Abstract
Passage in human blood of group A streptococcal isolate 64p was previously shown to result in the enhanced expression of M and M-related proteins. Similarly, when this isolate was injected into mice via an air sac model for skin infection, organisms recovered from the spleens showed both increased expression of M and M-related proteins and increased skin-invasive potential. We show that these phenotypic changes were not solely the result of increased transcription of the mRNAs encoding the M and M-related gene products. Rather, the altered expression was associated with posttranslational modifications of the M and M-related proteins that occur in this strain, based on the presence or absence of another virulence protein, the streptococcal cysteine protease SpeB. The phenotypic variability also correlates with colony size variation. Large colonies selected by both regimens expressed more hyaluronic acid, which may explain differences in colony morphology. All large-colony variants were SpeB negative and expressed three distinct immunoglobulin G (IgG)-binding proteins in the M and M-related protein family. Small-colony variants were SpeB positive and bound little IgG through their M and M-related proteins because these proteins, although made, were degraded or altered in profile by the SpeB protease. We conclude that passage in either human blood or a mouse selects for a stable, phase-varied strain of group A streptococci which is altered in many virulence properties.
Group A streptococci cause a wide range of human disease ranging from mild throat and skin infections to serious and life-threatening conditions of necrotizing fasciitis and a toxic shock-like syndrome (23, 58, 60). A number of potential virulence factors have been identified in different studies. These include surface M and M-related proteins (9, 45), fibronectin-binding proteins (43, 63), the hyaluronic acid capsule (18, 41, 56, 64), and a number of secreted products including the cysteine protease SpeB (17, 26–29, 33–35), streptokinase (37), and a variety of phage-encoded exotoxins (57).
Depending on the isolate studied and/or the model system used for virulence studies, the significance of a given putative virulence factor can vary from being great to nil. In many studies the antiphagocytic M protein has been shown to be the critical virulence factor (9, 45), while in other studies the hyaluronic capsule was found to be responsible for virulence irrespective of M protein expression (18, 64).
Similar differences have been noted in studies of the importance of SpeB in mouse infection models. Studies by Lukomski et al. (33–35) and others (29) provide evidence for SpeB as a virulence factor, while studies from our laboratory using a skin infection model (49, 50, 52) and studies by Ashbaugh et al. (2) in mouse model of intraperitoneal infection indicate that SpeB expression is not directly associated with a more virulent phenotype. These differences may reflect differences in isolates studied or in the precise animal model being used.
Interpretation of these divergent findings is further complicated by the observation that SpeB can modify other virulence factors such as streptolysin O (44) or M protein (6, 19, 53) to either increase or decrease their biological activities, respectively. In addition, cysteine protease can affect host receptors, activate cytokines, and metalloproteinases, and trigger various homeostatic pathways (14, 22, 27, 58, 65) and can potentially induce autoimmune postinfection sequelae (17) as well as influence invasion of epithelial cells (62).
Expression of virulence genes can also vary in cultured streptococci (7, 16, 38–40), and phenotypic changes in response to biological selection pressures in human blood or in mice are also well established (49, 50, 54). These phase variations as well as differences in genetic background could influence the effectiveness of a given putative virulence gene (45). Furthermore, preexisting immunity and difference in efficiency of innate immune responses in the host can also contribute to the outcome of the infection (23).
Our laboratory has studied one group A isolate, 64, extensively and found that stable phenotypic variants expressing enhanced surface immunoglobulin G (IgG)-binding proteins can be selected either in human blood or by passage in mice (49, 50, 54). These variants were found to be stable on subsequent subculture in the laboratory, in the absence of any biological selection pressure, for a period of over 5 years. Selected variants were clearly demonstrated to be more virulent when tested in a mouse model of skin infection (49, 50).
The selection of these stable variants of isolate 64 was not an all-or-nothing event but required multiple blood passages or passages in mice (49, 50, 54). In particular, the changes in expression of M and M-related IgG-binding proteins in isolate 64 passaged in human blood followed an interesting pattern. The parent isolate, 64p, expressed a predominant IgG-binding activity associated with the mrp gene product. Following sequential passage, three antigentically distinct IgG-binding proteins were identified. One is the Mrp protein expressed by the parent isolate; the other two IgG-binding proteins were found to be the products of the emm and enn genes (13). All three of these genes encoding IgG-binding proteins are known to be present in the coordinately regulated mga regulon. Thus, the pattern of differential gene expression between the parental strain and the strains derived from the mouse or human blood passage was intriguing.
In this study we have examined different selected variants of isolate 64 that demonstrate distinct IgG-binding protein phenotypes to determine the nature of the regulation that leads to the various IgG-binding phenotypes.
MATERIALS AND METHODS
Solubilization of IgG-binding surface proteins.
Proteins reactive in a nonimmune fashion with human immunoglobulins were extracted from the bacterial surface by treatment with CNBr as previously described (48). This procedure has been shown to be an efficient method to solubilize IgG-binding proteins from group A streptococci and results in solubilization of three distinct IgG-binding proteins from isolate 64/14 (13, 47).
Plasma proteins.
Human IgG1, IgG2, and IgG4 myeloma proteins were obtained from Calbiochem (San Diego, Calif.). Human IgG3 myeloma cryoglobulin was a gift from Richard Weber.
Labeling of proteins.
Human IgG1, IgG2, and IgG4 were labeled with horseradish peroxidase (HRP) by using an HRP labeling kit (Zymed Laboratories, San Francisco, Calif.). Human IgG3 cryoglobulin was labeled with biotin by using biotin-N-hydroxysuccinimide ester (Calbiochem, La Jolla, Calif.) according to the method of Bayer and Wilchek (4).
Analysis of IgG-binding proteins.
CNBr-extracted surface proteins, or sonicates of Escherichia coli containing recombinant proteins, were denatured and electrophoresed in 12% polyacrylamide minigels for 45 min at 200 V according to the method of Laemmli (30). Prestained molecular weight markers (low range; Bio-Rad, Richmond, Calif.) were included on each gel.
