ABSTRACT
Background
Inflammation linked to diabetic kidney disease (DKD) may affect white blood cell (WBC) counts and differentials. We examined the cross-sectional associations of total WBC count and WBC fractions with structural lesions of DKD in 108 Pima Indians with Type 2 diabetes who underwent research kidney biopsies. We also examined the longitudinal association of these WBC variables with renal function loss (RFL) in 941 Europeans with Type 2 diabetes from the SURDIAGENE study.
Methods
Associations of WBC variables with morphometric parameters were assessed by linear regression. RFL was defined as≥40% loss of estimated glomerular filtration rate from baseline. Associations with RFL were evaluated by Cox regression. Hazard ratios (HRs) were reported per standard deviation increment of each WBC variable.
Results
After multivariable adjustment, lymphocyte (r = −0.20, P = 0.043) and eosinophil (r = 0.21, P = 0.032) fractions in the Pima Indians correlated with glomerular basement membrane width. Eosinophil fraction also correlated with glomerular filtration surface density (r = −0.21, P = 0.031). Lymphocyte fraction (r = 0.25, P = 0.013), neutrophil fraction (r = −0.23, P = 0.021) and the neutrophil:lymphocyte ratio (r = −0.22, P = 0.024) correlated with percentage of normally fenestrated endothelial cells. During median follow-up of 4.5 years, 321 SURDIAGENE participants developed RFL. Lower lymphocyte fraction [HR = 0.67, 95% confidence interval (95% CI) 0.60–0.76] and higher neutrophil fraction (HR = 1.35, 95% CI 1.20–1.52), total WBC count (HR = 1.20, 95% CI 1.08–1.35) and neutrophil:lymphocyte ratio (HR = 1.44, 95% CI 1.28–1.62) each predicted RFL in this cohort.
Conclusions
WBC fractions associate with morphometric lesions of DKD and predict RFL in individuals with Type 2 diabetes.
Keywords: biomarkers, CKD, diabetic kidney disease, inflammation, kidney biopsy
INTRODUCTION
Diabetic kidney disease (DKD) is a leading cause of end-stage renal disease (ESRD) worldwide, and carries large human and societal costs [1]. The growing list of inflammatory markers associated with progressive kidney disease [2–6] suggests that inflammation plays a key role in DKD development and progression. A complete white blood cell (WBC) count is a low cost and widely available clinical test that reflects inflammation, and WBC counts and fractions have been linked to diabetes and its vascular complications [7–9]. These findings are thought to be related, at least in part, to the activation of WBCs by advanced glycation end-products. These activated cells in turn produce pro-inflammatory cytokines causing local tissue damage [10–12].
This study evaluated the cross-sectional relationship of WBCs with the underlying lesions of DKD in 108 Pima Indians who underwent research kidney biopsies. Also assessed was the predictive value of WBC variables for renal function loss (RFL) in a French cohort from the SURDIAGENE study. The longitudinal relationship with RFL was examined in SURDIAGENE because of the small number of events during follow-up in the Pima Indian cohort [13, 14].
MATERIALS AND METHODS
Study subjects and design
A total of 169 Pima Indian adults with Type 2 diabetes from the Gila River Indian Community participated in a 6-year randomized clinical trial testing the renoprotective efficacy of losartan versus placebo in early DKD (ClinicalTrials.gov number, NCT00340678). At the end of the trial, 111 participants underwent a research kidney biopsy to determine whether treatment was associated with preservation of kidney structure [13]. Thereafter, participants continued to be followed with annual measurements of glomerular filtration rate (GFR) [14].
For the present study, we selected the examination nearest to the date of the biopsy that had complete WBC data and we examined the associations between the WBC variables and kidney morphometric measurements. Of the 111 individuals who underwent biopsy, the 108 with complete WBCs and other relevant covariate measurements were included in the present analyses. This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each participant signed an informed consent document.
SURDIAGENE is a French single-center prospective cohort of patients with Type 2 diabetes who regularly visit the diabetes department at Poitiers University Hospital, Poitiers, France [15]. Patients were enrolled from 2002 to 2012. At baseline, 1468 patients were examined. Not all participants had a complete blood count at that visit, so the nearest available measurement was selected. A subset of the cohort (n = 941), with complete WBCs and other relevant covariate data, who also had subsequent visits were included in the present study. All but 31 (3.4%) of the participants in this cohort were Caucasians. Kidney function and vital status of all SURDIAGENE participants was confirmed through 31 December 2013. The Poitiers University Hospital Ethics Committee approved the study. Each participant signed an informed consent document.
Clinical and anthropometric measures
In both cohorts, five different subpopulations of WBCs were quantified; lymphocytes, neutrophils, monocytes, eosinophils and basophils. Participants were afebrile and free of acute illness at the time of examination, and all had a total WBC count within the normal range (3500–10 500 cells/mm³ in the Pima Indians and 4000–10 000 cells/mm³ in SURDIAGENE). Blood pressure was measured in both cohorts while subjects were seated; mean arterial pressure (MAP) was calculated as (2 × diastolic blood pressure + systolic blood pressure)/3. HbA1c was measured by high-performance liquid chromatography (HPLC). Iothalamate concentration for GFR determination in the Pima Indians was also measured by HPLC [16]. GFR was estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [17] in both cohorts. Urine albumin concentration was measured in both cohorts by immunoassay and serum/urine creatinine by a modified Jaffé reaction [18, 19]. Albumin excretion was assessed by the albumin:creatinine ratio (ACR). Urine albumin concentrations below the detection limits of the assays were set to those limits when computing ACR. Smoking data available in the SURDIAGENE cohort was reported as active smoker or non-smoker at the time of the research examination.
Morphometry of DKD
Kidney structural parameters, measured using quantitative morphometric methods [14, 20], included mean glomerular volume, glomerular basement membrane (GBM) width, mesangial fractional volume, glomerular filtration surface density, total filtration surface per glomerulus, cortical interstitial fractional volume, percent globally sclerotic glomeruli, number of podocytes per glomerulus, podocyte foot process width, percent podocyte detachment and percentage of normally fenestrated endothelium [14, 21–24]. An equation was used to calculate the percentage of sclerotic glomeruli [25]. An average ± standard deviation (SD) of 15 ± 6 glomeruli per biopsy were examined by light microscopy and 3 ± 1 by electron microscopy for the morphometric measurements.
