ABSTRACT
Aims
To explore the influence of nine healthy lifestyle factors on the risk of type 2 diabetes mellitus in adults in Guizhou, China.
Methods
Data were obtained from a large population‐based prospective cohort study in Guizhou Province, China. A total of 7,319 participants aged ≥18 years without diabetes at baseline were included in this study and were followed up from 2016 to 2020. A healthy lifestyle score was calculated based on the number of healthy lifestyle factors.
Results
During an average of 7.1 person‐years of follow‐up, 764 participants were diagnosed with type 2 diabetes mellitus. Compared with those of participants who scored 0–3 for a healthy lifestyle, the hazard ratios (95% confidence intervals) of those who scored 4, 5, 6, and ≥7 were 0.676 (0.523–0.874), 0.599 (0.464–0.773), 0.512 (0.390–0.673), and 0.393 (0.282–0.550), respectively, showing a gradual downward trend (P for trend <0.01). More importantly, they had lower fasting and 2 h post‐load plasma glucose levels and fewer changes in plasma glucose levels during follow‐up. If ≥7 healthy lifestyle factors were maintained, 33.8% of incident diabetes cases could have been prevented. Never smoking was the strongest protective factor against type 2 diabetes mellitus.
Conclusions
A healthy lifestyle can effectively decrease plasma glucose levels and reduce the incidence of type 2 diabetes mellitus in adults in Guizhou, China. In addition, not smoking may be an effective way to prevent type 2 diabetes mellitus.
Keywords: Healthy lifestyle, Incidence, Type 2 diabetes mellitus
The influence of each healthy lifestyle factor on the risk of Type 2 diabetes mellitus
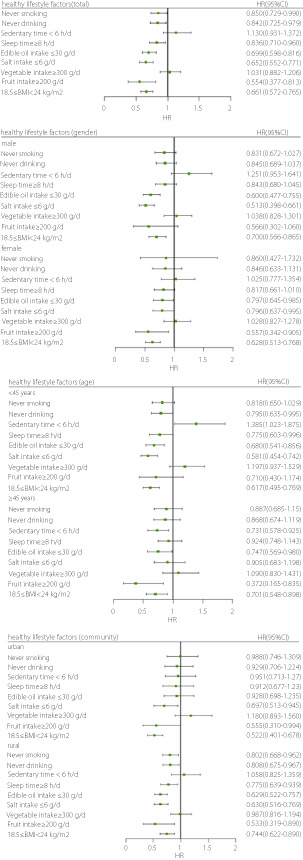
INTRODUCTION
Diabetes is one of the fastest‐growing diseases worldwide and poses a serious challenge to global public health 1 , 2 , with approximately 537 million adults (20–79 years) with diabetes in 2021 worldwide 3 . The largest number of patients with diabetes live in China, causing a heavy burden of the disease on the country. With economic development, lifestyle changes, and the acceleration of population aging, the prevalence of diabetes is continuously increasing in China. The latest research showed that its prevalence in adults in China was 11.2%, according to the World Health Organization 4 .
Studies have shown that a healthy lifestyle may reduce the risk of type 2 diabetes mellitus 5 , 6 . A large prospective cohort study observed that a healthy lifestyle increased life expectancy free of diabetes by 10.3 years for men and 12.3 years for women 7 . A cohort study in the United Kingdom showed that the risk of diabetes in men was reduced by half owing to a healthy lifestyle 8 . Another cohort study demonstrated that a healthy lifestyle might contribute to diabetes prevention among African Americans 9 . A prospective cohort study in China found that the combination of the amount of physical activity, diet, body mass index (BMI), and waist‐to‐hip ratio accounted for 72.6% of the etiology of diabetes 10 . Another study also established that lifestyle factors were associated with an increased diabetes burden in China 11 . The benefits of a healthy lifestyle on glucose metabolism have also been demonstrated in pregnant women 12 , 13 . Several randomized controlled trials have shown that the onset of type 2 diabetes mellitus can be delayed or prevented via lifestyle intervention among people with impaired glucose regulation, or even normal glucose tolerance can be restored 14 , 15 , 16 .
However, most previous studies only focused on diet, physical activity, smoking, alcohol intake, and a few other factors. Furthermore, little is known about the effects of lifestyle on glucose metabolism in ethnic minority regions in China. Therefore, this study aimed to identify the influence of nine lifestyle factors, such as smoking; alcohol intake; sedentary behavior; sleep time; BMI; and the intake of vegetables, fruits, edible oils, and salt, on the risk of type 2 diabetes mellitus in adults in Guizhou Province, which has the lowest prevalence of diabetes in China 4 . Furthermore, we assessed the contribution of healthy lifestyle factors to the incidence of diabetes and the importance of each constituent factor.
