Abstract
The interaction of commensal bacteria with immunocompetent cells may occur in definite compartments of the mucosal immune system, as limited translocation through the epithelial barrier cannot be excluded. In this study the stimulation of human peripheral blood mononuclear cells and purified lymphocyte subsets by nonpathogenic gram-positive lactobacilli (Lactobacillus johnsonii and Lactobacillus sakei) and gram-negative Escherichia coli was investigated. The various bacterial strains induced a differential cytokine pattern. Whereas L. johnsonii and L. sakei strongly induced gamma interferon (IFN-γ) and interleukin-12 (IL-12), E. coli and lipopolysaccharide (LPS) preferentially induced IL-10 after 16 h of stimulation. Expression of activation antigens CD69 and CD25 was observed on (CD3− CD56+) natural killer (NK) cells after stimulation of total human peripheral blood mononuclear cells. All bacteria mediated the proliferation of human peripheral blood mononuclear cells, and the strongest proliferative response was observed with L. johnsonii. Purified CD4+, CD8+, and CD19+ lymphocyte subsets were not activated upon bacterial stimulation but showed normal response to a mitogenic stimulus. In contrast, purified NK cells upregulated the IL-2Rα chain (CD25) and underwent proliferation when stimulated by L. johnsonii. E. coli and LPS were less effective in inducing proliferation. Expression of CD25 or secretion of IFN-γ from purified NK cells was significantly increased in the presence of bacterially primed macrophages, indicating that full activation required both bacterium- and cell contact-based signals derived from accessory cells.
The microflora is essential for immune education and amplification of lymphoid effector cells, mainly at the mucosal level. It is well documented that the efficacy of the mucosal immune system can be significantly impaired in germ-free animals (3, 20). Lactobacilli are members of the normal indigenous microflora (8, 23), and certain strains of Lactobacillus spp. have been used as probiotics (16). Recently, the modulation of human innate immune defenses following ingestion of specific lactic acid bacteria (LAB) was reported (25, 35, 37). Although the interaction between commensal, nonpathogenic bacteria and blood leukocytes seems to be an unusual event, it might occur in definite microenvironments of the mucosal immune system. The M-cell pockets harbor different types of immunocompetent cells, and a limited bacterial translocation through the epithelial barrier cannot be excluded (6, 32). The capacity to translocate enables bacteria to interact with cells of the immune system.
The investigation of interactions of nonpathogenic bacteria with leukocytes in vitro is of particular interest (i) to evaluate the immunomodulatory capacity of intestinal or food-fermenting bacteria on blood leukocytes and (ii) to investigate if a distinct pattern of immunomodulation can be established for different components of the microflora. In vitro, several species of lactobacilli have been shown to induce cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12) in human peripheral blood mononuclear cells (PBMC) (18, 28). IL-12, like gamma interferon (IFN-γ), is an important cytokine implicated in innate defense mechanisms in response to bacteria. Early production of IL-12 by macrophages contributes to effector cell maturation for both natural killer (NK) cells and CD8+ T cells, leading to a Th1-biased adaptive immune response (4, 5, 17, 26). The majority of circulating NK cells are CD3− CD56+ CD16+ (80 to 90%); a minority are CD3− CD56+ CD16− (19, 22). Morphologically they are very large granular lymphocytes and have the ability to migrate into tissues. The vast majority of NK cells are found in the liver, but they can also invade mucosal sites (34). NK cells provide a first line of defense against tumors, viral infections, and intestinal pathogens and thus have a key role in innate immunity (4, 21, 26).
This study provides data on the immunostimulatory effect of the nonpathogenic Escherichia coli and Lactobacillus (LAB) strains of human intestinal or fermented food origin, such as Lactobacillus johnsonii La 1 and Lactobacillus sakei LTH 681, on human peripheral blood mononuclear cells (PBMC) and purified leukocyte supopulations (CD4+, CD8+, CD19+, and CD56+ cells). We present results for a different cytokine patterns induced by gram-negative and gram-positive bacteria and show evidence that NK cells constitute primary targets for bacterially mediated activation.
MATERIALS AND METHODS
Bacteria.
The gram-negative, nonpathogenic E. coli K-12 LTH 634 (strain collection of the Institute of Food Technology, University of Hohenheim) was grown in brain heart infusion broth at 37°C. The gram-positive L. johnsonii La 1 (Nestlé strain collection) of human intestinal origin was cultivated in MRS broth (7) without acetate at 37°C. L. sakei LTH 681, isolated from fermented food, was grown in the same broth at 30°C. All bacteria were harvested by centrifugation (3,000 × g, 15 min) at stationary growth phase (24 h). Bacteria were washed three times with phosphate-buffered saline (PBS) (1×, pH 7.2; Gibco BRL) and subsequently diluted to final concentrations of 105 and 106 CFU/ml in RPMI 1640 (Gibco BRL) medium containing 20% native human AB serum (Sigma). Bacteria were either heat killed (100°C, 30 min) or used as live cells.
Isolation of human PBMC.
Human PBMC were purified from buffy coats (Blood Transfusion Centre, Lausanne, Switzerland) using Ficoll-Hypaque (1077; Pharmacia) gradient centrifugation. PBMC were harvested from the interface, washed five times with RPMI 1640, and diluted in RPMI 1640 containing 20% native human AB serum to a final concentration of 2 × 106/ml.
Purification of lymphocyte subpopulations.
