Abstract
Background
The closure of extensive burn wounds with widely expanded autologous split-thickness skin grafts (STSG) is associated with undesirable scar formation and contraction, due to the lack of dermis. Various materials for dermal replacement have been developed, either of xenogeneic, allogeneic or synthetic origin and are placed in the wound underneath a thin STSG in order to improve scar quality. In this study, a porcine wound model was used to compare several commercially available acellular dermal substitutes with an acellular dermal substitute prepared from glycerol preserved human skin: GlyadermⓇ.
Methods
Antigenic components of the allografts were removed by incubation in the 0.06 M NaOH solution. In the first experiments, the dermal substitutes were applied to full thickness wounds and covered simultaneously with STSG. Controls were covered with STSG only. The wound healing response was analyzed for 8 weeks, both macroscopically and histologically. The Mann-Whitney U test was used for statistical analysis. In the second series of experiments, GlyadermⓇ was applied in a two-stage procedure in comparison to Integra. The STSG was placed on the dermal substitutes one week later.
Results
In the first series, the inflammatory response and myofibroblast influx in GlyadermⓇ were limited, indicating possible beneficial outcomes on final wound healing results. The survival of the STSG on the acellular dermis was lower compared to the control wounds. Second series: the take of the STSG was the same as in the controls, but additionally wound contraction was reduced. The application of GlyadermⓇ was non-inferior to Integra.
Conclusion
GlyadermⓇ can be successfully used for the reduction of wound contraction when applied in a two-stage procedure.
Keywords: Dermal substitute, Burns, Glyaderm, Graft take, Surgery, Scar quality
Background
Extensive deep dermal or full thickness skin defects can be closed with widely expanded autologous split-thickness skin grafts (STSG).1 The results obtained with this standard surgical technique are less favorable in terms of contraction and scar quality, mainly due to the lack of dermis.2, 3, 4, 5, 31 Several materials for dermal replacement have been developed. These substitutes can be placed underneath the STSG and serve as a scaffold into which cells can infiltrate and repair the wound, ultimately resulting in less scar tissue formation and contractures.6, 7, 8
Nowadays, different dermal substitutes such as AlloDerm, Matriderm, and Integra are available on the market, but the benefit and cost-effectiveness of these materials are still under discussion.9, 10, 11, 12 AlloDerm (Lifecell Corp., Branchburg, NJ) is an acellular dermal substitute processed from cryopreserved human cadaver skin. When combined with a very thin STSG, the take rate of AlloDerm was improved and in the long term less scarring and contractures were reported.13 Integra (Lifesciences Corp, Inc, Plainsboro, NJ) consists of cross-linked bovine collagen and chondroitin-6-sulfate covered with a silicone layer to temporarily provide wound coverage. Integra is applied during the first operation after debridement and preparation of the wound bed. After a period of 2 to 4 weeks, the silicone layer is removed during a second operation and autografting is performed. The silicone layer serves as a barrier against bacteria, and it controls water evaporation and provides mechanical support. Several studies using Integra have reported less hypertrophic scar formation but also increasing the risk of infection.14 Matriderm, another commercially available dermal substitute, consists of a lattice of bovine collagen coated with elastin hydrolysate. Promising results were obtained, but in the long term no significant difference was observed when compared to wounds treated with STSG only except for a less visible meshed scar pattern.15 The possibility of MatriDerm to be used in a single-step procedure is a practical advantage,16 but comparative clinical data are limited. In a small clinical trial,17 improved scar elasticity was observed in the Matriderm group combined with sheet autografts compared to wounds treated with sheet autografts only.
To achieve optimal results, a substitute requires low antigenicity, stability as a dermal template, and the capacity for rapid vascularization to ensure survival of the overlying STSG. Dermal substitutes can be derived from xenogeneic tissue, allogeneic tissue from human skin, or synthesized with acellular materials from synthetic sources. Decellularized human donor skin ideally provides the natural three-dimensional collagen and elastin structure. All cells and appendages have to be removed in such a way that the structure of the collagen and elastin fibers is preserved. Several methods to remove antigenic structures have been described, using sodium dodecyl sulphate (SDS) and freeze drying techniques (Alloderm, Lifecell13,18) or Triton X-100 combined with Dispase.19 In the present study, we evaluated the use of a human dermal matrix prepared from glycerol preserved allograft skin (Glyaderm7) using low concentrations of NaOH in a porcine wound model. This NaOH solution treated with dermal substitute was compared to Integra, AlloDerm, and de-epidermized acellular dermis (DED).20, 21, 22
Methodology
Substitute materials and animals
Ethical Clearance
Human participants were not included. The experiments with animals were approved by the animal welfare committee of the Vrije Universiteit Medical Centre, Amsterdam. Human skin was obtained from donors with consent according to the Dutch Law on Organ donation.