Circulating WBCs within glomerular capillaries were identified and counted in electron microscopy images by a masked observer in 11 participants with few glomerular lesions and 9 with severe lesions. The number of WBCs per glomerular profile was divided by the number of glomerular capillary profiles, and the average count per biopsy was calculated. Participants with few glomerular lesions were selected from those in the lowest quartile of GBM width and highest quartile of glomerular filtration surface density and endothelial fenestration. Participants with severe lesions were selected from those in the highest quartile of GBM width and lowest quartile of glomerular filtration surface density and endothelial fenestration.
Statistical analyses
Data are presented as mean ± SD or median [interquartile range (IQR)]. Seven WBC variables were included in the analyses: total WBC count, lymphocyte fraction, neutrophil fraction, monocyte fraction, basophil fraction, eosinophil fraction and the neutrophil:lymphocyte ratio. Associations of WBC variables with clinical and morphometric measures were examined by Pearson correlations. Variables with non-normal distributions were either log2 or rank transformed, whichever was appropriate. WBCs and morphometric variables were standardized in all regression analyses. A rank transformation itself produces a standardized variable, but those that were not transformed or underwent log2 transformation were subsequently standardized for analysis. Stability of the WBC measurements over time was assessed by Pearson correlations between two sets of measurements in subsets of participants from both cohorts in whom repeat measurements were available.
Linear regression models were used to examine the association between WBCs and the morphometric variables adjusted for sex, treatment assignment during the clinical trial and baseline age, diabetes duration, HbA1c, MAP and GFR. ACR was not included in these models because it is highly correlated with the underlying structural lesions. Treatment assignment was included as this was an important exposure in the 6 years leading up to the present study. Regression model fit was assessed for normality and leverage with ‘Studentized’ residuals. Multicollinearity was assessed with eigenvalues and the condition index [26]. Each WBC variable was also tested for interaction with treatment assignment. Associations between WBCs and morphometric variables were illustrated by partial Pearson correlation coefficients.
Cox proportional hazards regression was used to examine the relationship between the baseline WBC variables and RFL, defined by a decline in estimated GFR during follow-up of ≥40% [27]. We reported previously that structural lesions strongly predicted this outcome [28]. Hazard ratios (HRs) were expressed for a 1 SD increment in the distribution of each WBC variable. Two models were described: (i) univariate and (ii) adjusted for age, sex, duration of diabetes, HbA1c, MAP, GFR and log2 ACR. The proportionality assumption for each covariate was assessed by the cumulative sums of Martingale residuals [29]. To assess the extent to which WBC variables enhanced prediction of RFL, generalized c-statistics were calculated for the fully adjusted models accounting for variable follow-up times [30]. Comparisons between nested models that included or excluded the analyte of interest were assessed by likelihood ratios tests [31, 32]. In addition, relative integrated discrimination improvement (rIDI) was calculated to assess the improvement in the 5-year RFL risk prediction of each WBC variable in addition to traditional DKD risk factors [33]; the 5-year risk was selected as it approximates the median follow-up time for the RFL outcome. The 95% CIs for the rIDIs were computed based on 1000 bootstrap samples.
RESULTS
Table 1 shows the clinical characteristics of the Pima Indian cohort at the research examination closest to the kidney biopsy and the SURDIAGENE cohort at baseline. SURDIAGENE participants were older, and had better glycemic control and lower estimated GFR than the Pima Indian cohort (76 ± 21 versus 108 ± 25 mL/min/1.73 m2). The median time between the research examination at which the WBC count was measured and the kidney biopsy in the Pima Indians was 0.2 years (IQR = 0.09–0.4 years); 795 SURDIAGENE participants had a WBC count measured apart from the baseline examination, so the median time between the baseline examination and WBC measurement was 1.6 years (IQR = 0.2–2.9 years).
Table 1.
Baseline characteristics in the Pima Indian and SURDIAGENE cohorts
| Pima Indian cohort (n =108) | SURDIAGENE cohort (n=941) | |
|---|---|---|
| Clinical measures | ||
| Age (years) | 45.6 ± 10.0 | 64.1 ± 10.6 |
| Sex (male), n (%) | 28 (26%) | 397 (42%) |
| Diabetes treatment, n (%) | 96 (89%) | 903 (96%) |
| Antihypertensive treatment, n (%) | 62 (57%) | 783 (83%) |
| Lipid-lowering treatment, n (%) | 27 (25%) | 566 (60%) |
| Treatment assignment (losartan versus placebo), n (%) | 61 (56%) | |
| Diabetes duration (years) | 14.0 (11.3–19.7) | 12 (6–20)a |
| HbA1c (%) | ||
| Percentage | 9.5 ± 2.2 | 7.9 ± 1.6 |
| mmol/mol | 80 ± 24 | 63 ± 18 |
| Blood pressure (mmHg) | ||
| Systolic | 124 ± 16 | 132 ± 17 |
| Diastolic | 78 ± 9 | 73 ± 11 |
| GFR | ||
| Measured (mL/min) | 145 ± 52 | |
| Measured (mL/min/1.73 m2) | 126 ± 43 | |
| Estimated (mL/min/1.73 m2) | 108 ± 25 | 76 ± 21 |
| Urine ACR (mg/g) | 27 (10–127) | 20 (9–85) |
| Serum creatinine concentration (mg/dL) | 0.73 ± 0.21 | 0.98 ± 0.27 |
| Complete blood count measures | ||
| WBC count (cell/mm3) | 6925 (5940–8100) | 6900 (5800–8000) |
| Lymphocyte count (cell/mm3) | 1986 (1545–2374) | 1911 (1448–2353) |
| Lymphocyte fraction (%) | 29.7 (23.8–33.5) | 28.5 (22.4–33.9) |
| Neutrophil count (cell/mm3) | 4093 (3424–4902) | 4092 (3247–5104) |
| Neutrophil fraction (%) | 60.2 (55.2–65.3) | 60.7 (54.0–66.7) |
| Monocyte count (cell/mm3) | 465 (390–554) | 485 (387–594) |
| Monocyte fraction (%) | 6.5 (5.8–7.7) | 7.2 (5.9–8.6) |
| Eosinophil count (cell/mm3) | 172 (118–261) | 172 (110–258) |
| Eosinophil fraction (%) | 2.5 (1.7–3.8) | 2.5 (1.7–3.8) |
| Basophil count (cell/mm3) | 53 (38–66) | 30 (20–43) |
| Basophil fraction (%) | 0.7 (0.5–0.9) | 0.4 (0.3–0.6) |
| Neutrophil:lymphocyte ratio | 2.00 (1.66–2.77) | 2.11 (1.60–2.97) |
Data are given as mean ± SD or median (IQR) unless otherwise indicated.
