Abstract
Salmonella Pullorum is one of the most important avian pathogenic bacteria due to widespread outbreaks accompanied by high mortality. It has been demonstrated that the Salmonella Enteritidis live vaccine strain Sm24/Rif12/Ssq is able to induce cross-immunity protection against Salmonella Gallinarum and Salmonella Infantis, however, it is unknown whether this vaccine is effective against Salmonella Pullorum infection. In the present study, the Hubbard parent chickens were orally administrated this vaccine at 1-day-old, 40-day-old, and 131-day-old respectively, and challenged by Salmonella Pullorum at 157-day-old to evaluate the protective effect of the Salmonella Enteritidis live vaccine strain Sm24/Rif12/Ssq. After each vaccination, the vaccine strain could be recovered from cloacal swabs within a week, whereas no vaccine strain was re-isolated from environmental samples throughout the experiment. Vaccination for the breeder chickens with Salmonella Enteritidis Sm24/Rif12/Ssq could relieve swollen liver (P = 0.0066) caused by Salmonella Pullorum infection and decrease Salmonella Pullorum colonization level in spleen (P = 0.0035), whereas no significant difference was found in the bacterial counts of liver, ovary and oviduct of vaccinated chickens. These results suggested that the Salmonella Enteritidis live vaccine strain Sm24/Rif12/Ssq was high safety and effective against Salmonella Pullorum infection to a certain extent.
Key words: Salmonella Enteritidis, live vaccine, Salmonella Pullorum, cross-protection, breeder chicken
INTRODUCTION
Pullorum disease is an acute septicaemic disease of avian species resulting from Salmonella enterica subsp. enterica serovar Gallinarum biovar Pullorum (S. Pullorum) infection. Newly hatched chicks show high susceptibility to S. Pullorum and infected chicks may manifest somnolence, depressed appetite, adherence of chalky white material to the vent (Shivaprasad, 2000). For growing and mature fowl, infected chickens may not exhibit any signs and cannot be detected by their physical appearance, which is by far the most important ways of perpetuation and spread of the organism. It is known that S. Pullorum could persists within macrophages in the spleen during the carrier state resulting in persistent infection and transmission to eggs or progeny (Berchieri et al., 2001; Wigley et al., 2001; Foster et al., 2021; Ijaz et al., 2021). Infected mature breeders will bring a set of problems including a drop in egg production, decreased fertility, diminished hatchability and high mortality of progeny (Shivaprasad, 2000). Benefiting from the great process of intensive poultry industry and the implementation of extensive eradication programs, S. Pullorum has been under control and not a primary concern in developed countries. However, it still persists in many countries in Africa, Asia, and South America, leading to severe economic losses (Barrow and Freitas Neto, 2011). In China, Salmonella infection in poultry is common and the main prevalent serovar isolated from chickens is S. Pullorum (Song et al., 2020).
There are several vaccine types for Salmonella immunization of poultry containing live-attenuated vaccines, inactivated vaccines and subunit vaccines. Compared with inactivated vaccines and subunit vaccines, live attenuated vaccines could induce protective immunity through activation of both antibody and cell-mediated immune responses (Desin et al., 2013; Acevedo-Villanueva et al., 2021). The Salmonella Enteritidis (S. Enteritidis) strain Sm24/Rif12/Ssq (AviPro Salmonella Vac E, ELANCO, Cuxhaven, Germany) is a live-attenuated vaccine strain of S. Enteritidis phage type 4, which is able to reduce the S. Enteritidis colonization in organs and decrease the egg contamination (Gantois et al., 2006; Atterbury et al., 2009; Huberman et al., 2019). Furthermore, due to its metabolic drift markers, this strain can be easily differentiated from field strains by its resistance to rifampicin and streptomycin and high susceptibility to quinolones, for instance to erythromycin, whereas any wild type strain of S. Enteritidis has the opposite pattern of sensibility (Chacana and Terzolo, 2006; Eeckhaut et al., 2018). The vaccination of first day of life protects chicks against the intestinal colonization of wild type S. Enteritidis by colonization-inhibition. Booster immunizations are used to decrease gut colonization, shedding, internal organ colonization, and egg contamination based on stimulation of cell-mediated and humoral immune responses. In addition, the vaccine strain Sm24/Rif12/Ssq shows cross-protection against other Salmonella serotypes, including S. Gallinarum and S. Infantis (Chacana and Terzolo, 2006; Eeckhaut et al., 2018), but it is unclear whether the vaccine is capable to protect chickens against S. Pullorum infection. In the present study, we evaluated whether this live-attenuated vaccine strain of S. Enteritidis was able to confer cross-protection against S. Pullorum infection.