Following electrophoresis, separated polypeptides were transferred to nitrocellulose (Bio-Rad) by electroblotting as described elsewhere (61) and incubated with an appropriate dilution of the indicated labeled probe. Nitrocellulose membranes probed with biotinylated probes were again washed and then reprobed with HRP-labeled streptavidin (Amersham Life Sciences, Chicago, Ill.). The membranes were developed by using an ECL (enhanced chemiluminescence) Western blotting kit (Amersham Life Sciences, Chicago, Ill.) according to the manufacturer's instructions and exposed to Kodak XAR-5 film for 5 to 60 s at ambient temperature.
Analysis of transcription of IgG-binding protein genes.
RNA was purified from 1 g (wet weight) of washed cells for each strain grown to an approximate optical density of 0.6 at 600-nm wavelength. RNA purification and Northern analysis were performed by methods described previously (66). The probes used for Northern hybridization were PCR-generated fragments containing only gene-specific regions of the three genes in the emm gene cluster of strain 64. These probes included bp 181 to 1091 of the SF4 gene (mrp64), 1643 to 2036 of the SF2 gene (emm64), and 3215-3472 of the SF3 gene (enn64) (13). Primers for probes were the following: mrp, 5′GGATCCCCGGGCATCCGTAGCAGTCGCT3′ and 5′TTCTTGGTTGGTTGCTGCTAATT3′; emm, 5′AATCTGCAGTATTCGCTTAGAAAATTAAAA3′ and 5′CCTAAAAGATTCCTATTAAGTCTA3′; enn, 5′ATGGCTAGCCACAACCAAGAAAAAT3′ and 5′GTTCTTGATAACGTTTTTCTACTTCTCG3′; and recA, 5′ACGAACGTCGAAAGCCCTTG3′ and 5′CGGTTTCTTCTGATGCTACTGCC3′.
Experimental procedures for labeling probes, running gels, and quantifying transcripts were previously described; results are reported as a simple ratio of M-related gene transcript over recA transcript on the same blot after correction for probe length and exposure time (66). The recA gene transcript is produced constitutively and has been shown to remain stable under conditions of the experiment (40). Thus, recA transcript is used as an internal control for RNA yield and loading. This control allows the comparison of relative levels of mrp, emm, and enn transcripts from strains emanating from each passage.
Isolation of extracellular streptococcal cysteine protease.
A cysteine protease was isolated from culture supernatants by a modification of the method described in reference 19. Isolate 64p was grown to stationary phase for 24 h at 37°C in Todd-Hewitt broth (THB). Centrifugation and filtration of the culture supernatant removed bacteria through a 0.22-μm-pore-size filter. The filtered culture supernatants were brought to 80% saturation with ammonium sulfate. Precipitated material was recovered by centrifugation at 4,000 × g for 20 min at 4°C. The pellet was resuspended in distilled water equal to 1% of the original culture volume and dialyzed extensively against distilled water. Preparations demonstrating proteolytic activity contained a single Mr-∼27,000 band in Coomassie blue-stained sodium dodecyl sulfate (SDS)-polyacrylamide gels.
Assay for functional cysteine protease activity.
Cysteine protease activity present in ammonium sulfate-precipitated culture supernatants was assayed following extensive dialysis against phosphate-buffered saline according to the method of North (42). Briefly, 50 μl of the concentrated culture supernatant, without or with 0.1 mM dithiothreitol, was added to wells of a microtiter plate. Following incubation for 30 min at 37°C, to allow for reduction of the enzyme, 150 μl of substrate-buffer solution was added to each well. The substrate-buffer solution consisted of 3.2 ml of 2.5 mM Benz-Pro-Phe-Arg-paranitroanilide (Sigma) dissolved in pH 4.0 distilled water plus 4.8 ml of 0.1 M sodium phosphate, pH 6.0. Cleavage of the substrate was monitored by measuring the A405 over time in a microtiter plate reader (BioTek, Winooska, Vt.). The cleavage of substrate and generation of product were determined to be linear with time to an A405 of 1.5. The cysteine protease-specific inhibitor E64 (Sigma) was included in parallel assays at a concentration of 1 μM to determine if all of the enzyme activity being measured could be attributed to the presence of a cysteine protease (3).
The antigenic form of SpeB was determined by Western blotting using a polyclonal antiserum to SpeB (Toxin Technologies, Sarasota, Fla.). Antibody bound to active SpeB (Mr ∼ 27,000) or the zymogen form of SpeB (Mr ∼ 48,000) was detected with a protein G-HRP reporter system. In all experiments, a parallel blot was probed with normal rabbit serum to control for any nonspecific binding proteins that might be present.
Treatment of recombinant proteins with SpeB.
Sonicates of E. coli expressing recombinant Emm64, Mrp64, or Enn64 protein were incubated at 37°C for the times indicated with the cysteine protease prepared from the culture supernatant of strain 64p in the absence or the presence of 1 μM E64. The reaction was stopped by the addition of SDS sample buffer and heating for 10 min at 100°C. Enzyme digests were resolved on SDS-polyacrylamide gels, electroblotted to nitrocellulose, and probed with labeled human IgG1 and IgG3. The blots were developed by using the Amersham ECL reporter system and exposed to Kodak XAR film for 5 to 60 s.
Casein overlay plate assay for detection of protease production.
Todd-Hewitt agar plates containing 100 to 200 individual colonies were overlaid with 5 ml of 0.8% agarose containing 1% skim milk and 1 mM dithiothreitol. Hydrolysis of casein was determined by examining plates for zones of clearing around individual colonies following 4 h of incubation at 37°C. Overlays were performed in duplicate in the absence or the presence of 1 μM E64 to ensure that casein hydrolysis was due to a cysteine protease.
Analysis of hyaluronic acid capsule.
Capsular hyaluronic acid was determined by a chemical method as described previously (64).
RESULTS
Analysis of effects of biological pressure on expression of M and M-related proteins.
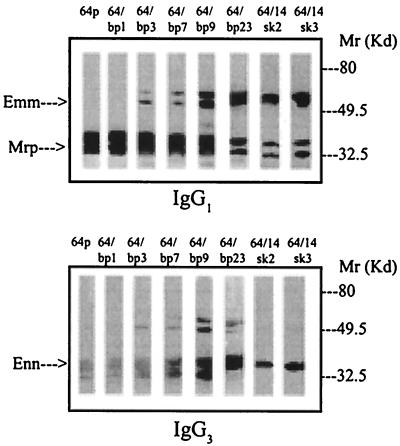
The pattern of expression of the M and M-related IgG-binding proteins varies as a function of blood or mouse passage. In the parent isolate (64p), a significant level of the Mrp gene product is identified, with no protein product from the emm or enn genes being detectable in CNBr extracts (Fig. 1). Following extensive passage of isolate 64 in human blood (64/14bp) or in mice (64/14sk), a variant which demonstrated changes in the profile of Emm- and Enn-binding proteins present in CNBr extract was selected (Fig. 1). In addition, quantitative changes in IgG binding were observed (Table 1).