PBMC were incubated in RPMI 1640–20% fetal calf serum (FCS) for 1.5 h at 37°C and 5% CO2 on 225-cm2 tissue culture plates (Costar) to allow adherence. Nonadherent peripheral blood lymphocytes (PBL) were separated from adherent cells by aspiration. Adherent cells were gently washed three times with prewarmed culture medium, harvested by using a rubber policeman (Costar), and used as monocytes/macrophages. Thereafter, PBL were washed with RPMI 1640. CD4+, CD8+, and CD19+ lymphocyte subsets were purified from PBL by magnetic cell sorting (MACS) using a positive selection technique. Briefly, the PBL suspension was labeled with CD4+, CD8+, and CD19+ MACS microbeads (Miltenyi Biotec) for 10 min at 4°C, washed once with 1× PBS–5% FCS, and dissolved in 1 ml of PBS–5% FCS. Labeled cells were then transferred to a high-gradient magnetic separation column placed in a magnetic field. After four washings with PBS–5% FCS, positive labeled cells remained on the column while nonlabeled cells passed through. After removal of the columns from the magnetic field, the labeled cells were recovered by elution. NK cells were isolated by an indirect magnetic labeling system according to the supplier's protocol (Miltenyi Biotec). The purity was controlled by flow cytometry analysis (FACScan; Becton Dickinson) analysis using fluorescein isothiocyanate (FITC)-phycoerythrin (PE)-labeled anti-CD4/CD8 (Becton Dickinson) monoclonal antibody (MAb), FITC-labeled anti-CD19 MAb (Becton Dickinson), FITC-labeled anti-CD56 MAb (Becton Dickinson), and PE-labeled anti-CD3 MAb (Becton Dickinson). The purities of isolated lymphocyte subpopulations ranged between 94 and 98%. Finally, purified lymphocyte subsets were diluted in cell culture medium as described above to a final density of 2 × 106/ml.
Stimulation of PBMC or lymphocyte subsets.
Freshly isolated PBMC or purified lymphocyte subsets were seeded at 2 × 106/ml (2 ml) into six-well tissue culture plates, and 2 ml of bacterial suspension was added to each well. For the stimulation, the final ratio between PBMC and bacteria (live or heat-killed) was 1:1 or 10:1. For control treatment, lipopolysaccharide (LPS; E. coli serotype O55:B5; 1 μg/ml; Sigma) or culture medium alone was added to the PBMC suspension. To determine the cytokine expression by PBMC, the samples were incubated in the absence of antibiotics for 2, 6, and 16 h at 37°C and 5% CO2. Subsequently, PBMC were collected, washed in cold PBS, and centrifuged and the cell pellet was lysed in guanidinium isothiocyanate denaturation solution. Cellular lysates were kept at −20°C until further processing. Cell culture supernatants were collected separately and kept at −20°C for cytokine analysis using the enzyme-linked immunosorbent assay (ELISA) technique. For flow cytometric analysis, PBMC were challenged by bacterial suspensions, LPS (E. coli serotype O55:B5; 1 μg/ml; Sigma), or phytohemagglutinin (PHA; 10 μg/ml; Sigma) for 3 and 5 days in the presence of gentamicin (125 μg/ml; Gibco BRL).
Expression of activation antigens on PBMC or lymphocyte subsets.
PBMC, purified CD4+, CD8+, and CD19+ lymphocytes, and CD3− CD56+ NK cells (106 cells/ml) were stimulated for 3 or 5 days with live bacteria (106 cells/ml), PHA (10 μg/ml), or LPS (1 μg/ml) as described above. To prevent bacterial overgrowth, gentamicin (125 μg/ml) was added to the culture medium. Thereafter, cells were washed (Cell Wash; Becton Dickinson) and double stained with directly conjugated MAb CD69-FITC or CD25-FITC and CD4− CD8− CD19− CD56− PE (Becton Dickinson) for 30 min on ice, washed two times, centrifuged, and resuspended in Cell Wash (Becton Dickinson). The percentage of CD69- or CD25-positive cells within the lymphocyte subpopulations was compared to that for nontreated cells using flow cytometry (FACScan; Becton Dickinson).
Priming of monocytes with nonpathogenic bacteria.
Monocytes/macrophages were incubated with 106 CFU of live E. coli, L. johnsonii, or L. sakei/ml for 12 h, harvested by scraping, and washed three times with PBS buffer. Cell viability was controlled by trypan blue exclusion and was generally >90%.
Cocultivation of purified NK cells with primed macrophages.
Purified CD56+ CD3− NK cells (106/ml) were incubated in the presence of gentamicin (125 μg/ml) with (i) bacterially primed monocytes (5 × 105 cells/ml), (ii) a mixture of nonprimed monocytes (5 × 105 cells/ml) and live bacteria (106 CFU/ml), or (iii) bacteria alone (106 CFU/ml). Incubation of NK cells and untreated monocytes (5 × 105 cells/ml) served as a control. To characterize to what extent cell-to-cell interaction is involved in the activation of NK cells by nonpathogenic bacteria, NK cells were coincubated with bacterially primed monocytes separated by cell culture inserts (pore size, 0.4 mm). After 3 and 5 days of stimulation, the percentage of CD69- or CD25-positive NK cells in the basolateral compartment was compared to that for noncocultured NK cells using flow cytometry. Supernatants were frozen for ELISA analysis.
RNA extraction and amplification by RT-PCR.