Duration reported to the nearest full year in the SURDIAGENE cohort.
Stability of the WBC measurements was examined by Pearson correlations between WBC variables measured at two separate times in subsets from each cohort. WBC counts measured twice over a median of 0.52 years (IQR = 0.13–0.96 years) in 96 (89%) of the Pima Indians were correlated (r = 0.64, P < 0.001), as were lymphocyte (r = 0.54, P < 0.001), neutrophil (r = 0.39, P < 0.001), monocyte (r = 0.66, P < 0.001), eosinophil (r = 0.56, P < 0.001) and basophil (r = 0.40, P < 0.001) fractions in the subset of 95 Pima Indians with data for the WBC fractions at both measurements. Similarly, WBC counts measured twice over a median of 0.60 years (IQR = 0.24–0.86) in 487 persons (52%) from the SURDIAGENE cohort were correlated (r = 0.41, P < 0.001), as were lymphocyte (r = 0.60, P < 0.001), neutrophil (r = 0.53, P < 0.001), monocyte (r = 0.49, P < 0.001), eosinophil (r = 0.79, P < 0.001) and basophil (r = 0.36, P < 0.001) fractions.
Pearson correlations of WBC variables with clinical characteristics in both cohorts are shown in Supplementary data, Table S1. None of the WBC variables correlated significantly with fasting plasma glucose concentration, HbA1c or GFR in the Pima Indians, but lymphocyte fraction correlated negatively with ACR. Except for a small but statistically significant correlation between basophil fraction and treatment with a diabetes medicine (r = 0.19, P = 0.046), WBC variables did not correlate with diabetes, antihypertensive or lipid lowering treatment, or with treatment assignment during the clinical trial preceding the kidney biopsy in the Pima Indians. Similarly, in the SURDIAGENE cohort, none of the WBC variables correlated with random plasma glucose concentration in the 421 participants with available glucose data. Several small but statistically significant correlations (ranging from −0.12 to 0.16) between some WBC variables and smoking or treatment with diabetes, lipid lowering or antihypertensive medicines were also found. Lymphocyte fraction correlated negatively with ACR, and positively with estimated GFR. In addition, neutrophil fraction, monocyte fraction and the neutrophil:lymphocyte ratio correlated negatively with estimated GFR, and neutrophil fraction and the neutrophil:lymphocyte ratio correlated positively with ACR. Lymphocyte fraction correlated negatively, and monocyte and basophil fractions correlated positively with serum creatinine concentration in both cohorts. When we examined the correlation between WBCs and estimated GFR in the Pima Indians, lymphocyte fraction correlated positively and monocyte, eosinophil and basophil fractions, and the neutrophil:lymphocyte ratio correlated negatively with estimated GFR (data not shown). These findings suggest that the WBC correlations with estimated GFR in the two cohorts were attributable largely to their correlations with serum creatinine concentration, as there were no statistically significant correlations with measured GFR in the Pima Indians.
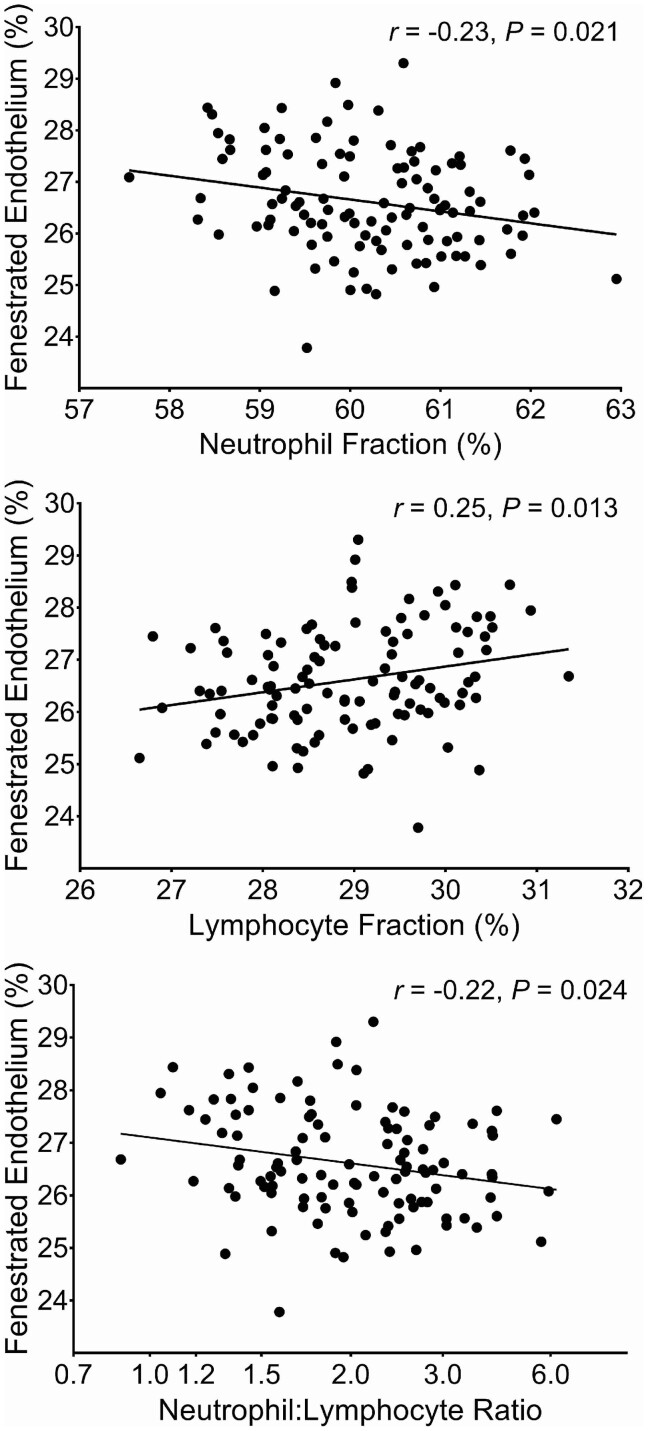
Morphometric parameters in the Pima Indians are shown in Table 2. Univariate Pearson correlations of WBC variables with morphometric parameters in the Pima Indians are shown in Supplementary data, Table S2. After adjustment for age, sex, diabetes duration, HbA1c, MAP, GFR and treatment assignment during the clinical trial, five of the eight statistically significant univariate correlations remained (Table 3). Lymphocyte fraction correlated positively, and neutrophil fraction and the neutrophil:lymphocyte ratio correlated negatively with the percentage of normally fenestrated endothelium. Lymphocyte fraction correlated negatively with GBM width, and eosinophil fraction correlated negatively with the glomerular filtration surface density. In addition, after adjustment for potential confounders, eosinophil fraction also correlated positively with GBM width. For illustration, partial residual regression plots of the adjusted relationship of the lymphocyte fraction, neutrophil fraction and neutrophil:lymphocyte ratio with the percentage of fenestrated endothelium are shown in Figure 1. When the analysis was restricted to the 100 participants in whom the WBCs were measured within 1 year of the baseline examination, statistically significant correlations remained between lymphocyte and neutrophil fractions and the percentage of normally fenestrated endothelium, and between eosinophil fraction and GBM width or glomerular filtration surface density (data not shown). In addition, eosinophil fraction correlated inversely with total filtration surface per glomerulus.
