Summary
Background
Booster vaccination is an efficient way to address the waning protection of vaccines and immune escape of SARS-CoV-2 variants. We aimed to assess the safety and immunogenicity of SCTV01C, a novel bivalent protein vaccine as a booster for people who previously received two doses of mRNA vaccine.
Methods
In this randomized, phase 1/2 trial, adults fully vaccinated with mRNA vaccines 3–24 month earlier were enrolled. Participants received SCTV01C at 20 μg, 40 μg or placebo. The primary endpoints were adverse reactions within 7 days and immunogenicity on Day 28 after vaccination. This trial was registered with ClinicalTrials.gov (NCT05043311).
Findings
Between January 27 and April 28, 2022, 234 adults were randomly assigned to receive SCTV01C or placebo. The most common solicited adverse events (AEs) were Grade 1 injection-site pain (10.7%) and pyrexia (6.3%). There were no reports of Grade 3 or above solicited AE, serious AEs or AEs of special interests. On Day 28 post the booster, the geometric mean concentrations (GMCs) of the specific binding IgG antibodies to spike protein for placebo, 20 μg and 40 μg SCTV01C were 1649, 4153 and 5354 BAU/mL, with fold of increase from baseline of 1.0, 2.8 and 3.4-fold, respectively. GMTs of neutralizing antibodies against live Delta variant were 1280, 3542, and 4112, with fold of increase of 1.1, 3.9 and 4.1-fold, respectively; GMTs of neutralizing antibodies against live Omicron variant were 218, 640, and 1083, with fold of increase of 1.1, 4.4 and 5.1-fold, respectively. Participants with low neutralizing antibody titers at baseline (below the lower limit of quantitation) had 64.0 and 49.4-fold of increase in GMTs for Delta and Omicron, respectively.
Interpretation
The heterologous booster of SCTV01C was safe, and induced uniformly high cross-neutralization antibody responses against Delta and Omicron variants.
Funding
Beijing Science and Technology Plan Project (Z221100007922012) and the National Key Research and Development Program of China (2022YFC0870600) supported this study.
Keywords: Safety, Immunogenicity, SARS-CoV-2, Bivalent protein vaccine, Booster, SCTV01C
Research in context.
Evidence before this study
With the emergence of SARS-CoV-2 variants, the effectiveness of the vaccines developed based on the original strain have dropped dramatically, although the protection against the severe disease from COVID-19 is still largely retained. Homologous or heterologous COVID-19 booster vaccinations may provide effective protection against Omicron variant. Several studies have systematically analyzed prime/booster regimens including 11 homologous prime/booster (3× BBIBP-CorV, 3× CoronaVac, 3× ADZ1222 vaccine, 3× BNT162b2 vaccine, 3× mRNA-1273 vaccine, 3× BriLife vaccine and 3× ZF2001 vaccine); and 4 heterologous prime/booster (2× BBIBP-CorV + 1× ZF2001 vaccine, 2× CoronaVac + 1× BNT162b2 vaccine, BNT162b2 vaccine + CoronaVac+BNT162b2 vaccine, and 2× BNT162b2 vaccine + 1× Ad26.COV2.S1 vaccine). The resultant fold-increase in neutralization GMTs ranged between 2.5 and 53.81 for Delta variant and 1.17–96.94 for Omicron after booster vaccination.
Added value of this study
A variant-adapted vaccine capable of inducing potent and broad immune responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a booster is currently being evaluated. SCTV01C, a bivalent protein vaccine based on the spike protein ectodomain (ECD) sequences of SARS-COV-2 Alpha (B.1.1.7) and Beta (B.1.351) variants, demonstrated favorable safety profile with low AE rates comparable to traditional inactivated viral vaccines. Importantly, SCTV01C boosting induced potent cross-strain neutralizing antibody responses to non-vaccine variants, Delta and Omicron. For the people with low levels of neutralizing antibody titers at baseline (below the lower limit of quantitation), SCTV01C boosting elevated their neutralizing antibody titers against both Delta and Omicron variants to high levels similar to titers observed in participants with high baseline titers. Hence, boosting with SCTV01C could induce uniformly high cross-neutralizing antibody responses regardless of the titers at baseline.
Implications of all the available evidence
With excellent thermostability, favorable safety profile, and cross-neutralization against non-vaccine variants, SCTV01C may play an important role in controlling the on-going Omicron pandemic. The use of vaccines that contain Alpha and Beta spike protein may be a promising strategy for broader protection against SARS-CoV-2 variants. SCTV01C may become a platform for the development of upgraded multi-variant vaccines (by adding or replacing with new variants) against future SARS-CoV-2 variants.
Introduction
The COVID-19 pandemic has evolved substantially ever since the first case of SARS-CoV-2 infection was reported in late 2019 and it is likely that this evolution will continue. Omicron variant (B.1.1.529) and its sublineages (BA.1, BA.2, BA.2.12.1, BA.3, BA.4 and BA.5), especially BA.4 and BA.5, are rapidly becoming the dominant variants in the U.S.1 Due to the highly transmissible Delta and Omicron variants and the waning immunity, there is an urgent need for the booster doses after the primary vaccination.
Homologous/heterologous mRNA vaccine boost after a primary series of BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) generated cross-neutralizing antibody responses to the Omicron variant.2, 3, 4, 5, 6, 7 However, the vaccine efficacy was much lower than the protection efficacy against the prototype strains, i.e., the protection efficacy of a third dose of BNT162b2 against Omicron infection waned from 53.4% to 16.5% in a span of three months after vaccination.8
The mRNA vaccine has a potential disadvantage in terms of storage and transportation. The stringent conditions under which the vaccine remains stable hinders its global availability. In addition, the lack of long-term safety record and potential side effects have all contributed to vaccine hesitancy. With the shift in global COVID-19 vaccination strategy as propelled by ultra-transmissible and immune escape variants, next generation vaccines based on classical technologies, but capable of offering cross-protection against existing and future variants are required to increase vaccination rates worldwide.
Bivalent or multivalent vaccines may represent an important strategy for developing broad-spectrum vaccines. Each variant contributes unique neutralizing epitope(s) that could expand the repertoire of neutralizing antibodies, and high frequent mutations possessed by multiple circulating variants would likely be present in the future variants. The WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) has recommended updating the composition of current COVID-19 vaccines when developing multivalent or broad-protective vaccines against SARS-CoV-2 current and future emerging variants.9 Trials based on broad-spectrum anti-coronavirus vaccines using multivalent design, including mRNA-1273.211 (bivalent wild type and Beta variants, Moderna),10 mRNA-1273.214 (bivalent wild type and omicron B.1.1.529 variants),5 NVSI-06-08 (recombinant protein vaccine, three heterologous RBDs from the prototype, Beta and Kappa SARS-CoV-2 strain, Sinopharm)11 and V-01D-351 (recombinant protein vaccine, bivalent from Beta and Delta, Livzon)12 are in progress.
SCTV01C contains two recombinant proteins, which are homotrimeric proteins, based on spike protein ectodomain (ECD) sequences of Alpha (B.1.1.7) and Beta (B.1.351) variants, and adjuvanted with SCT-VA02B, a squalene-based oil-in-water emulsion. The two monovalent vaccines induced potent neutralizing antibody responses against the antigen-matched variants, but drastic reductions in neutralizing antibody titers against antigen-mismatched variants were observed. In comparison, the bivalent vaccine SCTV01C induced relatively higher and broad-spectrum cross-neutralizing activities against various SARS-CoV-2 variants, including the D614G variant, variants of concern (VOCs) (B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.1.529), variants of interest (VOIs) (C.37, B.1.621), variants under monitoring (VUMs) (B.1.526, B.1.617.1, B.1.429, C.36.3) and other variants (B.1.618, 20I/484Q) in mouse immunogenicity studies.13,14 It possesses a trimerization auxiliary domain (T4-Foldon) which helps stabilize the trimeric protein conformation15 and boost the immune responses as well. Thermostable test showed that SCTV01C remained stable at 25 °C up to 6 months.13 Furthermore, a phase 1 study in previously unvaccinated participants showed that SCTV01C was well tolerated, and induced promising cross-strain neutralization against Alpha, Beta, Delta, Omicron variants and the sublineages of Omicron (NCT0514809, manuscript submitted).