METHODS
Study population
The data were obtained from the Guizhou Population Health Cohort Study (GPHCS), a large population database that aimed to investigate the epidemic of chronic diseases and risk factors. Details regarding the GPHCS cohort have been published previously 17 . The baseline survey covered 12 districts in Guizhou Province using multistage proportional stratified cluster sampling from 2010 to 2012. We included a total of 9,280 permanent residents aged ≥18 years in the study. The participants were followed up from 2016 to 2020. Of these, 8,163 participants completed the follow‐up (87.96%). In this study, we excluded those who had been diagnosed with diabetes (n = 809) or had missing diabetes data (n = 34) at baseline, were lost to follow‐up (n = 994), had died during the follow‐up period (n = 106), and had missing diabetes data at follow‐up (n = 18). Finally, the remaining 7,319 participants were included in the analysis. The flow chart of the study is shown in Figure 1. The study was approved by the Institutional Review Board of the Guizhou Center for Disease Control and Prevention (no. S2017‐02). In addition, written informed consent was obtained from all participants.
Figure 1.
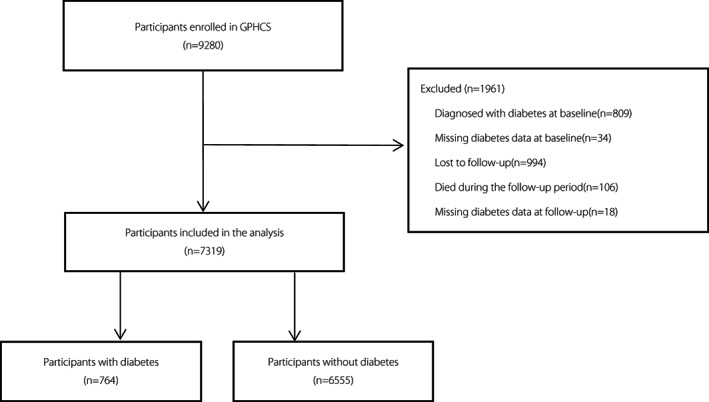
The flow chart of the study.
Assessment of lifestyle behaviors and other covariates
Information on smoking, alcohol consumption, educational background, sedentary behavior, sleep status, history of diseases, and family history of diabetes was collected from the self‐reported data of each participant. The food frequency questionnaire was used to assess the frequency and quantity of various foods consumed in the past 12 months. The intake of edible oil and salt in a family in the past 30 days was assessed using a family questionnaire and then converted into daily intake for each participant according to the number of people in a family.
After at least 10 h of overnight fasting, a 75 g oral glucose tolerance test was conducted for each participant. A venous blood sample was collected before and 2 h after glucose administration. Plasma glucose was detected using the hexokinase method within 4 h. After centrifugation, serum separated from the remaining blood samples was stored at –20°C and transferred to Guizhou Center for Disease Control and Prevention to detect levels of triglycerides, total cholesterol, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol (Olympus 400 Analyzer; Beckman Coulter, Brea, CA, USA).
Body height, weight, and blood pressure were measured by strictly and uniformly trained staff. BMI was calculated by dividing the weight by height squared (kg/m2).
According to the recommendations of Chinese dietary guidelines 18 and previous studies 19 , 20 , 21 , 22 , healthy lifestyle factors include never smoking, never drinking, sedentary time <6 h/day, sleeping time 7–9 h/day, having a BMI between 18.5 and 23.9 kg/m2, vegetable intake ≥300 g/day, fruit intake ≥200 g/day, edible oil intake ≤30 g/day, and salt intake ≤6 g/day. A healthy lifestyle score was constructed based on these nine lifestyle factors. For each healthy lifestyle, participants who met the standard were assigned 1 point. Higher scores indicated healthier lifestyles than those with lower scores. Due to the small score range, participants with a score of ≤3 were combined into one group, and those with a score of ≥7 were combined into another group.
Diabetes was defined as a fasting plasma glucose concentration of ≥7.0 mmol/L, 2 h glucose concentration of ≥11.1 mmol/L, or having been diagnosed with diabetes by township or community and above hospitals. According to the 1999 World Health Organization criteria, impaired glucose tolerance is defined as a fasting plasma glucose concentration of <7.0 mmol/L and a 2 h glucose concentration of 7.8–11.0 mmol/L; impaired fasting glucose is defined as a fasting plasma glucose concentration of 6.1–6.9 mmol/L and 2 h glucose concentration of <7.8 mmol/L 23 , 24 . Lastly, hypertension included those with a systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or self‐reported hypertension 25 .
Statistical analysis
The Statistical Package for the Social Sciences (version 23.0; IBM Corporation, Armonk, NY, USA) and R (version 4.1.2; Lucent Technologies Inc., Murray Hill, NJ, USA) were used to perform statistical analyses. Person‐years were used as the time variable. The person‐years were calculated from the baseline survey to the onset of diabetes or the end of follow‐up, and the incidence density of different healthy lifestyle groups was calculated. A Cox proportional hazards regression model was used to evaluate the risk of type 2 diabetes mellitus. The hazard ratio and 95% confidence interval (CI) were calculated with a healthy lifestyle score of 0–3 as reference.