Dermal substitutes
Glyaderm was prepared from donated human skin by the Euro Skin Bank as described earlier7 using low concentrations of NaOH (0,06 M). DED was prepared according to the method in literature7,20,21 using incubation in phosphate buffered saline (PBS). Acellular dermal tissue was prepared by repeated washing of glycerol preserved donor skin (Euro Skin Bank, Beverwijk, the Netherlands) in sterile PBS supplemented with 50 IU/ml−1 penicillin G and 50 µg/ml−1 streptomycin (Gibco, Paisley, U.K.) for 3 weeks at 37°C and further stored at 4°C (no longer than 6 months). AlloDerm was ordered from Lifecell (Lifecell Corp., Branchburg, NJ) and Integra from Lifesciences (Lifesciences Corp., Inc, Plainsboro, NJ).
Animals
Twelve female Yorkshire pigs (weight 30-35 kg) were used. The same animal model as described earlier.7 A grid was tattooed one week prior to the first operation, by cutting the skin with a scalpel till subepidermal depth and applying tattoo ink, allowing measurement of wound contraction and applying a natural growth correction. The tattooed grid of deposited ink particles in the deeper part of the dermis is macroscopically clearly visible and microscopically also in the biopsies taken.
Experimental procedures
This study was conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
One-stage procedure
Four full thickness excision wounds of 4 × 4 cm were inflicted on each flank of the animals under general anaesthesia. The dermal matrices that were tested (Glyaderm, Alloderm, and DED) were meshed 1:1.5 and sutured into the wounds. An Aesculap dermatome (B. Braun) was used to harvest autologous split skin (0.2-0.3 mm thickness) from the animal's dorsum. The autologous skin was meshed 1:3 and sutured on top of the dermal matrix. Further wound dressing was performed as described in the reference.7 In short, grafts were covered with Surfasoft. Wounds were covered with NaCl soaked gauzes, fixated with adhesive bandages, and protected with elastic bandage. Dressings were changed on days 4, 7, and weekly from then on.
Two-stage procedure
The animal model was similar to 2.2.1, again 4 full thickness excision wounds of 4 × 4 cm were prepared under anaesthesia on each side of the animal, but then the control wounds were directly covered with STSG meshed 1:3. Glyaderm, and Integra were meshed 1:1.5 and sutured into the wounds with Surfasoft on top of the Glyaderm. Further wound dressing was performed as described earlier.7 Seven days later, the second operation was performed. STSG's were harvested from the dorsum, meshed 1:3, and sutured on top of the dermal substitutes.
Evaluation of wound healing
Dressings were changed on days 4 and 7 postsurgery and assessed for any signs of infection. Thereafter, wound dressings were weekly changed until the wounds were completely closed. We removed Surfasoft at day 7, and the take rate was assessed. Biopsies (4 mm) were taken at days 7 and 14. Wounds colonized with bacteria were excluded from analysis.
The pigs were sacrificed 8 weeks after surgery. After macroscopic inspection of the scars and measurement of wound contraction using planimetry, we excised large biopsies covering the full wound.
Digital photographs were taken to evaluate wound healing evaluation at days 7, 14, and 56.
Planimetry
The planimetry, which is the measurement of distances and angles on a distinct plane, was performed as described in earlier studies.7 Briefly, wound contraction was measured by tracing the edges of the wound and the tattoo grid on transparent film. Visitrak was used to measure contraction.
(Immuno)-histochemistry
Sections were prepared of 5 µm thickness and stained using the following methods:
-
1.
Hematoxylin-Eosin (Gurr, BDH Ltd, Poole, UK), for standard morphology of the wounds and the cells present. 2. Elastica von Giesson (Merck, Darmstadt, Germany), to stain collagen and elastin. 3. α-smooth muscle actin (α-SMA) antibody (Sigma) to stain pericytes and myofibroblasts, which are present in blood vessels and scars, respectively.
After 10 min fixation in acetone, slides were incubated with the sections with the α-SMA antibody diluted in PBS for 45 min at room temperature. Thereafter, the slides were washed thrice with PBS followed by incubation with a secondary antibody conjugated with horseradish-peroxidase (rabbit anti mouse, Dako, Glostrub, Denmark) for 1 hour at room temperature. After a washing step using PBS, the slides were incubated with diaminobenzidine (Dako) to stain the positive cells.
Two independent observers analyzed the stained sections and scored the influx of cells. The hematoxylin sections were used to analyze the influx of inflammatory cells. In the biopsies taken at the end of the experiment, an ocular grid was used in the microscope to quantify the areas in the dermal matrices with inflammatory cells. Myofibroblasts were quantified on digital images of the stained sections and analyzed using Lucia G software.