MATERIALS AND METHODS
Chickens and Experimental Groups
A total of 96 newly hatched Hubbard parent chicks were obtained from Yisheng (Yisheng Livestock and Poultry Breeding Co., Ltd., Shandong, China) and randomly divided into 4 groups, which were housed in separate rooms more than 50 meters apart with wood shavings and waterlines by different experimental personnel to avoid cross-contamination. The details of group information and treatment were shown in Table 1. All chicks were confirmed to be Salmonella-free by bacteriological analysis of cloacal swabs according to a previously described method (Yang et al., 2019). In addition, to avoid chickens overweighting, the Hubbard Breeder Management Manual and the Hubbard Breeder Nutrition Guide (www.hubbardbreeders.com/documentation) were referred to make feed programs. All procedures used in this study were approved by the Animal Care Committee of Shandong Agricultural University (P. R. China) and were carried out in accordance with the guidelines for experimental animals of the Ministry of Science and Technology (Beijing, P. R. China).
Table 1.
Group information and treatment.
| Age of vaccination (days old) |
Age of challenge (days old) |
||||
|---|---|---|---|---|---|
| Group | Number | 1 | 40 | 131 | 157 |
| Control | 18 | - | - | - | - |
| Only vaccinated | 30 | Yes | Yes | Yes | - |
| Vaccinated + SP | 30 | Yes | Yes | Yes | Yes |
| Nonvaccinated + SP | 18 | - | - | - | Yes |
Vaccination
According to the manufacturer's instructions, the live commercial attenuated S. Enteritidis vaccine strain Sm24/Rif12/Ssq resistant to rifampicin and streptomycin (AviPro Salmonella Vac E, ELANCO) was diluted in distilled water to give a final concentration of between 1 and 8 × 108 CFU per dose. Then chicks of the only vaccinated group and the vaccinated + SP (S. Pullorum challenged chickens after vaccination) group were vaccinated by oral gavage at the 1-day-old, 40-day-old, and 131-day-old, respectively. Meanwhile, the control group and the nonvaccinated + SP group was orally inoculated with the same volume of distilled water.
Challenge Strains and Challenge Procedure
The S. Pullorum standard strain CVCC526 resistant to clarithromycin and sensitive to rifampicin was used for challenge in this study. The strain was grown at 37°C in Luria-Bertani (LB) liquid media (Haibo Biotechnology, Qingdao, China) for 12 h, then bacterial cells were centrifuged and adjusted to a dose of 1.8 × 109 CFU in a volume of 200 μL phosphate-buffered saline solution (PBS, Solarbio, Beijing, China) for challenge. Chickens of the nonvaccinated + SP group and the vaccinated + SP group were administered by injection into the breast muscle at 157-day-old, whereas the control group and the only vaccinated group was inoculated the same volume of bacteria free PBS buffer.
Re-isolation of the Vaccine Strain
To monitor the shedding of the vaccine strain, cloacal swabs were taken individually from the chicks of the only vaccinated group and the vaccinated + SP group (n = 60). After each vaccination, cloacal swabs were taken every 3 d for 3 wk and then taken weekly till next vaccination. A total of 2,114 cloacal swabs from 35 batches of samples were taken from 4-day-old to 178-day-old. Besides, 390 environmental samples of the only vaccinated group and the vaccinated + SP group including wood shavings, waterline nipple swabs and the remaining feed in the feeding trough were collected every 7 d to examine whether the live vaccine strain survived in the environment. The swabs, wood shavings, or feed were incubated overnight at 37°C in buffered peptone water (BPW, Haibo Biotechnology, Qingdao, China) and afterwards a loopful was plated on brilliant-green phenol-red lactose sucrose (BPLS) agar (Haibo Biotechnology) plates supplemented with 200 μg/mL streptomycin (Sigma, Shanghai, China) and 100 μg/mL rifampicin (Sigma), which was used as selective medium to detect the vaccine strain (Gantois et al., 2006).