The population attributable risk percentage (PAR%) was calculated to evaluate the effect of an increasing number of healthy lifestyle factors on the incidence of type 2 diabetes mellitus under the assumption of causality. Considering the distribution of the number of healthy lifestyle factors in our study population, PAR% and 95% CI for participants maintaining ≥4, ≥5, ≥6, and ≥7 healthy lifestyle factors were estimated respectively.
In addition, we subtracted one of the nine healthy lifestyle factors at a time and calculated the hazard ratio and 95% CI for incident type 2 diabetes mellitus to assess the importance of each healthy lifestyle factor.
Several sensitivity analyses were conducted: (i) participants who had been diagnosed with diabetes for more than 5 years at follow‐up were excluded; (ii) participants with prediabetes at follow‐up were excluded; (iii) participants with hypertension at baseline were excluded; (vi) the cutoff value of sedentary time from 6 to 8 h/day was adjusted; (v) ethnicity was adjusted as a confounding factor. A two‐sided P‐value <0.05 was considered statistically significant.
RESULTS
Baseline characteristics of participants
A total of 7,319 participants with an average age of 43.7 ± 14.9 years were included in this study. The average follow‐up was 7.1 ± 1.3 person‐years. During the follow‐up period, 764 participants were diagnosed with type 2 diabetes mellitus. According to whether diabetes occurs at follow‐up and considering healthy lifestyle scores, the baseline characteristics of participants are shown in Tables 1 and 2, respectively. Among those diagnosed with diabetes during the follow‐up period, the proportion of young people; urban population; and participants with appropriate sleep time, never smoking, having optimal BMI, meeting the intake standards of fruit, edible oil, and salt was lower, while the proportion of participants with a low education level accompanied with dyslipidemia and hypertension was higher (P < 0.05 or 0.01). There was no significant difference in the proportion of participants with sedentary time <6 h/day, high vegetable intake, never drinking, and a family history of diabetes, regardless of whether diabetes occurred (P > 0.05). With the increase in healthy lifestyle scores, the proportion of men, urban population, combined dyslipidemia, and hypertension decreased, and the proportion of young people, participants with a low education level, sedentary time <6 h/day, appropriate sleep time, optimal BMI, never smoking, never drinking, and meeting the intake standards of vegetable, fruit, edible oil, and salt gradually increased (all P < 0.01).
Table 1.
Baseline characteristics of participants according to whether diabetes occurs at follow‐up. Values are numbers (percentages)
| Characteristic | Total (n = 7,319) | Diabetes (n = 764) | Non‐diabetes (n = 6,555) | P value |
|---|---|---|---|---|
| Men | 3427 (46.8) | 371 (48.6) | 3056 (46.6) | 0.309 |
| Age <45 years | 4043 (55.2) | 339 (44.4) | 3704 (56.5) | <0.01 |
| Urban | 2496 (34.1) | 225 (29.5) | 2271 (34.6) | 0.004 |
| Education years <9 years | 4164 (56.9) | 494 (64.7) | 3670 (56.0) | <0.01 |
| Sedentary behavior <6 h/day | 5922 (80.9) | 627 (82.1) | 5295 (80.8) | 0.391 |
| Sleep time 7–9 h/day | 5723 (78.2) | 565 (74.0) | 5158 (78.7) | 0.003 |
| Never smoking | 5240 (71.6) | 522 (68.3) | 4718 (72.0) | 0.034 |
| Never drinking | 5002 (68.3) | 508 (66.5) | 4494 (68.6) | 0.245 |
| 18.5 ≤BMI ≤23.9 kg/m2 | 4668 (63.8) | 428 (56.0) | 4240 (64.7) | <0.01 |
| Vegetable intake ≥300 g/day | 4949 (67.6) | 538 (70.4) | 4411 (67.3) | 0.080 |
| Fruit intake ≥200 g/day | 424 (5.8) | 27 (3.5) | 397 (6.1) | 0.005 |
| Edible oil intake ≤30 g/day | 2542 (34.7) | 226 (29.6) | 2316 (35.3) | 0.002 |
| Salt intake ≤6 g/day | 2110 (28.8) | 182 (23.8) | 1928 (29.4) | 0.001 |
| Dyslipidemia | 4103 (56.1) | 464 (60.7) | 3639 (55.5) | 0.006 |
| Hypertension | 1756 (24) | 223 (29.2) | 1533 (23.4) | <0.01 |
| Family history of diabetes | 98 (1.8) | 11 (2.0) | 87 (1.8) | 0.754 |
The information on family history of diabetes was missing in 1925 participants. BMI, body mass index.
Table 2.