Statistical analysis
Statistical analysis was performed using Graphpad Prism version 9.0.2 (San Diego, CA, USA). Normality of data was assessed using the Shapiro-Wilk test. Significant differences between treatments were assessed with the Mann-Whitney test. A priori, values of p < 0.05 were considered to be statistically significant.
Results
One-stage procedure
Survival of the autologous split thickness skin graft
The Surfasoft was removed at day 7 post-operative, and the take rate was scored as the percentage that was still viable. Table 1 shows that the take rate on a dermal substitute in a one-stage procedure is lower compared to the control wounds. AlloDerm showed the best result, followed by Glyaderm and DED.
Table 1.
Take rate of autologous split skin in the one stage procedure. *The percentage of the autologous skin that was still viable (take rate) at day 7 after operation is higher in control wounds with no acellular dermis. † De-epidermized acellular dermis.
| Number of wounds | Mean take rate* | Range take rate | |
|---|---|---|---|
| Control | 12 | 92 | 75-100 |
| Glyaderm | 12 | 65 | 25-100 |
| AlloDerm | 6 | 75 | 35-100 |
| DED† | 8 | 50 | 20-100 |
Inflammatory response in the dermal matrices
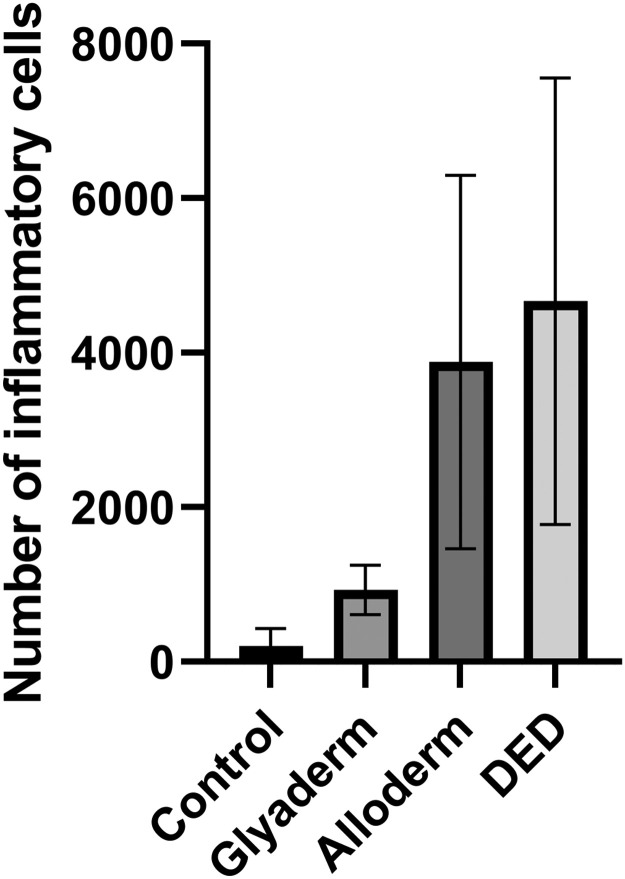
In the sections of the biopsies from days 7 and 14, numerous immune cells were seen in the dermal matrices compared to the control. The highest numbers seemed to be present in the DED, followed by Alloderm. The number of cells that could be observed in the Glyaderm matrix was relatively low but higher in comparison with the control wounds. In the sections from the biopsies taken 8 weeks after operation, the matrices could be observed in the formed scar tissue. Elastin fibers surrounded with large collagen fibers were present that could be easily distinguished from the new thin fibers produced by fibroblasts migrated into the matrices. Macrophages and lymphocytes were also present in the dermal matrices, sometimes large accumulations were observed, especially in the DED matrix. Since the week 8 sections were taken from biopsies covering the whole wound area, it was possible to quantify the areas within the dermal matrices covered with inflammatory cells. As can be seen in Figure 1, the inflammatory response was significantly higher in DED and Alloderm compared to Glyaderm.
Figure 1.
The influx of inflammatory cells in the scar at day 56 is higher when acellular dermis is applied to the wound in comparison to controls. In addition, the type of acellular dermis has a clear effect on the inflammatory response.
Eight weeks after surgery, the area with inflammatory cells in Glyaderm-treated scars was significantly lower compared to wounds treated with Alloderm or DED (p < 0.05).
Effect of the matrices on scar formation and wound contraction
In case the take of the autologous skin on the Glyaderm was > 70%, the quality of the scar seemed better (Figure 2), but there were no significant differences in wound contraction between controls and wounds treated with a dermal substitute in the one-stage procedure.