Bacterial Burden of Organs
At the d 21 post-challenge, 6 randomly selected chickens from each group were weighted and euthanized. The liver, spleen and heart of chicken were weighted for calculating the organ/body weight ratio. Besides, samples of liver, spleen, ovary, oviduct, and cecal content of infected chickens were weighted and homogenized by automatic sample fast grinder (Shanghai Jingxin, Shanghai, China) in 1 mL of PBS, then serial dilutions of the homogenates were plated onto xylose lysine desoxycholate (XLD) agar (Haibo Biotechnology) plates (Haibo Biotechnology) supplemented with 50 μg/mL clarithromycin (Sigma) for determining the number of S. Pullorum. Three suspected S. Pullorum colonies for each sample were identified by PCR assays using a specific target gene ipaJ (Xu et al., 2018).
Statistical Analysis
GraphPad Prism 8 software was used for statistical analysis. One-way ANOVA analysis was used to determine statistical differences in the organ/body weight ratio. Bacterial counts/gram tissue was log transformed and normality of the data was tested with the t test. Differences with P-values below 0.05 were considered to be significant.
RESULTS AND DISCUSSION
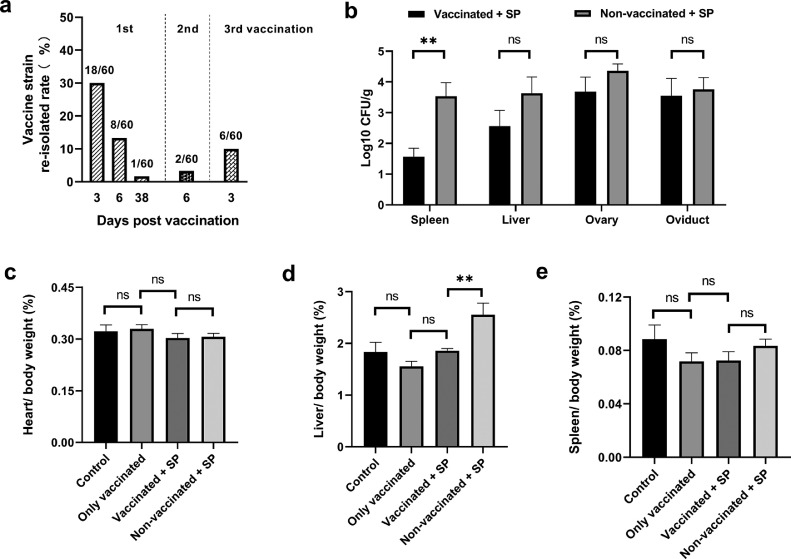
In general, it is very important that live vaccines should be safe as well as efficient and at the same time do not interfere with the existing systems for monitoring infection (Zhang-Barber et al., 1999). To monitor the shedding of the vaccine strain, 2,114 cloacal swabs covering from the period of chick-rearing to sexual maturity were collected. Vaccine strain was detected on the 3, 6, 38 d post first vaccination, 6 d post second vaccination and 3 d post third, and not detected at other time (Figure 1A). It was within a week after vaccination that the vaccine strain could be recovered from cloacal swabs with the highest probability (Figure 1A), which coincided with the previous reports (Chacana and Terzolo, 2006; Huberman et al., 2019). Interestingly, the vaccine strain could be still re-isolated at 38 d post first vaccination, indicating that the excretion period of the vaccine strain could persist longer than we thought before. No vaccine strain was detected (0/390) in samples from wood shavings, waterline nipples swabs, and the remaining feed in the feeding trough for both the only vaccinated group and the vaccinated + SP group throughout the experiment, even during the excretion period of the vaccine strain in chickens. The result indicated that the vaccine strain could not survive in the environment. Besides, no any clinical signs of depression or disease were observed in the chicks after vaccination. In summary, the S. Enteritidis strain Sm24/Rif12/Ssq with metabolic drift mutantation and reduced virulence showed good safety profile as a live vaccine, making vaccination at the first day of life was feasible.
Figure 1.
(A) The re-isolation rate of the vaccine strain from the positive cloacal swabs in both the only vaccinated group and the vaccinated + SP group throughout the whole experiment. (B) The bacterial loads of S. Pullorum in spleen, liver, ovary, and oviduct on 21 d postchallenge. The effect of the S. Enteritidis vaccine on the heart/body weight ratio (C), liver/body weight ratio (D) and spleen /body weight ratio (E) on 21 d postchallenge.
