early macular RFI volatility, defined as an increase macular RFI of > 2.5 points between months 1 and 2 (during the loading phase), was associated with increased risk of rebounding with transition to PRN treatment. In the rebounder group, 50% of eyes demonstrated early macular RFI volatility compared to 0% of eyes in the Nonrebounder group (p=0.02).
DISCUSSION
This study evaluated the longitudinal dynamics of aqueous humor cytokine concentrations in eyes undergoing anti-VEGF therapy with ranibizumab, as well as the link between cytokine levels and therapeutic response defined by reduction in macular edema. In this analysis, anatomical Super Responders and Early Responders to anti-VEGF therapy had significantly higher intraocular mean VEGF at baseline than Non-Responders as well as decreased mean MCP-1. Distinct longitudinal cytokine dynamics was observed between anatomical Responders and Non-Responders in VEGF, LIF, and IL-6 with both LIF and IL-6 increasing in Non-Responders.
In addition, this study investigated the link between higher-order imaging biomarkers from OCT in predicting treatment response in eyes receiving anti-VEGF therapy, as well as the biological underpinnings of the imaging parameters through correlations with aqueous humor cytokine concentrations. Anatomical Super Responders and Responders overall had greater IRF, RFI, and retinal thickness throughout the entire macula and within the central subfield compared to Nonresponders at baseline. Panmacular retinal volume correlated with increased VEGF and IL-6 and decreased MCP-1; PECAM-1 correlated with reduced IRF feature parameters, and MMP-1 correlated with increased SRF parameters. Levels of TGFa, uPAR, and LIF at baseline differentiated eyes more likely to experience rebounding of disease. Glycemic control as measured by HA1c correlated with I-309, MCP-4, and TIMP-1. These findings support the possible utility of baseline aqueous humor cytokine levels in optimizing treatment selection in DME management for predicting anatomic resolution.
Expanding knowledge of biomarkers for treatment response in DME may be important for patient outcomes, treatment burden, and cost effectiveness. The gold standard treatment of DME validated by numerous clinical trials is anti-VEGF therapy, yet substantial proportions of DME patients fail to respond completely functionally or anatomically, represented by 21% of the patients in the current analysis.4,5 Prior studies have reported up to 31–46% of patients with incomplete functional improvement with anti-VEGF therapy.4,6 Time to treatment with anti-VEGF therapy has been associated with extent of visual recovery.16 The cost of ophthalmic use of anti-VEGF therapy to the Medicare Part B budget alone is over $2 billion annually.17 A thorough understanding of an individual patient’s disease phenotype through pre-treatment intraocular cytokine evaluation may engender optimal treatment regimen selection, reducing less effective and costly treatment burden.
Though several studies have assessed the predictive value of aqueous humor cytokines in determining clinical response to anti-VEGF therapy in DME, these reports have yielded heterogeneous and often conflicting results. While Shimura et al8 and Udaondo et al11 reported, similarly to the current analysis, that increased mean VEGF concentration at baseline predicted treatment response, both Felfeli et al18 and Kwon et al9 did not identify baseline VEGF as a predictive factor. Moreover, Hillier et al10 reported that decreased mean VEGF levels were associated with favorable anatomic response to ranibizumab. The causes of these inconsistencies are likely multifactorial including variability in responder categorization, baseline disease severity, analytical power, and heterogeneity in disease phenotypes.
Udaondo et al11 raised the possible impact of baseline and treatment course glycemic control on anti-VEGF therapy response and levels of pre-treatment VEGF. In the current set of patients, there was no significant increase in HA1c at baseline between the anatomical Responders and Nonresponders. Rather, the Nonresponders had a reduced mean HA1c at baseline compared to Responders though this difference was not statistically significant. In contrast to both Udaondo et al11 and Shimura et al8 whose baseline mean VEGF were under 200pg/mL, this cohort overall had a mean VEGF concentration of 631pg/mL, more similar to Hillier et al’s10 and Felfeli et al’s18 cohorts with higher average VEGF levels that yet yielded differing conclusions.