Table 2.
Morphometric parameters in the Pima Indian cohort
| Morphometric parameters | |
|---|---|
| Global glomerular sclerosis (%) | 5.2 (0.0–17.2) |
| Mean glomerular volume (×106 µm3) | 5.7 (4.8–6.9) |
| Glomerular basement membrane width (nm) | 508 (420–603) |
| Cortical interstitial fractional volume (%) | 29.5 (24.4–33.1) |
| Mesangial fractional volume (%) | 18.3 (14.2–24.6) |
| Glomerular filtration surface density (µm2/µm3) | 0.07 (0.06–0.09) |
| Total filtration surface/glomerulus (×105 µm2) | 4.0 (3.2–5.2) |
| Podocyte number per glomerulus | 615 (473–775) |
| Foot process width (nm) | 453 (405–524) |
| Podocyte detachment (%) | 0.30 (0.00–1.12) |
| Fenestrated endothelium (%) | 26.2 (21.8–31.6) |
Data are given as median (IQR).
Table 3.
Pearson correlations of WBC variables with morphometric variables adjusted for age, sex, treatment assignment, duration of diabetes, HbA1c, MAP and GFR
| Morphometric variables | WBC count | Lymphocyte fraction | Neutrophil fraction | Monocyte fraction | Eosinophil fraction | Basophil fraction | NLR |
|---|---|---|---|---|---|---|---|
| Global glomerular sclerosis | 0.01 | −0.06 | 0.04 | 0.12 | 0.07 | −0.03 | 0.05 |
| 0.896 | 0.536 | 0.700 | 0.225 | 0.503 | 0.782 | 0.630 | |
| Mean glomerular volume | −0.01 | −0.14 | 0.10 | 0.09 | 0.05 | −0.01 | 0.13 |
| 0.930 | 0.150 | 0.317 | 0.351 | 0.590 | 0.896 | 0.185 | |
| GBM width | −0.02 | −0.20 | 0.09 | 0.17 | 0.21 | −0.08 | 0.15 |
| 0.878 | 0.043 | 0.385 | 0.081 | 0.032 | 0.433 | 0.142 | |
| Cortical interstitial fractional volume | −0.08 | 0.05 | −0.05 | 0.14 | 0.06 | 0.00 | −0.07 |
| 0.452 | 0.599 | 0.595 | 0.167 | 0.577 | 0.978 | 0.492 | |
| Mesangial fractional volume | −0.02 | −0.11 | 0.05 | 0.16 | 0.14 | −0.11 | 0.07 |
| 0.842 | 0.264 | 0.644 | 0.103 | 0.174 | 0.294 | 0.500 | |
| Glomerular filtration surface density | 0.01 | 0.01 | 0.05 | −0.11 | −0.21 | −0.00 | 0.03 |
| 0.927 | 0.949 | 0.624 | 0.276 | 0.031 | 0.975 | 0.735 | |
| Total filtration surface/glomerulus | −0.03 | −0.09 | 0.12 | −0.04 | −0.16 | −0.03 | 0.12 |
| 0.752 | 0.361 | 0.222 | 0.687 | 0.116 | 0.737 | 0.234 | |
| Podocyte number per glomerulus | −0.07 | −0.001 | −0.05 | 0.05 | 0.09 | 0.05 | 0.00 |
| 0.511 | 0.955 | 0.626 | 0.643 | 0.363 | 0.635 | 1.000 | |
| Foot process width | 0.03 | −0.18 | 0.12 | 0.10 | 0.00 | −0.02 | 0.16 |
| 0.756 | 0.072 | 0.232 | 0.332 | 0.978 | 0.845 | 0.109 | |
| Podocyte detachment | −0.02 | 0.12 | −0.15 | −0.10 | 0.18 | 0.11 | −0.15 |
| 0.820 | 0.232 | 0.123 | 0.317 | 0.075 | 0.272 | 0.140 | |
| Fenestrated endothelium | −0.17 | 0.25 | −0.23 | −0.06 | −0.02 | 0.10 | −0.22 |
| 0.095 | 0.013 | 0.021 | 0.550 | 0.873 | 0.322 | 0.024 |
The correlation is shown on top, and the P-value below. Statistically significant correlations (P < 0.05) are shown in bold. NLR = neutrophil:lymphocyte ratio.
FIGURE 1.
Partial residual regression plots showing the relationships of neutrophil and lymphocyte fractions and the neutrophil:lymphocyte ratio with the percentage of normally fenestrated endothelium, adjusted for age, sex, diabetes duration, HbA1c, MAP, GFR and treatment assignment during the clinical trial.
A significant interaction with treatment assignment was found in the relationship between monocyte fraction and cortical interstitial fractional volume, mesangial fractional volume and podocyte number per glomerulus. When analyzed separately by treatment assignment, monocyte fraction correlated positively with podocyte number per glomerulus in those who were randomized to receive placebo during the clinical trial (r = 0.35, P = 0.025), but not in those who were randomized to receive losartan (r = −0.17, P = 0.217). Conversely, monocyte fraction correlated positively with cortical interstitial fractional volume (r = 0.29, P = 0.034) and mesangial fractional volume (r = 0.33, P = 0.013) in those randomized to receive losartan but not in those who received placebo (r = −0.05, P = 0.768 for cortical interstitial fractional volume; r = −0.03, P = 0.845 for mesangial fractional volume).