Poultry meat and eggs are a major source of S. Enteritidis, but since the introduction of the Salmonella vaccines including S. Enteritidis live vaccine (AviPro Salmonella Vac E) in the European Union and other regions worldwide, the S. Enteritidis contamination rate of poultry flocks and products has reduced significantly. Vaccination with the live vaccine at 1-day-old and followed by booster immunizations, have become standard practice in the European. Moreover, this vaccine is capable to protect chickens against a broad range of Salmonella enterica serotypes, including S. Gallinarum and S. Infantis (Chacana and Terzolo, 2006; Eeckhaut et al., 2018). In China, due to the complex composition of breeds, the elimination of S. Pullorum is a tough task and the government is promoting this project to be fastened and widen. Considering this vaccine could induce cross-protection against other Salmonella serotypes, in the present study we evaluated whether the only approved Salmonella vaccine for chickens on the Chinese market so far was able to confer cross-protection against S. Pullorum infection. On 21 d postchallenge, the vaccination significantly decreased the liver/body weight ratio (P = 0.0066), whereas no significant effect was observed on the spleen/body weight ratio (P = 0.4081) and heart/body weight ratio (P = 0.9775; Figures 1C–1E). These data suggested that the vaccination was effective in relieving swollen liver caused by S. Pullorum infection. Bacteriological analysis of the spleen in the vaccinated + SP group showed that the colonization level of S. Pullorum significantly decreased (P = 0.0035) and the bacterial load of S. Pullorum in the liver was 0.36-fold lower (P = 0.1761) than the non-vaccinated + SP group (Figure 1B). However, the number of S. Pullorum in the ovary and oviduct of chickens in the vaccinated + SP group were similar with that of the nonvaccinated + SP group (Figure 1B), which was consist with the previous study of Huberman et al. (2019). The sexual maturity of experimental chickens may be related to this result. Although the exact mechanism regarding persistent infection and carriage which result in infection of the reproductive tract is still unclear, the increase of sex hormones could reduce capacity of T cells to respond specifically and nonspecifically to antigens, which probably enables the bacteria to spread to the reproductive tract (Wigley et al., 2005). It has been known that the S. Pullorum has a preference to survive and proliferate in the macrophage of liver and spleen, which will contribute to persistent infection and dissemination to other organs. Therefore, alleviation the invasion and reduction of bacterial colonization levels in the liver and spleen are important for protection against S. Pullorum infection (Foster et al., 2021). In general, the above data suggested that the S. Enteritidis vaccine was able to relieve swollen liver caused by S. Pullorum infection and reduce the colonization level of S. Pullorum in spleen of chickens. However, the vaccination could not prevent bacterial translocation into reproductive system of chickens in the period of sexual maturity.
CONCLUSIONS
This work demonstrated that the live-attenuated S. Enteritidis strain Sm24/Rif12/Ssq was able to relieve organ damage caused by S. Pullorum infection and reduce the bacterial loads of S. Pullorum in spleen.
ACKNOWLEDGMENTS
This work was supported by Key Research and Development Program of Shandong Province China (2022CXGC010606), Shandong Provincial Agricultural Animal Breeding Project of China (2020LZGC013) and the Talents Development Project of Zaozhuang (BH023005). We thank Longzong Guo from Yisheng Livestock and Poultry Breeding Co., Ltd, for his professional advice on rearing the Hubbard parent chicks
DISCLOSURES
The authors have declared no conflicts of interest.