Baseline characteristics of participants according to the healthy lifestyle scores. Values are numbers (percentages)
| Characteristic | Healthy lifestyle score | P value | ||||
|---|---|---|---|---|---|---|
| 0–3 (n = 1,087) | 4 (n = 1,534) | 5 (n = 1,874) | 6 (n = 1,780) | 7– (n = 1,044) | ||
| Men | 853 (78.47) | 880 (57.37) | 813 (43.38) | 584 (32.81) | 297 (28.45) | <0.01 |
| Age <45 years | 531 (48.85) | 772 (50.33) | 1004 (53.58) | 1060 (59.55) | 676 (64.75) | <0.01 |
| Urban | 458 (42.13) | 570 (37.16) | 642 (34.26) | 520 (29.21) | 306 (29.31) | <0.01 |
| Education years <9 years | 511 (47.01) | 851 (55.48) | 1107 (59.07) | 1082 (60.79) | 613 (58.72) | <0.01 |
| Sedentary behavior <6 h/day | 811 (74.61) | 1313 (85.59) | 1699 (90.66) | 1672 (93.93) | 1009 (96.65) | <0.01 |
| Sleep time 7–9 h/day | 565 (51.98) | 1078 (70.27) | 1514 (80.79) | 1579 (88.71) | 987 (94.54) | <0.01 |
| Never smoking | 256 (23.55) | 811 (52.87) | 1360 (72.57) | 1562 (87.75) | 1013 (97.03) | <0.01 |
| Never drinking | 307 (28.24) | 875 (57.04) | 1460 (77.91) | 1595 (89.61) | 1003 (96.07) | <0.01 |
| 18.5 ≤BMI ≤23.9 kg/m2 | 431 (39.83) | 815 (53.3) | 1121 (59.88) | 1390 (78.18) | 911 (87.26) | <0.01 |
| Vegetable intake ≥300 g/day | 457 (42.24) | 868 (56.66) | 1282 (68.81) | 1418 (80.98) | 924 (91.21) | <0.01 |
| Fruit intake ≥200 g/day | 25 (2.32) | 42 (2.77) | 90 (4.84) | 124 (7.07) | 143 (13.94) | <0.01 |
| Edible oil intake ≤30 g/day | 108 (9.94) | 265 (17.28) | 583 (31.11) | 785 (44.1) | 801 (76.72) | <0.01 |
| Salt intake ≤6 g/day | 86 (7.92) | 223 (14.54) | 402 (21.45) | 626 (35.17) | 773 (74.04) | <0.01 |
| Dyslipidemia | 700 (64.4) | 916 (59.71) | 1051 (56.08) | 921 (51.74) | 515 (49.33) | <0.01 |
| Hypertension | 320 (29.44) | 440 (28.68) | 455 (24.28) | 364 (20.45) | 177 (16.95) | <0.01 |
| Family history of diabetes | 21 (2.49) | 19 (1.72) | 24 (1.74) | 17 (1.31) | 17 (2.21) | 0.317 |
The information on family history of diabetes was missing in 1925 participants. BMI, body mass index.
The influence of each healthy lifestyle factor on the risk of type 2 diabetes mellitus
The influence of each healthy lifestyle factor on the risk of type 2 diabetes mellitus is shown in Figure 2. Never smoking, never drinking, appropriate sleep time, optimal BMI, low intake of edible oil and salt, and high fruit intake were protective factors against type 2 diabetes mellitus. Subgroup analysis showed that optimal BMI and low intake of edible oil and salt were protective factors for men; appropriate sleep time, optimal BMI, low intake of edible oil and salt, and high fruit intake were protective factors for women; and optimal BMI, low salt intake, and high fruit intake were protective factors for urban participants. The protective effect of a healthy lifestyle was consistent between the total participants and rural participants.
Figure 2.