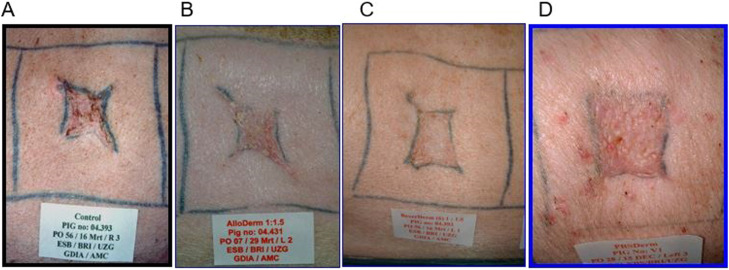
Figure 2.
Macroscopic aspects of control wound (A) and wounds treated with AlloDerm (B), Glyaderm (C), or DED (D). Pictures of representative scars at 56 days after injury. Wounds treated with Glyaderm scored higher in terms of absence of erythematous appearance.
Wounds with a take > 75% treated with Glyaderm had the best score with respect to color and smoothness. No significant differences in wound contraction were observed between groups at 8 weeks after operation.
Effect of the matrices on the numbers of myofibroblasts
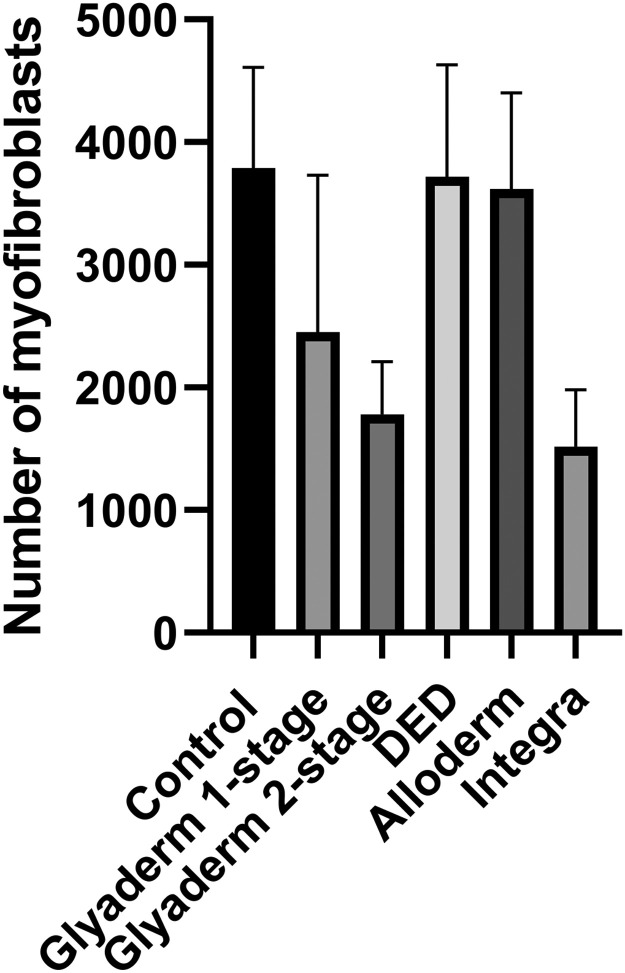
Myofibroblasts are associated with wound contraction and hypertrophic scarring.23,24 Although the sections from biopsies of the control wounds showed high numbers of strongly positive myofibroblasts in the new scar tissue, this was not significantly different compared to wounds treated with a dermal substitute. There were also no significant differences in myofibroblast numbers between wounds treated with Glyaderm, AlloDerm, or DED (Figure 3).
Figure 3.
Application of Glyaderm or Integra in a two-stage procedure resulted in lower numbers of myofibroblasts in the scar (week 8).
Two-stage procedure
Effect of a two-stage procedure on graft survival
Glyaderm was tested in a two-stage procedure to optimize graft take. Autografting took place 1 week postimplantation. After dressing removal, the Glyaderm had a slightly red appearance if no infection or dehydration had taken place. The thickness of Glyaderm had to be < 0.6 mm to enable blood vessel ingrowth within one week.
The take rate of the autologous skin on the dermal substitute is much higher compared to the one-stage procedure (Table 1) and comparable to control wounds and Integra in a two-stage procedure (Table 2).
Table 2.