REFERENCES
- Acevedo-Villanueva K.Y., Akerele G.O., Al Hakeem W.G., Renu S., Shanmugasundaram R., Selvaraj R.K. A novel approach against Salmonella: a review of polymeric nanoparticle vaccines for broilers and layers. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atterbury R.J., Carrique-Mas J.J., Davies R.H., Allen V.M. Salmonella colonisation of laying hens following vaccination with killed and live attenuated commercial Salmonella vaccines. Vet. Rec. 2009;165:493–496. doi: 10.1136/vr.165.17.493. [DOI] [PubMed] [Google Scholar]
- Barrow P.A., Freitas Neto O.C. Pullorum disease and fowl typhoid–new thoughts on old diseases: a review. Avian Pathol. 2011;40:1–13. doi: 10.1080/03079457.2010.542575. [DOI] [PubMed] [Google Scholar]
- Berchieri A., Jr, Murphy C.K., Marston K., Barrow P.A. Observations on the persistence and vertical transmission of Salmonella enterica serovars Pullorum and Gallinarum in chickens: effect of bacterial and host genetic background. Avian Pathol. 2001;30:221–231. doi: 10.1080/03079450120054631. [DOI] [PubMed] [Google Scholar]
- Chacana P.A., Terzolo H.R. Protection conferred by a live Salmonella Enteritidis vaccine against fowl typhoid in laying hens. Avian Dis. 2006;50:280–283. doi: 10.1637/7463-102705R.1. [DOI] [PubMed] [Google Scholar]
- Desin T.S., Koster W., Potter A.A. Salmonella vaccines in poultry: past, present and future. Expert Rev. Vaccines. 2013;12:87–96. doi: 10.1586/erv.12.138. [DOI] [PubMed] [Google Scholar]
- Eeckhaut V., Haesebrouck F., Ducatelle R., Van Immerseel F. Oral vaccination with a live Salmonella Enteritidis/Typhimurium bivalent vaccine in layers induces cross-protection against caecal and internal organ colonization by a Salmonella Infantis strain. Vet. Microbiol. 2018;218:7–12. doi: 10.1016/j.vetmic.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Foster N., Tang Y., Berchieri A., Geng S., Jiao X., Barrow P. Revisiting persistent Salmonella infection and the carrier state: what do we know? Pathogens. 2021;10 doi: 10.3390/pathogens10101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I., Ducatelle R., Timbermont L., Boyen F., Bohez L., Haesebrouck F., Pasmans F., van Immerseel F. Oral immunisation of laying hens with the live vaccine strains of TAD Salmonella vac E and TAD Salmonella vac T reduces internal egg contamination with Salmonella Enteritidis. Vaccine. 2006;24:6250–6255. doi: 10.1016/j.vaccine.2006.05.070. [DOI] [PubMed] [Google Scholar]
- Huberman Y.D., Velilla A.V., Terzolo H.R. Evaluation of different live Salmonella enteritidis vaccine schedules administered during layer hen rearing to reduce excretion, organ colonization, and egg contamination. Poult. Sci. 2019;98:2422–2431. doi: 10.3382/ps/pez003. [DOI] [PubMed] [Google Scholar]
- Ijaz A., Veldhuizen E.J.A., Broere F., Rutten V., Jansen C.A. The interplay between Salmonella and intestinal innate immune cells in chickens. Pathogens. 2021;10 doi: 10.3390/pathogens10111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaprasad H.L. Fowl typhoid and Pullorum disease. Rev. Sci. Tech. 2000;19:405–424. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- Song Y., Wang F., Liu Y., Song Y., Zhang L., Zhang F., Gu X., Sun S. Occurrence and characterization of Salmonella isolated from chicken breeder flocks in nine Chinese provinces. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley P., Berchieri A., Jr, Page K.L., Smith A.L., Barrow P.A. Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infect. Immun. 2001;69:7873–7879. doi: 10.1128/IAI.69.12.7873-7879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley P., Hulme S.D., Powers C., Beal R.K., Berchieri A., Jr, Smith A., Barrow P. Infection of the reproductive tract and eggs with Salmonella enterica serovar Pullorum in the chicken is associated with suppression of cellular immunity at sexual maturity. Infect. Immun. 2005;73:2986–2990. doi: 10.1128/IAI.73.5.2986-2990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Liu Z., Li Y., Yin C., Hu Y., Xie X., Li Q., Jiao X. A rapid method to identify Salmonella enterica serovar Gallinarum biovar Pullorum using a specific target gene ipaJ. Avian Pathol. 2018;47:238–244. doi: 10.1080/03079457.2017.1412084. [DOI] [PubMed] [Google Scholar]
- Yang J., Ju Z., Yang Y., Zhao X., Jiang Z., Sun S. Serotype, antimicrobial susceptibility and genotype profiles of Salmonella isolated from duck farms and a slaughterhouse in Shandong province, China. BMC Microbiol. 2019;19:202. doi: 10.1186/s12866-019-1570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang-Barber L., Turner A.K., Barrow P.A. Vaccination for control of Salmonella in poultry. Vaccine. 1999;17:2538–2545. doi: 10.1016/s0264-410x(99)00060-2. [DOI] [PubMed] [Google Scholar]