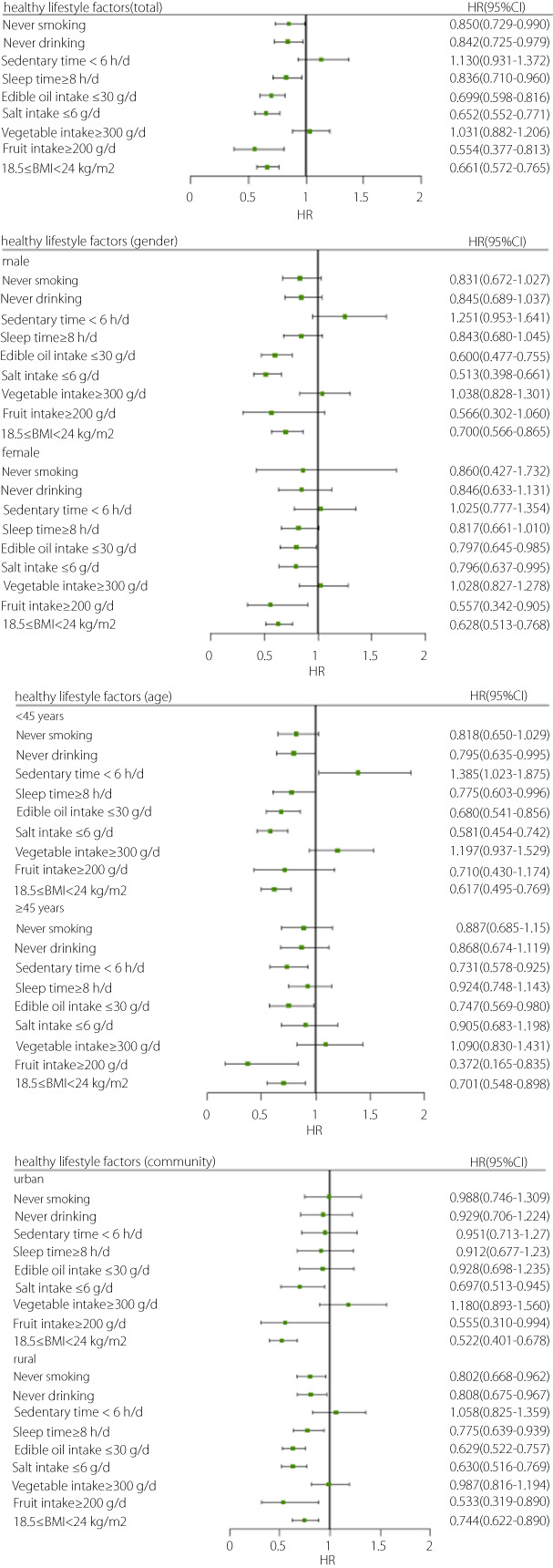
The influence of each healthy lifestyle factor on the risk of type 2 diabetes mellitus.
Effect of healthy lifestyle score on type 2 diabetes mellitus
The results of Cox proportional hazards regression are presented in Table 3. In this study, the incidence of type 2 diabetes mellitus was 14.7/1,000 person‐years. With the increasing number of healthy lifestyle factors, the incidence of diabetes gradually decreased. Compared with those of participants who scored 0–3 for a healthy lifestyle, the hazard ratios of those who scored 4, 5, 6, and ≥7 were 0.676 (95% CI: 0.523–0.874), 0.599 (95% CI: 0.464–0.773), 0.512 (95% CI: 0.390–0.673), and 0.393 (95% CI: 0.282–0.550), respectively, showing a gradual downward trend (P for trend <0.01). Compared with that in participants with a score of only 0–3 healthy lifestyle factors, the risk of developing diabetes in participants with a score of ≥7 healthy lifestyle factors decreased by 60.7%. The PAR% of the incidence of type 2 diabetes mellitus was 10.5% (95% CI: 5.7–15.0%) if all participants maintained ≥4 healthy lifestyle factors. When the number of healthy lifestyle factors was increased to ≥5, ≥6, or ≥7, the PAR% was 15.9% (95% CI: 8.8–22.5%), 23.3% (95% CI: 12.5–32.7%), and 33.8% (95% CI: 15.1–48.4%), respectively. Subgroup analysis showed that higher healthy lifestyle scores indicated a lower risk of type 2 diabetes mellitus regardless of sex, age, and community. Furthermore, the nine healthy lifestyle factors included in this study appeared to be more effective in preventing type 2 diabetes mellitus in men than in women (Figure 3). To assess the importance of each healthy lifestyle factor, the hazard ratio and 95% CI for incident type 2 diabetes mellitus were calculated after subtracting one of the nine healthy lifestyle factors at a time. The results showed that the hazard ratio changed with each factor subtracted; never smoking was the strongest protective factor against type 2 diabetes mellitus (Figure 4).
Table 3.
Hazard ratios (95% confidence intervals) of incident diabetes and the healthy lifestyle scores
| Healthy lifestyle score | Cases | Person‐years of follow‐up | Incident density (/1,000 PYs) | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|
| Total | 764 | 51,924.01 | 14.71 | NA | NA |
| 0–3 | 154 | 7,491.4 | 20.56 | 1.000 (ref) | NA |
| 4 | 181 | 10,684.93 | 16.94 | 0.676 (0.523–0.874) | 0.003 |
| 5 | 183 | 13,247.53 | 13.81 | 0.599 (0.464–0.773) | <0.01 |
| 6 | 160 | 12,805.24 | 12.49 | 0.512 (0.390–0.673) | <0.01 |
| 7– | 86 | 7,694.91 | 11.18 | 0.393 (0.282–0.550) | <0.01 |
| P for trend | NA | NA | NA | NA | <0.01 |
Adjusted for age (<45/≥45 years), gender, community, education years (≥9/<9 years), family history of diabetes (no/yes), hypertension (no/yes), dyslipidemia (no/yes).
Figure 3.