FIG. 1.
Western blot analysis of the heterogeneity of IgG-binding proteins in CNBr extracts of various blood- and mouse-passaged isolates of strain 64. The upper and lower panels were probed with IgG1 and IgG3, respectively, as described in Materials and Methods. Samples were adjusted for approximately equivalent IgG1-binding activity. For quantitative differences in IgG-binding proteins among bacterial variants, see Table 1.
TABLE 1.
SpeB production and expression of IgG-binding proteins by variants of isolate 64
| Isolate | SpeB activity of bacteria shown in the absence of E64
|
IgG-binding protein expressiona
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mrp
|
Emm
|
Enn
|
||||||
| Antigenicb | Functionalc | Medium | Medium + E64 | Medium | Medium + E64 | Medium | Medium + E64 | |
| 64p | + (a) | + | ++ | ++ | − | ++ | − | + |
| 64/bp1 | + (a) | + | ++ | ++ | − | ++ | − | + |
| 64/bp3 | + (a) | + | ++ | ++ | − | + | − | + |
| 64/bp7 | + (a) | + | ++ | ++ | − | + | − | + |
| 64/bp9 | + (a) | + | ++ | ++ | + | + | − | + |
| 64/bp12 | − | − | ++ | ++ | ++ | ++ | ++ | ++ |
| 64/bp23 | − | − | ++ | ++ | ++ | ++ | ++ | ++ |
| 64/14sk/2 | − | − | ++ | ++ | ++ | ++ | ++ | ++ |
| 64/14sk/3 | − | − | ++ | ++ | ++ | ++ | ++ | ++ |
IgG-binding proteins present in CNBr extracts of bacteria grown in either the presence or absence of 1 μM E64. Activity was detected by Western blot analysis using an ECL system as described in Materials and Methods. ++, reactive bands observed following 5 s of exposure to X-ray film; +, bands observed following 10 to 30 s of exposure; −, no reactivity observed following 30 to 200 s of exposure.
The presence of either the zymogen SpeB protein (Mr ∼ 48,000) or the active enzyme (Mr ∼ 28,000) was measured by Western blotting as described in Methods. (a) indicates low-Mr active form. No significant binding to normal rabbit serum was observed under the assay conditions used.
SpeB activity of culture supernatants measured after reduction, using the synthetic substrate assay described in Materials and Methods. +, detectable substrate cleavage; −, no detectable substrate cleavage.
To determine whether the differences in IgG-binding protein expression corresponded to changes in mRNA levels in the biologically selected isolates, quantitative Northern blotting analysis was performed. Total RNA was recovered from bacterial populations of isolate 64 following 1, 3, 7, 9, or 23 sequential blood passages or passage through mouse skin and recovery from the spleen (64/14sk2 and -3). The purified RNA was probed for the presence of message for each M and M-related protein and compared to message for recA in the same RNA preparation as an internal control for RNA recovery.
The results presented in Table 2 demonstrate that emm and mrp messages were roughly equivalent in all variants despite the major differences in the corresponding proteins associated with variants (Fig. 1 and Table 1). In the parental isolate, the enn gene (encoding an Mr-∼32,000 IgG3-binding protein) was not transcribed as actively as the emm or mrp gene (encoding Mr-∼50,000 and Mr-∼35,000 IgG1-, IgG2-, and IgG4-binding proteins, respectively). However, following passage of this isolate through human blood on nine or more occasions, the level of enn message increased (Table 2). This increase in enn gene expression is accompanied by increased levels of the Mr-∼32,000 IgG3-binding Enn protein in CNBr extracts (Fig. 1). Thus, the differences in Enn protein expression among variants could be related, at least in part, to regulation at the transcriptional level.
TABLE 2.
Ratios of emm gene transcript to recA control transcript
| Gene probe | Ratio, IgG-binding protein-specific transcript/recA-specific transcripta
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 64/p | 64/bp1 | 64/bp3 | 64bp7 | 64/bp9 | 64/bp23 | 64/14sk2 | 64/14sk3 | |
| SF4 gene (mrp64) | 22 | 34 | 11 | 17 | 7 | 11 | 17 | 21 |
| SF1 gene (emm64) | 44 | 9 | 26 | 20 | 39 | 50 | 41 | 52 |
| SF3 gene (enn64) | 2.0 | 1.0 | 1.4 | 1.1 | 4.6 | 7.8 | 6.2 | 7.0 |
Ratio of transcripts in the same sample and on the same blot.
An increase in transcriptional levels, however, would not account for the differences in expression of Emm and Mrp proteins (Fig. 1 and Table 1) compared to message levels of the corresponding genes shown in Table 2. Taken together, the data suggest that some form of posttranslational modification of Emm and/or Enn might contribute to the IgG-binding phenotypes of the variants detected in CNBr extracts.
Previous studies of IgG-binding protein expression by M1 isolates had identified an effect of a cysteine protease SpeB on the quantity and immunoglobulin-binding properties of the IgG-binding M1 protein (51, 53). Consequently, in the next series of experiments the production of SpeB by the blood- and mouse-passaged variants of isolate 64 was tested. This analysis was carried out both for expression of functional enzyme in a synthetic substrate assay and for protein production as measured by Western immunoblotting. This latter approach had the advantage of detecting production of both active enzyme (Mr ∼ 28,000) and the zymogen form of SpeB (Mr ∼ 48,000). In all of these studies, only the low-Mr form of SpeB was detected. If the cultures were grown in the presence of iodoacetic acid, the zymogen form of SpeB was readily detectable (data not shown).
The results of this analysis demonstrated differences in the production of SpeB between parental and passaged isolates. In the parent and early blood passages of isolate 64 (bp3, bp7, and bp9), there was a demonstrable quantity of the active form of SpeB detected in culture supernatants by both antigenically and functional assays. SpeB protein could not be detected in the culture supernatants of isolates that had been passaged in human blood on more than 12 occasions (Table 1).
