Objectives of the study
There is little guidance regarding the impact of alcohol and cannabis on the clinical course of inflammatory bowel disease. The aim of this study was to assess the prevalence, sociodemographic characteristics and impact of alcohol and cannabis use on the clinical course of the disease.
Methods
We performed an analysis of prospectively collected data within the Swiss Inflammatory Bowel Disease Cohort Study with yearly follow-ups and substance-specific questionnaires. We analyzed the prevalence of use, the profile of users at risk for addiction and the impact of alcohol and cannabis on the course of the disease.
Results
We collected data of 2828 patients included between 2006 and 2018 and analyzed it according to their completion of specific surveys on alcohol and cannabis use. The prevalence of patient-reported active use was 41.3% for alcohol and 6% for cannabis. Heavy drinkers were over-represented among retired, married smokers receiving mostly aminosalicylates and less immunosuppression. In ulcerative colitis patients, low-to-moderate drinking was associated with less extensive disease. Cannabis users were often students with ileal Crohn’s disease.
Conclusion
A significant proportion of patients with inflammatory bowel disease consume alcohol or cannabis. Heavy alcohol consumption is most likely in male smokers >50 years, whereas young men with ileal disease rather use cannabis.
Keywords: addiction, alcohol, cannabis, epidemiology, inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) describes two main disorders involving chronic inflammation of the digestive tract: Crohn’s disease and ulcerative colitis. The extrapolated prevalence of IBD in Switzerland by the end of 2014 was up to 0.37% in 2014 [1].
Environmental factors play an important role in the pathogenesis of IBD. They modify the risk of developing the disease and influence its course. The most deleterious environmental factors reported so far are smoking and a diet rich in fatty acids, red meat and refined sugars but poor in fibers and fruit [2,3].
Addictive behavior is not more frequent in IBD patients than in the general population, except for nicotine use [4]. There is a scarcity of data on two common recreational and frequently consumed agents in our society, alcohol and cannabis. The goal of this study is to contribute to a better knowledge of these two substances, which have been less associated with the pathogenesis of IBD [5] and thus have not been the focus of research on environmental factors in IBD.
In Europe, rates of alcohol consumption and attributable burden of disease are high in comparison to global worldwide averages [6,7]. IBD patients seem to have similar rates and patterns of alcohol consumption compared to the general population [8]. In 2012, a survey on substance abuse among the Swiss population reported that 84.6% of men and 66% of women consumed alcohol regularly, and overall 10% did so on a daily basis [6,9]. In 2012, 6.3% of the Swiss population reported active cannabis consumption and up to 20% among <25 years old individuals [6].
Alcohol intake increases intestinal permeability by disrupting the gut-barrier function and inhibits the immune system by decreasing T-cell activity and interleukin (IL)-12 levels [10,11]. Chronic alcohol abuse can cause intestinal bacterial overgrowth and increases Kupffer cell activity in the liver with elevated production of proinflammatory mediators such as tumor necrosis factor alpha (TNF-α, IL-1 and IL-6 [12]. Studies investigating alcohol as an etiologic factor for developing IBD notoriously showed mixed and inconsistent results [5].
In the last couple of years, the use of medical cannabis has gained popularity after the discovery of the cannabinoid receptors CB1 and CB2 [13,14]. The latter has a key role in maintaining gut homeostasis by modulating motility, inflammation, perception of visceral pain and immune tolerance, notably by altering proinflammatory cytokine-induced chemotaxis (TNF-α, IL-6 and IL-8) [15,16]. Data suggest that exogenous cannabis reduces symptoms associated with IBD but a recent randomized controlled trial shows that this improvement is not associated with a decrease in inflammatory activity [17–20]. Solid data concerning the prevalence, the patterns of consumption and the effects of alcohol and cannabis use on the clinical course of IBD patients are still lacking, including large prospective studies. Therefore, the aims of this study were to determine the prevalence of alcohol and cannabis consumption among IBD patients in Switzerland and to cross-sectionally and prospectively evaluate its influence on the disease.
Materials and methods
Study population and design
Our work is an analysis of prospective data collected between 2006 and 2018 on patients enrolled in the Swiss IBD cohort study (SIBDCS). This nationwide multicentered effort includes patients of all ages and sex with a diagnosis of IBD (>4 months) and permanent residence status in all parts of Switzerland. At enrollment and yearly follow-ups, data were collected on clinical, socioeconomic and psychosocial aspects during a clinical physician evaluation and from an annual patients’ questionnaire. Disease phenotype, complications and severity, current and past therapies and surgeries, factors that influence disease course (e.g., smoking) and blood test results were collected. The data were then entered into a Microsoft Access database (Access; Microsoft, Redmond, Washington, USA) and manually validated for quality control at the SIBDCS datacenter, located at the Institute of Social and Preventive Medicine, Lausanne University Hospital [21].