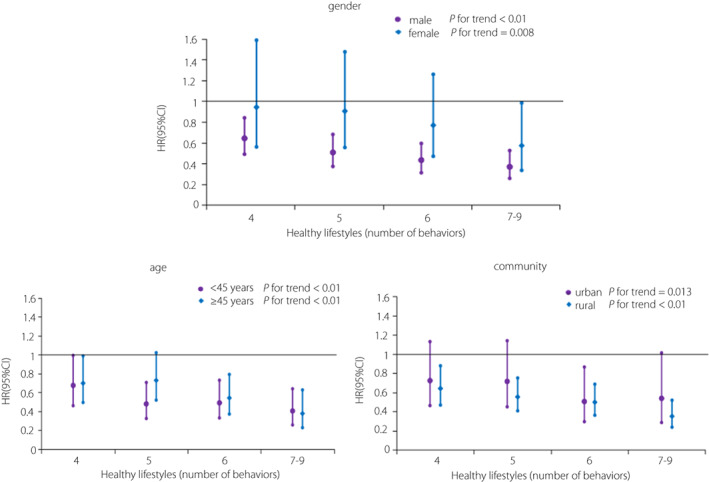
Subgroup analysis of the association between healthy lifestyle scores and the risk of type 2 diabetes mellitus.
Figure 4.
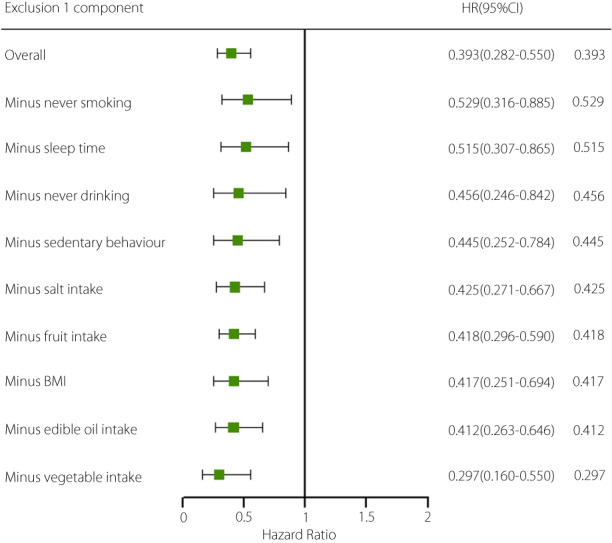
Hazard ratios (95% confidence intervals) associated with extreme categories (0–3 vs 7–9) of the nine healthy lifestyle factors after subtraction of one factor at a time. Adjusted for age (<45/≥45 years), gender, community, education years (≥9/<9), family history of diabetes (no/yes), hypertension (no/yes), dyslipidemia (no/yes), and corresponding subtracted components.
Effect of healthy lifestyle score on plasma glucose levels
The association between healthy lifestyle scores and changes in plasma glucose levels from baseline to follow‐up is presented in Table 4. No differences in fasting plasma glucose and 2 h post‐load plasma glucose were observed in each group at baseline. Participants with a healthy lifestyle score of ≥4 had lower fasting and 2 h post‐load plasma glucose levels and fewer changes in plasma glucose levels during follow‐up than participants with a healthy lifestyle score of 0–3.
Table 4.
The association between healthy lifestyle scores and the changes in plasma glucose levels during follow‐up
| Healthy lifestyle score | Baseline | Follow‐up | Change in plasma glucose levels† | |||
|---|---|---|---|---|---|---|
| FPG | 2hPG | FPG | 2hPG | FPG | 2hPG | |
| 0–3 | 5.03 ± 0.75 | 5.52 ± 1.44 | 5.72 ± 1.80 | 7.11 ± 2.62 | 0.71 ± 1.92 | 1.59 ± 2.88 |
| 4 | 5.04 ± 0.72 | 5.54 ± 1.38 | 5.62 ± 1.58* | 6.89 ± 2.32* | 0.59 ± 1.71* | 1.36 ± 2.60 |
| 5 | 5.03 ± 0.73 | 5.48 ± 1.33 | 5.57 ± 1.59* | 6.89 ± 2.63* | 0.54 ± 1.76* | 1.43 ± 2.84 |
| 6 | 5.07 ± 0.75 | 5.49 ± 1.31 | 5.51 ± 1.48* | 6.59 ± 2.13* | 0.45 ± 1.69* | 1.10 ± 2.39* |
| 7– | 5.05 ± 0.74 | 5.57 ± 1.25 | 5.42 ± 1.73* | 6.64 ± 2.54* | 0.38 ± 1.94* | 1.04 ± 2.83* |
| P value | 0.539 | 0.360 | 0.001 | <0.01 | <0.01 | <0.01 |
*P < 0.05, Compared with healthy lifestyle score of 0–3. †Changes in plasma glucose levels: plasma glucose levels at followed up minus plasma glucose levels at baseline. 2hPG, 2 h post‐load plasma glucose; FPG, fasting plasma glucose.
Sensitivity analysis
In this study, sensitivity analysis showed that the results obtained with each method were consistent with the main analysis results (Table S1).