The current study also evaluated cytokine dynamics related to eyes that initially responded to therapy but proceeded to experience a clinically meaningful amount of rebound DME when transitioned to a PRN treatment schedule. At baseline this work identified multiple cytokines involved in inflammation and/or interact with VEGF that differentiated eyes associated with DME rebound including uPAR and LIF. The uPAR protein has been implicated as a regulator in VEGF mediated BRB disruption.19 VEGF levels at the rebound time-points had a substantial effect size difference compared to VEGF levels at time-points associated with anatomic response, though the small sample size impeded the meeting of significance thresholds using non-parametric statistics. Additional investigations into the underpinnings of DME rebounding involving larger numbers of patients may be able to provide more clarity on which patients may be able to be shifted from fixed monthly injections to less frequent, as needed retreatment.
Nearly 2/3 of patients may not achieve a clinically meaningful vision improvement with anti-VEGF therapy highlighting the mixture of drivers underlying the pathophysiology of DME.4–7 In the IMAGINE analysis, the anatomical Responders and the Super Responders more so represent eyes with a more VEGF driven phenotype, while the Nonresponders and to a lesser extent the Slow Responders likely represent a more multifactorial, inflammation-driven phenotype that would benefit from alternate therapy. These results provide evidence of this as anatomical Nonresponders had increased baseline MCP-1, a mediator of inflammation which has been implicated in DME disease progression.20 Moreover, Nonresponders had statistically significant increases in IL-6 and leukemia inhibitory factor (LIF). The former is one of the key activators of proinflammatory pathways, while LIF has notably been implicated in both inflammation and VEGF modulation.21,22
The current study found mixed concordance with prior data on inflammatory drivers. Shimura et al8 found good responders had increased IL-6 and MCP-1 at baseline compared to poor responders contrasting with our results, while Felefil et al18 demonstrated a significant decrease in MCP-1 following therapy. Though MCP-1 decreased in this dataset following treatment, this difference was not statistically significant. Kwon et al9 on the other hand reported that while VEGF did not predict treatment response to anti-VEGF therapy, IL-8 levels did. Multiple studies also identified the possible role of ICAM-1 as an early biomarker of treatment response though this work did not detect ICAM-1 in sufficient quantities for analysis.8,10
Prior investigations into the predictive capacity of OCT in DME outcomes after anti-VEGF therapy have highlighted several imaging features of interest. These include the association between ganglion cell layer thinning and presence of hyperreflective foci.23,24 The depth of information available for phenotype characterization through OCT imaging is rich, and deep learning models have been applied with some success in predicting response to treatment in DME.25,26 To date, no investigation has combined higher order quantitative OCT image analysis with intraocular cytokine assessment.
This study explored the association between imaging biomarkers and underlying signaling molecules at baseline. Several cytokines were associated with either an increased retinal thickness or fluid parameters. In contrast to a prior study,27 VEGF concentrations correlated with baseline CST and trended toward association with fluid indices. IL-6 increased with worsened edema, while MCP-1 decreased. Both have been associated with DME pathogenesis through an increase in retinal permeability, and both IL-6 and MCP-1 levels correlated with response to treatment in a prior study.8,20 Hyperglycemia leads to loss of PECAM-1 in the retinal endothelium which aligns with this data as worse disease by fluid parameters correlated with decreased PECAM-1 whose functions include maintenance of endothelial cell junction integrity.28 Matrix metalloproteinases (MMPs) are hypothesized to mediate that specific effect as well as contribute to blood-retinal-barrier disruption generally.1 MMP-1 in this study correlated with increased SRF with no correlation to IRF providing possible evidence of anatomically localized pathogenic activity.