Isolates that had SpeB activity could be completely inhibited by addition of 1 μM E64, a cysteine protease inhibition (3). Growth in the presence of the inhibitor also correlated with a shift in the expression pattern for IgG-binding proteins Mrp, Emm, and Enn (Table 1). When this isolate was grown in the absence of E64, the CNBr extracts contained a lower quantity of IgG-binding activity and a markedly different profile. Under these culture conditions, the emm gene product and the enn gene product were markedly reduced or absent, while minimal change was observed for the mrp gene product (Table 1). These results suggest that SpeB might degrade Emm and Enn proteins while having minimal effects on Mrp.
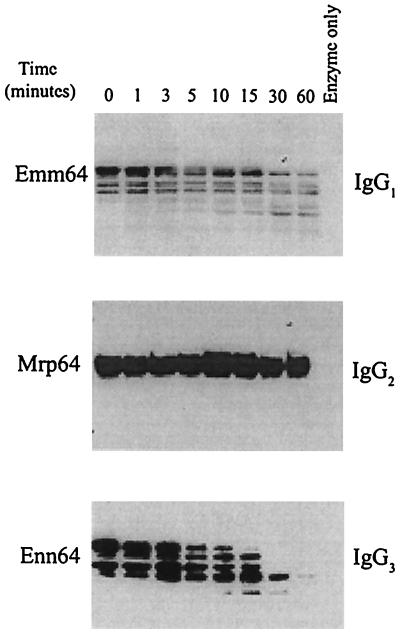
To test the hypothesis that SpeB protease activity was involved in posttranslational modification of M and M-related proteins, recombinant Emm 64/14, Mrp 64/14, or Enn 64/14 was incubated with cysteine protease prepared from the culture supernatant of isolate 64p as described in Materials and Methods. The results of these studies indicated that the cysteine protease could preferentially destroy recombinant Emm and Enn protein while the Mrp protein was relatively resistant to degradation with the enzyme (Fig. 2). These studies provide an explanation for the differences in surface IgG-binding protein profiles for isolates grown in presence or absence of the cysteine protease inhibitor E64 (Table 1).
FIG. 2.
Treatment of recombinant Emm, Mrp, or Enn 64 with a streptococcal cysteine protease and effect on IgG-binding properties. Sonicates of E. coli expressing recombinant Emm, Mrp, or Enn protein were incubated at 37°C for the times indicated with the cysteine protease prepared from the culture supernatant of strain 64p. The reaction was stopped by addition of sample buffer and boiling. The immunoglobulin reactivity remaining following immunoblotting was measured as described in Materials and Methods. Blots were developed by an ECL procedure and exposed to Kodak XAR film for 30 to 60 s.
Isolate 64, following nine passages in human blood (64/bp9), demonstrated a change in the profile of IgG-binding proteins, with all three IgG-binding proteins being detectable (Table 1). The IgG-binding profiles in CNBr extracts of these isolates were intermediate between those for the parental (64p) and an extensively mouse selected variant (64/14sk) or a variant selected following 12 or more sequential passages in human blood (64/bp12) (Table 1).
To determine whether this effect was due to a mixed population of cysteine protease-positive and -negative organisms within the population, individual colonies of isolate 64/bp9 were grown on Todd-Hewitt agar and replica plates overlaid with casein with or without E64 to detect cysteine protease-producing colonies. In this assay, two distinct phenotypes of casein hydrolysis could be distinguished. One group of colonies failed to cause casein hydrolysis, while the second group showed efficient clearing of casein around the colonies. This activity was dependent on production of a cysteine protease, since inclusion of E64 totally inhibited the activity.
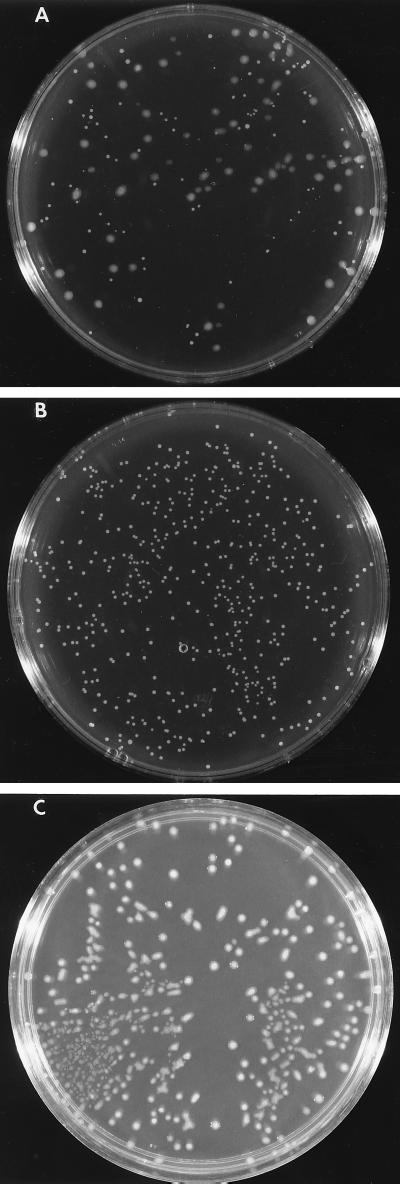
In addition to differences in cysteine protease production, bacteria grown on Todd-Hewitt agar plates showed two morphologically distinct types of colonies (Fig. 3A). These colonies could be separated and maintained their morphological characteristics (Fig. 3B and C). The small and large colonies were analyzed for hyaluronic acid content, and small colonies were found to contain <50% of the hyaluronic acid content of the large variants. This was consistent with the colony size morphology being the result of a phase variant in capsular content. When tested for production of a casein-hydrolyzing enzyme by using a casein overlay technique, all of the small colonies were positive, while no hydrolysis was observed surrounding the large colonies. Studies using E64 and analysis of antigenic SpeB protein expression confirmed that the casein hydrolysis associated with the small colonies was mediated by SpeB. The large colonies failed to secrete either the zymogen or active form of SpeB (data not shown).
FIG. 3.