Take of the autologous skin in the two stage procedure. *The percentage of the autologous skin that was still viable (take rate) at day 7 after application of acellular dermis or Integra is comparable to control wounds (directly closed).
| Number of wounds | Mean take rate * | Range take rate | |
|---|---|---|---|
| Control | 12 | 95 | 75-100 |
| Glyaderm | 8 | 90 | 60-100 |
| Integra | 6 | 96 | 55-100 |
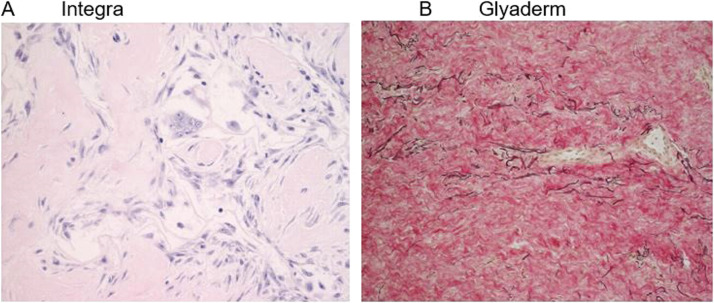
Compared to the one-stage procedure, the number of inflammatory cells in the wounds treated with Glyaderm was lower at days 7 and 14 after wounding. The influx of immune cells in Integra was comparable, but some giant cells, which are multinucleated fusion cells and mostly present at sites of granulomatous conditions or chronic inflammations,25 were present (Figure 4A). Eight weeks after injury, elastin fibers could still be observed in the Glyaderm, surrounded by newly produced collagen fibers (Figure 4B) of which the majority was already replaced.
Figure 4.
a) Presence of multi-nucleated giant cells with Integra. b) In the Glyaderm treated site, donor elastin fibers are present after 8 weeks and newly produced collagen fibers are visible.
In the Glyaderm matrix, only a few accumulations of macrophages and lymphocytes could be observed, but around the Integra fibers some giant cells were observed 8 weeks post surgery.
Effect of Glyaderm in a two-stage procedure on contraction and scarring
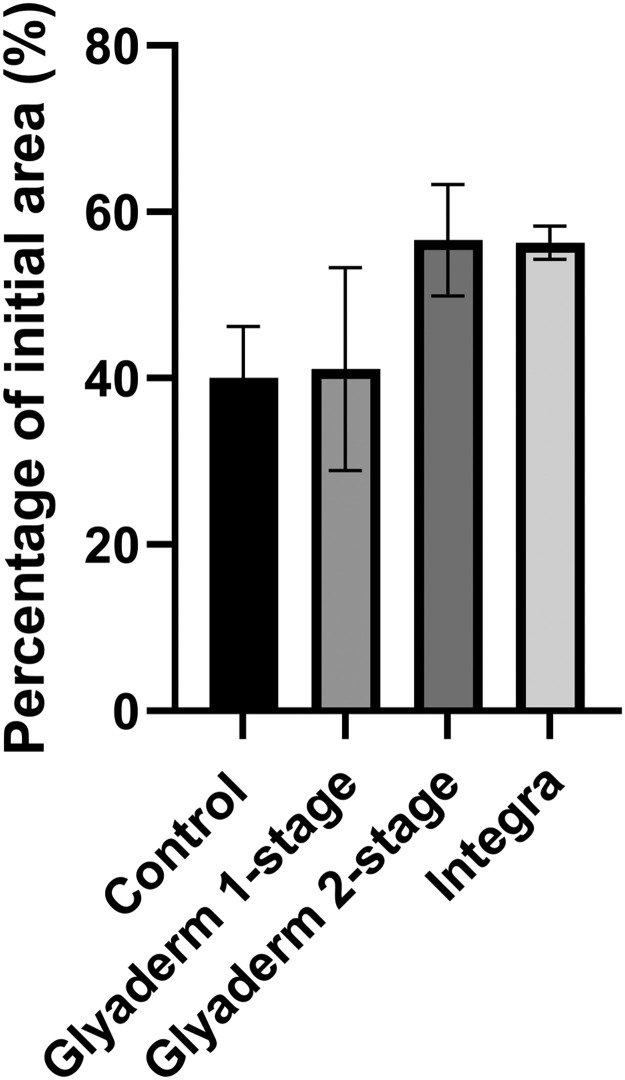
Contraction of wounds treated with Glyaderm in a two-stage procedure is reduced compared to Glyaderm in one-stage procedure (Figure 5). Wounds treated with Integra showed the same contraction.
Figure 5.
Wound contraction was measured at 8 weeks after the second operation (n=8 wounds).