Questionnaire-based evaluation of prevalence
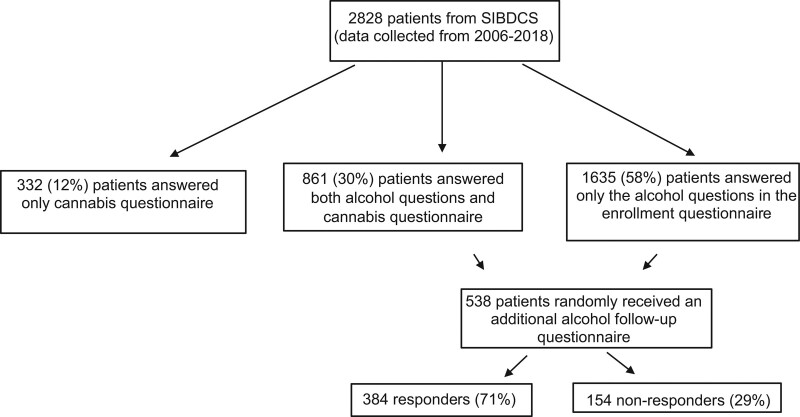
We included all adult patients who completed at least one patient enrollment questionnaire including questions on alcohol intake between 2006 and 2013 and subsequently answered the additional questionnaire on cannabis consumption in 2018 and were thus followed up for at least 5 years (2013–2018), see Fig. 1. We excluded patients who did not complete the self-reported questionnaires, which contained a set of questions on cannabis use and other possible confounding factors such as the consumption of tobacco, alcohol or other drugs. Some questions were derived from the cohort study on substance use risk factors (C-Surf), a bigger project investigating substance consumption by young men in Switzerland. We reviewed data on the prevalence of alcohol and cannabis consumption in the general Swiss population from an official addiction monitoring report in 2016 financed by the Swiss federal office of public health [6].
Fig. 1.
Flowchart of the study for alcohol and cannabis.
Questionnaire on alcohol
To account for a possible change of drinking habit during the follow-up period, 538 patients of the cohort randomly received an additional alcohol questionnaire in 2014 to reassess and refine their drinking habits in terms of frequency, quantity (including frequency of binge drinking), type of alcohol consumed and its effect on IBD symptoms. In total 384 patients (71%) replied to this additional questionnaire.
Questionnaire on cannabis
To better assess the amount of cannabis consumed and to evaluate the consumption of other illegal drugs among IBD patients, we sent 2030 additional questionnaires to the patients from the SIBDCS in 2018. We received 1193 answers (response rate of 58.8%).
Data analysis and definitions
Stratification of patients in terms of abstainers and current consumers
-
(1)
Regarding alcohol, three categories have been created: abstainers (nondrinkers or drinking ≤1× weekly), low-to-moderate drinkers (drinking >1× weekly to ≤1× daily) and heavy drinkers (drinking >1× daily). Binge drinking was defined as consuming six or more drinks on one occasion.
-
(2)
For cannabis a distinction was made between active users, previous users and nonusers. For the clarity of the analysis the denomination ‘cannabis user’ vs. ‘never used cannabis’ was chosen.
Pattern of disease according to consumption
We performed a cross-sectional and prospective comparison of IBD disease patterns among the three groups of alcohol consumption and the two groups of cannabis users. In the cross-sectional analysis, we used information on current disease characteristics available from the physician and patient enrollment questionnaires such as demographics (age at visit, age at disease onset, diagnosis, sex), lifestyle factors (tobacco and alcohol consumption and BMI), disease pattern at the time of diagnosis (IBD subtype, disease location and extent, disease behavior according to the Montréal classification and extraintestinal manifestations), current therapy, past disease status including the history of hospitalizations and surgical procedures, and history of IBD-related complications including dysplasia, cancer, presence of stenosis or fistula. We also evaluated the need for steroid use, conventional immunosuppressants and biologic agents, as well as medication-related adverse events/intolerance. The severity of the underlying IBD was assessed according to the Crohn’s Disease Activity Index for patients with Crohn’s disease and the Modified Truelove and Witts Activity Index for patients with ulcerative colitis.
Evaluation of disease course
For the follow-up analysis, all patients with the use of cannabis or alcohol and a follow-up of more than 1 year were included. In the prospective analysis, we compared the disease course of the above-defined groups based on the data from annual follow-up visits, in terms of occurrence of flares, need for steroid therapy, escalating immunosuppressive therapy, as well as disease activity, the occurrence of IBD-related complications, hospitalizations, surgeries and change of disease location.
Statistical analysis
We used the statistical software Stata, release 16 (StataCorp LLC, College Station, Texas, USA) for data analysis. Normally distributed continuous variables were expressed using means, SDs and range. We made comparisons between groups using the two-sided t-test and used analysis of variance for three groups or more. For skewed continuous variables, we indicated median, interquartile ranges and ranges. We used the Kruskal-Wallis test to compare groups. Categorical variables were expressed in percentages and compared using the chi-square test or Fisher’s exact test in case of insufficient sample size. Logistic regression analysis was used to analyze the association between alcohol/cannabis consumption and course of disease summarized as binary variables (e.g. occurrence of complications yes/no, need for surgery yes/no), which allowed correcting for the influence of other confounders (smoking, alcohol, other illicit drug use and depressive symptoms).
Ethical considerations
The study was approved by a central national ethics committee (Zurich BASEC-Nr: 2018-02068) and all local ethics committees of each participating center in Switzerland. Two additional projects related to the specific questions on Alcohol (KEK-Nr: BE 298/15) and Cannabis (Bern BASEC-Nr: 2017-00977) were approved by a local ethics committee.
Results
Prevalence of alcohol and cannabis consumption among the SIBDCS in comparison to the Swiss population
Alcohol
Data were analyzed from 2019 Swiss IBD patients (56% Crohn’s disease, 51.3% female) who were included in the cohort between July 2006 and May 2013, when information on alcohol consumption was available. Overall, 870 (43·1%) of Swiss IBD patients consumed alcohol and 57% were nondrinkers (≤1× weekly), whereas 40.6% were low-to-moderate drinkers (>1× weekly to ≤1× daily) and only 2·5% were heavy drinkers (>1× daily)
Cannabis
In 2018, 1193 IBD patients (54% Crohn’s disease, 46% ulcerative colitis) answered the supplementary cannabis questionnaire. Overall, 72 (6·1%) IBD patients actively consumed cannabis with 7% Crohn’s disease patients and 5% ulcerative colitis patients. Additionally, 160 (13·4%) IBD patients reported previous use of cannabis.