Colonial morphology of strain 64p following passage through human blood on nine consecutive occasions. A stationary culture of blood-passaged strain 64/bp9 was diluted in phosphate-buffered saline and spread on Todd-Hewitt agar. Following overnight incubation at 37°C, plates with ∼100 colonies were examined for colonial morphology. (A) Day 9 of blood passage demonstrating the presence of two colony types; (B) a small colony selected and expanded from the plate in panel A. The small-colony variants secreted an E64-inhibitable cysteine protease that led to hydrolysis of overlaid casein. (C) A large colony selected and expanded from the plate in panel A. The large-colony variants did not generate a cysteine protease.
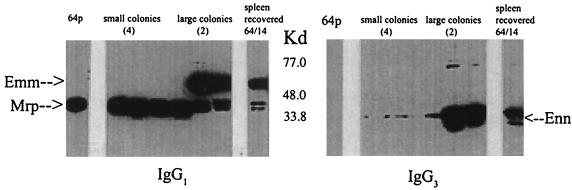
Representative small and large colonies were expanded in culture, and their surface IgG-binding proteins were analyzed following CNBr extraction. The IgG-binding protein profile of the cysteine protease-producing small isolates was similar to that of extracts of the parent 64p isolate, while those extracted from the larger, cysteine protease-negative colonies demonstrated the IgG-binding profile of the extensively mouse- or blood-passaged isolates (Fig. 4). Analysis of individual colonies of bacteria recovered following extensive passage of isolate 64 through human blood on 12 or more occasions consisted entirely of the large-colony phenotype, and all colonies failed to produce cysteine protease activity. These observations suggest that isolates with the large-colony morphology were stable.
FIG. 4.
Immunoglobulin-binding reactivity of surface proteins expressed by representative small and large colonies. Single-colony isolates of blood-passaged strain 64/bp9 demonstrating either the small- or the large-colony morphology were grown to stationary phase in THB. Surface proteins following extraction with CNBr were separated by SDS-PAGE, electroblotted to nitrocellulose, and probed as indicated. A representative extract from the parent isolate 64p and an extract from the isolate passaged 14 times in mice (64/14) are included for reference. Blots were developed by an ECL procedure and exposed to Kodak XAR film for 30 to 60 s.
To test this prediction, three arbitrarily selected colonies of either the small- or large-colony morphology were passaged in THB and screened for morphological type and cysteine protease activity following each passage. The results of these studies (Table 3) demonstrate that the large colonies retained their morphological appearance and high level of hyaluronic acid capsule on subculture. By contrast, there was a significant and measurable rate of conversion from small colonies to large colonies with concomitant loss of cysteine protease activity and acquisition of a larger hyaluronic acid capsule (Table 3).
TABLE 3.
Stability of representative colonies of 64/bp9 grown sequentially in THBa
| Selected colony | Colony morphology | SpeB | % Large
|
|
|---|---|---|---|---|
| 1 day | 10 days | |||
| 1 | Small | + | 0 | 5 |
| 2 | Small | + | 0 | 10 |
| 3 | Small | + | 0 | 2 |
| 4 | Large | − | 100 | 100 |
| 5 | Large | − | 100 | 100 |
| 6 | Large | − | 100 | 100 |
Single colonies were grown sequentially overnight in THB as stationary cultures and scored for capsule size by visual observation of colony morphology on Todd-Hewitt agar plates after 1 and 10 days of subculture in THB.
The resulting large colonies selected from the small colonies in these experiments retained their morphology and lack of secretion of cysteine protease on subculture on 10 additional occasions. This finding suggests that the cysteine protease-negative, large-colony phenotype is more stable. Growth of small colonies in the presence of E64 failed to convert them to large colony types. Similarly, incubation of large colonies with purified SpeB failed to convert them to small colonies. These results suggest that the association between capsule production and SpeB activity cannot be attributed to a direct effect of the enzyme activity on a capsule synthetic pathway but would be consistent with some form of coregulation of gene expression.
DISCUSSION
Regulation of expression of virulence factors by Streptococcus pyogenes is complex. In addition to multiple serotypic and genetic variation in group A isolates (7–12, 23), phenotypic variation can occur as a result of transcriptional control of individual virulence genes (16, 20, 38–40) as well as through posttranslational modification of exposed proteins by bacterial proteases like SpeB (6, 53, 58). SpeB expression can, in turn, be regulated by the rgg gene (16) and potentially influenced by the activity of the oligopeptide permease and transport mechanisms (36, 46). In addition, our laboratory has described a global regulating gene, pel, which can influence secretion of SpeB as well as influencing surface M and M-related proteins (32).
It is also clear from many in vitro and in vivo studies of group A isolates that either M protein or the hyaluronic acid capsule can play a direct role as a major virulence factor 2, 18, 41, 55, 56; C. D. Ashbaugh, M. H. Shearer, R. C. Kennedy, G. C. White, and M. R. Wessels, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. D/B-160, p. 240, 1999). Recent studies on the regulation of capsule synthesis by a number of investigators have identified a two-component regulatory system (1, 5, 20). This regulatory activity may also control additional phenotypic characteristics of the organism, possibly including SpeB (21). At this time, the nature of the sensory signal triggering the two-component system has not been described.
In this study we have observed variants of isolate 64 in which SpeB, M, and M-related proteins and capsular phenotype could vary. These variants were selected by passage of group A isolate 64 in human blood or mice. Although the precise biological pressure responsible for selecting the variant forms has not been identified, similar phenotypes were selected in each of the biological systems. The results summarized in Table 1 demonstrated that SpeB-positive variants expressed lower levels of Emm and Enn relative to Mrp, while the SpeB-negative variants showed approximately equivalent levels of all three IgG-binding molecules. Analysis of the differences indicates that SpeB could degrade the Emm and Enn proteins while having minimal effect on Mrp (Fig. 2). Comparison of SpeB-producing and nonproducing isolates also demonstrated significant differences in capsule morphology. SpeB-negative variants were associated with a larger capsule than their isogenic SpeB-positive variants (Fig. 3).
Taken together, all of our observations suggest that biological pressures in human blood or in a mouse can select for a phase variation that shifts a small-capsule, SpeB-positive bacterium into a large-capsule, SpeB-negative bacterium. The SpeB-negative large-colony variant of isolate 64 appears to be better adapted to survival against biological pressures in either human blood or in mice. Whether the small-colony variant has a superior adaptive capability within a different environment is not known.