Total RNA from PBMC or leukocyte subpopulations was isolated using the acid guanidinium thiocyanate-phenol-chloroform method (Micro RNA isolation kit; Stratagene). cDNA was synthesized from RNA by reverse transcription (RT) of 0.5 μg of total RNA at 42°C for 30 min using specific 3′ priming, a 1 mM concentration of each deoxynucleoside triphosphate, and 2.5 U of murine leukemia virus reverse transcriptase (Perkin-Elmer)/ml. PCR amplification was performed using Taq polymerase (Perkin-Elmer) and specific primers coding for the human cytokines TNF-α, IL-12, IL-10, IFN-γ, and β-actin in a total volume of 50 μl. Thermocycles were run at 94°C (1 min), 94°C (1 min), 60°C (1 min), and 72°C (1 min) for 30 cycles. PCR products were analyzed on 2% agarose gels. The oligonucleotide primers used were as follows: TNF-α (5′), 5′-CAGAGGGAAGAGTTCCCCAG-3′ and (3′) 5′-CCTTGGTCTGGTAGGAGACG-3′ (product length, 324 bp); IL-12 p40 (5′), 5′-CGTAGAATTGGATTGGTATCCGG-3′ and (3′) 5′-GCTCTTGCCCTGGACCTGAACGC-3′ (product length, 702 bp); IFN-γ (5′), 5′-ATATCTTGGCTTTTCAGCTC-3′ and (3′) 5′-CTCCTTTTTCGCTTCCCTGT-3′ (product length, 489 bp); IL-10 (5′), 5′-TGATGTCTGGGTCTTGGTTC-3′ and (3′) 5′-GCCTAACATGCTTCGAGATC-3′ (product length, 204 bp); and β-actin (5′), 5′-GGCGACGAGGCCCAGGAGCAAGAGAGGCATC-3′ and (3′) 5′-CGATTTCCCGCTCGGCCGTGGTG-GTGAAGC-3′ (product length, 460 bp).
Proliferation assay.
PBMC or purified lymphocyte subsets were diluted in complete RPMI 1640 medium to a final concentration of 105/ml. The PBMC suspension was then transferred to 96-well flat-bottom culture plates (Costar), and 100 μl of RPMI 1640 medium containing 2 × 104 to 2 × 107 CFU of bacteria/ml, LPS (2 μg/ml), or PHA (20 μg/ml) was added. Cells were stimulated in the presence of gentamicin (125 μg/ml) for 3 and 5 days at 5% CO2 and 37°C. Finally, the cells were pulsed with 1 μCi of [3H]thymidine for 18 h before being harvested on filter mats. Analysis of [3H]thymidine incorporation was by liquid scintillation counting (TopCount; Packard).
ELISA.
Cytokine concentration in cell culture supernatants (IFN-γ, IL-10, IL-12 p70, and TNF-α) was determined after 16 h of bacterial stimulation using ELISA (ImmunoKontact). Dose-response experiments performed for each cytokine indicated that maximal secretion was obtained with 106 CFU of bacteria/ml, corresponding to a ratio of 1:1 (bacteria to PBMC). This concentration was used in further experiments.
Statistics.
Values are given as the means of triplicate measurements ± standard deviations (SD). Results were confirmed for at least three different blood donors in independent experiments. The significance was tested by applying the Mann-Whitney U test.
RESULTS
Gram-positive and gram-negative nonpathogenic bacteria differentially induce cytokine expression in human PBMC.
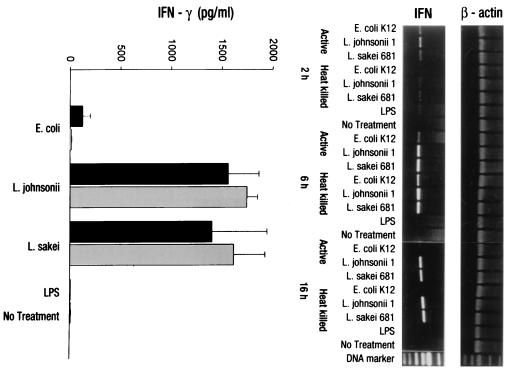
Freshly isolated PBMC were stimulated by nonpathogenic gram-positive L. johnsonii or L. sakei or gram-negative E. coli at a ratio of 1:1 (live or heat-killed bacteria to PBMC) for 2, 6, and 16 h in RPMI 1640–10% human AB serum. No bacterial overgrowth of the antibiotic-free cultures was detected at the given time point as controlled by the plating or nonacidification of the culture medium (phenol red indicator). RT-PCR analysis of total RNA extracted from PBMC after stimulation showed an early induction (2 h) of gene transcripts for IFN-γ by both Lactobacillus strains. The level of induction was increased within 6 h and stayed high at 16 h. In contrast, the E. coli-mediated IFN-γ mRNA induction was very low at the early time points and was completely abrogated at 16 h. LPS did not induce IFN-γ mRNA at any time point investigated (Fig. 1, right). The amount of IFN-γ secreted into cell culture supernatants was determined after 16 h of bacterial stimulation using ELISA techniques. The predominant induction of IFN-γ in human PBMC by the two LAB strains was also confirmed at the secretory level, where significant amounts of IFN-γ (1,500 pg/ml) were detectable in cell culture supernatants compared to the amounts induced in E. coli- (200 pg/ml) or LPS-stimulated PBMC. Soluble LPS was unable to induce IFN-γ, confirming the results obtained by RT-PCR analysis (Fig. 1, left).
FIG. 1.
Expression of IFN-γ by PBMC upon stimulation with nonpathogenic bacteria. RT-PCR and ELISA analyses were used to determine IFN-γ expression by PBMC (106/ml) upon stimulation with heat-killed (gray bars) and live (black bars) bacterial cells (106 CFU/ml) of E. coli, L. johnsonii, or L. sakei or LPS (1 μg/ml). Gene transcription (IFN-γ, IL-12 p40) was determined after 2, 6, and 16 h. Protein secretion was analyzed after 16 h of stimulation. No antibiotics were added to the cultures. Values are means ± SD of triplicate measurements and represent one of three independent experiments.