Partial correlations between differential counts (rather than fractions) and the morphometric parameters after additional adjustment for total WBC count are shown in Supplementary data, Table S3. Lymphocyte count correlated positively with the percentage of normally fenestrated endothelium and negatively with GBM width.
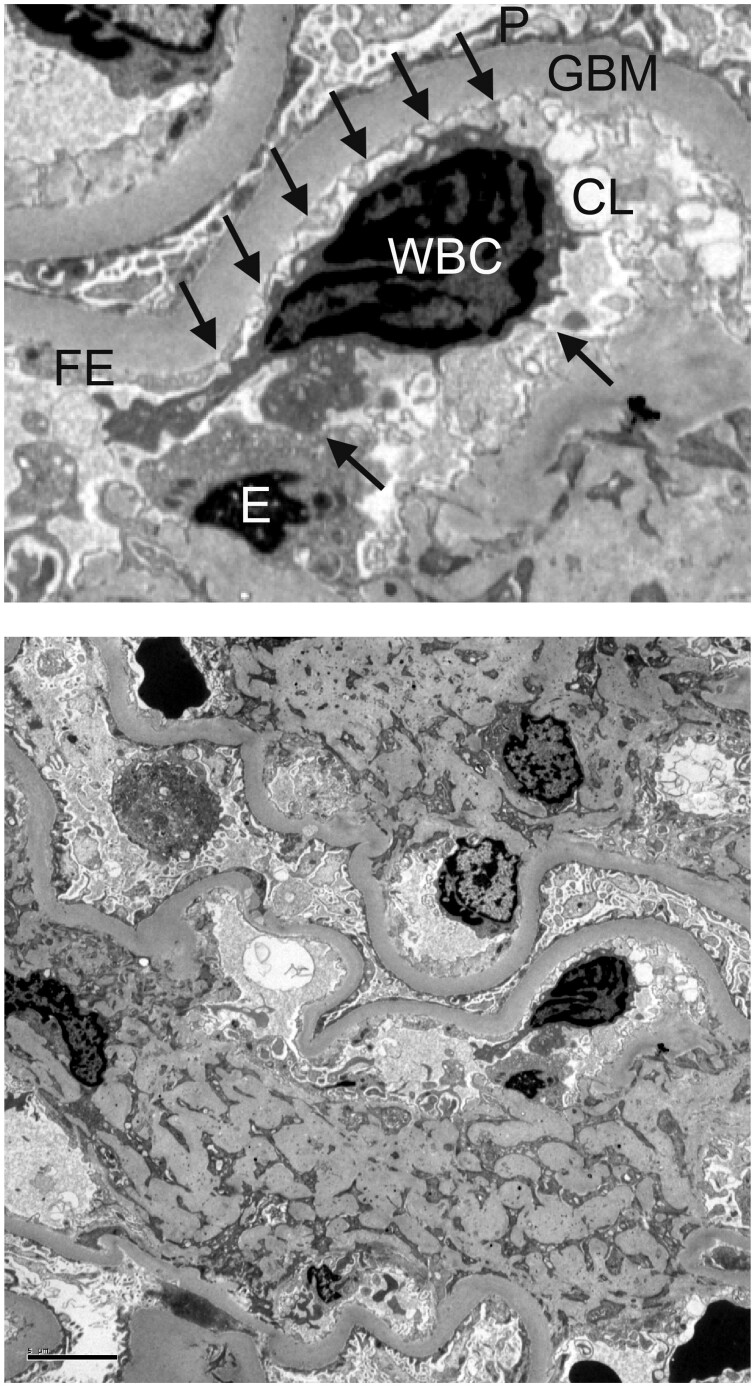
The number of WBCs per glomerular capillary profile in the subset of nine participants with the most severe structural injury (0.08 ± 0.02) was 2.7 times as high as in the 11 participants with the least structural injury (0.03 ± 0.02, P < 0.001). The correlation between the number of WBCs per glomerular capillary and the total WBC count was not statistically significant (r = −0.03, P = 0.900), suggesting that the increased number of WBCs per glomerular capillary was not related to increased numbers of circulating WBCs. We frequently observed leukocyte projections touching endothelial cells within the glomerular capillary in the electron microscopy images, suggesting signaling between these two cell types (Figure 2).
FIGURE 2.
Peripheral glomerular capillaries from a Pima Indian with Type 2 diabetes. In the upper panel, arrows point to projections extending from the WBC to the adjacent endothelium within the glomerular capillary lumen. Transmission electron microscopy ×3000. P, podocyte foot processes; E, endothelial cell body; FE, fenestrated endothelium; CL, capillary lumen. Complete image is shown in the lower panel. Scale bar illustrates a length of 5 µm.
In the SURDIAGENE cohort, baseline WBC parameters were assessed as predictive variables for follow-up RFL. During median follow-up of 4.5 years (IQR = 2.6–7.4 years), 321 individuals developed RFL. After adjustment for age, sex, diabetes duration, HbA1c, MAP, GFR and ACR, total WBC count (HR = 1.20, 95% CI 1.08–1.35), lymphocyte fraction (HR = 0.67, 95% CI 0.60–0.76), neutrophil fraction (HR = 1.35, 95% CI 1.20–1.52) and the neutrophil:lymphocyte ratio (HR = 1.44, 95% CI 1.28–1.62) were each associated with RFL (Table 4). The c-statistic for the fully adjusted model predicting RFL was 0.707 when it did not include a WBC variable. The addition of the lymphocyte fraction increased the c-statistic to 0.728 (likelihood ratio test P < 0.001, rIDI 23.4%, 95% CI 7.6–44.0); the addition of the neutrophil fraction increased the c-statistic to 0.717 (likelihood ratio test P < 0.001, rIDI 14.9%, 95% CI 3.6–29.8); and the addition of the neutrophil:lymphocyte ratio increased the c-statistic to 0.725 (likelihood ratio test P < 0.001, rIDI 20.1%, 95% CI 6.0–37.7). Associations of differential counts (rather than fractions) and the neutrophil:lymphocyte ratio after additional adjustment for total WBC count are shown in Supplementary data, Table S4. Statistically significant relationships of lymphocytes and neutrophils with RFL were present with both fractions and counts, and the relationship between the neutrophil:lymphocyte ratio and RFL was largely unchanged when adjusted additionally for total WBC count. Conclusions were unchanged when analysis was restricted to the 346 individuals in whom the WBC count was measured within 1 year of the baseline examination. When restricted to the 910 Caucasians in the SURDIAGENE cohort, the relationship between total WBC count and RFL in the fully adjusted model was no longer statistically significant (HR = 1.10, 95% CI 0.98–1.23), but the overall conclusions were otherwise unchanged.