Leonard et al. recently described small- and large-colony variants of M2 and M49 isolates (31). The small-colony types were associated with high-density, low-nutrient-flow conditions that exist in prolonged stationary-phase cultures. In their study, the small-colony variants were found to be deficient in Mga and gene products under its control. For isolate 64, small-colony variants produced equivalent or greater levels of transcript from genes in the mga region. Furthermore, the large-colony variants of the M2 and M49 isolates derived under nutrient-poor conditions produced SpeB, while the large-colony variants of 64, selected by biological pressure in human blood or in mice, failed to secrete either the zymogen or functionally active enzyme. In both cases, however, the large-colony variant appeared to be more stable than the small-colony variant under nutrient-rich conditions.
Analysis of the different variants in a mouse model of skin infection demonstrates that the SpeB-negative, large-capsule form is more invasive (52). Thus, SpeB expression is associated with decreased virulence potential in this system. Since SpeB can posttranslationally degrade surface M proteins on strain 64 (53), the decreased invasive potential may be indirectly attributable to the small amount of capsule or the loss of M protein, or possibly a combination of the two. Studies by Wessels and colleagues on the role of hyaluronic acid capsule or M protein expression in the virulence of isogenic streptococcal strains in mouse and baboon models are in basic concordance with the direction of virulence potentiation reported here (2, 41, 64; Ashbaugh et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). They reported that a large capsule and the presence of M protein both increase virulence potential. In the studies by Wessels and colleagues, the presence or absence of SpeB did not vary the virulence potential of an isolate.
Lukomski and colleagues (33–35) and others (29) also performed studies using SpeB-negative isogenic mutants and found that SpeB-expressing strains were more virulent than their SpeB-negative counterparts. Vaccination of mice with SpeB could also prevent subsequent death following a lethal infection in this case (26). In contrast with our findings and those of Wessels and colleagues (2, 41, 64; Ashbaugh et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.), the findings of Lukomski et al. suggested a role for SpeB in increasing the virulence potential of the associated strain. Lukomski et al. also demonstrated that differences between the wild type and an isogenic SpeB-negative mutant were dependent on the growth phase of the mutant used for infection (34).
Thus, there is contrasting evidence for the role of SpeB in virulence. This could reflect properties of the genetic background of the strains under study or differences in the susceptibility of virulence factors (e.g., M protein) to degradation by SpeB, potential differences in isoenzyme forms of SpeB itself (59), or subtle differences in the animal models (i.e., strains, site of infection, etc.) being analyzed.
Isolate 64 is an M-nontypeable isolate, and its mga regulon has a structure of a type called pattern D (8, 24, 25). Patterns in the mga regulon are based on characteristics of the genes clustered that reflect the evolution of this cluster by gene duplication (8). These patterns function as genetic markers for strains with known epidemiological properties: pattern A to C strains are highly associated with a throat tissue site of colonization, pattern D strains are highly associated with a skin tissue site of colonization, and pattern E strains are intermediate (24, 25). Thus, genetic pattern may mark lineages that are adaptive for a particular colonization site, although as yet particular properties that target a strain to a particular site are unknown.
Isolates in the pattern D group, like 64, are associated with impetigo and invasive skin infections (10–12). Although we have not examined the specific strains used by other investigators in previous studies, serotype M1 and M3 strains are generally pattern A and serotype M49 and M2 are generally pattern E. It is possible that the genetic background of strain 64 differs in substantial ways from backgrounds of the other strains in which SpeB expression and virulence have previously been tested. SpeB expression may have quite different consequences in this background and indeed appears to phase vary in the opposite direction from strains previously examined (31).
The association described between SpeB production, capsule, and the quantity and property of M and M-related proteins described here for isolate 64 following exposure to biological pressures that can be encountered during infection underscores the complexities of the pathogenic process. Thus, the relative contributions of different virulence factors should always be considered in a comprehensive manner, including a consideration of such variables as genetic background, capsule status, and potential for posttranslational modification by bacterial enzymes.
In this study, the large-colony SpeB phenotype forms was selected naturally in response to biological pressures and appeared to be more stable than the small-colony SpeB+ variant. This phase variation may be an important component of the dynamic host-pathogen interaction that determines whether a carrier, a local, or an invasive infection results and may in turn be influenced by the site of initial colonization by the bacteria.
ACKNOWLEDGMENTS
We thank Carol Hepner for typing the manuscript.
This work was supported by grant AI43474 from the National Institutes of Health.