Aligning with multiple previous studies, the current work affirmed the importance of IRF, particularly within the fovea, as an important marker for response to anti-VEGF.26,29,30 Additionally, prior studies have highlighted the importance of microlocalization and morphology of IRF in relation to predicting impact on responsiveness; IRF height and localization to the outer nuclear layer around the fovea were strongly predictive. The current report adds to these findings by emphasizing the possible utility of IRF indices both throughout the macula and within the central subfield in predicting which eyes will anatomically respond to anti-VEGF. RFI has been shown to correlate with functional outcomes in prior work, and this study further demonstrated its baseline predictive capacity.13 In addition, affirming prior investigations using both qualitative and quantitative methodologies, SRF trended toward differentiation of anatomical Responders and Nonresponders.26,29,31
In our review of the literature, no study to date has correlated outer retina integrity parameters with intraocular cytokines. In this analysis using quantitative EZ features, several cytokines correlated with increased disruption to EZ integrity including VEGF, ANGPTL4, LIF, and HGF. These cytokines in particular have been previously implicated in DME pathogenesis often cooperating with VEGF to promote microvascular permeability.21,32,33 One cytokine with functions in cell survival and cell migration, bFGF, correlated with improved EZ integrity. Whether these correlations are the result of a more general inflammatory perturbation to the outer retina is unclear, but IL-6 and several other mediators of inflammation did not correlate with any EZ parameter. Though no literature was found regarding intraocular cytokines, serum levels of VEGF, anti-myeloperoxidase (MPO) antibody, and I-CAM1 have correlated with EZ disruption in diabetic retinopathy.34,35 The serum VEGF correlation with EZ disruption aligned with this study’s aqueous humor findings. Further investigations into these associations may continue to provide additional insights into DME pathophysiology.
Some eyes also undergo rebounding of disease as treatment durations are increased. In contrast to tortuosity metrics from ultra-widefield fluorescein angiography,36 OCT features at baseline in this study was unable to distinguish these eyes. However, higher order assessment of early fluid volatililty on OCT, specifically an increase in Macular IRF Index between months 1 and 2, may differentiate eyes unable to tolerate treatment interval increases. This notably aligns with a prior analysis of patients enrolled in the VISTA Trial.37 Exploring the mechanisms behind this early treatment response volatility would require a more comprehensive sampling of intraocular cytokines than was performed in this investigation. Specifically, evaluating cytokine dynamics between months 1 and 2 and their association with RFI volatility could provide clinically meaningful insights. This intriguing finding should be further explored in additional datasets as a potential marker for predicting treatment burden and for exploring new therapeutic alternatives in those eyes that do not tolerate treatment interval extension
Though the current study reported multiple findings of interest, there are a number of limitations worth acknowledging. Firstly, the use of aqueous humor cytokine assessment may not completely reflect changes in the posterior segment. However, several studies have shown correlation with vitreous and aqueous humor cytokine levels previously.38–40 As with most of the current reports in the literature evaluating aqueous cytokine data, this study was limited by available sample size and therefore had limited statistical power especially in subgroup assessments; in particular, there were only 5 Nonresponders. For example, ANGPTL4, a molecule that cooperates with VEGF in inducing DME, had a significant effect size difference between anatomical Responders and Nonresponders that did not achieve significance using non-parametric testing.32 Similarly there were insufficient eyes to perform multivariable regression that could control for possible confounding parameters. The pre-defined categorization of treatment response generated Responder groups with significantly more fluid volume than the Nonresponders. Whether this reflects increased fluid on presentation in VEGF driven DME or is a source of bias is unclear. There is some evidence against this being a source of bias as Felfeli et al’s18 cohort found anatomical Nonresponders had increased macular volume compared to Responders. The categorization system also defined response status solely by anatomic features which may not consistently correlate with visual recovery if persistent macular edema caused irrecoverable vision loss. In addition, not all eyes had cytokines available at each time-point. The DAVE trial used ranibizumab which may limit the generalizability of these findings to other VEGF targeting therapies, such as aflibercept and bevacizumab.15,18 Relatedly, multiple isoforms/subtypes of VEGF exist and heterogeneity in assay isoform detection method as the assay used in this study specifically detected the VEGF-A isoforms, VEGF165 and VEGF121. Differences in the isoform specificity of detection platforms, like the multiplexed ELISA immunoassay utilized in this study vs. multiplexed bead based assay methodologies, could explain some of the observed variability in the literature. However, even using the same general detection approach does not account for contrasting literature; for example, both this study and Hillier et al10 utilized a similar multiplex ELISA-based approach with distinct results. Whether VEGF-A bound to ranibizumab is detected by this assay remains unclear which is another important limitation, though there was a significant and intuitive drop in detected VEGF after treatment initiation.