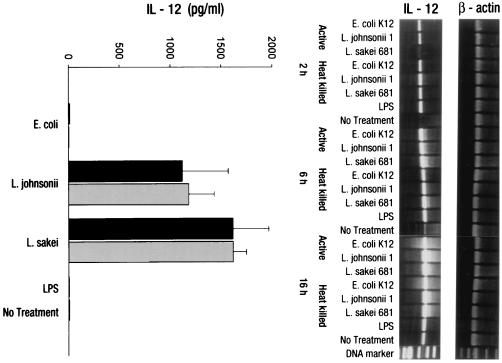
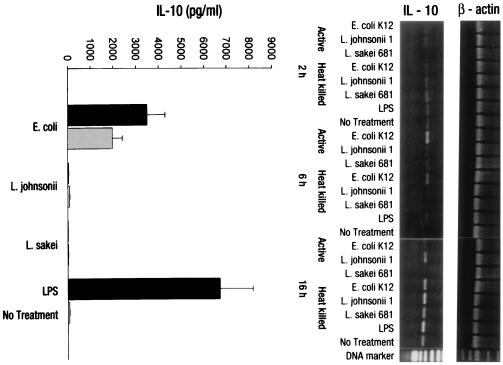
In contrast to IFN-γ, IL-12 p40 mRNA was strongly induced by all bacterial treatments after 6 and 16 h, with higher levels of induction for lactobacilli. E. coli and LPS, although weaker, induced significant IL-12 p40 mRNA levels compared to the untreated control (Fig. 2, right). However, IFN-γ secretion was exclusively induced by LAB. Neither E. coli nor soluble LPS (1 μg/ml) induced any secretion of IL-12 p70 above background levels (Fig. 2, left). A distinct induction of IL-10 mRNA was observed only at 6 h with the E. coli treatment. At 16 h, IL-10 mRNA was induced by all bacteria except L. sakei. Notably, IL-10 transcripts were also expressed in untreated PBMC. The analysis of secreted IL-10 in cell culture supernatants provided more conclusive results: only the gram-negative E. coli and gram-negative bacterium-derived soluble factor LPS (P < 0.001) were potent stimulators of IL-10 secretion, probably by direct activation of macrophages (Fig. 3). Whereas for the other cytokines no differences in the stimulatory activities of live and heat-killed bacteria used at the same concentration could be observed, IL-10 secretion by PBMC was significantly greater with live than with heat-killed E. coli (P < 0.01). No differences in the amounts of TNF-α production (7,000 pg/ml for bacteria, 3,500 pg/ml control) following the stimulation of PBMC with the different bacterial strains could be observed (data not shown).
FIG. 2.
Expression of IL-12 by PBMC upon stimulation with nonpathogenic bacteria. RT-PCR and ELISA analyses were used to determine IL-12 expression by PBMC (106/ml) upon stimulation with heat-killed (gray bars) and live (black bars) bacterial cells (106 CFU/ml) of E. coli, L. johnsonii, or L. sakei or LPS (1 μg/ml). Gene transcription (IL-12 p40) was determined after 2, 6, and 16 h. Protein secretion (IL-12 p70) was analyzed after 16 h of stimulation. No antibiotics were added to the cultures. Values are means ± SD of triplicate measurements and represent one of three independent experiments.
FIG. 3.
Expression of IL-10 by PBMC upon stimulation with nonpathogenic bacteria. RT-PCR and ELISA analyses were used to determine IL-10 expression by PBMC (106/ml) upon stimulation with heat-killed (gray bars) and live (black bars) bacterial cells (106 CFU/ml) of E. coli, L. johnsonii, or L. sakei or LPS (1 μg/ml). Gene transcription was determined after 2, 6, and 16 h. Protein secretion was analyzed after 16 h of stimulation. No antibiotics were added to the cultures. Values are means ± SD of triplicate measurements and represent one of three independent experiments.
Expression of activation antigens CD69 and CD25 (IL-2Rα chain) on PBMC upon bacterial challenge.
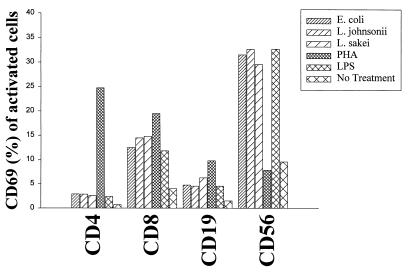
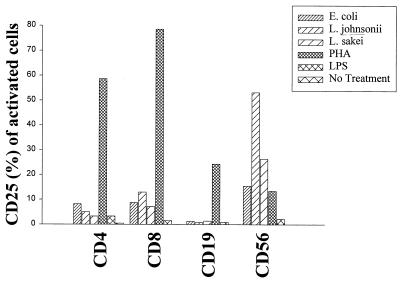
CD69, a marker of leukocyte activation, was determined on lymphocyte subsets (CD4+, CD8+, and CD19+) and natural killer (NK) cells (CD3− CD56+) at day 3 after stimulation of bulk cultures (gentamicin at 125 μg/ml) using flow cytometry. All bacterial strains (106 CFU/ml; live cells) and LPS (1 μg/ml) induced CD69 expression preferentially on NK cells (<30% CD69+ cells) and to a lesser extent on CD8+ cells (12 to 15%). CD4+ and CD19+ cells remained unresponsive to bacterial stimulation. The mitogenic stimulus PHA (10 μg/ml) preferentially activated CD4+ and CD8+ T cells, demonstrating the general responsiveness of T lymphocytes (Fig. 4). The induction of the IL-2Rα chain (CD25) constitutes an important step in the generation of high-affinity IL-2R, necessary to enter cell cycling, followed by proliferation. As for the expression of CD69, we detected the expression of CD25 after bacterial treatment only on NK cells at day 5 poststimulation. The highest level of expression was observed with L. johnsonii (P < 0.05 compared to that for E. coli). T and B cells remained unresponsive to bacterial activation, although the response to PHA was normal (Fig. 5).