REFERENCES
- 1.Alberti S, Ashbaugh C D, Wessels M R. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Mol Microbiol. 1998;28:343–353. doi: 10.1046/j.1365-2958.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh D D, Warren H B, Carey V J, Wessels M R. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Investig. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett A, Kembhavi J, Brown A A, Kirschke H, Knight C G, Tami M, Kanada K. l-trans-Epoxysuccinyl-leucylamido (4-guanidino) butane (E-64) and its analogues as inhibitors for cysteine proteinases including cathepsins B, H, and L. Biochem J. 1982;2301:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer E A, Wilchek M. Protein biotinylation. Methods Enzymol. 1990;185:138–160. doi: 10.1016/0076-6879(90)84268-l. [DOI] [PubMed] [Google Scholar]
- 5.Bernish B, van de Rijn I. Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J Biol Chem. 1999;274:4786–4793. doi: 10.1074/jbc.274.8.4786. [DOI] [PubMed] [Google Scholar]
- 6.Berge A, Björck L. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J Biol Chem. 1995;270:9862–9867. doi: 10.1074/jbc.270.17.9862. [DOI] [PubMed] [Google Scholar]
- 7.Bessen D E, Fischetti V A. Nucleotide sequences of two adjacent M or M-like protein genes of group A streptococci: different RNA transcript levels and identification of a unique immunoglobulin A-binding protein. Infect Immun. 1992;60:124–135. doi: 10.1128/iai.60.1.124-135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bessen D E, Hollingshead S K. Allelic polymorphism of emm loci provides evidence for horizontal gene spread in group A streptococci. Proc Natl Acad Sci USA. 1994;91:3280–3284. doi: 10.1073/pnas.91.8.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bessen D E, Veasy L G, Hill H R, Augustine N H, Fischetti V A. Serologic evidence for class I group A streptococcal infection among rheumatic fever patients. J Infect Dis. 1995;172:1608–1611. doi: 10.1093/infdis/172.6.1608. [DOI] [PubMed] [Google Scholar]
- 10.Bessen D E, Sotir C M, Readdy T L, Hollingshead S K. Genetic correlates of throat and skin isolates of group A streptococci. J Infect Dis. 1996;173:896–900. doi: 10.1093/infdis/173.4.896. [DOI] [PubMed] [Google Scholar]
- 11.Bessen D E, Fiorentino T R, Hollingshead S K. Molecular markers for throat and skin isolates of group A streptococci. Adv Exp Med Biol. 1997;418:537–543. doi: 10.1007/978-1-4899-1825-3_125. [DOI] [PubMed] [Google Scholar]
- 12.Bessen D E, Izzo M W, Fiorentino T R, Caringal R M, Hollingshead S K, Beall B. Genetic linkage of exotoxin alleles and emm gene markers for tissue tropism in group A streptococci. J Infect Dis. 1999;179:627–636. doi: 10.1086/314631. [DOI] [PubMed] [Google Scholar]
- 13.Boyle M D P, Hawlitzky J, Raeder R, Podbielski A. Analysis of the genes encoding two unique type IIa immunoglobulin G-binding proteins expressed by a single group A streptococcus. Infect Immun. 1994;62:1336–1347. doi: 10.1128/iai.62.4.1336-1347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns E H, Marciel A M, Musser J M. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chausee M S, Ajdic D, Ferretti J J. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect Immun. 1999;67:1715–1722. doi: 10.1128/iai.67.4.1715-1722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chausee M S, Phillips E R, Ferretti J J. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect Immun. 1996;65:1956–1959. doi: 10.1128/iai.65.5.1956-1959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cu G A, Mezzano S, Bannan J D, Zabriskie J B. Immunohistochemical and serological evidence for the role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Kidney Int. 1998;54:819–826. doi: 10.1046/j.1523-1755.1998.00052.x. [DOI] [PubMed] [Google Scholar]
- 18.Dale J B, Washburn R G, Marques M B, Wessels M R. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun. 1996;64:1495–1501. doi: 10.1128/iai.64.5.1495-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott S D. A proteolytic enzyme produced by group A streptococci with special reference to its effect on the type specific M antigen. J Exp Med. 1945;81:573–591. doi: 10.1084/jem.81.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federle M J, McIver K S, Scott J R. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J Bacteriol. 1999;181:3649–3657. doi: 10.1128/jb.181.12.3649-3657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heath A, DiRita V J, Barg N L, Engleberg N C. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect Immun. 1999;67:5298–5305. doi: 10.1128/iai.67.10.5298-5305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herwald H, Collin M, Werner-Esterl W, Björck L. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J Exp Med. 1996;184:1–9. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holm S E, Norby A, Bergholm A-M, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden 1988–1989. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Hollingshead S K, Readdy T L, Yung D L, Bessen D E. Structural heterogeneity of the emm gene cluster in group A streptococci. Mol Microbiol. 1993;8:707–717. doi: 10.1111/j.1365-2958.1993.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 25.Hollingshead S K, Arnold J, Readdy T L, Bessen D E. Molecular evolution of a multigene family in group A streptococci. Mol Biol Evol. 1994;11:208–219. doi: 10.1093/oxfordjournals.molbev.a040103. [DOI] [PubMed] [Google Scholar]
- 26.Kapur V, Maffei J T, Greer R S, Li L-L, Adams G J, Musser J M. Vaccination with streptococcal extracellular cysteine protease (interleukin 1β convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 27.Kapur V, Majesky M W, Li L-L, Black R A, Musser J M. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapur V, Topouzis S, Majesky M W, Li L L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 29.Kuo C-F, Wu J-J, Lin K-Y, Tsai P-J, Lee S-C, Jin Y-T, Lei H Y, Lin Y-S. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun. 1998;66:3931–3935. doi: 10.1128/iai.66.8.3931-3935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Leonard B A, Woischnik M, Podbielski A. Production of stabilized virulence factor-negative variants by group A streptococci during stationary phase. Infect Immun. 1998;66:3841–3847. doi: 10.1128/iai.66.8.3841-3847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Sledjeski D D, Kreikemeyer B, Podbielski A, Boyle M D P. Identification of pel, a Streptococcus pyogenes locus that affects both surface and secreted proteins. J Bacteriol. 1999;181:6019–6027. doi: 10.1128/jb.181.19.6019-6027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukomski S, Burns E H, Jr, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukomski S, Montgomery C A, Rurngirwa J, Geske R S, Barrish J P, Adams G J, Musser J M. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect Immun. 1999;67:1779–1788. doi: 10.1128/iai.67.4.1779-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukomski S, Sreevatsan S, Reichardt W, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Investig. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyon W R, Gibson C M, Caparon M. A role for trigger factor and an Rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 1998;17:6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malke H. Polymorphism of the SK gene: implications for the pathogenesis of post-streptococcal glomerulonephritis. Zentbl Bakteriol. 1993;278:246–257. doi: 10.1016/s0934-8840(11)80842-x. [DOI] [PubMed] [Google Scholar]