Overall, this work assessed aqueous humor cytokine expression as predictive biomarkers for anatomic treatment response to intravitreal ranibizumab in DME. These results indicate both VEGF and MCP-1 pre-treatment concentrations differ between eyes likely to experience anatomic response to anti-VEGF therapy. This study further characterized the anatomic-biologic bridge identifying correlations between signaling molecules and higher order imaging features from OCT such as RFI. Further research is needed to validate and provide enhanced characterization of the link between OCT features and underlying cytokine expression. The characterization of imaging biomarkers that provide a link to the primary underlying biologic phenotype for a given patient’s DME could engender cost-effective, timely personalized treatment regimens for eyes with DME as additional therapeutic options become available.
Supplementary Material
Acknowledgements/Disclosures
a). Funding/Support:
This work was supported by Regeneron [REGE1901], RPB Unrestricted Grant to the Cole Eye Institute [RPB1508DM], the National Institutes of Health [K23 -EY022947], and the Betty J Powers Retina Research Fellowship. All study decisions were made independently of funding sources.
b). Financial disclosures:
CCW has research support from the following: Adverum, Allergan, Apellis, Clearside, EyePoint, Genentech/Roch, Neurotech, Novartis, Opthea, Regeneron, Regenxbio, Samsung, Santen; is a consultant for the following: Alimera Sciences, Allegro, Allergan, Alynylam, Apellis, Bayer, Clearside, D.O.R.C., EyePoint, Genentech/Roche, Kodiak, Notal Vision, Novartis, ONL Therapeutics, PolyPhotonix, RecensMedical, Regeneron, Regenxbio, Santen; and is a speaker for Regeneron. SKS has research support from Regeneron, Allergan, and Gilead; is a consultant for Bausch and Lomb, Novartis, and Regeneron. DMB has research support from the following: Adverum, Allergan, Apellis, Clearside, Genentech/Roch, Novartis, Opthea, Regeneron, Regenxbio, Samsung, Santen; is a consultant for the following: Regeneron, Bayer, Senju, Allergan, Optos, Zeiss, Heidelberg, OHR, Biotime, Gemini, Genentech/Roche, Novartis, Apellis, Regenxbio, Chengdu Kanghong Biotechnology. JPE has research support from the following: Aerpio, Alcon, Thrombogenics/Oxurion, Regeneron, Genentech, Novartis, Allergan; is a consultant for the following: Aerpio, Alcon, Allegro, Allergan, Genentech/Roche, Novartis, Thrombogenics/Oxurion, Leica, Zeiss, Regeneron, Santen; and holds a patent with Leica. JRA, SA, LL, HJY, MH, JR have no financial disclosures to report.
All authors attest that they meet the current CRediT criteria for authorship.
Biographies
Justis P. Ehlers, MD is the Norman C. and Donna Harbert Endowed Chair for Ophthalmic Research at the Cole Eye Institute at the Cleveland Clinic. He is the Director of the Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research at the Cleveland Clinic.

Joseph Abraham MD is an incoming resident physician at the Cole Eye Institute of Cleveland Clinic. He completed his medical school training at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve School of Medicine and was a Betty J. Powers Retina Research Fellow in the lab of Dr. Justis P. Ehlers.

Footnotes
CRediT author statement
Joseph Abraham: Formal Analysis; Investigation; Writing – Original Draft, Project Administration. Charles Wykoff: Resources; Investigation; Supervision; Writing – Review and Editing. Sruthi Arepalli: Formal Analysis; Writing – Review and Editing; Leina Lunasco: Formal Analysis; Investigation; Project Administration; Writing – Review and Editing; Hannah Yu: Investigation; Writing – Review and Editing. Jamie Reese: Supervision; Project Administration. Sunil Srivastava: Resources; Writing – Review and Editing; David Brown: Resources; Investigation; Writing – Review and Editing Justis Ehlers: Conceptualization; Resources; Methodology; Software; Writing – Review and Editing. Supervision. Project Administration. Funding Acquisition.