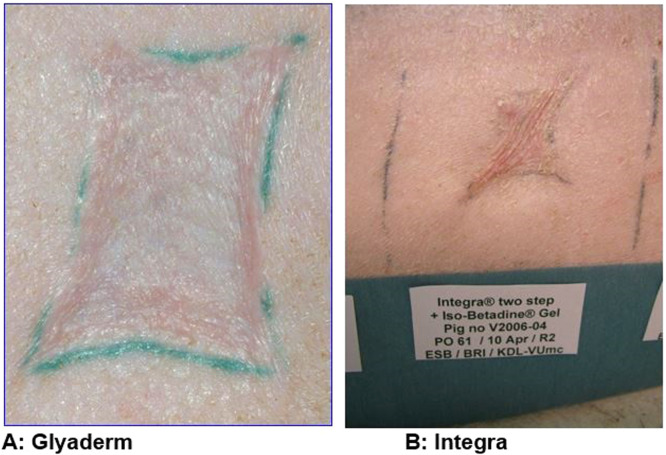
The scar quality of the wounds treated with Glyaderm in the two-stage procedure seemed better compared to Integra, though not statistically significant (Figure 6).
Figure 6.
Scar quality after Glyaderm seems superior compared to the use of Integra.
Discussion
The aim of this study was to compare Glyaderm with other human dermal matrices; AlloDerm and DED. The NaOH method to decellularize human donor skin (Glyaderm) is more thorough. Less cells and remnants of appendages could be detected in sections of the material. This could explain the milder inflammatory response in the wounds treated with Glyaderm compared to wounds treated with Alloderm or DED. As a consequence, wounds treated with Glyaderm showed better results with respect to color and smoothness of the scar. Nevertheless, this could only be observed in wounds with an adequate take rate.
The survival of STSG decreased when placed on a dermal matrix compared to the control wounds covered with only autologous STSG. The take rate on Glyaderm was lower than on Alloderm but higher compared to DED. The NaOH solution may have caused some damage to the basal membrane molecules, which are important for outgrowth of the epithelial cells, as has been shown in vitro21 and vivo.18 The method for processing DED preserves intact basal membrane molecules,20 but the inflammatory response in the matrix may hamper the ingrowth of blood vessels from the wound bed. Although the inflammatory response was lower with Glyaderm, no significant differences were observed with respect to wound contraction. The number of myofibroblasts, which are known to be related to contraction and scarring, did not differ.23,24 For the initial survival during the first days, the autologous skin graft depends on the diffusion of nutrients from the wound fluid before newly formed blood vessels are connected. It takes at least 4 days for the endothelial cells to invade the relatively tightly woven collagen and elastin fibers from the human skin-derived matrices.
Thereafter, we tested Glyaderm in a two-stage procedure with a one week interval between the dermal implantation and autografting. In our porcine model, this time period was sufficient to reach a take rate comparable to control wounds without Glyaderm as well as wounds treated with Integra. The good survival of the STSG indicated a fast ingrowth of fibroblasts and blood vessels into the Glyaderm matrix, leading to reduced wound contraction compared to the control wounds. Interestingly, the numbers of inflammatory cells were also lower in wounds treated with Glyaderm in the two-stage procedure compared to the one-stage procedure, both early after wounding (day 7) and at day 56. In addition, the numbers of myofibroblasts in the scars at day 56 were significantly lower when compared to control wounds, covered with only autologous split skin. As expected, wounds treated with Integra also showed improved results compared to the controls. There were no significant differences in wound contraction and numbers of myofibroblasts between Integra or Glyaderm in the two-stage procedure, only the presence of giant cell formation in wounds treated with Integra was observed, potentially caused by glutaraldehyde crosslinking.
Druecke et al.26 have described the use of Integra in a one-stage procedure in a porcine model and did not observe differences in contraction between control and Integra-treated wounds. Thus, although two operations are needed, the final results with respect to wound contraction and scar formation are much better if a dermal substitute like Integra or Glyaderm is used in a two-stage procedure. Only thinner dermal substitutes with a more open structure that allow faster ingrowth of blood vessels may be used in a one-stage procedure.27 These types of dermal substitutes lack the natural structures of collagen and elastin fibers present in Glyaderm, which can modulate fibroblasts to produce more randomly organized collagen fibers. Open pore structure matrices are more vulnerable to early degradation by matrix-metallo-proteinases (MMP's) produced by infiltrating fibroblasts and macrophages.
These good results obtained with Glyaderm in the two stages lead to a pilot study with a group of 12 burn patients.28 The growth of blood vessels from the wound bed into Glyaderm was assessed using laser Doppler imaging. An interval of 5-7 days between the first operation and the second operation was sufficient to achieve a take rate > 95%. Thereafter, an intra-individual comparative clinical study was performed to evaluate the long-term effects. The elasticity of the scar was significantly improved at 1 year follow-up when using Glyaderm.8 Additionally, several layers of Glyaderm could be applied on exposed bone, and the wound could be successfully closed with STSG.29 Biopsies taken 7 days after implantation clearly showed new collagen in the Glyaderm,30 confirming the observation in the porcine model. The human donor derived, native elastin fibers serve as a scaffold for autologous fibroblasts, resulting in scar tissue with improved elasticity.