- 38.McIver K, Heath A S, Green B D, Scott J R. Specific binding of the activator Mga to promoter sequences of the emm and scpA genes in the group A streptococcus. J Bacteriol. 1995;177:6619–6624. doi: 10.1128/jb.177.22.6619-6624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIver K, Heath A S, Scott J R. Regulation of virulence by environmental signals in group A streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect Immun. 1995;63:4540–4542. doi: 10.1128/iai.63.11.4540-4542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIver K, Scott J R. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J Bacteriol. 1997;179:5178–5187. doi: 10.1128/jb.179.16.5178-5187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moses A E, Wessels M R, Zalcman K, Alberti S, Natanson-Yaron S, Menes T, Hanski E. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A streptococcus. Infect Immun. 1997;65:64–71. doi: 10.1128/iai.65.1.64-71.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.North M J. Cysteine endopeptidases of parasitic protozoa. Methods Enzymol. 1994;244:523–539. doi: 10.1016/0076-6879(94)44038-7. [DOI] [PubMed] [Google Scholar]
- 43.Okada N, Pentland A P, Falk P, Caparon M G. M protein and protein F act as important determinants of cell-specific tropism of streptococcus pyogenes in skin tissue. J Clin Investig. 1994;94:965–977. doi: 10.1172/JCI117463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinkney M, Kapur V, Smith J, Weller U, Palmer M, Glanville M, Messner M, Musser J M, Bhakdi S, Chow M A. Different forms of streptolysin O produced by Streptococcus pyogenes and by Escherichia coli expressing recombinant toxin: cleavage by streptococcal cysteine protease. Infect Immun. 1995;63:2776–2779. doi: 10.1128/iai.63.7.2776-2779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Podbielski A, Schnitzler N, Beyhs P, Boyle M D P. M-related protein (Mrp) contributes to group A streptococcal resistance to phagocytosis by human granulocytes. Mol Microbiol. 1996;19:429–441. doi: 10.1046/j.1365-2958.1996.377910.x. [DOI] [PubMed] [Google Scholar]
- 46.Podbielski A, Leonard B A. The group A streptococcal dipeptide permease (Dpp) is involved in the uptake of essential amino acids and affects the expression of cysteine protease. Mol Microbiol. 1998;28:1323–1334. doi: 10.1046/j.1365-2958.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 47.Podbielski A, Hawlitzky J, Pack T D, Flosdorff A, Boyle M D P. A group A streptococcal Enn protein potentially resulting from intergenomic recombination exhibits atypical immunoglobulin-binding characteristics. Mol Microbiol. 1994;12:725–736. doi: 10.1111/j.1365-2958.1994.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 48.Raeder R, Otten R A, Chamberlin L, Boyle M D P. Functional and serological analysis of type II IgG-binding proteins expressed by pathogenic group A streptococci. J Clin Microbiol. 1992;30:3074–3081. doi: 10.1128/jcm.30.12.3074-3081.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raeder R, Boyle M D P. Association of type II immunoglobulin G-binding protein expression and survival of group A streptococci in human blood. Infect Immun. 1993;61:3696–3702. doi: 10.1128/iai.61.9.3696-3702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raeder R, Boyle M D P. Association between expression of immunoglobulin G-binding proteins by group A streptococci and virulence in a mouse skin infection model. Infect Immun. 1993;61:1378–1384. doi: 10.1128/iai.61.4.1378-1384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raeder R, Boyle M D P. Distinct profiles of IgG-binding expression by invasive M1 serotype isolates of Streptococcus pyogenes. Clin Diagn Lab Immunol. 1995;2:478–483. doi: 10.1128/cdli.2.4.478-483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raeder R, Boyle M D P. Properties of IgG-binding proteins expressed by Streptococcus pyogenes isolates are predictive of invasive potential. J Infect Dis. 1996;173:888–895. doi: 10.1093/infdis/173.4.888. [DOI] [PubMed] [Google Scholar]
- 53.Raeder R, Woischnik M, Podbielski A, Boyle M D P. A secreted streptococcal cysteine protease can cleave a surface expressed M1 protein and alter the immunoglobulin-binding properties. Res Microbiol. 1998;149:539–548. doi: 10.1016/s0923-2508(99)80001-1. [DOI] [PubMed] [Google Scholar]
- 54.Reis K J, Yarnall M, Ayoub E M, Boyle M D P. Effect of mouse passage on Fc receptor expression by group A streptococci. Scand J Immunol. 1984;20:433–439. doi: 10.1111/j.1365-3083.1984.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 55.Schrager H M, Rheinwald J G, Wessels M. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Investig. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrager H M, Alberti S, Cywes C, Dougherty G J, Wessels M R. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A streptococcus to CD44 on human keratinocytes. J Clin Investig. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlievert P M, Assimacopoulos A P, Cleary P P. Severe invasive group A streptococcal disease: clinical description and mechanisms of pathogenesis. J Lab Clin Med. 1996;127:13–22. doi: 10.1016/s0022-2143(96)90161-4. [DOI] [PubMed] [Google Scholar]
- 58.Shanley T P, Schrier D, Kapur V, Kehoe M, Musser J M, Ward P A. Streptococcal cysteine protease augments lung injury induced by products of group A streptococci. Infect Immun. 1996;64:870–877. doi: 10.1128/iai.64.3.870-877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stockbauer K E, Magoun L, Liu M, Burns E H, Jr, Gubba S, Renish S, Pan X, Bodary S C, Baker E, Coburn J, Leong J M, Musser J M. A natural variant of the cysteine protease virulence factor of group A Streptococcus with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins αvβ3 and αIIbβ3. Proc Natl Acad Sci USA. 1999;96:242–247. doi: 10.1073/pnas.96.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talkington D F, Schwartz B, Black C M, Todd J K, Elliot J, Breiman R F, Facklam R R. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of toxic shock syndrome. Infect Immun. 1993;61:3369–3374. doi: 10.1128/iai.61.8.3369-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai P J, Juo C F, Lin K Y, Lin Y S, Lei H Y, Chen F F, Wang J R, Wu J J. Effect of group A streptococcal cysteine protease on invasion of epithelial cells. Infect Immun. 1998;66:1460–1466. doi: 10.1128/iai.66.4.1460-1466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van-Heyningen T, Fogg G, Yates D, Yanski E, Caparon M. Adherence and fibronectin-binding are environmentally regulated in the group A streptococcus. Mol Microbiol. 1993;9:1213–1222. doi: 10.1111/j.1365-2958.1993.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 64.Wessels M R, Moses A E, Goldberg J B, DiCesare T J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolf B B, Gibson C A, Kapur V, Hussaine I M, Musser J M, Gonias S L. Proteolytically active streptococcal pyrogenic exotoxin B cleaves monocytic cell urokinase receptor and releases an active fragment of the receptor from the cell surface. J Biol Chem. 1994;268:30682–30687. [PubMed] [Google Scholar]
- 66.Yung D-L, Hollingshead S K. DNA sequencing and gene expression of the emm gene cluster in an M50 group A streptococcus strain virulent for mice. Infect Immun. 1996;64:2193–2200. doi: 10.1128/iai.64.6.2193-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]