FIG. 4.
Expression of the cellular activation antigen CD69 on lymphocyte subsets (CD4+, CD8+, and CD19+) and NK cells (CD3− CD56+). PBMC (106/ml) were stimulated with live E. coli, L. johnsonii, and L. sakei cells (106 CFU/ml) and LPS (1 μg/ml). PHA (10 μg/ml) and culture medium were used as controls. FACS analysis was performed to determine CD69 expression on lymphocyte subsets (CD4+, CD8+, and CD19+) and NK cells (CD3− CD56+) after 3 days of stimulation. Gentamicin (125 μg/ml) was added to the cultures. Values are means of duplicate measurements and represent one of three independent experiments.
FIG. 5.
Expression of the cellular activation antigen CD25 (IL-2Rα chain) on lymphocyte subsets (CD4+, CD8+, and CD19+) and NK cells (CD3− CD56+). PBMC (106/ml) were stimulated with live E. coli, L. johnsonii, and L. sakei cells (106 CFU/ml) and LPS (1 μg/ml). PHA (10 μg/ml) and culture medium were used as controls. FACS analysis was performed to determine CD69 expression on lymphocyte subsets (CD4+, CD8+, and CD19+) and NK cells (CD3− CD56+) after 5 days of stimulation. Gentamicin (125 μg/ml) was added to the cultures. Values are means of duplicate measurements and represent one of three independent experiments.
Bacterially mediated induction of proliferation in human PBMC and purified NK cells.
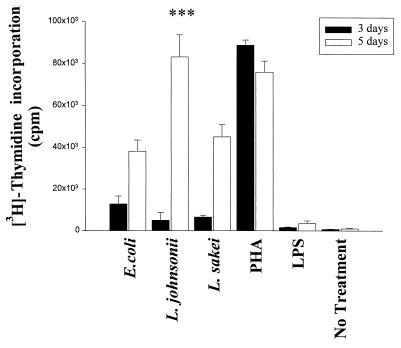
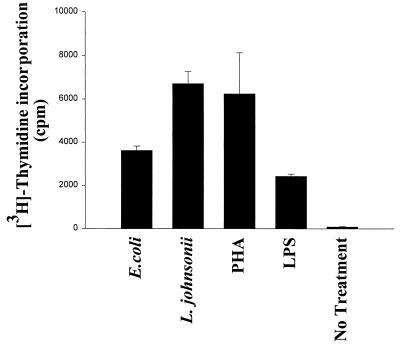
Proliferation of PBMC was determined after 3 and 5 days of stimulation with LAB strains, E. coli, LPS (1 μg/ml), or PHA (10 μg/ml) by measuring [3H]thymidine incorporation. As shown in Fig. 6, all bacteria (live cells) induced significant proliferation of PBMC after 5 days of culture. L. johnsonii was the strongest effector, producing levels comparable with the levels obtained with the mitogenic stimulus. For the stimulation of lymphocyte subsets L. johnsonii and E. coli were chosen as representative gram-positive and gram-negative strains, respectively. Stimulation of purified CD4+, CD8+, and CD19+ lymphocytes did not result in proliferation with any of these strains (data not shown). However, purified CD3− CD56+ NK cells proliferated upon bacterial stimulation for 5 days, although the proliferative response was 10 times lower than that for total PBMC, suggesting that (i) only a subpopulation of NK cells was stimulated by bacteria or LPS or (ii) stimulation with bacteria alone was suboptimal. L. johnsonii had a significantly greater stimulatory effect than E. coli and LPS (P < 0.05) (Fig. 7). Experiments performed with heat-killed bacteria in the absence of gentamicin exhibited results similar to those for live bacteria (data not shown).
FIG. 6.
Proliferative response of PBMC. PBMC (106/ml) were stimulated with live E. coli, L. johnsonii, and L. sakei cells (106 CFU/ml) and LPS (1 μg/ml). PHA (10 μg/ml) and culture medium were used as controls. Proliferation was indicated by [3H]thymidine (1 μg/well) uptake after 3 and 5 days of stimulation. Gentamicin (125 μg/ml) was added to the cultures. Values are means ± SD obtained in triplicate. ∗∗∗, P < 0.001.
FIG. 7.
Proliferative response of purified NK cells (CD3− CD56+). Purified NK cells (106/ml) were stimulated with live E. coli and L. johnsonii cells (106 CFU/ml) and LPS (1 μg/ml). PHA (10 μg/ml) and culture medium were used as controls. Proliferation was indicated by [3H]thymidine (1 μg/well) uptake after 5 days of stimulation. Gentamicin (125 μg/ml) was added to the cultures. Values are means ± SD obtained in triplicate.
Activation of human NK cells by bacteria requires direct contact and is dependent on the presence of accessory cells.