Here, we report the safety and immunogenicity results of SCTV01C as a booster in adults previously vaccinated with two doses (primary series) of mRNA vaccine.
Methods
Ethics
The trial complied with the ethical requirements of Good Clinical Practice and the Declaration of Helsinki. The survey and study design were reviewed and approved by the Ministry of Health and Prevention (reference number: RCMOHP/CT1/0123/2021). All trial participants enrolled voluntarily and signed the inform consent prior to any study procedure.
Study design and participants
This was a randomized, double-blinded, placebo-controlled, phase 1/2 trial of a booster vaccination of SCTV01C (a bivalent SARS-CoV-2 trimeric spike protein vaccine) conducted at a single center, the Al Kuwait Hospital (Al Baraha Hospital) in Dubai, United Arab Emirates (UAE). All eligible participants were 18–59 years old and fully vaccinated with mRNA anti-SARS-CoV-2 vaccine (Pfizer-BioNTech or Moderna) 3–24 months earlier. Health status and history of the infections with other coronaviruses and/or infections with SARS-CoV-2 were assessed based on the medical history and clinical laboratory findings, vital sign and physical examination during the screening visits. Participants with a history of SAS-CoV-2 or COVID-19 tested positive with nasopharyngeal/nasal/throat swabs (by real-time polymerase chain-reaction assay), high-risk populations to COVID-19 infection (such as medical workers, close contacts of patients with COVID-19 infection, etc.), individuals with fever, and those with a history of severe acute respiratory syndrome (SARS), Middle East Respiratory Syndrome (MERS) or other coronavirus infection were excluded.
Randomization and masking
The Interactive Network Response System (IWRS) was used to randomize eligible participants prior to study vaccination. The randomization codes were generated via block randomization using SAS software (version 9.4). During the phase 1 trial, participants in each cohort were randomly assigned in a 4:1 ratio to receive an intramuscular injection of SCTV01C (20 μg or 40 μg) or saline placebo; whereas participants were assigned in a 2:1 ratio for each cohort of the phase 2 trial. The vaccine, adjuvant or placebo were identical in appearance. All participants, investigators, clinical research associates, data analyst and laboratory staff were masked to group allocation.
Procedures
SCTV01C is a recombinant protein vaccine developed and manufactured according to good manufacturing practice guidelines by Sinocelltech Ltd., with genetic engineering technology adopted to express in CHO cells. Main active ingredients of SCTV01C comprise of trimeric spike extracellular domain (S-ECD) of SARS-CoV-2 variants Alpha (B.1.1.7) and Beta (B.1.351), and adjuvanted with SCT-VA02B, an oil-in-water emulsion. SCTV01C was supplied in single use vials as a sterile, emulsified, white solution, 0.5 mL/vial, with low (20 μg) and high dose (40 μg), stored and transported at 2–8 °C protected from light, with a validity period of 24 months. The placebo in this trial was 0.9% sodium chloride (normal saline) injection and the dosage form, package and route of administration were consistent with those of the study vaccine.
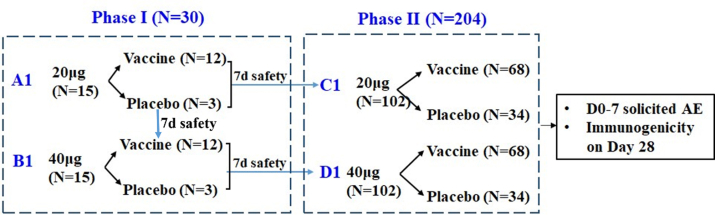
This seamless Phase 1/2 clinical study was composed of a dose-escalation phase I stage (cohort A1 and cohort B1) and a dose exploration phase II stage (cohort C1 and cohort D1). The enrollment started with cohort A1. Fifteen participants aged from 18 to 59 years were randomly assigned to receive 20 μg SCTV01C or saline in a ratio of 4:1. Upon completing 7 days safety observation, enrollment began in cohort B1 and cohort C1 simultaneously: fifteen participants in cohort B1 were randomly assigned to receive 40 μg SCTV01C or saline in a ratio of 4:1 and 102 participants in cohort C1 were randomized to receive 20 μg SCTV01C or saline in a ratio of 2:1. Following 7 days safety observation of cohort B1, enrollment of Cohort D1 was initiated: 102 participants were randomized to receive 40 μg of SCTV01C or saline in a ratio of 2:1 (Supplementary Fig. S1).
Post booster vaccination, solicited AEs within 7 days; unsolicited AEs (adverse event) within 28 days; SAEs, AESIs, MAAEs within 365 days were monitored and recorded. AEs and abnormal changes in laboratory tests were graded according to the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers in Preventive Vaccine Clinical Trial - FDA Standard.16
Serum samples were collected to evaluate the geometric mean titer (GMT) of the specific binding-IgG antibodies against the spike protein of SARS-CoV-2 on D0 and D28 by enzyme-linked immunosorbent assay (ELISA), following the manufacturer's instructions of LIAISON® SARS-CoV-2 TrimericS IgG assay.17 GMTs of IgG antibodies were normalized according to the First WHO International Standard for anti-SARS-CoV-2 immunoglobulin (NIBSC code: 20/268),18 and converted to geometric mean concentration (GMC) using WHO assigned International Binding Antibody Units (BAU). GMTs of neutralizing antibody activities against live SARS-CoV-2 Delta and Omicron variants were measured using plaque reduction neutralization test (PRNT) with the test kits shown in previous study.19 The peripheral blood mononuclear cells were collected to assess specific T-helper-1(Th1, interferon gamma (IFN-γ) release) and T-helper-2 (Th2, interleukin-4 (IL-4) release) responses before vaccination, and on day 14 after booster vaccination (D14) using T-SPOTⓇ.COVID test and enzyme-linked immunospot (ELISpot) IL-4 COVID TEST assay, reported in previous studies.20 The ELISA IgG test, ELISpot assay and live virus neutralization assay were performed according to the manufacturer's guidelines (Biogenix, Abu Dhabi, United Arab Emirates) at G42 LABORATORY LLC in Abu Dhabi, United Arab Emirates.
Outcome
The endpoints for safety were the incidence and severity of adverse reactions (ARs) within 7 days after vaccination; adverse events (AEs) within 7 days; unsolicited AEs within 28 days; laboratory abnormalities related AEs within 14 days; serious adverse events (SAEs); AEs of special interest (AESIs) and medically attended AEs (MAAEs) within 365 days, after vaccination. The endpoints for immunogenicity were geometric mean concentration (GMC) of the specific binding-IgG antibodies against spike protein of wild-type SARS-COV-2 strain on day 28 after vaccination (D28); GMTs of neutralizing antibodies (live virus neutralization assay) to Delta (B.1.617.2) and Omicron (B.1.1.529) variants on D28; the fold increase of these GMC and GMT from baseline (before vaccination). The exploratory endpoint of the specific T-cell response induced by SCTV01C at day 14 after booster vaccination was measured only in the phase 1 trial.