Alcohol and cannabis consumption rates according to sex, age and ethnicity in the SIBDCS in comparison to the Swiss population
Alcohol
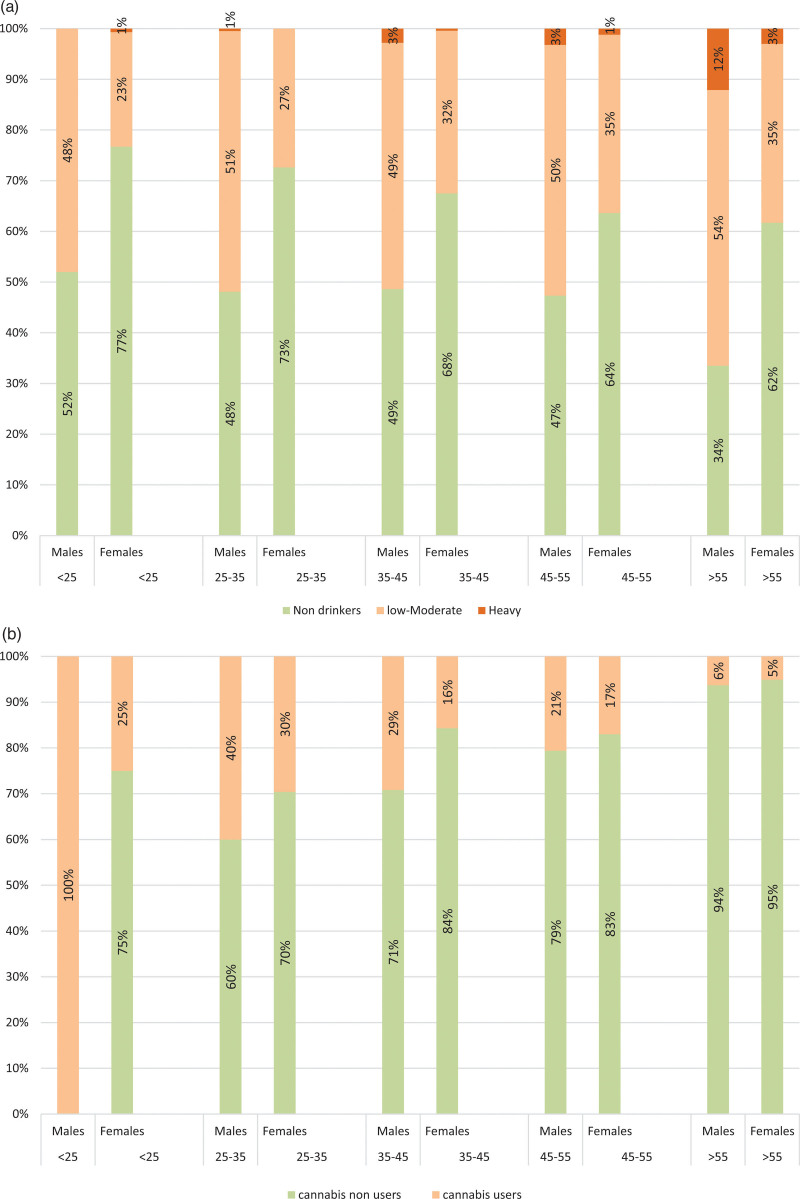
Trends in alcohol consumption in IBD patients according to age and sex were roughly similar to the Swiss population, with men drinking greater quantities and more frequently than women. The proportion of men among heavy and low-to-moderate drinkers in the SIBDCS was 72% and 59% with a median age of 72 and 54 years, respectively (Fig. 2a and Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A782).
Fig. 2.
Alcohol (a) and Cannabis (b) consumption rates according to age groups and sex in the Swiss inflammatory bowel disease cohort study (SIBDCS).
Cannabis
The gender distribution was balanced (51% males). IBD cannabis users were younger than the other patients from the SIBDCS (controls) with a median age of 43 years, mostly driven by men under 25 years (Fig. 2b).
Patient characteristics associated with alcohol and cannabis consumption
Alcohol
IBD heavy drinkers had a higher BMI (26 kg/m2 vs. 23 kg/m2), were more often active smokers (22% vs. 20%), retired (45% vs. 13%) and married (72% vs. 54%) (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A782).
Cannabis
The percentage of active tobacco smokers was higher among cannabis users (46% vs. 20% in controls) and there was a higher proportion of active cannabis users among students (20% vs. 9%) (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A782).
Disease characteristics associated with alcohol and cannabis consumption pattern
Alcohol
The activity and location of the disease in IBD patients were similar among the different alcohol drinking pattern groups. There was no significant complication associated with alcohol consumption in Crohn’s disease patients (Table 1).