Supplemental Material Available at AJO.com
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Das A, McGuire PG, Rangasamy S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology. 2015;122(7):1375–1394. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J Diabetes Res. 2016;2016:2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong TY, Cheung CMG, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015;122(10):2044–2052. [DOI] [PubMed] [Google Scholar]
- 5.Heier JS, Korobelnik J-F, Brown DM, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology. 2016;123(11):2376–2385. [DOI] [PubMed] [Google Scholar]
- 6.Reddy RK, Pieramici DJ, Gune S, et al. Efficacy of Ranibizumab in Eyes with Diabetic Macular Edema and Macular Nonperfusion in RIDE and RISE. Ophthalmology. 2018;125(10):1568–1574. [DOI] [PubMed] [Google Scholar]
- 7.Wykoff CC, Elman MJ, Regillo CD, Ding B, Lu N, Stoilov I. Predictors of Diabetic Macular Edema Treatment Frequency with Ranibizumab During the Open-Label Extension of the RIDE and RISE Trials. Ophthalmology. 2016;123(8):1716–1721. [DOI] [PubMed] [Google Scholar]
- 8.Shimura M, Yasuda K, Motohashi R, Kotake O, Noma H. Aqueous cytokine and growth factor levels indicate response to ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101(11):1518–1523. [DOI] [PubMed] [Google Scholar]
- 9.Kwon J-w, Jee D. Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PLoS One. 2018;13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillier RJ, Ojaimi E, Wong DT, et al. Aqueous Humor Cytokine Levels and Anatomic Response to Intravitreal Ranibizumab in Diabetic Macular Edema. JAMA Ophthalmol. 2018;136(4):382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udaondo P, Hernández C, Briansó-Llort L, García-Delpech S, Simó-Servat O, Simó R. Usefulness of Liquid Biopsy Biomarkers from Aqueous Humor in Predicting Anti-VEGF Response in Diabetic Macular Edema: Results of a Pilot Study. J Clin Med. 2019;8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh Y, Vasanji A, Ehlers JP. Volumetric ellipsoid zone mapping for enhanced visualisation of outer retinal integrity with optical coherence tomography. British Journal of Ophthalmology. 2015:bjophthalmol-2015–307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers JP, Uchida A, Hu M, et al. Higher-Order Assessment of OCT in Diabetic Macular Edema from the VISTA Study: Ellipsoid Zone Dynamics and the Retinal Fluid Index. Ophthalmol Retina. 2019;3(12):1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu D, Yuan A, Kaiser PK, et al. A novel segmentation algorithm for volumetric analysis of macular hole boundaries identified with optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(1):163–169. [DOI] [PubMed] [Google Scholar]
- 15.Brown DM, Ou WC, Wong TP, et al. Targeted Retinal Photocoagulation for Diabetic Macular Edema with Peripheral Retinal Nonperfusion: Three-Year Randomized DAVE Trial. Ophthalmology. 2018;125(5):683–690. [DOI] [PubMed] [Google Scholar]
- 16.Boyer DS, Nguyen QD, Brown DM, et al. Outcomes with As-Needed Ranibizumab after Initial Monthly Therapy: Long-Term Outcomes of the Phase III RIDE and RISE Trials. Ophthalmology. 2015;122(12):2504–2513.e2501. [DOI] [PubMed] [Google Scholar]
- 17.Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR. Cost-Effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema. JAMA Ophthalmol. 2016;134(8):888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felfeli T, Juncal VR, Hillier RJ, et al. Aqueous Humor Cytokines and Long-Term Response to Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema. Am J Ophthalmol. 2019;206:176–183. [DOI] [PubMed] [Google Scholar]
- 19.El-Remessy AB, Franklin T, Ghaley N, et al. Diabetes-induced superoxide anion and breakdown of the blood-retinal barrier: role of the VEGF/uPAR pathway. PLoS One. 2013;8(8):e71868. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 20.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73–79. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Zhang X, Liao N, Wen F. Assessment of biomarkers using multiplex assays in aqueous humor of patients with diabetic retinopathy. BMC Ophthalmol. 2017;17(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota Y, Hirashima M, Kishi K, Stewart CL, Suda T. Leukemia inhibitory factor regulates microvessel density by modulating oxygen-dependent VEGF expression in mice. J Clin Invest. 2008;118(7):2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshitake T, Murakami T, Suzuma K, Dodo Y, Fujimoto M, Tsujikawa A. Hyperreflective Foci in the Outer Retinal Layers as a Predictor of the Functional Efficacy of Ranibizumab for Diabetic Macular Edema. Sci Rep. 2020;10(1):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnin S, Tadayoni R, Erginay A, Massin P, Dupas B. Correlation between ganglion cell layer thinning and poor visual function after resolution of diabetic macular edema. Invest Ophthalmol Vis Sci. 2015;56(2):978–982. [DOI] [PubMed] [Google Scholar]