Statistics
Statistical analyses were done with SAS software (version 9.4). A sample size of 240 assuming 20% of the participants being none valuable or withdrawing was determined mainly by referring to the ‘Technical Guidance for Prophylactic Vaccine Clinical Trials’ published by China health authority. This sample size would allow to achieve at least 5-fold increase over control group in the GMTs of neutralizing antibodies to Omicron, assuming a true increase of 8-fold, standard deviation of 0.4 (in scale) and a power of 80%. The 8-fold increase after booster vaccination was estimated based on the pre-clinical animal studies and human studies on SCTV01C, and the published data of the neutralizing antibody titers in mRNA COVID-19 vaccine recipients. The safety and immunogenicity analyses were performed based on intention to treat population. The comparison was performed from high dose group to low dose group in a hierarchical order to account for multiple comparisons. For the safety analysis, all participants who received booster vaccines were analyzed based on solicited AEs (local and systemic) within 7 days and unsolicited AEs within 28 days after booster vaccination. The proportion of participants with at least one solicited AE of Grade ≥3 was reported for each group. Unsolicited adverse events were coded by MedDRA version 24.1 and tabulated by primary system organ class (SOC) and preferred term (PT) for each group. The immunogenicity analysis included the full analysis set of participants with valid immunogenicity test results before and after booster vaccination. In the analysis of immunogenicity, data reported as below the lower limit of detection were imputed as half of the threshold. The comparison between SCTV01C and placebo groups were performed using Analysis of Covariance (ANCOVA) based on log-transformed data.
Role of the funding source
The funder of the study had no role in data collection, data analysis, data interpretation, or writing of the article. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study participants
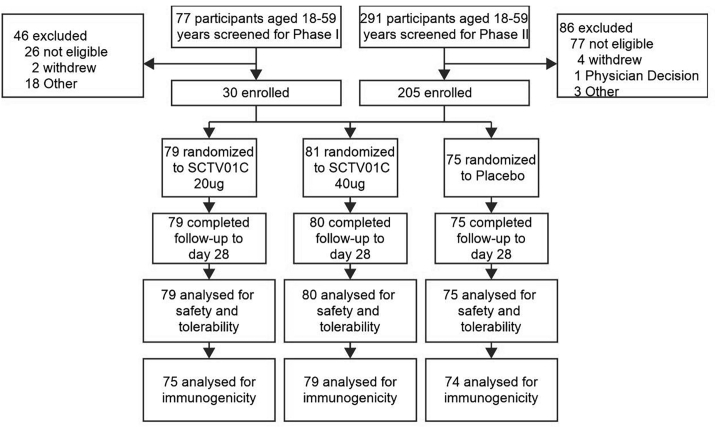
From January 27, 2022 to April 28, 2022, 368 participants aged 18–59 years were screened, of whom 30 and 205 participants were eligible and randomized into the phase I and phase II trials respectively. The data form 234 participants were used for the safety analysis and the immunogenicity evaluations were performed on the data of 232 participants; therefore, the target sample size (240 with a 20% dropout) was reached. Specifically, 75, 79 and 80 participants received placebo (normal saline), 20 μg and 40 μg SCTV01C, respectively (Fig. 1). Three participants were excluded from analysis for either missing baseline or post-baseline immunogenicity data: one participant was due to blood sample being lipemic at baseline, and the other two for missing blood collections at Day 28 after the vaccination. Participants and investigators were blinded to the treatment when the missing data occurred, and there were no informative clue that this missing could introduce bias to the immunogenicity assessment, so the data missing was considered to be in a random mechanism. The demographic and baseline characteristics were generally balanced between groups in terms of age, ethnicity, sex, allergic and vaccination history (Table 1). All participants previously received mRNA anti-SARS-CoV-2 vaccine and the median interval from last vaccination was 6.7 months. One participant in the 40 μg SCTV01C group withdrew from the study for personal reason after booster vaccination.
Fig. 1.
Study profile.
Table 1.
Baseline characteristics of participants.
| SCTV01C |
||||||
|---|---|---|---|---|---|---|
| Saline (N = 75) | 20 μg (N = 79) | 40 μg (N = 80) | Total (N = 159) | Overall (N = 234) | p valuea | |
| Age (years) | 0.58 | |||||
| N (missing) | 75 (0) | 79 (0) | 80 (0) | 159 (0) | 234 (0) | |
| Mean (SD) | 29.1 (7.73) | 28.8 (6.97) | 29.4 (7.88) | 29.1 (7.43) | 29.1 (7.51) | |
| Median | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | |
| Range | (19, 58) | (19, 51) | (19, 53) | (19, 53) | (19, 58) | |
| Sex | 0.77 | |||||
| Female n (%) | 0 | 0 | 2 (2.5) | 2 (1.3) | 2 (0.9) | |
| Male n (%) | 75 (100) | 79 (100) | 78 (97.5) | 157 (98.7) | 232 (99.1) | |
| Race | 0.32 | |||||
| Asian n (%) | 75 (100) | 78 (98.7) | 79 (98.8) | 157 (98.7) | 232 (99.1) | |
| Black n (%) | 0 | 1 (1.3) | 0 | 1 (0.6) | 1 (0.4) | |
| Other n (%) | 0 | 0 | 1 (1.3) | 1 (0.6) | 1 (0.4) | |
| BMI (kg/m2) | 0.17 | |||||
| N (missing) | 75 (0) | 78 (1) | 79 (1) | 157 (2) | 232 (2) | |
| Mean (SD) | 24.3 (4.16) | 24.2 (4.31) | 22.8 (3.59) | 23.5 (4.01) | 23.8 (4.07) | |
| Median | 23.7 | 23.65 | 22.2 | 23.2 | 23.4 | |
| Range | (17.0, 36.1) | (15.8, 40.4) | (16.2, 34.7) | (15.8, 40.4) | (15.8, 40.4) | |
| Allergic history | 0.54 | |||||
| Yes n (%) | 1 (1.3) | 0 | 2 (2.5) | 2 (1.3) | 3 (1.3) | |
| No n (%) | 74 (98.7) | 79 (100.0) | 78 (97.5) | 157 (98.7) | 231 (98.7) | |
| History of SARS-COV-2 vaccination | – | |||||
| Yes n (%) | 75 (100) | 79 (100) | 80 (100) | 159 (100) | 234 (100) | |
| No n (%) | 0 | 0 | 0 | 0 | 0 | |
| Interval from last vaccine (months) | 0.12 | |||||
| N (missing) | 74 (1) | 79 (0) | 76 (4) | 155 (4) | 229 (5) | |
| Mean (SD) | 6.9 (2.0) | 6.2 (2.0) | 6.9 (2.3) | 6.6 (2.2) | 6.7 (2.1) | |
| Median | 7.0 | 5.8 | 6.9 | 6.5 | 6.7 | |
| Min, Max | 3.2, 10.9 | 3.3, 12.0 | 2.2, 10.9 | 2.2, 12 | 2.2, 12 | |
| Comorbidities n (%) | – | |||||
| Hypertension | 0 | 1 (1.3) | 0 | 1 (0.6) | 1 (0.4) | |
| Diabetes mellitus | 0 | 2 (2.5) | 0 | 2 (1.3) | 2 (0.9) | |
| Glucose tolerance impaired | 0 | 1 (1.3) | 0 | 1 (0.6) | 1 (0.4) | |
| Gastrointestinal disorders | 0 | 0 | 1 (1.3) | 1 (0.6) | 1 (0.4) | |
| Malignancy | 0 | 0 | 0 | 0 | 0 | |
| Hepatitis E | 0 | 1 (1.3) | 0 | 1 (0.6) | 1 (0.4) | |
BMI = Body Mass Index; SD = Standard Deviation.
Statistical analysis between saline group and total (SCTV01C 20 μg group + SCTV01C 40 μg group) group.
All participants completed at least a 4-week follow-up after vaccination and up to day 120.