Conclusions
An acellular dermal substitute such as Glyaderm can be successfully used to reduce wound contraction in the porcine wound model. Glyaderm with a thickness > 0.5 mm should be used in a two-step procedure for optimal results. During the interval between the first and second step, blood vessels and fibroblasts will infiltrate the Glyaderm. In this way, the survival of the STSG is improved, resulting in a better quality of the final scar.
Acknowledgments
Ethics approval and consent to participate
Human participants were not involved in this study. The experiments with animals were approved by the animal welfare committee of the Vrije Universiteit Medical Centre, Amsterdam.
Consent for publication
All authors of this study gave consent for publication.
Availability of data and materials
All data are presented in the main manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was financially supported by the Dutch Burn Foundation & Euro Skin Bank.
Authors' contributions
All authors have made substantial contribution to:
1) The conception and design of the study: AP, HH, MH, SM, NP, CR
2) Acquisition of data: AP, CR, HH, MH, SM, NP
3) Analysis and interpretation of data: AP, CR, HH, MH, SM, NP, IDD, KC
4) Drafting the article: AP, CR, HH, MH, SM, NP, IDD, KC
5) Revising the article critically for important intellectual content: AP, CR, HH, MH, SM, NP, IDD, KC, BVDL
6) Final approval of the version to be submitted: AP, CR, HH, MH, SM, NP, IDD, KC, BVDL
Acknowledgements
Not applicable.
Authors' information (optional)
Not applicable.
References
- 1.Rijpma D, Claes KEY, De Decker I, Verbelen J, Pijpe A, Van Zuijlen P. The Meek micrograft technique for burns; review on its outcomes. Searching for the superior skin grafting technique. Burns. 2022 doi: 10.1016/j.burns.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 2.De Decker I, De Graeve L, Hoeksema H, Monstrey S, De Coninck P, Vanlerberghe E, et al. Enzymatic debridement : past, present, and future. Acta Chir Belg. 2022;0:1–17. doi: 10.1080/00015458.2022.2068746. [DOI] [PubMed] [Google Scholar]
- 3.De Decker I, Hoeksema H, Vanlerberghe E, Beeckman A, Verbelen J, De Coninck P, et al. Occlusion and hydration of scars: moisturizers versus silicone gels. Burns. 2022:1–15. doi: 10.1016/j.burns.2022.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Claes KEY, De Decker I, Monstrey S, Shoham Y, Vyncke T, Depypere B, et al. Helpful hints in deciding what and when to operate after enzymatic debridement. Burns. 2022:1–11. doi: 10.1016/j.burns.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 5.De Decker I, Hoeksema H, Verbelen J, Vanlerberghe E, De Coninck P, Speeckaert MM, et al. The use of fluid silicone gels in the prevention and treatment of hypertrophic scars: a systematic review and meta-analysis. Burns. 2022:1–19. doi: 10.1016/j.burns.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Mohd Hilmi AB, Halim AS, Jaafar H, Asiah AB, Hassan A. Chitosan dermal substitute and Chitosan skin substitute contribute to accelerated full-thickness wound healing in irradiated rats. Biomed Res Int. 2013:2013. doi: 10.1155/2013/795458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richters CD, Pirayesh A, Hoeksema H, Kamperdijk EWA, Kreis RW, Dutrieux RP, et al. Development of a dermal matrix from glycerol preserved allogeneic skin. Cell Tissue Bank. 2008;9:309–315. doi: 10.1007/s10561-008-9073-4. [DOI] [PubMed] [Google Scholar]
- 8.Pirayesh A, Hoeksema H, Richters C, Verbelen J, Monstrey S. Glyaderm dermal substitute : Clinical application and long-term results in 55 patients. Burns. 2014;41:132–144. doi: 10.1016/j.burns.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Kearney JN. Clinical evaluation of skin substitutes. Burns. 2001;27:545–551. doi: 10.1016/S0305-4179(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 10.Shakespeare P. Skin substitutes–benefits and costs. Burns. 2001;27:vii–viii. [PubMed] [Google Scholar]
- 11.Jones I, Currie L, Martin R. A guide to biological skin substitutes The function of normal skin. Br J Plast Surg Br Assoc Plast Surg. 2002;55:185–193. doi: 10.1054/hips.2002.3800. [DOI] [PubMed] [Google Scholar]
- 12.Brusselaers N, Pirayesh A, Hoeksema H, Richters CD, Verbelen J, Beele H, et al. Skin replacement in burn wounds. J Trauma - Inj Infect Crit Care. 2010;68:490–501. doi: 10.1097/TA.0b013e3181c9c074. [DOI] [PubMed] [Google Scholar]
- 13.Wainwright D, Madden M, Luterman A, Hunt J, Monafo W, Heimbach D, et al. Clinical Evaluation of an Acellular Allograft Dermal Matrix in Full-Thickness Burns. J Burn Care Rehabil. 1996;17:124–136. doi: 10.1097/00004630-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 14.00000658-198809000-00008.pdf n.d.