- 25.Rasti R, Allingham MJ, Mettu PS, et al. Deep learning-based single-shot prediction of differential effects of anti-VEGF treatment in patients with diabetic macular edema. Biomed Opt Express. 2020;11(2):1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerendas BS, Bogunovic H, Sadeghipour A, et al. Computational image analysis for prognosis determination in DME. Vision Res. 2017;139:204–210. [DOI] [PubMed] [Google Scholar]
- 27.Hillier RJ, Ojaimi E, Wong DT, et al. AQUEOUS HUMOR CYTOKINE LEVELS AS BIOMARKERS OF DISEASE SEVERITY IN DIABETIC MACULAR EDEMA. Retina (Philadelphia, Pa). 2017;37(4):761–769. [DOI] [PubMed] [Google Scholar]
- 28.Eshaq RS, Harris NR. Loss of Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) in the Diabetic Retina: Role of Matrix Metalloproteinases. Invest Ophthalmol Vis Sci. 2019;60(2):748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerendas BS, Prager S, Deak G, et al. Predictive imaging biomarkers relevant for functional and anatomical outcomes during ranibizumab therapy of diabetic macular oedema. Br J Ophthalmol. 2018;102(2):195–203. [DOI] [PubMed] [Google Scholar]
- 30.Itoh Y, Petkovsek D, Kaiser PK, Singh RP, Ehlers JP. Optical Coherence Tomography Features in Diabetic Macular Edema and the Impact on Anti-VEGF Response. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bressler SB, Odia I, Maguire MG, et al. Factors Associated With Visual Acuity and Central Subfield Thickness Changes When Treating Diabetic Macular Edema With Anti-Vascular Endothelial Growth Factor Therapy: An Exploratory Analysis of the Protocol T Randomized Clinical Trial. JAMA Ophthalmol. 2019;137(4):382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sodhi A, Ma T, Menon D, et al. Angiopoietin-like 4 binds neuropilins and cooperates with VEGF to induce diabetic macular edema. J Clin Invest. 2019;129(11):4593–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campochiaro PA, Hafiz G, Mir TA, et al. Pro-permeability Factors in Diabetic Macular Edema; the Diabetic Macular Edema Treated With Ozurdex Trial. Am J Ophthalmol. 2016;168:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain A, Saxena S, Khanna VK, Shukla RK, Meyer CH. Status of serum VEGF and ICAM-1 and its association with external limiting membrane and inner segment-outer segment junction disruption in type 2 diabetes mellitus. Mol Vis. 2013;19:1760–1768. [PMC free article] [PubMed] [Google Scholar]
- 35.Sinha S, Saxena S, Prasad S, et al. Association of serum levels of anti-myeloperoxidase antibody with retinal photoreceptor ellipsoid zone disruption in diabetic retinopathy. J Diabetes Complicat. 2017;31(5):864–868. [DOI] [PubMed] [Google Scholar]
- 36.Moosavi A, Figueiredo N, Prasanna P, et al. Predicting Tolerance to Extended Interval Dosing in Diabetic Macular Edema and Retinal Vein Occlusion via Subvisual Feature Assessment of Ultra-widefield Angiography: Preliminary Findings in the PERMEATE study. Invest Ophthalmol Vis Sci. 2019;60(9):1437–1437. [Google Scholar]
- 37.Ehlers JP. “Higher Order Assessment of OCT for Quantitative Retinal Features in the VISTA DME Study.” Angiogenesis; February 2019; Miami, FL. [Google Scholar]
- 38.Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243(1):3–8. [DOI] [PubMed] [Google Scholar]
- 39.Noma H, Funatsu H, Yamasaki M, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond). 2008;22(1):42–48. [DOI] [PubMed] [Google Scholar]
- 40.Noma H, Funatsu H, Mimura T, Harino S, Hori S. Aqueous humor levels of vasoactive molecules correlate with vitreous levels and macular edema in central retinal vein occlusion. Eur J Ophthalmol. 2010;20(2):402–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.