Safety
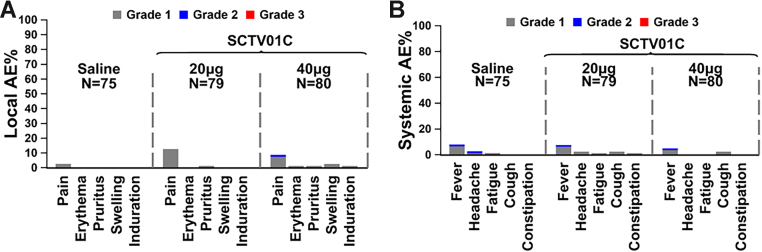
The overall occurrence of injection related AEs was 32.7% within 28 days after injection. Altogether, 30/79 (38.0%) in the 20 μg SCTV01C group, and 22/80 (27.5%) in the 40 μg SCTV01C group reported at least one injection related AE within 28 days. One (1.3%) related Grade 3 hyperglycemia was reported in the 20 μg SCTV01C group. The incidences of solicited AEs were 13.3%, 25.3% and 16.3% in the placebo, 20 μg and 40 μg SCTV01C groups, respectively. No deaths or hospitalizations, SAEs, AESIs, MAAEs or Grade ≥3 vaccine-related solicited AEs were reported. There were no significant differences in routine clinical laboratory values across groups. In the SCTV01C group, the most common: solicited local AEs with incidence ≥1% were injection site pain (10.7%), pruritus (1.3%), and swelling (1.3%); solicited systemic AEs with incidence ≥1% were pyrexia (6.3%), cough (2.5%), and headache (1.3%). There was significant increase in the occurrence of solicited local AEs in SCTV01C group compared to the placebo group (p = 0.0373). Likewise, the most common unsolicited AEs in SCTV01C group with incidence ≥2% were pyrexia (4.4%), blood creatine phosphokinase increased (3.1%), glycosuria (2.5%) and headache (2.5%), All these unsolicited AEs were Grade 1–2, except one report of Grade 3 elevated CPK in the 20 μg SCTV01C group that was identified as un-related to the injection (Table 2 and Fig. 2).
Table 2.
Adverse events and reactions after the booster vaccination.
| AE | SCTV01C |
||||
|---|---|---|---|---|---|
| Saline (N = 75) n (%) |
20 μg (N = 79) n (%) |
40 μg (N = 80) n (%) |
Total (N = 159) n (%) |
p valuea | |
| TEAEs | 26 (34.7) | 32 (40.5) | 31 (38.8) | 63 (39.6) | 0.7431 |
| Vaccine-related TEAEs | 22 (29.3) | 30 (38.0) | 22 (27.5) | 52 (32.7) | |
| AEs within 0–7 days | 11 (14.7) | 18 (22.8) | 13 (16.3) | 31 (19.5) | |
| AEs within 0–28 days | 22 (29.3) | 30 (38.0) | 22 (27.5) | 52 (32.7) | |
| Grade ≥3 AEs | 0 | 1 (1.3) | 0 | 1 (0.6) | |
| Grade ≥3 vaccine-related AEs | 0 | 1 (1.3) | 0 | 1 (0.6) | |
| Solicited AEs | |||||
| Any | 10 (13.3) | 20 (25.3) | 13 (16.3) | 33 (20.8) | 0.1416 |
| Grade ≥3 | 0 | 0 | 0 | 0 | |
| Solicited local AEs | |||||
| Any | 2 (2.7) | 11 (13.9) | 8 (10.0) | 19 (11.9) | 0.0373 |
| Grade ≥3 | 0 | 0 | 0 | 0 | |
| Injection site pain | 2 (2.7) | 10 (12.7) | 7 (8.8) | 17 (10.7) | |
| Injection site pruritus | 0 | 1 (1.3) | 1 (1.3) | 2 (1.3) | |
| Injection site swelling | 0 | 0 | 2 (2.5) | 2 (1.3) | |
| Injection site erythema | 0 | 0 | 1 (1.3) | 1 (0.6) | |
| Injection site induration | 0 | 0 | 1 (1.3) | 1 (0.6) | |
| Solicited systemic AEs | |||||
| Any | 8 (10.7) | 11 (13.9) | 6 (7.5) | 17 (10.7) | 0.4313 |
| Grade ≥3 | 0 | 0 | 0 | 0 | |
| Pyrexia | 6 (8.0) | 6 (7.6) | 4 (5.0) | 10 (6.3) | |
| Cough | 0 | 2 (2.5) | 2 (2.5) | 4 (2.5) | |
| Headache | 2 (2.7) | 2 (2.5) | 0 | 2 (1.3) | |
| Constipation | 0 | 1 (1.3) | 0 | 1 (0.6) | |
| IP-related solicited AEs | 9 (12.0) | 18 (22.8) | 12 (15.0) | 30 (18.9) | 0.1826 |
| Unsolicited AEs | 21 (28.0) | 17 (21.5) | 21 (26.3) | 38 (23.9) | 0.6532 |
| Vaccine-related unsolicited AEs | 14 (18.7) | 15 (19.0) | 12 (15.0) | 27 (17.0) | |
| Pyrexia | 2 (2.7) | 4 (5.1) | 3 (3.8) | 7 (4.4) | |
| CPK increased | 1 (1.3) | 3 (3.8) | 2 (2.5) | 5 (3.1) | |
| Glycosuria | 2 (2.7) | 0 | 4 (5.0) | 4 (2.5) | |
| Headache | 0 | 1 (1.3) | 3 (3.8) | 4 (2.5) | |
| SAEs | 0 | 0 | 0 | 0 | |
| AESI | 1 (1.3) | 0 | 0 | 0 | |
AESI = adverse event of special interest; CPK = creatine phosphokinase; IP = investigational product; SAE = serious adverse event; TEAE = treatment-emergent adverse event.
Statistical comparison of AEs between saline and total SCTV01C.
Fig. 2.
Incidence of local and systemic solicited AEs after booster injection. The grading scales are derived from the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (Grade 1: mild, Grade 2: moderate or Grade 3: severe). The percentages of participants in each group with adverse events during the 7 days after vaccination are plotted for solicited local (Panel A) and systemic (Panel B) adverse events. Among all participants who received SCTV01C, the most frequent solicited local and systemic AEs were Grade 1 injection-site pain and pyrexia. There were no Grade 3 (severe) events. Participants with zero adverse events make up the remainder of the 100% calculation (not shown).
Immunogenicity
The humoral immune responses were examined on the day before SCTV01C vaccination (D0, baseline) and 28 days after the booster injection (D28). One booster dose of SCTV01C induced significant specific spike binding IgG and neutralizing antibody responses to both Delta and Omicron variants, and the immune responses at the two dose levels (20 μg and 40 μg) were similar. The immune response to the placebo was minimal.