DISCUSSION
In this study, we comprehensively explored the influence of nine lifestyle factors on the risk of type 2 diabetes mellitus in adults in Guizhou, China. From the results, we observed that never smoking, never drinking, appropriate sleep time, optimal BMI, low intake of edible oil and salt, and high fruit intake were protective factors against type 2 diabetes mellitus. With the increase in the number of healthy lifestyle factors, the risk of type 2 diabetes mellitus gradually decreased, especially among men. Compared with those in participants with a healthy lifestyle score of only 0–3, the plasma glucose levels in participants with a score of ≥4 were lower at follow‐up, and the risk of developing diabetes in participants with a score of ≥7 decreased by 60.7%. If ≥7 healthy lifestyle factors were maintained, 33.8% of incident diabetes cases could have been prevented. The strongest protective factor against diabetes was never smoking.
Our results confirm that a healthy lifestyle can reduce the risk of type 2 diabetes mellitus, which is consistent with previous studies 6 , 7 , 8 , 9 , 10 , 26 . A large prospective cohort study examined the effects of five lifestyle factors (smoking, alcohol intake, BMI, physical activity, and diet) on the risk of type 2 diabetes mellitus. The results showed that participants with more healthy lifestyle factors had a lower risk of type 2 diabetes mellitus 7 . A 10 year cohort study showed that increased lifestyle scores, especially dietary fiber intake, were associated with a lower risk of diabetes 27 . Li et al. 6 analyzed two independent cohort studies involving 558,302 Chinese participants. It was found that compared with participants with an unhealthy lifestyle, those with a healthy lifestyle had a significantly lower risk of type 2 diabetes mellitus. The adjusted hazard ratio was 0.30 and 0.41, respectively, and 0.30 for the combined cohort. Another community‐based study involving 11,596 Chinese adults aged ≥40 years found that the risk of diabetes in participants with a high healthy lifestyle score decreased by 32–44% when compared with participants with a low score 26 .
Our findings showed that most of the nine healthy lifestyle factors were protective against diabetes. In addition to never smoking, never drinking, appropriate sleep time, high fruit intake, and maintaining optimal weight, low intake of edible oil and salt were also beneficial for glucose metabolism. However, a sedentary time <6 h/day and vegetable intake ≥300 g/day had no significant protective effect. Although sedentary behavior is considered a risk factor for diabetes, some studies have reported inconsistent results. For example, a case–control study found that there was no significant difference in sedentary time between patients with type 2 diabetes mellitus and healthy controls after adjusting for BMI 28 . Another study showed that the total sedentary time was not associated with the development of gestational diabetes mellitus 29 . Hsueh et al. also found no significant correlation between total sedentary time and the risk of type 2 diabetes mellitus. However, after specifying sedentary behaviors, watching television ≥2 h/day was associated with a high risk of type 2 diabetes mellitus 30 . In our study, we did not observe that vegetable intake was associated with a lower risk of diabetes, which is inconsistent with previous studies 31 , 32 . An earlier meta‐analysis showed that consuming more green leafy vegetables reduces the risk of diabetes by 14% 33 . However, Hamer et al. 34 showed that an increase in vegetable intake did not reduce the risk of diabetes. Another meta‐analysis also found no association between total vegetable intake and diabetes, but intake of root vegetables was associated with a lower risk of diabetes 35 . A recent study also showed that vegetable intake did not alter the gut microbiota or related metabolites to reduce the risk of type 2 diabetes mellitus 36 . Although both sedentary time <6 h/day and vegetable intake ≥300 g/day were not independently associated with the development of type 2 diabetes mellitus, it was found that the risk of type 2 diabetes mellitus gradually decreased with the increase in the number of healthy lifestyle factors based on the analysis of any combination of various healthy lifestyle factors. The possible reason was that a certain healthy lifestyle factor had no significant effect on diabetes, but synergy appeared to decrease the risk of type 2 diabetes mellitus. The exact mechanism was unclear.
Interestingly, our results showed that the nine healthy lifestyle factors appeared to be more effective in preventing type 2 diabetes mellitus in men than in women. A possible reason is that the progression of diabetes in men is more susceptible to lifestyle. Previous studies have shown that there is a significant difference in the risk of diabetes between men and women. While the specific pathogenesis has not yet been elucidated, it may be related to the different levels of sex hormones, nutritional factors, and social and psychological factors 37 , 38 .
Notably, our results showed that never smoking was the strongest protective factor against type 2 diabetes mellitus among all healthy lifestyle factors. A large number of studies have demonstrated that active and passive smoking are both closely related to a higher risk of type 2 diabetes mellitus 39 , 40 , 41 . Cigarettes may change body composition, cause adverse fat distribution, reduce insulin sensitivity, and damage pancreatic β‐cell function, thereby affecting glucose homeostasis 40 . A previous study found a linear dose–response relationship between smoking and the risk of type 2 diabetes mellitus. Smoking accounted for 18.8% and 5.4% of the etiology of type 2 diabetes mellitus in men and women, respectively 42 . A recent cohort study in Korea showed that after adjustment for multiple factors, compared with that among current smokers, the risk of diabetes among quitters and non‐smokers decreased by 14.2% and 38.4%, respectively 43 . Pan et al. 39 found that the risk of diabetes decreased with an increase in smoking cessation time among former smokers.