Table 4.
Unadjusted and multivariable Cox models are shown for the association of the total WBC count, differential fractions and the neutrophil:lymphocyte ratio with ≥40% decline in estimated GFR from the baseline value in the SURDIAGENE cohort
| Variable | Unadjusted |
Adjusted |
c-Statistic with WBC | Difference in c-Statistic (95% CI) | Likelihood ratio P-value | rIDI (95% CI) | P-value for rIDI | ||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||||||
| Total WBC count | 1.18 (1.05–1.31) | 0.004 | 1.15 (1.02–1.28) | 0.018 | 0.710 | 0.003 (0.003–0.004) | 0.026 | 0.038 (0.000–0.136) | 0.054 |
| Lymphocyte fraction | 0.61 (0.54–0.68) | <0.001 | 0.71 (0.63–0.80) | <0.001 | 0.728 | 0.021 (0.020–0.021) | <0.001 | 0.234 (0.076–0.440) | <0.001 |
| Neutrophil fraction | 1.47 (1.32–1.65) | <0.001 | 1.30 (1.16–1.46) | <0.001 | 0.717 | 0.011 (0.011–0.011) | <0.001 | 0.149 (0.036–0.298) | <0.001 |
| Monocyte fraction | 1.15 (1.03–1.30) | 0.017 | 1.08 (0.96–1.23) | 0.203 | 0.709 | 0.003 (0.002–0.003) | 0.131 | 0.009 (−0.001–0.082) | 0.452 |
| Eosinophil fraction | 0.94 (0.84–1.05) | 0.287 | 0.92 (0.82–1.03) | 0.126 | 0.707 | 0.000 (0.000–0.000) | 0.155 | 0.015 (−0.001–0.081) | 0.250 |
| Basophil fraction | 0.95 (0.84–1.06) | 0.348 | 0.93 (0.83–1.04) | 0.217 | 0.707 | 0.000 (0.000–0.001) | 0.260 | 0.001 (−0.007–0.050) | 0.942 |
| NLR | 1.59 (1.42–1.78) | <0.001 | 1.38 (1.23–1.55) | <0.001 | 0.725 | 0.018 (0.018–0.018) | <0.001 | 0.201 (0.060–0.377) | <0.001 |
The multivariable model was adjusted for age, sex, diabetes duration, HbA1c, MAP, estimated GFR and ACR. The HRs are given for a 1 SD difference in each white cell variable. C-statistic, P-value for the likelihood ratio test and the 5-year rIDI for prediction of RFL from the fully adjusted Cox models are shown with and without each white cell variable. NLR = neutrophil:lymphocyte ratio.
DISCUSSION
Several WBC fractions and the neutrophil:lymphocyte ratio are modestly but statistically significantly associated with DKD lesions in Pima Indians with Type 2 diabetes. Specifically, lower lymphocyte fraction, higher neutrophil fraction and higher neutrophil:lymphocyte ratio were each associated with a lower percentage of endothelial fenestrations. We and others reported previously that loss of endothelial fenestrae correlates with elevated albuminuria [22, 34], and the percentage of normally fenestrated endothelium predicts RFL in Pima Indians [28]. Further examination of a subset of kidney biopsies suggested that extensive glomerular injury was associated with increased numbers of WBCs per glomerular capillary. These WBCs often had projections touching the glomerular capillary endothelial cells, suggesting that signaling may be occurring between them. The nature and impact of this signaling is unknown, but may be important given the association between the WBCs and the percentage of endothelial fenestrations in this study. In addition, higher eosinophil fraction and lower lymphocyte fraction were each associated with higher GBM width, another structural parameter predicting RFL in this population [28]. Separately, in the SURDIAGENE cohort, total WBC count, lymphocyte and neutrophil fractions and the neutrophil:lymphocyte ratio were each significantly associated with RFL, even after ACR was included in the multivariate analysis. Each of these WBC variables significantly improved the accuracy of RFL prediction when considered in addition to traditional renal risk factors. These findings suggest that modest differences in several WBC fractions reflect and may contribute to the underlying tissue injury that leads to progressive GFR loss in DKD.
Higher total WBC counts are associated with the development of and the microvascular and macrovascular complications that accompany Type 2 diabetes [7, 8, 35]. In a cross-sectional study of 1480 Chinese persons with Type 2 diabetes [35], participants in the highest versus the lowest quartile of WBC counts had a 4.2-fold increased odds of DKD, defined by an ACR ≥30 or a serum creatinine concentration >1.5 mg/dL. In addition, participants in the highest quartile of neutrophil counts had a 5.5-fold increased odds and those in the highest quartile of monocyte counts had a 3.5-fold increased odds of DKD. On the other hand, lower lymphocyte counts were associated with higher risk of DKD (odds ratio of highest versus lowest quartile = 0.6). Although there was no relationship with eosinophil counts after adjustment for potential confounders, eosinophils may play an important role in DKD. In 783 Japanese persons with Type 2 diabetes, eosinophil counts were associated with albumin excretion rate in men [36]. Moreover, aggregates of interstitial eosinophils were more common in kidney biopsies in persons with DKD than in other types of kidney disease, including allergic interstitial nephritis, and these interstitial eosinophilic aggregates were associated with more interstitial fibrosis and tubular atrophy [37].
In a longitudinal study of 1 594 700 veterans in the USA (28.6% with diabetes), higher baseline monocyte counts in the peripheral blood were associated with higher risk of incident chronic kidney disease (estimated GFR <60 mL/min/1.73 m2) and ESRD during median follow-up of 9.2 years [38]. In the kidneys, monocytes may become activated macrophages secreting pro-inflammatory factors such as tumor necrosis factor α (TNFα), interluekin-1, interleukin-6 and reactive oxygen species [39], which may contribute to this observation. Relationships between monocyte fraction and structural parameters in the present study were influenced by the study treatment during the 6-year trial that preceded the kidney biopsy. We found no relationship between monocyte fractions and RFL.