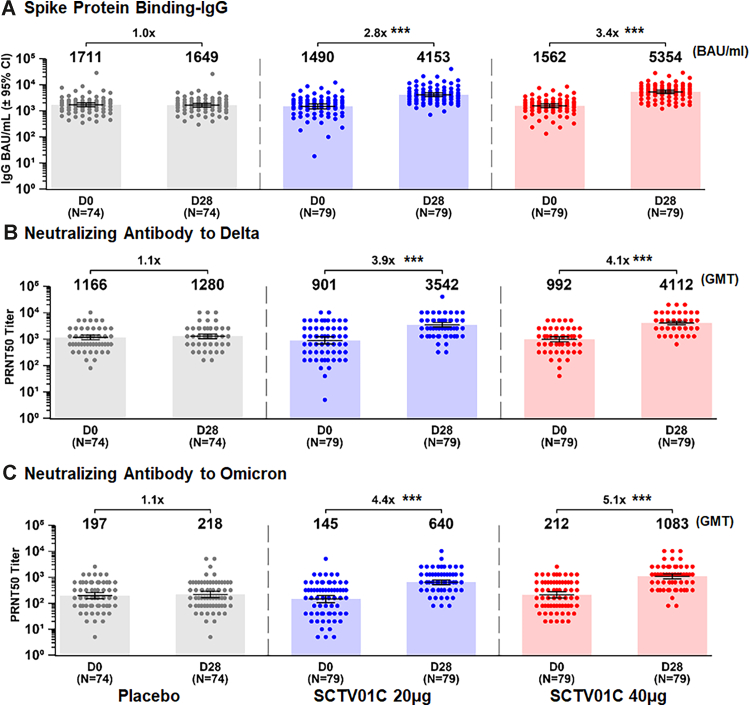
On Day 28 post booster vaccination, the GMCs of specific spike protein binding-IgG (converted to WHO International Binding Antibody Units, BAU) were 1649 (95% CI: 1402–1940), 4153 (95% CI: 3526–4891), and 5354 (95% CI: 4587–6251) BAU/mL, with 1.0 (p = 0.7580), 2.8 (p < 0.0001) and 3.4 (p < 0.0001)-fold increase over the baseline (D0), for placebo, 20 μg and 40 μg groups respectively (Fig. 3A). The GMTs of neutralizing antibody responses to live SARS CoV-2 Delta variant were 1280 (95% CI: 1045–1567), 3542 (95% CI: 2922–4293), and 4112 (95% CI: 3545–4768), with 1.1 (p = 0.5195), 3.9 (p < 0.0001) and 4.1 (p < 0.0001)-fold increase over baseline (D0) for the placebo, 20 μg and 40 μg groups, respectively (Fig. 3B). One booster dose of SCTV01C also elicited potent neutralizing antibody responses to live Omicron variant. The GMTs of neutralizing antibody responses to Omicron BA.1 were 218 (95% CI: 164–290), 640 (95% CI: 520–788), and 1083 (95% CI: 868–1352), with 1.1 (p = 0.6015), 4.4 (p < 0.0001) and 5.1 (p < 0.0001)-fold increase for the placebo, 20 μg and 40 μg groups, respectively (Fig. 3C).
Fig. 3.
GMC (BAU/mL) of anti-spike protein IgG (A) and GMTs of neutralizing antibodies against live SARS-CoV-2 Delta variant (B) and Omicron variant (C). Specific binding-IgG antibody titers were measured using enzyme-linked immunosorbent assay (ELISA) and converted to geometric mean concentration (GMC) using WHO assigned International Binding Antibody Units (BAU). GMTs of neutralizing antibody responses were measured using 50% plaque reduction neutralization test (PRNT50). Bars show the GMCs and GMTs with 95% CIs at Day 0 and Day 28. Dots represent the values for individual participants. Note: Only those with available baseline and post-baseline data were included; ∗∗∗p < 0.0001, paired statistical comparison over time.
For the combined 20 μg and 40 μg groups of SCTV01C, the specific binding-IgG to spike protein was 4716 (95% CI: 4211–5281), with a 3.1-fold increase to baseline (Table 3). Likewise, the GMTs of neutralizing antibodies to Delta and Omicron variants were 3816 (95% CI: 3382–4305) and 833 (95% CI: 713–973), with a fold increase of 4.0 and 4.7, respectively (Table 3).
Table 3.
Anti-spike protein IgG and neutralizing antibodies against live SARS-CoV-2 Delta variant and Omicron variant.
| Day |
Saline |
SCTV01C |
|||
|---|---|---|---|---|---|
| N = 74 | 20 μg, N = 79 | 40 μg, N = 79 | Total N = 158 | ||
| IgG (BAU/mL) | |||||
| Day 0 | GMC (95% CI) | 1711 (1434, 2042) | 1490 (1195, 1857) | 1562 (1320, 1849) | 1525 (1330, 1750) |
| Day 28 | GMC (95% CI) | 1649 (1402, 1940) | 4153 (3526, 4891) | 5354 (4587, 6251) | 4716 (4211, 5281) |
| Fold-Increase (95% CI) | 1.0 (0.9, 1.0) | 2.8 (2.2, 3.6) | 3.4 (2.8, 4.2) | 3.1 (2.6, 3.6) | |
| GMR (95% CI) | 2.6 (2.1, 3.2) | 3.3 (2.7, 4.1) | 2.9 (2.4, 3.5) | ||
| p value | <0.0001 | <0.0001 | <0.0001 | ||
| Delta live virus neutralizing antibody PRNT50 | |||||
| Day 0 | GMT (95% CI) | 1166 (948, 1433) | 901 (666, 1219) | 992 (805, 1224) | 946 (788, 1135) |
| Day 28 | GMT (95% CI) | 1280 (1045, 1567) | 3542 (2922, 4293) | 4112 (3545, 4768) | 3816 (3382, 4305) |
| Fold-Increase (95% CI) | 1.1 (1.0, 1.2) | 3.9 (2.8, 5.5) | 4.1 (3.3, 5.2) | 4.0 (3.3, 5.0) | |
| GMR | 2.9 (2.3, 3.7) | 3.3 (2.6, 4.2) | 3.1 (2.5, 3.8) | ||
| p value | <0.0001 | <0.0001 | <0.0001 | ||
| Omicron live virus neutralizing antibody PRNT50 | |||||
| Day 0 | GMT (95% CI) | 197 (150, 258) | 145 (106, 200) | 212 (161, 278) | 175 (142, 216) |
| Day 28 | GMT (95% CI) | 218 (164, 290) | 640 (520, 788) | 1083 (868, 1352) | 833 (713, 973) |
| Fold-Increase (95% CI) | 1.1 (0.9, 1.3) | 4.4 (3.2, 6.2) | 5.1 (3.9, 6.8) | 4.7 (3.8, 5.9) | |
| GMR (95% CI) | 3.2 (2.4, 4.4) | 4.8 (3.6, 6.5) | 4.0 (3.1, 5.1) | ||
| p value | <0.0001 | <0.0001 | <0.0001 | ||
Fold-Increase = Fold increase over baseline; GMT = Geometric Mean Titer; GMC = Geometric Mean Concentration; GMR = fold increase over control (saline).
p value was calculated by comparison of control group and SCTV01C group, through an ANCOVA model adjusted by log-transformed baseline and interval of vaccination.
For each type of variant, only those with available baseline and post-baseline data are included (iFAS).
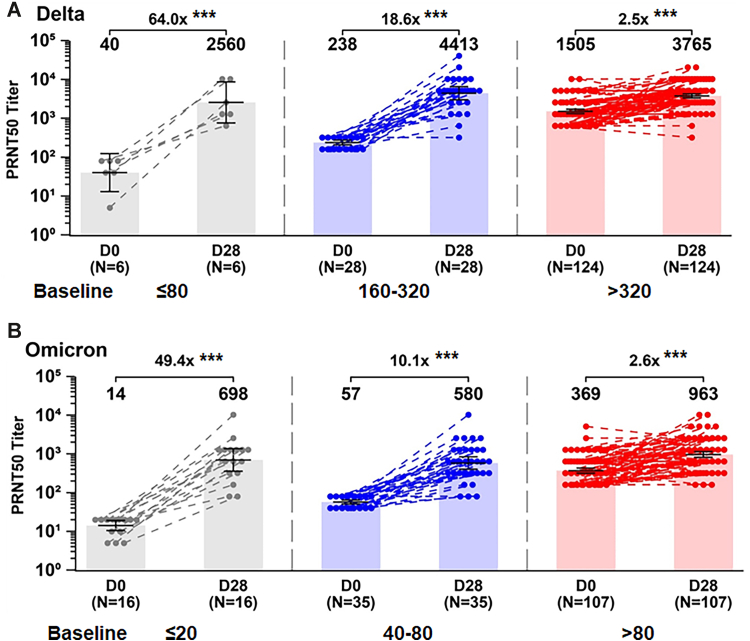
The impact of the pre-existing SARS-COV-2 immunity on SCTV01C booster was analyzed. The participants were assigned to different groups - low baseline titer group, medium baseline titer group, and high baseline titer group based on the GMT levels of the neutralizing antibody before booster vaccination. For the Omicron variant, participants with GMTs at D0 equal to or lower than the lower limit of quantitation (LLOQ: 20), in the range of 40–80 and over 80 were considered as low, medium and high baseline titers, respectively. Similarly, for the Delta variant, GMTs at D0 equal to or lower than 80 (four times the LLOQ), in the range of 160–320 and over 320 were considered as low, medium and high baseline titers, respectively (Fig. 4). For combined data of 20 μg and 40 μg of SCTV01C, the Day 28 GMTs of the cross-neutralizing antibody to Delta variant were from 2560 to 3765, with a fold increase of 64.0 (p < 0.0001), 18.6 (p < 0.0001), and 2.5 (p < 0.0001)-fold for the low, medium and high baseline titer groups, respectively. Likewise, the GMTs to Omicron variants were from 698 to 963 with a fold increase of 49.4 (p < 0.0001) 10.1(p < 0.0001) and 2.6-fold (p < 0.0001) for the low, medium and high baseline titer groups, respectively.