Table 1.
inflammatory bowel disease patients’ disease characteristics according to alcohol drinking pattern and cannabis consumption
| Nondrinkers N = 390 |
Low-to-moderate drinkers N = 314 |
Heavy drinkers N = 18 |
Cannabis user N = 133 |
Control group N = 722 |
||
|---|---|---|---|---|---|---|
| Type of IBD | ||||||
| Crohn’s disease (%) | 212 (54%) | 164 (52%) | 6 (33%) | 80 (60%) | 382 (53%) | |
| Colitis ulcerosa (%) | 178 (46%) | 150 (45%) | 12 (67%) | 53 (40%) | 340 (47%) | |
| Disease activity (Median + IQR) | ||||||
| Crohn’s disease Activity Index | 66 (31–111) |
54 (26–98) |
99 (34–197) |
70 (42–105) |
63 (31–107) |
|
| Modified Truelove & Witts activity index | 2 (1–6) | 2 (1–4) | 2 (1.5–3.5) | 3 (1–5) | 2 (1–5) | |
| Disease location Crohn’s disease | ||||||
| L1 | 63 (30%) | 42 (26%) | 2 (33%) | 27 (34%) | 106 (28%) | |
| L2 | 66 (31%) | 58 (35%) | 2 (33%) | 3 (4%) | 125 (31%) | |
| L3 | 71 (33%) | 51 (31%) | 2 (33%) | 29 (36%) | 126 (33%) | |
| L4 (in total) | 12 (6%) | 13 (8%) | 0 (0) | 8 (10%) | 27 (8%) | |
| Disease behavior (Crohn’s disease) | ||||||
| B1 | 96 (45%) | 83 (51%) | 2(33%) | 45 (56%) | 181 (48%) | |
| B2 | 48 (23%) | 29 (18%) | 0 (0) | 13 (16%) | 77 (20%) | |
| B3 | 68 (32%) | 52 (32%) | 4(66%) | 22 (28%) | 124 (32%) | |
| Disease location ulcerative colitis | ||||||
| Pancolitis | 68 (32%) | 41 (25%) | 6 (50%) | 20 (38%) | 115 (34%) | |
| Left-sided colitis | 69 (43%) | 75 (50%) | 4 (33%) | 22 (41%) | 148 (44%) | |
| Proctitis | 34 (16%) | 30 (20%) | 2 (17%) | 11 (21%) | 66 (20%) | |
| Anemia (%) | ||||||
| Crohn’s disease | 54 (27%) | 33 (22 %) | 1 (20%) | 18 (23%) | 106 (24%) | 0.748 |
| Ulcerative colitis | 42 (26%) | 24 (18%) | 1 (9%) | 15 (32%) | 82 (23%) | 0.107 |
| Extraintestinal manifestations | ||||||
| All IBD patients | 250 (64%) | 167 (53%) | 13 (72%) | 103 (55%) | 407 (56%) | 0·020 |
| Crohn’s disease | 152 (72%) | 94 (57%) | 5 (83%) | 80 (60%) | 228 (60%) | 0·016 |
| Ulcerative colitis | 98 (55%) | 73 (49%) | 8 (67%) | 23 (43%) | 179 (53%) | 0·275 |
| Current therapy | ||||||
| Crohn disease | ||||||
| Oral 5-ASA | 47 (22%) | 21 (13%) | 3 (50%) | 18 (23%) | 71 (19%) | 0·021 |
| Topical 5-ASA | 3 (1%) | 4 (2%) | 1 (17%) | 3 (4%) | 8 (2%) | 0·08 |
| Immunomodulators | 118 (56%) | 81 (49%) | 0 | 35 (44%) | 199 (52%) | 0·019 |
| Anti-TNF Agents | 70 (33%) | 40 (24%) | 0 | 28 (35%) | 110 (29%) | 0·075 |
| Systemic steroids | 20 (9%) | 21 (13%) | 0 | 12 (15%) | 41 (11%) | 0·405 |
| Budesonide | 23 (11%) | 11 (7%) | 0 | 8 (10%) | 34 (9%) | 0·457 |
| Ulcerative colitis | ||||||
| Oral 5-ASA | 83 (47%) | 92 (61%) | 10 (83%) | 32 (60%) | 185 (54%) | 0·007 |
| Topical 5-ASA | 47 (26%) | 43 (29%) | 2 (17%) | 15 (28%) | 92 (27%) | 0·820 |
| ImmunomodulatorsAnti-TNF | 66 (37%) | 46 (31%) | 3 (25%) | 21 (40%) | 115 (34%) | 0·460 |
| Agents | 21 (12%) | 9 (6%) | 0 | 5 (9%) | 30 (9%) | 0·205 |
| Systemic Steroids | 31 (17%) | 26 (17%) | 2 (17%) | 6 (11%) | 59 (17%) | 0·750 |
| Topical Steroids | 8 (4.5%) | 8 (6%) | 0 | 2 (4%) | 17 (5%) | 0·747 |
| Fistulas, abscesses or stenosis in Crohn’s disease | ||||||
| Perianal fistula | 0 | 1 (1%) | 0 | 0 | 1 (0.5%) | 0·654 |
| Other fistula | 2 (1%) | 1 (1%) | 0 | 0 | 3 (1%) | 0·844 |
| Stenosis | 12 (7%) | 12 (8%) | 0 | 4 (8%) | 24 (6%) | 0·765 |
| Need for surgery | ||||||
| Crohn disease | ||||||
| Overall | 141 (67%) | 107 (65%) | 4 (67%) | 52 (65%) | 252 (66%) | 0·992 |
| Small bowel surgery | 106 (27%) | 66 (21%) | 5 (28%) | 27 (20%) | 177 (46%) | 0·184 |
| Colon surgery | 31 (15%) | 22 (13%) | 1 (17%) | 10 (13%) | 54 (14%) | 0·961 |
| Ulcerative colitis | 43 (24%) | 29 (19%) | 2 (17%) | 8 (15%) | 74 (21%) | 0·461 |
| At least one hospitalization during last 12 months related to IBD | ||||||
| All IBD | 76 (20%) | 50 (16%) | 2 (13%) | 25 (19%) | 128 (18%) | 0·604 |
| Crohn disease | 42 (20%) | 34 (21%) | 1 (20%) | 16 (20%) | 77 (20%) | 0·996 |
| Ulcerative colitis | 34 (19%) | 16 (11%) | 1 (9%) | 9 (18%) | 51 (15%) | 0·181 |
ASA, aminosalicylique acid; IBD, inflammatory bowel disease ;TNF-α, tumor necrosis factor alpha.