We examined the relationships of cell fractions rather than cell counts with morphometric variables and RFL in the present study, because the fractions include information about the relative abundance of each leukocyte species. In the Supplementary tables, we have also reported relationships with WBC counts. Whereas the relationship of counts and fractions with RFL were consistent, fractions were more strongly associated with the morphometric variables. We also evaluated the neutrophil:lymphocyte ratio, as this ratio may also be a better predictor of inflammation than counts alone [40]. Factors such as the level of hydration or the handling of blood samples may affect the absolute counts more than either the fractions or the ratio. In addition, the neutrophil:lymphocyte ratio integrates non-specific inflammation marked by elevated neutrophils with signs of physiological stress indicated by lymphopenia. Indeed, the neutrophil:lymphocyte ratio is the strongest WBC predictor of adverse outcomes in coronary artery syndromes [41]. In DKD, the neutrophil:lymphocyte ratio was associated with 24-h urine protein and albumin excretion in 80 Turkish patients with newly diagnosed Type 2 diabetes [42], and correlated positively with the presence of microalbuminuria and inversely with the estimated GFR in 114 Turkish patients with Type 2 diabetes of longer duration [43]. In our study, the neutrophil:lymphocyte ratio was associated with structural lesions of DKD and with progressive GFR loss that occurs in the presence of those lesions.
Strengths of this study include the availability of research kidney biopsies and the morphometric methods used to assess kidney structure. Demonstrating relationships between WBC fractions and RFL in a separate cohort provides independent confirmation for WBCs’ role in progressive DKD, and the consistency of the findings across racial groups suggest these results can be generalized. Weaknesses include the cross-sectional morphometric analysis, which precludes assessment of predictive associations between WBCs and kidney structure. The relatively small cohort and loss to follow-up from progressive kidney disease limited more robust examination of relationships between WBCs and RFL in the Pima Indians. Although we do not have complete data for measurements of other pro-inflammatory markers in these cohorts, a recent SURDIAGENE study, including some participants from the present study, found a relationship between elevated serum TNF receptor 1 and RFL [44]. Moreover, the combination of serum TNF receptor 1, N-terminal prohormone brain natriuretic peptide and midregional-proadrenomedullin, which each may increase inflammation in certain settings, yielded the strongest association with RFL [44]. In subsets of the present Pima Indian cohort, we found that elevated serum TNF receptors 1 and 2 [45] and advanced glycation end-products [46] were associated with the structural determinants of RFL. Together, these findings suggest a substantial role for inflammation in the development of the structural lesions that ultimately lead to progressive DKD.
Our findings are based on a single measurement of the WBC variables in each cohort. Moreover, WBC measurements in the Pima Indians were not performed at the time of the kidney biopsy, but at an examination near the time of biopsy, and the median interval between the baseline examination and measurement of the WBC variables in the SURDIAGENE cohort was 1.6 years. Hence, variability of these measurements may influence our findings. Little is known about the stability of these measurements. After normalizing for population variance, the average fluctuation of WBC counts measured four times over 6 weeks in 45 patients in a Canadian study was 4.2% [47]. In the Pima and SURDIAGENE cohorts, correlations between WBC measurements made a median of 0.52 and 0.60 years apart, respectively, ranged from 0.39 to 0.66, suggesting modest stability of these measures over time. We did not adjust for multiple comparisons in our analyses because this was an exploratory study, and we wanted to avoid masking relationships of potential interest. The consistency of the neutrophil and lymphocyte relationships with morphometric variables and with the risk of RFL across cohorts provides some assurance against false-positive results.
In conclusion, a routine WBC count and differential provide clinically relevant information about DKD risk in Pima Indians and Caucasians with Type 2 diabetes. Lymphocytes and neutrophils, which together represent ∼90% of WBCs, had the strongest associations with structural lesions of DKD and with RFL. Neutrophil and lymphocyte fractions and the neutrophil:lymphocyte ratio each modestly but significantly improved prediction of RFL over traditional risk factors. The value of these WBC parameters may be enhanced by combining them with other markers of inflammation, especially those localized predominantly to the kidneys.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the members of Gila River Indian Community and the SURDIAGENE cohort patients for participation in these studies and the staff of the NIDDK Phoenix Branch and the Poitiers Biological Resource Center (CRB BB-0033-00068, Poitiers, France) for assistance.
FUNDING
This research was sponsored by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and by the American Diabetes Association [Clinical Science Award (1-08-CR-42)]. The SURDIAGENE study was supported by grants from PHRC-Poitiers 2004 and AFD (Research Grant 2003) and by the Groupe d’Etude des Maladies Métaboliques et Systémiques (Poitiers, France).
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract form.
Contributor Information
Kevin M Wheelock, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA.
Pierre-Jean Saulnier, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA; CHU Poitiers Inserm, Clinical Investigation Center CIC1402, Poitiers, France.
Stephanie K Tanamas, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA.
Pavithra Vijayakumar, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA.
E Jennifer Weil, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA.
Helen C Looker, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA.
Robert L Hanson, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA.
Kevin V Lemley, Department of Pediatrics, University of Southern California Keck School of Medicine, Los Angeles, CA, USA.
Berne Yee, Southwest Kidney Institute, Phoenix, AZ, USA.
William C Knowler, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA.
Samy Hadjadj, CHU Poitiers Inserm, Clinical Investigation Center CIC1402, Poitiers, France.
Behzad Najafian, Department of Pathology, University of Washington, Seattle, WA, USA.
Michael Mauer, Department of Nephrology, University of Minnesota, Minneapolis, MN, USA.
Robert G Nelson, Phoenix Epidemiology and Clinical Research Branch, Phoenix, AZ, USA.