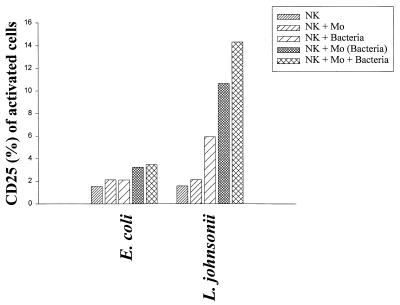
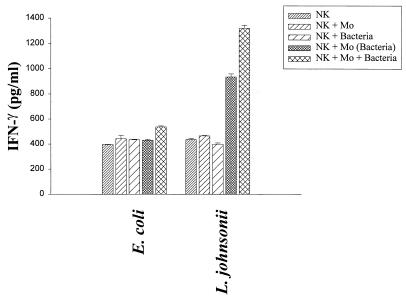
The results for activation antigens and proliferation demonstrated that NK cells can in fact be partially activated by direct contact to bacteria. However, the weak proliferative response observed in the purified NK cell population could reflect the need for accessory cells, such as macrophages, which were present in the PBMC bulk cultures. Therefore, to study the dependence of NK cell activation by bacteria on accessory cells, purified CD3− CD56+ NK cells (106/ml) were stimulated (i) directly with L. johnsonii or E. coli (106 CFU/ml), (ii) with macrophages previously primed (12 h) with the same bacterial strains, and (iii) with a mixture of macrophages and bacteria. Finally, the percentages of activated NK cells expressing CD25 were determined after 3 and 5 days of culture (gentamicin at 125 μg/ml) (Fig. 8). Whereas E. coli did not stimulate NK cells above the levels of control treatments (untreated cells, NK cells, and macrophages), L. johnsonii alone induced suboptimal expression of CD25 on NK cells. This expression was increased in the presence of macrophages (primed or in combination with live bacteria). Selective activation of NK cells by L. johnsonii could be further documented by the production of IFN-γ following stimulation with primed macrophages (936 pg/ml) or macrophages and live bacteria (1,321 pg/ml). In contrast, E. coli remained unable to stimulate IFN-γ secretion in NK-macrophage cocultures above control levels (400 pg/ml). It is noteworthy that the induction of the cytokine response was dependent on the presence of macrophages and bacteria, as direct stimulation of NK cells with bacteria alone did not result in IFN-γ secretion (Fig. 9). Separation of primed macrophages and bacteria from NK cells by filter inserts did not result in the induction of CD25 on NK cells, demonstrating that activation of NK cells is not exclusively based on monocyte-derived cytokines but requires cell-to-cell contact (data not shown). These results clearly demonstrate that activation of NK cells by nonpathogenic bacteria required the help of accessory, antigen-presenting cells, such as macrophages.
FIG. 8.
Expression of the cellular activation antigen CD25 (IL-2Rα chain) on purified NK cells (CD3− CD56+). Purified NK cells (106/ml) were incubated with (i) nonprimed macrophages (Mo) (5 × 105/ml), (ii) live bacteria (106 CFU/ml; E. coli or L. johnsonii), (iii) bacterially primed macrophages, or (iv) a mixture of bacteria and macrophages. FACS analysis was performed to determine CD25 expression on lymphocyte NK cells (CD3− CD56+) after 5 days of stimulation. Gentamicin (125 μg/ml) was added to the cultures. Values are means of duplicate measurements and represent one of three independent experiments.
FIG. 9.
Secretion of IFN-γ by purified NK cells (CD3− CD56+). Purified NK cells (106/ml) were incubated with (i) nonprimed macrophages (Mo) (5 × 105/ml), (ii) bacteria (106 CFU/ml; E. coli or L. johnsonii), (iii) bacterially primed macrophages, or (iv) a mixture of bacteria and macrophages. ELISA analysis was performed to determine IFN-γ secretion by purified NK cells (CD3− CD56+) after 5 days of stimulation. Gentamicin (125 μg/ml) was added to the cultures. Values are means of triplicate measurements and represent one of three independent experiments.
DISCUSSION
The present study on the direct interaction of nonpathogenic bacteria with human PBMC is based on the assumption that bacteria and immunocompetent cells may physically interact in definite mucosal environments. The epithelial compartment, the lamina propria, and M-cell pockets are potential sites where commensal, nonpathogenic bacteria may encounter immunocompetent cells. It is well documented that M cells promote the interaction between luminal antigens, including bacteria, and immunocompetent cells (32). The occurrence of limited bacterial translocation to the lamina propria in humans has also been reported (6, 38). Although PBMC are only partially representative of immunocompetent cells in intestinal mucosal compartments, phenotypical similarities with respect to the germ line-encoded receptors involved in the recognition of bacterial antigens on lymphocytes and macrophages, such as pattern recognition receptors (27, 39), could constitute the link between both populations and thus may provide important indications of the functional aspects of the mucosal immune response to luminal bacteria. We showed that gram-positive and gram-negative nonpathogenic bacteria induced different cytokine patterns in human PBMC. Whereas all bacteria induced TNF-α secretion, differences with respect to the induction of the Th1-like cytokines IL-12 and IFN-γ and the inhibitory cytokine IL-10 were observed. L. johnsonii and L. sakei strongly induced IFN-γ and IL-12 but not IL-10. In contrast, the gram-negative E. coli and the gram-negative bacterium-derived soluble immunomodulator LPS stimulated preferentially the synthesis of IL-10 and had little or no capacity to induce IFN-γ or IL-12 in human PBMC. These results are in agreement with reports by Miettinen et al. (28) and Muller-Alouf et al. (31) comparing different nonpathogenic and pathogenic gram-positive bacteria with respect to the induction of cytokines in PBMC. These in vitro data may also reflect the common immunosuppression observed in patients undergoing endotoxemia (2, 14).