Extraintestinal manifestations development in Crohn’s disease patients was associated with heavy drinking habits. However, heavy drinking IBD patients tended to receive less immunomodulators, anti-TNF agents and steroids, but rather more oral 5-aminosalicylique acid ASA (50% for Crohn’s disease; P = 0.02 and 83% for ulcerative colitis.
There were no differences in terms of the need for surgery or hospitalizations among the different drinking pattern groups in both Crohn’s disease and ulcerative colitis patients (Tables 1.
Cannabis
Ileal involvement was more often associated with cannabis use in Crohn’s disease patients, whereas in ulcerative colitis patients no disease location was linked with cannabis consumption. There was no difference in terms of the need for surgery and hospitalization in the last 12 months (Tables 1). Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A782.
Effects of alcohol and cannabis consumption on gastrointestinal symptoms
Alcohol
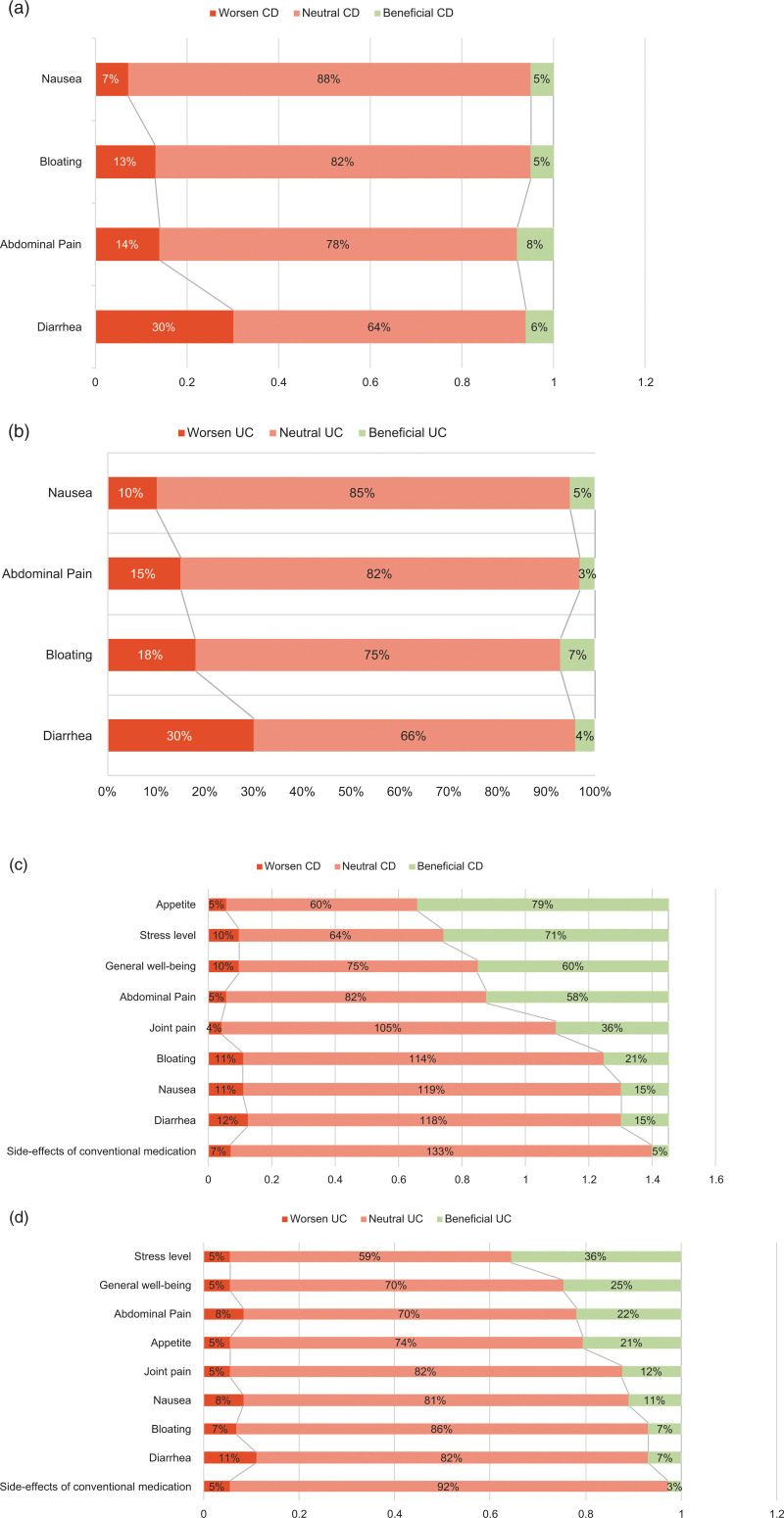
Overall alcohol consumption was associated with an increase in diarrhea, abdominal pain and bloating in both diseases (Fig. 3a,b). Diarrhea was the major symptom increased by alcohol with 30% of patients reporting a worsening.
Fig. 3.
Influence of alcohol and cannabis consumption on gastro-intestinal symptoms in CD (3a/3c) and UC (3b/3d).
