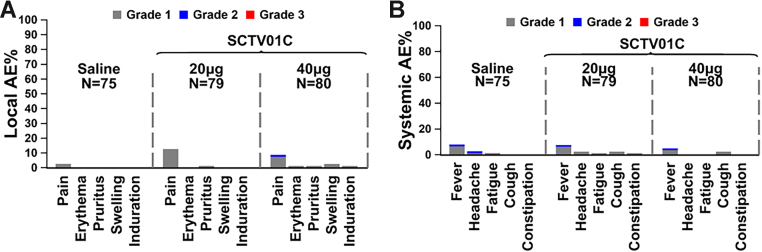
Fig. 2.
Incidence of local and systemic solicited AEs after booster injection. The grading scales are derived from the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (Grade 1: mild, Grade 2: moderate or Grade 3: severe). The percentages of participants in each group with adverse events during the 7 days after vaccination are plotted for solicited local (Panel A) and systemic (Panel B) adverse events. Among all participants who received SCTV01C, the most frequent solicited local and systemic AEs were Grade 1 injection-site pain and pyrexia. There were no Grade 3 (severe) events. Participants with zero adverse events make up the remainder of the 100% calculation (not shown).