Our interest was then focused on the identification of the cellular subpopulations activated by different bacterial strains. The analysis of activation antigens CD69 and CD25 (IL-2Rα chain) in stimulated PBMC bulk cultures indicated that only NK cells upregulating both markers were activated by bacterial treatment, although the PHA control suggested that all lymphocyte subsets (CD4+, CD8+, and CD19+) responded normally to a mitogenic stimulus. Low expression of CD69 on CD8+ cells after bacterial treatment could be attributed to a contamination with CD8dim NK cells rather than a specific activation of CD3+ CD8+ T cells.
Proliferation of PBMC following bacterial stimulation was demonstrated for all bacteria after 5 days in culture. However, proliferation of isolated lymphocyte subsets was only observed with CD3− CD56+ NK cells. The fact that the proliferative response with purified NK cells was 10 times lower than with total PBMC could be due to (i) a selective stimulation of a particular NK cell subset or (ii) the dependence on accessory cells for complete activation. A variety of NK cell receptors, implicated in activation or inhibition of NK cell effector functions, e.g., proliferation and cytolytic activity, have been described recently (22, 29, 36). Thus, the phenotypic characterization of the responsive NK subpopulation will provide further information on the specificity of the interaction with bacteria.
Coculturing purified NK cells with bacterially primed macrophages revealed that expression of CD25 is strongly promoted in the presence of an accessory cell, indicating the requirement for cell contact-based signals for activation. This could be mediated by the interaction of costimulatory molecules, such as CD28, CD16, or the CD94 receptor complex, which were shown to be expressed on human NK cells and which have key roles in expansion and effector functions (12, 41, 42). The dependence on accessory cell function was also reflected by the selective induction of IFN-γ secretion from NK cells in the presence of L. johnsonii-primed macrophages or in coculture with macrophages and L. johnsonii. The synergistic effect on NK cell activation observed in the combination of macrophages and bacteria is likely to be based on the additional secretion of monokines, which engage constitutively expressed monocyte-derived cytokine receptors on NK cells (9).
Although the secretion of cytokines required the presence of accessory cells, a direct interaction between bacteria and NK cells, leading to activation, was demonstrated. This interaction was more intense with L. johnsonii than with E. coli and could be linked to different bacterial cell surface determinants, which may constitute the molecular basis for specific immunomodulatory properties. It is reported that lactobacilli interact with asialo-GM1 receptors on epithelial cells (11, 43). Expression of this receptor on murine NK cells is also reported (33), and it may also constitute a putative receptor on human NK cells to mediate activation by bacteria (30). Furthermore, the oral administration of L. johnsonii to healthy volunteers increased the phagocytic activity of PBMC, suggesting that this stimulation could take place within the normal homeostasis of the immune system (37). Thus, activation of monocytes/macrophages seems a common denominator for both the in vivo observation following LAB ingestion and the in vitro data showing that L. johnsonii-primed monocytes mediate NK cell activation and subsequent IFN-γ secretion.
NK cells play an important role in innate immune resistance, particularly through synthesis of the proinflammatory cytokine IFN-γ. Recently, only a small role for NK cells in the early production of IFN-γ after infection of mice with Listeria monocytogenes was claimed (33). Our data suggest that NK cells, as well as macrophages, constitute primary targets for bacterial stimulation. This is consistent with previous observations that IFN-γ production in response to bacteria requires NK cells but not T cells (1). IFN-γ production in vitro by activated NK cells is highly dependent on the presence of IL-12, which induces effector maturation and expansion of NK cells and CD8+ T cells. Those cells which encounter first a foreign antigen play an important role in determining whether a Th1- or Th2-biased immune response is mounted to an antigenic challenge. IL-12 is implicated in the mechanisms of an innate immune response and, at the same time, shifts a developing immune response towards the “on” set of cell-mediated immunity, which constitutes one major part of acquired immunity (17). It has been clearly established that IL-12 and IFN-γ mediate protective functions against intracellular pathogens by inducing monocyte/macrophage activation (24, 40).
It is essential that NK cell activity remain under stringent and finely tuned control. The system of inhibitory and stimulatory receptors and the cytokine microenvironment allow the control of NK cell responses (10, 19). The role of NK cells in the recognition of commensal bacterial signals, in part mediated by monocytes, has not been established. The fact that we demonstrated the induction of a distinct immune response in human PBMC by nonpathogenic bacteria should encourage further work to understand the physiology of bacterial interaction with host cells.