Fig. 4.
Fold increase of neutralizing antibodies against live SARS-CoV-2 Delta (A) Omicron (B) variants in groups with low, medium and high baseline titers. The participants were assigned to three groups based on the GMT levels at baseline. For the Omicron variant, GMTs at D0 equal to or lower than the lower limit of quantitation (LLOQ: 20), in the range of 40–80 and over 80 were considered as low, medium and high baseline titers, respectively. For the Delta variant, GMTs at D0 equal to or lower than 80 (four times the LLOQ), in the range of 160–320 and over 320 were considered as low, medium and high baseline titers, respectively. Note: The combined data of 20 μg and 40 μg of SCTV01C were used; ∗∗∗p < 0.0001, paired statistical comparison over time.
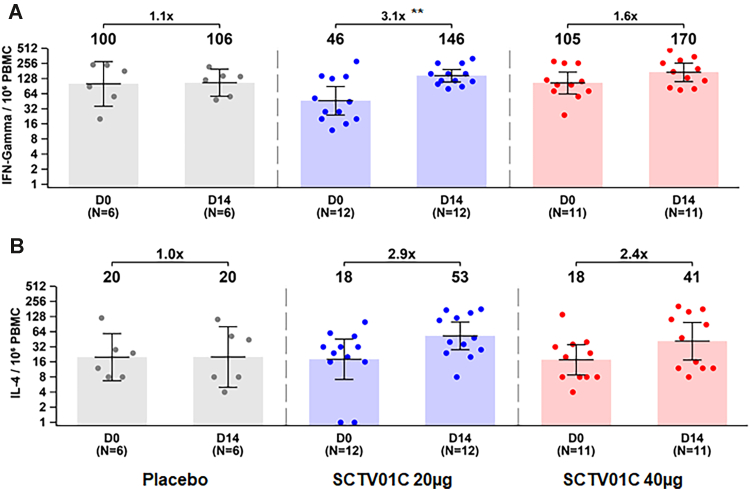
The peripheral blood mononuclear cells were collected to assess specific Th1 (IFN-γ release) and Th2 (IL-4 release) responses before vaccination and 14 days (D14) after booster vaccination. For saline, 20 μg SCTV01C and 40 μg SCTV01C groups, number of specific T-cells with secretion of IFN-γ (Th1) increased 1.1 (p = 0.9022), 3.1 (p = 0.0019) and 1.6 (p = 0.0614)-fold from the baseline respectively, and number of IL-4 (Th2) secreting T-cells increased by 1.0 (p = 0.9891), 2.9 (0.1916) and 2.4 (p = 0.0996)-fold from the baseline, respectively On D14. The numbers of IFN-γ (Th1) secreting T cells and IL-4 (Th2) secreting T-cells were increased after SCTV01C booster, however, the statistically significant change was only observed for IFN-γ levels and only in the 20 μg SCTV01C group (Fig. 5 and Table 4).
Fig. 5.
Th1 (A. IFN-γ release) and Th2 (B. IL-4 release) responses. The peripheral blood mononuclear cells (PBMC) were collected from the participants of phase 1 before vaccination, and on day 14 after booster vaccination (D14). The number of specific T cells with secretion of IFN-γ (Th1) and IL-4 (Th2) were measured with spot per 10⁶ PBMC using enzyme-linked immunospot (ELISpot) assay. Note: Only those with available baseline and post-boosting data were included; ∗∗p < 0.01, paired statistical comparison over time.
Table 4.
Th1 (A. IFN-γ release) and Th2 (B. IL-4 release) responses.
| Day | Saline |
SCTV01C |
|||
|---|---|---|---|---|---|
| N = 6 | 20 μg, N = 12 | 40 μg, N = 11 | Total, N = 23 | ||
| T cell INF-gamma (spot per 10⁶ PBMC) | |||||
| Day 0 | GMC (95% CI) | 100 (36, 280) | 46 (24, 89) | 105 (64, 172) | 68 (45, 104) |
| Day 14 | GMC (95% CI) | 106 (57, 198) | 146 (110, 195) | 170 (111, 260) | 157 (125, 198) |
| Fold-Increase (95% CI) | 1.1 (0.6, 1.8) | 3.1 (1.6, 6.3) | 1.6 (0.9, 2.9) | 2.3 (1.5, 3.6) | |
| GMR (95% CI) | 1.5 (0.9, 2.7) | 1.6 (0.9, 2.7) | 1.6 (1.0, 2.6) | ||
| p value | 0.1208 | 0.0924 | 0.0753 | ||
| T cell IL-4 (spot per 10⁶ PBMC) | |||||
| Day 0 | GMC (95% CI) | 20 (7, 59) | 18 (7, 46) | 18 (9, 35) | 18 (10, 31) |
| Day 14 | GMC (95% CI) | 20 (5, 81) | 53 (28, 99) | 41 (18, 98) | 47 (29, 76) |
| Fold-Increase (95% CI) | 1.0 (0.3, 3.6) | 2.9 (1.0, 8.3) | 2.4 (0.6, 8.6) | 2.6 (1.2, 5.6) | |
| GMR (95% CI) | 2.6 (0.8, 8.4) | 2.1 (0.6, 6.7) | 2.3 (0.8, 6.8) | ||
| p value | 0.1054 | 0.2292 | 0.1198 | ||
Fold-Increase = Fold increase over baseline; GMC = Geometric Mean Concentration; GMR = Fold increase over control (saline).
p value was calculated by comparison of control group and SCTV01C group, through an ANCOVA model adjusted by log-transformed baseline and interval of vaccination.
For each type of variant, only those with available baseline and post-baseline data are included (iFAS).
Discussion
We conducted a randomized, double-blind phase 1/2 trial to assess the reactogenicity and immunogenicity of SCTV01C, a novel bivalent COVID-19 vaccine as a booster in adults previously vaccinated with two doses of mRNA vaccines.
The demographic and characteristics of the participants in terms of age, BMI and health condition generally reflected those of the study's population. However, male and Asian constituted a large portion of participants, so the female and other ethnicities were not well represented. This study aimed to assess the reactogenicity and immunogenicity of SCTV01C in the healthy adults. The immunocompromised patients and/or people with severe or uncontrollable medical conditions were excluded from enrollment, so, the number of the participants with the comorbidities was small. During the course of the trial, we did not observe the impact of comorbidities or pre-existing health conditions on the immune responses induced by SCTV01C vaccination.