- 15.00006534-200112000-00014.pdf n.d.
- 16.Ryssel H, Gazyakan E, Germann G, Öhlbauer M. The use of MatriDermⓇ in early excision and simultaneous autologous skin grafting in burns-A pilot study. Burns. 2008;34:93–97. doi: 10.1016/j.burns.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Haslik W, Kamolz LP, Manna F, Hladik M, Rath T, Frey M. Management of full-thickness skin defects in the hand and wrist region: first long-term experiences with the dermal matrix MatridermⓇ. J Plast Reconstr Aesthetic Surg. 2010;63:360–364. doi: 10.1016/j.bjps.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Livesey SA, Herndon DN, Hollyoak MA, Atkinson YH, Nag A. Transplanted acellular allograft dermal matrix: Potential as a template for the reconstruction of viable dermis. Transplantation. 1995;60:1–9. doi: 10.1097/00007890-199507150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Walter RJ, Matsuda T, Reyes HM, Walter JM, Hanumadass M. Characterization of acellular dermal matrices (ADMs) prepared by two different methods. Burns. 1998;24:104–113. doi: 10.1016/S0305-4179(97)00110-1. [DOI] [PubMed] [Google Scholar]
- 20.Breetveld M, Richters CD, Rustemeyer T, Scheper RJ, Gibbs S. Comparison of wound closure after burn and cold injury in human skin equivalents [4] J Invest Dermatol. 2006;126:1918–1921. doi: 10.1038/sj.jid.5700330. [DOI] [PubMed] [Google Scholar]
- 21.Ralston DR, Layton C, Dalley AJ, Boyce SG, Freedlander E, Mac Neil S. The requirement for basement membrane antigens in the production of human epidermal/dermal composites in vitro. Br J Dermatol. 1999;140:605–615. doi: 10.1046/j.1365-2133.1999.02758.x. [DOI] [PubMed] [Google Scholar]
- 22.Gibbs S, Van Den Hoogenband HM, Kirtschig G, Richters CD, Spiekstra SW, Breetveld M, et al. Autologous full-thickness skin substitute for healing chronic wounds. Br J Dermatol. 2006;155:267–274. doi: 10.1111/j.1365-2133.2006.07266.x. [DOI] [PubMed] [Google Scholar]
- 23.Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 24.Gabbiani G. [The myofibroblast. A key cell in wound healing and in fibro- contractive diseases of the connective tissue] Schweiz Rundsch Med Prax. 1984;73:939–941. [PubMed] [Google Scholar]
- 25.Gupta G, Athanikar SB, Pai VV, Naveen KN. Giant cells in dermatology. Indian J Dermatol. 2014;59:481–484. doi: 10.4103/0019-5154.139887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Druecke D, Lamme EN, Hermann S, Pieper J, May PS, Steinau HU, et al. Modulation of scar tissue formation using different dermal regeneration templates in the treatment of experimental full-thickness wounds. Wound Repair Regen. 2004;12:518–527. doi: 10.1111/j.1067-1927.2004.012504.x. [DOI] [PubMed] [Google Scholar]
- 27.van der Veen VC, van der Wal MBA, van Leeuwen MCE, Ulrich MMW, Middelkoop E. Biological background of dermal substitutes. Burns. 2010;36:305–321. doi: 10.1016/j.burns.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Swart D. The development of a national household energy surveillance system—Challenges and opportunities. Burns. 2007;33:S134. doi: 10.1016/j.burns.2006.10.311. [DOI] [Google Scholar]
- 29.Verbelen J, Hoeksema H, Pirayesh A, Van Landuyt K, Monstrey S. Exposed tibial bone after burns: Flap reconstruction versus dermal substitute. Burns. 2016;42:e31–e37. doi: 10.1016/j.burns.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Oostendorp C, Uijtdewilligen PJE, Versteeg EM, Hafmans TG, Van Den Bogaard EH, De Jonge PKJD, et al. Visualisation of newly synthesised collagen in vitro and in vivo. Sci Rep. 2016;6:1–7. doi: 10.1038/srep18780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Decker I., Szabó A., Hoeksema H., Speeckaert M., Delanghe J.R., Blondeel P., et al. Treatment of hypertrophic scars with corticoid-embedded dissolving microneedles. J Burn Care Res. 2022 doi: 10.1093/jbcr/irac165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the main manuscript.