REFERENCES
- 1.Bancroft G J, Schreiber R D, Bosma G C, Bosma M J, Unanue E R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987;139:1104–1107. [PubMed] [Google Scholar]
- 2.Berg D J, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Investig. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg R D, Savage D C. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun. 1975;11:320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohn E, Autenrieth I B. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J Immunol. 1996;156:1458–1468. [PubMed] [Google Scholar]
- 5.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 6.Deitch E A, Xu D, Qi L, Berg R D. Bacterial translocation from the gut impairs systemic immunity. Surgery. 1991;109:269–276. [PubMed] [Google Scholar]
- 7.De Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 8.Dubos R, Schaedler R W, Costello R, Hoet P. Indigenous, normal and autochthonous flora of the gastrointestinal tract. J Exp Med. 1965;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehniger T A, Shah M H, Turner M J, VanDeusen J B, Whitman S P, Cooper M A, Suzuki K, Wechser M, Goodsaid F, Caligiuri M A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 10.Fort M M, Leach M W, Rennick D M. A role for NK cells as regulators of CD4+ T cells in a transfer model of colitis. J Immunol. 1998;161:3256–3261. [PubMed] [Google Scholar]
- 11.Fujiwara S, Hashiba H, Hirota T, Forstner J F. Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide. Appl Environ Microbiol. 1997;63:506–512. doi: 10.1128/aem.63.2.506-512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galea-Lauri J, Darling D, Gan S U, Krivochtchapov L, Kuiper M, Gaken J, Souberbielle B, Farzaneh F. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol. 1999;163:62–70. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 14.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson P R, Verhaar H J, Selby W S, Jewell D P. The mononuclear cells of human mesenteric blood, intestinal mucosa and mesenteric lymph nodes: compartmentalization of NK cells. Clin Exp Immunol. 1984;56:445–452. [PMC free article] [PubMed] [Google Scholar]
- 16.Guarner F, Schaafsma G J. Probiotics. Int J Food Microbiol. 1998;39:237–238. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 17.Hall S S. IL-12 at the crossroads. Science. 1995;268:1432–1434. doi: 10.1126/science.7770767. [DOI] [PubMed] [Google Scholar]
- 18.Hessle C, Hanson L A, Wold A E. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–282. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogan P G, Hapel A J, Doe W F. Lymphokine-activated and natural killer cell activity in human intestinal mucosa. J Immunol. 1985;135:1731–1738. [PubMed] [Google Scholar]
- 20.Kenworthy R. Observations on the reaction of the intestinal mucosa to bacterial challenge. J Clin Pathol. 1971;24:138–142. [Google Scholar]
- 21.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 22.Lanier L L. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 23.Lidbeck A, Nord C E. Lactobacilli and the normal human anaerobic microflora. Clin Infect Dis. 1993;16(Suppl. 4):S181–S187. doi: 10.1093/clinids/16.supplement_4.s181. [DOI] [PubMed] [Google Scholar]
- 24.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 25.Marteau P, Verman J P, Dehennin J P, Bord S, Brassart D, Pochart P, Desjeux J F, Rambaud J C. Effects of intrajejunal perfusion and chronic ingestion of Lactobacillus johnsonii strain La 1 on serum concentration and jejunal secretions of immunoglobulins and serum proteins in healthy humans. Gastroenterol Clin Biol. 1997;21:293–298. [PubMed] [Google Scholar]
- 26.Mastroeni P, Harrison J A, Chabalgoity J A, Hormaeche C E. Effect of interleukin-12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect Immun. 1996;64:189–196. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medzhitov R, Preston-Hurlburt P, Janeway C A J. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto M, Julkunen I. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–6062. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari M C, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 30.Muller C, Szangolies M, Kukel S, Kiehl M, Sorice M, Griggi T, Lenti L, Bauer R. Characterization of autoantibodies to natural killer cells in HIV-infected patients. Scand J Immunol. 1996;43:583–592. doi: 10.1046/j.1365-3083.1996.d01-80.x. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Alouf H, Alouf J E, Gerlach D, Ozegowski J H, Fitting C, Cavaillon J M. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigenic erythrogenic toxins, heat killed streptococci and lipopolysaccharide. Infect Immun. 1994;62:4915–4921. doi: 10.1128/iai.62.11.4915-4921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neutra M R. M cells in antigen sampling in mucosal tissues. Curr Top Microbiol Immunol. 1999;236:17–32. doi: 10.1007/978-3-642-59951-4_2. [DOI] [PubMed] [Google Scholar]
- 33.Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, Koyasu S. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J Exp Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang G, Buret A, Batey R T, Chen Q Y, Couch L, Cripps A, Clancy R. Morphological, phenotypic and functional characteristics of a pure population of CD56+ CD16− CD3− large granular lymphocytes generated from human duodenal mucosa. Immunology. 1993;79:498–505. [PMC free article] [PubMed] [Google Scholar]
- 35.Perdigon G, Alvarez S, Rachid M, Aguero G, Gobbato N. Immune system stimulation by probiotics. J Dairy Sci. 1995;78:1597–1606. doi: 10.3168/jds.S0022-0302(95)76784-4. [DOI] [PubMed] [Google Scholar]
- 36.Ross G D, Vetvicka V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993;92:181–184. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiffrin E J, Rochat F, Link-Amster H, Aeschlimann J M, Donnet-Hughes A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J Dairy Sci. 1995;78:491–497. doi: 10.3168/jds.S0022-0302(95)76659-0. [DOI] [PubMed] [Google Scholar]
- 38.Sedman P C, Macfie J, Sagar P, Mitchell C J, May J, Mancey-Jones B, Johnstone D. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643–649. doi: 10.1016/0016-5085(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 39.Stahl P D, Ezekowitz R A. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 41.Voss S D, Daley J, Ritz J, Robertson M J. Participation of the CD94 receptor complex in costimulation of human natural killer cells. J Immunol. 1998;160:1618–1626. [PubMed] [Google Scholar]
- 42.Warren H S, Kinnear B F. Quantitative analysis of the effect of CD16 ligation on human NK cell proliferation. J Immunol. 1999;162:735–742. [PubMed] [Google Scholar]
- 43.Yamamoto K, Miwa T, Taniguchi H, Nagano T, Shimamura K, Tanaka T, Kumagai H. Binding specificity of Lactobacillus to glycolipids. Biochem Biophys Res Commun. 1996;228:148–152. doi: 10.1006/bbrc.1996.1630. [DOI] [PubMed] [Google Scholar]