SCTV01C was safe, with low reactogenicity. Among 159 participants who received SCTV01C, the incidence rate of solicited ARs was 18.9%. No hospitalizations, SAEs, AESIs, MAAEs, Grade ≥3 solicited or unsolicited treatment related AEs. Furthermore, SCTV01C associated ARs that occurred in ≥5% subjects were mild injection-site pain (10.7%) and pyrexia (6.3%). Compared to the saline control group, the statistical significant increase was only shown in the solicited local AEs in SCTV01C group. The overall safety and reactogenicity profile of one SCTV01C boost was similar to that of reported homologous prime/booster with inactivated vaccines (CoronaVac showed 6–18% solicited ARs and 1–16% of injection-site pain. BBIBP-CorV showed 12.72% solicited ARs, 3.98% of injection-site pain and 4.2% of headaches),11,21 and comparable to the AEs of the primary 2 dose series of inactivated vaccines.22,23 Contrastingly, greater reactogenicity, such as fatigue, headache, arthralgia, myalgia, nausea, diarrhea, fever, chill, irritability, loss of appetite and pain at the injection-site were commonly observed in homologous/heterologous boosting with adenoviral vectored vaccines or mRNA vaccines.24
Generally, the heterologous booster regimens were immunogenically superior to homologous prime/booster with the same COVID-19 vaccines.4, 5, 6, 7,25 Heterologous booster of one dose of SCTV01C in previous mRNA vaccine recipients resulted in substantial cross-neutralizing antibody responses against both Delta and Omicron variants. The PRNT50 values compared favorably to the homologous prime/booster of three doses of mRNA vaccines,4,6 although direct comparison of neutralizing titers obtained from different labs was hampered by the variabilities of the assays. For the combined 20 μg and 40 μg SCTV01C groups, GMTs of neutralizing antibodies against live Delta and Omicron variants were 4716 (95% CI: 4211–5281) and 833 (95% CI: 713–973) on D28, respectively. Table 5 compared the GMTs results of this study with those from the previous studies with the Pfizer BNT162b2 booster.26, 27, 28
Table 5.
Comparison of neutralizing antibody against Omicron between Pfizer mRNA booster and SCV01C booster.
| SCTV01C boosterd |
||||||||
|---|---|---|---|---|---|---|---|---|
| PRNT50 (GMT) | Pfizer mRNA booster |
Low baseline |
Medium baseline |
High baseline |
Overall |
|||
| N = 2026 | N = 2027 | N = 2028 | N = 16 | N = 35 | N = 107 | N = 158 | ||
| Delta | Pre-booster | 86 | NA | 40 | 238 | 1505 | 946 | |
| Post-booster | 2315a | 2560 | 4413 | 3765 | 3816 | |||
| Fold-change | 26.9 | 64 | 18.6 | 2.5 | 4 | |||
| Omicron | Pre-booster | 35 | 5 | 13 | 14 | 57 | 369 | 175 |
| Post-booster | 628a | 67b | 336c | 698 | 580 | 963 | 833 | |
| Fold-change | 17.9 | 13 | 25 | 49.4 | 10.1 | 2.6 | 4.7 | |
GMT = Geometric Mean Titer.
Tested at 2–3 weeks after the booster dose.
Tested at 28 days after the booster dose.
Tested at 1 month after the booster dose.
Tested measured at 28 days after the booster dose.
This clinical trial started when the COVD-19 pandemic was evolving rapidly with the Omicron variant gradually overtaking Delta variant and a significant portion of the population either vaccinated or infected with SARS-CoV-2 in UAE. According to published reports, asymptomatic infections accounted for approximately 32.4%–45% of Omicron infections.29,30 We observed highly diversified baseline neutralizing antibody titers to both Delta and Omicron variants prior to boosting with SCTV01C. About 90% of the participants showed positive PRNT50 (above lower limit of quantitation LLOQ: 20) of neutralizing antibody to live Omicron, which were much higher than previously reported for people vaccinated with two doses of mRNA vaccines. A BNT162b2 study showed that 3–5 weeks post second dose, the neutralizing GMTs against Omicron was 731 and likewise mRNA 1273 preprint study showed neutralizing GMTs of 14 against Omicron four weeks post second dose.32
Consequentially, we assessed the impact of the heterogeneities of the pre-existing SARS-CoV-2 immunity on the immunogenicity of SCTV01C booster. Participants were assigned to different groups - low baseline titer group, medium baseline titer group, and high baseline titer group - based on the GMT levels of the neutralizing antibody before SCTV01C booster vaccination (Fig. 4). The Day 28 GMTs of the neutralizing antibodies to Delta variant were 2560, 4413 and 3765 with corresponding fold increase of 64.0, 18.6 and 2.5, for the low, medium and high baseline titer groups, respectively. GMTs to Omicron variant were 698, 580 and 963 with a fold increase of 49.4, 10.1 and 2.6, respectively, for the low, medium and high baseline titer groups. Notably, the participants with low levels of neutralizing antibodies at baseline reached high PRNT50 values after SCTV01C boosting, with the highest fold increase achieved in the low baseline titer group.
The findings in this study has limitations. First, the data showed unexpected high titers of neutralizing antibody present in a large portion of the trial participants at baseline, which may relate to the asymptomatic infection. However, the impact of previous infection and/or asymptomatic infection that occurred during the observation period could not be analyzed in-depth. Second, the immunogenicity of booster vaccination was assessed in a short period, and as result, the immune persistence data is not yet available. Furthermore, subgroup analysis provided some insights on the fold-of-increase for those with low baseline titers, but this population may not be well presented due to the small sample size. . Finally, this trial initially estimated an 8-fold increase in the neutralizing antibody titers against Omicron after SCTV01C booster vaccination, however, the unexpected high baseline neutralizing antibody titers to both Delta and Omicron, compromised the overall fold-of-increase, as a result, the pre-defined the fold of increase in the neutralizing antibody did not reach.
Including this study, SCTV01C has been evaluated in three separate phase 1/2 clinical trials. A phase 1 trial assessing SCTV01C in vaccine naïve people in China with a two-dose regimen (NCT05148091) has shown favorable safety profiles and potent immunity against Alpha, Beta, Delta and Omicron variants (manuscript submitted). In another phase 1/2 booster study in people previously vaccinated with inactivated vaccine (NCT05043285), similar safety profiles and immunogenicity to this booster study were observed (manuscript submitted), further confirming the potential application of SCTV01C booster as an important tool for controlling Omicron pandemic. A phase 3 immunogenicity and safety study comparing SCTV01C in head-to-head with mRNA vaccine is on-going (NCT05323461).
In summary, the current data showed that heterologous booster of SCTV01C in mRNA vaccine recipients was safe and well tolerated with a reactogenicity profile comparable to that of inactivated vaccines. Most importantly, SCTV01C booster vaccination induced significant neutralization activities against Delta and Omicron variants. Even participants with low level of neutralizing antibody at baseline, SCTV01C boosting elicited a high fold of increase in the neutralizing antibody responses for both Delta and Omicron variants.
Contributors
Dr. Suad Hannawi was the study site principal investigator, responsible for the supervision of the study, the coordination of resources, data analysis, data verification and interpretation. Dr. Linda Saifeldin, Dr. Alaa Abuquta, Dr. Aala Hassan and Dr. Ahmad Alamadi contributed to participant management and implementation of the study (vaccine management, vaccination, participant screening management, communication and coordination with the sponsor, CRO, and ethics). Dr. Sally A. Mahmoud was responsible for laboratory testing and assay development. Dr. Dongfang Liu and Lixin Yan contributed to the medical monitoring and management, protocol writing and statistical analysis. Dr. Liangzhi Xie contributed to the study conception, study design, project management, data interpretation and manuscript writing. Dr. Suad Hannawi and Dr. Liangzhi Xie verified the underlying data. All authors critically reviewed and approved the final version of the manuscript.