Cannabis
A majority (66.6 % in Crohn’s disease and 80.2% in ulcerative colitis) of IBD patients did not report any difference in symptoms with cannabis consumption. However, when a positive impact was described, it was improving appetite and well-being, as well as reducing abdominal pain and stress. This was more pronounced in Crohn’s disease patients (Fig. 3c,d).
Modification of drinking habits since diagnosis
In general, most of our IBD patients maintained their alcohol consumption between enrollment and follow-up. In total 33% of men and 36% of women reported a reduction or cessation of alcohol consumption between the diagnosis and the reassessment questionnaire (Supplementary Table 2, Supplemental digital content 1, http://links.lww.com/EJGH/A782).
Possible protective effect of alcohol on ulcerative colitis activity
In ulcerative colitis patients, low-to-moderate alcohol consumption is associated with a decrease in steroid use, immunomodulatory use and elevated inflammatory markers (i.e. CRP >5 mg/L) suggesting a U-shaped model. No similar association was seen in cannabis users.
Discussion
This study is the first to evaluate the prevalence of cannabis use as well as alcohol consumption with various drinking patterns in such a large cohort of well-phenotyped IBD patients. We found a prevalence of 41·3% of regular alcohol consumption (≥1 drink per week) and 6·1% of active cannabis users.
The rate of alcohol consumption in the SIBDCS was lower than that among the general Swiss population [6]. The last population survey in 2016 showed that 50.9% of the general Swiss population consumed at least one drink per week, with the proportion of heavy drinkers increasing with age (at-risk behavior, up to 7·1% of the >65 year old) [6]. The latter was also slightly lower in IBD patients. Few other studies have analyzed alcohol consumption. In a survey comprising 90 IBD patients in 2010, Swanson et al. [8] published rates of alcohol consumption among IBD patients similar to the levels observed in the US population. According to a survey on dietary habits of 165 Dutch IBD patients, alcohol consumption was less frequent among IBD patients than among their non-IBD compatriots but no prevalence has been described [22]. Concerning cannabis consumption, the lifetime prevalence was lower in IBD patients in the SIBDCS compared to the general Swiss population (19·4% versus 31·6%). However, the active use of cannabis was similar between both populations with 6% in the SIBDCS and 6·8% in the general Swiss population. This is also consistent with international studies [18].
Furthermore, our study evaluated alcohol and cannabis consumption rates by age group and sex. Overall, alcohol consumption significantly increased with age and male sex in the SIBCDS, which was also observed in the general Swiss population. However, the alcohol consumption habits of younger people (<25 years old) were different in comparison to those of older age groups. The reassessment questionnaire allowed us to specify the presence and frequency of binge drinking among the different age groups. We observed a higher number of binge drinkers among young IBD males, in agreement with the observations made in the general Swiss population (Supplementary Table 3, Supplemental digital content 1, http://links.lww.com/EJGH/A782) [6]. Having a professional activity was a protective factor with regard to heavy drinking and should therefore be maintained to the greatest extent possible. On the other hand, cannabis consumption was more often observed in young male students and less in married or retired patients. This confirms that cannabis use is more popular, available and accepted among younger male adults [6].
We also assessed the effect of alcohol and cannabis on gastrointestinal symptoms. A third of the SIBDCS patients experienced inconveniences associated with alcohol consumption such as diarrhea, abdominal pain and bloating. Notably, 33% of men and 36% of women reported a reduction or cessation of alcohol consumption. The patients who most decreased their alcohol intake were heavy drinkers. In general, patterns of alcohol consumption tended to equalize among the different drinking pattern groups during follow-up. This could be explained by progressing age and counseling by the treating physicians. In comparison, a Canadian study from 2016 showed that 31% of IBD patients completely avoided alcohol and 42% of chronic alcohol consumers were avoiding it while the disease was active [23]. In our study, we did not evaluate the activity of the disease in those patients who reduced their alcohol consumption.
Symptomatic benefits associated with cannabis consumption varied according to IBD type and were more pronounced in patients with Crohn’s disease. Cannabis was used to relieve abdominal pain and stress as well as to improve appetite and general well-being among IBD patients, which has already been described in the literature [15]. Indeed, upregulation of the CB2R expression in the gut can alleviate abdominal pain [24] and a phase IIa study using Olorinab (APD371), a peripheral agonist of CB2a, showed a reduction of abdominal pain without psychotropic effects in Crohn’s disease patients [25]. Furthermore, an improvement in appetite was also consistently shown in previous studies [26]. In fact, cannabis promotes anabolic processes and energy storage via central (leptin and ghrelin secretion) and peripheral pathways (insulin resistance) [27].
In our study, alcohol consumption was associated with less prescriptions of immunomodulators, steroids and anti-TNF among ulcerative colitis and Crohn’s disease patients. It is unclear, if this results from the fact that patients consuming alcohol have less severe disease, to begin with, or if increasing age linked with increasing alcohol consumption often involves a milder course of the disease with less need for advanced therapy [28]. Another hypothesis is that patients consuming alcohol, and especially heavy drinkers, might receive less aggressive IBD treatments because of noncompliance or reluctance from the treating physician to use such therapies. Of note, data from the literature did not show a worse outcome in socio-economically deprived IBD patients [29].