REFERENCES
- 1. Levin A, Tonelli M, Bonventre J et al.; ISN. Global Kidney Health Summit participants. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017. doi: 10.1016/S0140-6736(17)30788-2 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2. Fufaa GD, Weil EJ, Nelson RG et al. Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol Dial Transplant 2015; 30: 599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aso Y, Yoshida N, Okumura K et al. Coagulation and inflammation in overt diabetic nephropathy: association with hyperhomocysteinemia. Clin Chim Acta 2004; 348: 139–145 [DOI] [PubMed] [Google Scholar]
- 4. Pavkov ME, Nelson RG, Knowler WC et al. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int 2015; 87: 812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gohda T, Niewczas MA, Ficociello LH et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 2012; 23: 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pezzolesi MG, Satake E, McDonnell KP et al. Circulating TGF-beta1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes 2015; 64: 3285–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong PC, Lee KF, So WY et al. White blood cell count is associated with macro- and microvascular complications in chinese patients with type 2 diabetes. Diabetes Care 2004; 27: 216–222 [DOI] [PubMed] [Google Scholar]
- 8. Vozarova B, Weyer C, Lindsay RS et al. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002; 51: 455–461 [DOI] [PubMed] [Google Scholar]
- 9. Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm 2012; 2012: Article ID 146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pertynska-Marczewska M, Kiriakidis S, Wait R et al. Advanced glycation end products upregulate angiogenic and pro-inflammatory cytokine production in human monocyte/macrophages. Cytokine 2004; 28: 35–47 [DOI] [PubMed] [Google Scholar]
- 11. Shurtz-Swirski R, Sela S, Herskovits AT et al. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care 2001; 24: 104–110 [DOI] [PubMed] [Google Scholar]
- 12. Hofmann MA, Schiekofer S, Isermann B et al. Peripheral blood mononuclear cells isolated from patients with diabetic nephropathy show increased activation of the oxidative-stress sensitive transcription factor NF-kappaB. Diabetologia 1999; 42: 222–232 [DOI] [PubMed] [Google Scholar]
- 13. Weil EJ, Fufaa G, Jones LI et al. Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes 2013; 62: 3224–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanamas SK, Saulnier PJ, Fufaa GD et al. Long-term effect of losartan on kidney disease in American Indians with type 2 diabetes: a follow-up analysis of a randomized clinical trial. Diabetes Care 2016; 39: 2004–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hadjadj S, Fumeron F, Roussel R et al. Prognostic value of the insertion/deletion polymorphism of the ACE gene in type 2 diabetic subjects: results from the Non-insulin-dependent Diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events, and Ramipril (DIABHYCAR), Diabete de type 2, Nephropathie et Genetique (DIAB2NEPHROGENE), and Survie, Diabete de type 2 et Genetique (SURDIAGENE) studies. Diabetes Care 2008; 31: 1847–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myers BD, Nelson RG, Tan M et al. Progression of overt nephropathy in non-insulin-dependent diabetes. Kidney Int 1995; 47: 1781–1789 [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vasquez B, Flock EV, Savage PJ et al. Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following diet-induced reduction of hyperglycaemia. Diabetologia 1984; 26: 127–133 [DOI] [PubMed] [Google Scholar]
- 19. Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol 1960; 30: 207–212 [PubMed] [Google Scholar]
- 20. Weibel ER. Stereological Methods, Vol. 1: Practical Methods for Biological Morphometry. London, UK: Academic Press, 1979 [Google Scholar]
- 21. Pagtalunan ME, Miller PL, Jumping-Eagle S et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 1997; 99: 342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weil EJ, Lemley KV, Mason CC et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int 2012; 82: 1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mauer SM, Steffes MW, Ellis EN et al. Structural-functional relationships in diabetic nephropathy. J Clin Invest 1984; 74: 1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 1994; 43: 1358–1364 [DOI] [PubMed] [Google Scholar]
- 25. Tan JC, Busque S, Workeneh B et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int 2010; 78: 686–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belsley DA, Kuh E, Welsch RE. Regression Diagnostics: Identifying Influential Observations and Sources of Collinearity. Hoboken, NJ: John Wiley and Sons, 1980 [Google Scholar]
- 27. Coresh J, Turin TC, Matsushita K et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fufaa GD, Weil EJ, Lemley KV et al. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol 2016; 11: 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin DY, Wei L-J, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 1993; 80: 557–572 [Google Scholar]
- 30. Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004; 23: 2109–2123 [DOI] [PubMed] [Google Scholar]
- 31. Demler OV, Pencina MJ, D’Agostino RB. Misuse of DeLong test to compare AUCs for nested models. Stat Med 2012; 31: 2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pepe MS, Kerr KF, Longton G, Wang ZY. Testing for improvement in prediction model performance. Stat Med 2013; 32: 1467–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27: 157–172 [DOI] [PubMed] [Google Scholar]
- 34. Toyoda M, Najafian B, Kim Y et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes 2007; 56: 2155–2160 [DOI] [PubMed] [Google Scholar]
- 35. Chung FM, Tsai JC, Chang DM et al. Peripheral total and differential leukocyte count in diabetic nephropathy: the relationship of plasma leptin to leukocytosis. Diabetes Care 2005; 28: 1710–1717 [DOI] [PubMed] [Google Scholar]
- 36. Fukui M, Tanaka M, Hamaguchi M et al. Eosinophil count is positively correlated with albumin excretion rate in men with type 2 diabetes. Clin J Am Soc Nephrol 2009; 4: 1761–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dai DF, Sasaki K, Lin MY et al. Interstitial eosinophilic aggregates in diabetic nephropathy: allergy or not? Nephrol Dial Transplant 2015; 30: 1370–1376 [DOI] [PubMed] [Google Scholar]
- 38. Bowe B, Xie Y, Xian H et al. Association between monocyte count and risk of incident CKD and progression to ESRD. Clin J Am Soc Nephrol 2017. doi: 10.2215/CJN.09710916 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 2006; 17: 368–377 [DOI] [PubMed] [Google Scholar]
- 40. Bhat T, Teli S, Rijal J et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther 2013; 11: 55–59 [DOI] [PubMed] [Google Scholar]
- 41. Azab B, Zaher M, Weiserbs KF et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 2010; 106: 470–476 [DOI] [PubMed] [Google Scholar]
- 42. Afsar B. The relationship between neutrophil lymphocyte ratio with urinary protein and albumin excretion in newly diagnosed patients with type 2 diabetes. Am J Med Sci 2014; 347: 217–220 [DOI] [PubMed] [Google Scholar]
- 43. Ciray H, Aksoy AH, Ulu N et al. Nephropathy, but not angiographically proven retinopathy, is associated with neutrophil to lymphocyte ratio in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2015; 123: 267–271 [DOI] [PubMed] [Google Scholar]
- 44. Saulnier PJ, Gand E, Velho G et al. Association of circulating biomarkers (adrenomedullin, TNFR1, and NT-proBNP) with renal function decline in patients with type 2 diabetes: a French prospective cohort. Diabetes Care 2017; 40: 367–374 [DOI] [PubMed] [Google Scholar]
- 45. Pavkov ME, Weil EJ, Fufaa GD et al. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int 2016; 89: 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saulnier PJ, Wheelock KM, Howell S et al. Advanced glycation end products predict loss of renal function and correlate with diabetic nephropathy lesions in American Indians with type 2 diabetes. Diabetes 2016; 65: 3744–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnston KW, Hosang MY, Andrews DF. Reproducibility of noninvasive vascular laboratory measurements of the peripheral circulation. J Vasc Surg 1987; 6: 147–151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.