Data sharing statement
Anonymized participant data will be made available when the trials are complete, upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement. All data will be made available for a minimum of 5 years from the end of the trial.
Declaration of interests
Dr. Liangzhi Xie, Dr. Dongfang Liu, and Lixin Yan are employees of Sinocelltech Ltd. and have ownership or potential stock option interests in the company. All authors declare no other conflicts of interest.
Acknowledgments
We thank the CRO team of PDC FZ-LLC, for their hard work, support, and guidance of the trial; Mr. Bo Zhong, the project manager of SCTV01C-01-2 trial operation teams, for his committed dedication to managing and running of the trial; Mr. Adham Rezk and Revonbio B.V for their vaccine consultancy, strategy and coordination. We also acknowledge Dr. Adam Abdul Hakeem Baidoo, Dr. Miaomiao Zhang and Dr. Yuanxin Chen for their medical writing and editorial support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104386.
Appendix A. Supplementary data
Supplementary Figure S1.
Trial procedure. 30 and 205 participants were eligible and randomized into the phase I and phase II trials respectively. During the phase 1 trial, participants in each cohort were randomly assigned in a 4:1 ratio to receive an intramuscular injection of SCTV01C (20 μg or 40 μg) or saline placebo; whereas participants were assigned in a 2:1 ratio for each cohort of the phase 2 trial. The enrollment started with cohort A1. Upon completing 7 days safety observation, enrollment began in cohort B1 and cohort C1 simultaneously. Following 7 days safety observation of cohort B1, enrollment of Cohort D1 was initiated.
References
- 1.Centers for Disease Control and Prevention COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 2.Ariën K.K., Heyndrickx L., Michiels J., et al. Three doses of BNT162b2 vaccine confer neutralising antibody capacity against the SARS-CoV-2 Omicron variant. NPJ Vaccines. 2022;7:35. doi: 10.1038/s41541-022-00459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pajon R., Doria-Rose N.A., Shen X., et al. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launay O., Cachanado M., Luong Nguyen L.B., et al. Immunogenicity and safety of beta-adjuvanted recombinant booster vaccine. N Engl J Med. 2022;387:374–376. doi: 10.1056/NEJMc2206711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalkias S., Harper C., Vrbicky K., et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med. 2022;387:1279–1291. doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyke K.E., Atmar R.L., Islas C.D., et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med. 2022;3:100679. doi: 10.1016/j.xcrm.2022.100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X., Munro A.P.S., Feng S., et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: three month analyses of the COV-BOOST trial. J Infect. 2022;84:795–813. doi: 10.1016/j.jinf.2022.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patalon T., Saciuk Y., Peretz A., et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun. 2022;13:3203. doi: 10.1038/s41467-022-30884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Interim statement on COVID-19 vaccines in the context of the circulation of the omicron SARS-CoV-2 variant from the WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) 11 January 2022. https://www.who.int/news/item/11-01-2022-interim-statement-on-covid-19-vaccines-in-the-context-of-the-circulation-of-the-omicron-sars-cov-2-variant-from-the-who-technical-advisory-group-on-covid-19-vaccine-composition
- 10.Chalkias S., Eder F., Essink B., et al. Safety, immunogenicity and antibody persistence of a bivalent beta-containing booster vaccine against COVID-19: a phase 2/3 trial. Nat Med. 2022;28(11):2388–2397. doi: 10.1038/s41591-022-02031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaabi N.A., Yang Y.K., Du L.F., et al. Safety and immunogenicity of a hybrid-type vaccine booster in BBIBP-CorV recipients in a randomized phase 2 trial. Nat Commun. 2022;13:3654. doi: 10.1038/s41467-022-31379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z., He Q., Zhao W., et al. A heterologous V-01 or variant-matched bivalent V-01D-351 booster following primary series of inactivated vaccine enhances the neutralizing capacity against SARS-CoV-2 delta and omicron strains. J Clin Med. 2022;11:4164. doi: 10.3390/jcm11144164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R., Huang X., Cao T., et al. Development of a thermostable SARS-CoV-2 variant-based bivalent protein vaccine with cross-neutralizing potency against Omicron subvariants. Virology. 2022;576:61–68. doi: 10.1016/j.virol.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R., Sun C., Ma J., et al. A bivalent COVID-19 vaccine based on alpha and beta variants elicits potent and broad immune responses in mice against SARS-CoV-2 variants. Vaccines (Basel) 2022;10:702. doi: 10.3390/vaccines10050702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier S., Güthe S., Kiefhaber T., Grzesiek S. Foldon, the natural trimerization domain of T4 fibritin, dissociates into a monomeric A-state form containing a stable beta-hairpin: atomic details of trimer dissociation and local beta-hairpin stability from residual dipolar couplings. J Mol Biol. 2004;344:1051–1069. doi: 10.1016/j.jmb.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Food and Drug Administration. 2007. https://www.fda.gov/media/73679/download
- 17.Bonelli F., Blocki F.A., Bunnell T., et al. Evaluation of the automated LIAISON® SARS-CoV-2 TrimericS IgG assay for the detection of circulating antibodies. Clin Chem Lab Med. 2021;59:1463–1467. doi: 10.1515/cclm-2021-0023. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization First WHO international standard for anti-SARS-CoV-2 immunoglobulin (human) 2020. https://www.nibsc.org/documents/ifu/20-136.pdf
- 19.Mahmoud S., Ganesan S., Al Kaabi N., et al. Immune response of booster doses of BBIBP-CORV vaccines against the variants of concern of SARS-CoV-2. J Clin Virol. 2022:150–151. doi: 10.1016/j.jcv.2022.105161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruse M., Dark C., Aspden M., et al. Performance of the T-SPOTⓇ.COVID test for detecting SARS-CoV-2-responsive T cells. Int J Infect Dis. 2021;113:155–161. doi: 10.1016/j.ijid.2021.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng G., Wu Q., Pan H., et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomized, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22:483–495. doi: 10.1016/S1473-3099(21)00681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X., Wei D., Xu W., et al. Neutralizing activity of BBIBP-CorV vaccine-elicited sera against beta, delta and other SARS-CoV-2 variants of concern. Nat Commun. 2022;13:1788. doi: 10.1038/s41467-022-29477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanriover M.D., Doğanay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munro A.P.S., Janani L., Cornelius V., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa Clemens S.A., Weckx L., Clemens R., et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi J.Y., Lee Y.J., Ko J.H., et al. Neutralizing activity against SARS-CoV-2 delta and omicron variants following a third BNT162b2 booster dose according to three homologous or heterologous COVID-19 vaccination schedules. Front Cell Infect Microbiol. 2022;12:948014. doi: 10.3389/fcimb.2022.948014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung N.H.L., Cheng S.M.S., Cohen C.A., et al. Homologous and heterologous boosting with CoronaVac and BNT162b2: a randomized trial (the Cobovax study). medRxiv. 2022. https://www.medrxiv.org/content/10.1101/2022.08.25.22279158v2
- 28.Xia H., Zou J., Kurhade C., et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe. 2022;30(4):485–488.e3. doi: 10.1016/j.chom.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shang W., Kang L., Cao G., et al. Percentage of asymptomatic infections among SARS-CoV-2 omicron variant-positive individuals: a systematic review and meta-analysis. Vaccines (Basel) 2022;10(7):1049. doi: 10.3390/vaccines10071049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection. A narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng S.M.S., Mok C.K.P., Leung Y.W.Y., et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022;28(3):486–489. doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doria-Rose N.A., Shen X., Schmidt S.D., et al. Booster of mRNA-1273 strengthens SARS-CoV-2 omicron neutralization. medRxiv. 2021 doi: 10.1101/2021.12.15.21267805. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.