The risk of addiction, including alcohol, is increased in IBD patients because they are prone to stigmatization, depression and suicide [30]. Current smoking was associated with cannabis consumption and heavy drinking in the SIBDCS, and overall, patients in the SIBDCS smoked more than the Swiss population in 2013 (28–33% and 25.9%, respectively). Tobacco consumption biases the effect of alcohol on the risks and symptoms of IBD because it is linked to an increased relative risk and complicated course of Crohn’s disease, whereas it is a protective factor regarding the development of ulcerative colitis [31]. Among other addictions, high rates of narcotic use and long-term prescription of narcotics have been described, mostly in Crohn’s disease patients [32]. In our practice, this is much smaller and beyond the scope of this study.
The strengths of our study include: (1) a prospective design of a large patient cohort. (2) The collected data are patient-related. Indeed, we deliberately abstained from using data from the physicians’ questionnaires as patients may disclose their consumption more accurately in an anonymous way. (3) It is the first study with a reassessment of alcohol consumption and behavior a few years after enrollment with a focus on the change of alcohol consumption and its effect on symptoms. (4) We had a high response rate in our additional survey on alcohol and cannabis consumption (response rate of 71% for alcohol and 59% for cannabis).
The main limitations of this study are: (1) anonymous questionnaires have inherent limitations relating to sampling bias as those who have an interest in the subject may be more likely to respond, skewing the sample. Due to the nature of the questions (alcohol, cannabis and illicit substances), patients are less likely to respond, creating a risk of reporting bias. (2) SIBDCS patients attend annual follow-up visits and thus ongoing treatment as well as cannabis and alcohol consumption patterns were considered valid until the next follow-up visit. Therefore, short-term changes in IBD therapy (such as on-off steroids or antibiotics) may not have been taken into account, which could limit the generalizability of our results. (3) There is a risk of selection bias because patients evaluated in the yearly follow-ups are often included in periods of relatively low disease activity with the risk of masking any significant differences regarding inflammation and disease activity or behavior. (4) The SIBDCS is not a population-based study and as such, our results are subject to a referral center bias. (5) Questionnaire surveys often have missing data. (6) The risk of a confounding factor by the well-studied deleterious effect of tobacco consumption as cannabis was mostly mixed with tobacco. (7) The lack of information in the enrollment and follow-up questionnaires of the SIBDCS concerning the quantity of alcohol consumption in terms of standardized units.
Conclusion
Our prospective study showed similar rates of cannabis consumption and lower rates of alcohol consumption in Swiss IBD patients, compared to the general Swiss population. IBD patients consuming alcohol were more likely to be older men with ulcerative colitis whereas patients consuming cannabis tended to be younger men. This observation will help the clinician to be aware of possible addictions and the need for active assessment in the outpatient clinic.
Because alcohol consumption is overall linked to reduced use of advanced therapies (immunomodulators, anti-TNF, steroids), it is of utmost importance to evaluate if any patients consuming alcohol and especially heavy drinkers are suboptimally treated for the underlying IBD.
Acknowledgements
This work was supported by grants from the Swiss National Science Foundation (SNF) for the Swiss IBD Cohort, (grant no. 33CS30_148422).
De-identified participant data will be made available to others upon request following the Study Steering Group discussion and signing of a data access agreement. Requests for access to data should be made to the corresponding author and the first authors via the corresponding email given.
Conflicts of interest
M.M. has received consultant fees from Vifor, Abbvie, UCB, MSD, Lilly, Janssen, Takeda. He also received grants from UCB, Abbvie, Vifor, MSD, Takeda. He received speaker fess from Vifor, Janssen, Abbvie, MSD, Pfizer, UCB and Takeda. P.M. has received consultant fees from AstraZeneca, AbbVie, Ferring Pharmaceuticals, Janssen, MSD, Nestlé Health Sciences, Pfizer, Takeda, UCB Pharma, and Vifor. Lecture fees: AbbVie, Ferring Pharmaceuticals, Janssen, Hospira, MSD, Pfizer, Takeda, UCB Pharma, and Vifor. Research grants: iQone. R.V.K. has received consultant fees from Vifor. For the remaining authors, there are no conflicts of interest.
Supplementary Material
Footnotes
Dr. Maude Martinho-Grueber and Dr. Ioannis Kapoglou contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.eurojgh.com.
References
- 1.Bähler C, Vavricka SR, Schoepfer AM, Brüngger B, Reich O. Trends in prevalence, mortality, health care utilization and health care costs of Swiss IBD patients: a claims data based study of the years 2010, 2012 and 2014. BMC Gastroenterol 2017; 17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosnes J. Smoking, physical activity, nutrition and lifestyle: environmental factors and their impact on IBD. Dig Dis 2010; 28:411–417. [DOI] [PubMed] [Google Scholar]
- 3.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 2011; 106:563–573. [DOI] [PubMed] [Google Scholar]
- 4.Hirschmann S, Koehnen J, Schuster B, Atreya R, Krauss N, Mudter J, et al. P763 Addiction in IBD patients: more than just smoke? J Crohn’s Colitis 2019; 13(Suppl 1):S502–S3. [Google Scholar]
- 5.Georgiou AN, Ntritsos G, Papadimitriou N, Dimou N, Evangelou E. Cigarette smoking, coffee consumption, alcohol intake, and risk of crohn’s disease and ulcerative colitis: a mendelian randomization study. Inflamm Bowel Dis 2021; 27:162–168. [DOI] [PubMed] [Google Scholar]
- 6.Gmel G, Kuendig H, Notari L, C G. Monitorage suisse des addictions: consommation d’alcool, tabac et drogues illégales en Suisse en 2016. Addiction Suisse, Lausanne, Suisse 2017. [Google Scholar]
- 7.Laramée P, Kusel J, Leonard S, Aubin HJ, François C, Daeppen JB. The economic burden of alcohol dependence in Europe. Alcohol Alcohol 2013; 48:259–269. [DOI] [PubMed] [Google Scholar]
- 8.Swanson GR, Sedghi S, Farhadi A, Keshavarzian A. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol 2010; 44:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehm J, Taylor B, Roerecke M, Patra J. Alcohol consumption and alcohol-attributable burden of disease in Switzerland, 2002. Int J Public Health 2007; 52:383–392. [DOI] [PubMed] [Google Scholar]
- 10.Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol 1994; 89:2205–2211. [PubMed] [Google Scholar]
- 11.Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol 2004; 173:3398–3407. [DOI] [PubMed] [Google Scholar]
- 12.Marques-Vidal P, Bochud M, Bastardot F, von Känel R, Ferrero F, Gaspoz JM, et al. Associations between alcohol consumption and selected cytokines in a Swiss population-based sample (CoLaus study). Atherosclerosis 2012; 222:245–250. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346:561–564. [DOI] [PubMed] [Google Scholar]
- 14.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365:61–65. [DOI] [PubMed] [Google Scholar]
- 15.Abalo R, Vera G, López-Pérez AE, Martínez-Villaluenga M, Martín-Fontelles MI. The gastrointestinal pharmacology of cannabinoids: focus on motility. Pharmacology 2012; 90:1–10. [DOI] [PubMed] [Google Scholar]
- 16.Di Marzo V, Izzo AA. Endocannabinoid overactivity and intestinal inflammation. Gut 2006; 55:1373–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naftali T, Bar-Lev Schleider L, Sklerovsky Benjaminov F, Lish I, Konikoff FM, Ringel Y. Medical cannabis for inflammatory bowel disease: real-life experience of mode of consumption and assessment of side-effects. Eur J Gastroenterol Hepatol 2019; 31:1376–1381. [DOI] [PubMed] [Google Scholar]
- 18.Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, Steinhart H. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2011; 23:891–896. [DOI] [PubMed] [Google Scholar]
- 19.Naftali T, Bar-Lev Schleider L, Almog S, Meiri D, Konikoff FM. Oral CBD-rich cannabis induces clinical but not endoscopic response in patients with Crohn’s Disease, a randomised controlled trial. J Crohns Colitis 2021; 15:1799–1806. [DOI] [PubMed] [Google Scholar]
- 20.Naftali T, Bar-Lev Schleider L, Scklerovsky Benjaminov F, Konikoff FM, Matalon ST, Ringel Y. Cannabis is associated with clinical but not endoscopic remission in ulcerative colitis: a randomized controlled trial. PLoS One 2021; 16:e0246871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittet V, Michetti P, Mueller C, Braegger CP, von Känel R, Schoepfer A, et al.; Swiss IBD Cohort Study Group. Cohort profile update: the swiss inflammatory bowel disease cohort study (SIBDCS). Int J Epidemiol 2019; 48:385–386f. [DOI] [PubMed] [Google Scholar]
- 22.Opstelten JL, de Vries JHM, Wools A, Siersema PD, Oldenburg B, Witteman BJM. Dietary intake of patients with inflammatory bowel disease: a comparison with individuals from a general population and associations with relapse. Clin Nutr 2019; 38:1892–1898. [DOI] [PubMed] [Google Scholar]
- 23.Vagianos K, Clara I, Carr R, Graff LA, Walker JR, Targownik LE, et al. What are adults with inflammatory bowel disease (IBD) eating? A closer look at the dietary habits of a population-based Canadian IBD cohort. JPEN J Parenter Enteral Nutr 2016; 40:405–411. [DOI] [PubMed] [Google Scholar]
- 24.Fioramonti J, Bueno L. Role of cannabinoid receptors in the control of gastrointestinal motility and perception. Expert Rev Gastroenterol Hepatol 2008; 2:385–397. [DOI] [PubMed] [Google Scholar]
- 25.Yacyshyn BR, Hanauer S, Klassen P, English BA, Stauber K, Barish CF, et al. Su1930 – Safety and Efficacy of Olorinab, a Peripherally Restricted, Highly Selective, Cannabinoid Receptor 2 Agonist in a Phase 2A Study in Chronic Abdominal Pain Associated with Crohn’s Disease. Gastroenterology 2019; 156(6, Suppl 1):S–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benson MJ, Abelev SV, Connor SJ, et al. Medicinal cannabis for inflammatory bowel disease: a survey of perspectives, experiences, and current use in Australian Patients. Crohn’s Colitis 360 2020; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatta-Cherifi B, Cota D. New insights on the role of the endocannabinoid system in the regulation of energy balance. Int J Obes (Lond) 2016; 40:210–219. [DOI] [PubMed] [Google Scholar]
- 28.Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018; 16:343–356.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahon S, Lahmek P, Macaigne G, Faurel JP, Sass C, Howaizi M, et al. Socioeconomic deprivation does not influence the severity of Crohn’s disease: results of a prospective multicenter study. Inflamm Bowel Dis 2009; 15:594–598. [DOI] [PubMed] [Google Scholar]
- 30.Carson HJ, Dudley MH, Knight LD, Lingamfelter D. Psychosocial complications of Crohn’s disease and cause of death. J Forensic Sci 2014; 59:568–570. [DOI] [PubMed] [Google Scholar]
- 31.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc 2006; 81:1462–1471. [DOI] [PubMed] [Google Scholar]
- 32.Mantzouranis G, Fafliora E, Saridi M, Tatsioni A, Glanztounis G, Albani E, et al. Alcohol and narcotics use in inflammatory bowel disease. Ann Gastroenterol 2018; 31:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.