The major advantages of our study are the large sample size, relatively long follow‐up time, and high follow‐up rate. To our knowledge, this is the first study to explore the influence of nine healthy lifestyle factors on the risk of type 2 diabetes mellitus in an ethnic minority region in China. More importantly, we analyzed the importance of each lifestyle factor, and five different methods were used for the sensitivity analysis to maximize the robustness of our results.
Nevertheless, this study has several limitations. First, lifestyle factors depend on participants' self‐reports; therefore, measurement errors are inevitable. Second, other lifestyle factors such as intake of red meat, milk, seafood, and beverages were not analyzed. Finally, our participants were permanent residents of Guizhou Province, China, and the results may not be applicable to other populations.
In summary, a healthy lifestyle can reduce the incidence of type 2 diabetes mellitus in adults in Guizhou, China. With the increasing number of healthy lifestyle factors, the incidence of diabetes gradually decreased. A healthy lifestyle, especially not smoking, may be an effective way to prevent type 2 diabetes mellitus.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The study was approved by the Institutional Review Board of the Guizhou Center for Disease Control and Prevention (no. S2017‐02).
Informed consent: written informed consent was obtained from all participants.
Registry and the registration no. of the study/trial: 20170314.
Animal studies: N/A.
Supporting information
Table S1 | Sensitivity analysis of the hazard ratios (95% confidence intervals) of incident diabetes with healthy lifestyle scores
ACKNOWLEDGMENTS
Funding for this study came from the Guizhou Provincial Science and Technology Fund (Contract Number: [2018]2819).
Contributor Information
Tao Liu, Email: liutaombs@163.com.
Jianhua Luo, Email: luojianhua_gy@163.com.
DATA AVAILABILITY STATEMENT
Application for datasets generated during and/or analyzed during the current study may be considered by the corresponding author on reasonable request.
REFERENCES
- 1. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol 2020; 16(7): 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Standl E, Khunti K, Hansen TB, et al. The global epidemics of diabetes in the 21st century: current situation and perspectives. Eur J Prev Cardiol 2019; 26(2_suppl): 7–14. [DOI] [PubMed] [Google Scholar]
- 3. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country‐level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022; 183: 109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ 2020; 369: m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber MB, Hassan S, Quarells R, et al. Prevention of Type 2 diabetes. Endocrinol Metab Clin North Am 2021; 50(3): 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H, Khor CC, Fan J, et al. Genetic risk, adherence to a healthy lifestyle, and type 2 diabetes risk among 550,000 Chinese adults: results from 2 independent Asian cohorts. Am J Clin Nutr 2020; 111(3): 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Schoufour J, Wang DD, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ 2020; 368: l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elwood P, Galante J, Pickering J, et al. Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the Caerphilly cohort study. PLOS One 2013; 8(12): e81877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joseph JJ, Echouffo‐Tcheugui JB, Talegawkar SA, et al. Modifiable lifestyle risk factors and incident diabetes in African Americans. Am J Prev Med 2017; 53(5): e165–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lv J, Yu C, Guo Y, et al. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int J Epidemiol 2017; 46(5): 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Wang DD, Ley SH, et al. Time trends of dietary and lifestyle factors and their potential impact on diabetes burden in China. Diabetes Care 2017; 40(12): 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badon SE, Enquobahrie DA, Wartko PD, et al. Healthy lifestyle during early pregnancy and risk of gestational diabetes mellitus. Am J Epidemiol 2017; 186(3): 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juan J, Yang H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health 2020; 17(24):9517. 10.3390/ijerph17249517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong Q, Zhang P, Wang J, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30‐year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol 2019; 7(6): 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6): 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindström J, Ilanne‐Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow‐up of the Finnish Diabetes Prevention Study. Lancet 2006; 368(9548): 1673–1679. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Wang Y, Chen Y, et al. Associations of Dietary Patterns and Risk of Hypertension in Southwest China: A Prospective Cohort Study. Int J Environ Res Public Health 2021; 18: 12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chinese Nutrition Society . Chinese Dietary Guidelines. Beijing, China: People's Medical Publishing House Press, 2016. [Google Scholar]
- 19. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol 2021; 9(6): 373–392. [DOI] [PubMed] [Google Scholar]
- 20. Matthews CE, Keadle SK, Troiano RP, et al. Accelerometer‐measured dose‐response for physical activity, sedentary time, and mortality in US adults. Am J Clin Nutr 2016; 104(5): 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health 2015; 1(1): 40–43. [DOI] [PubMed] [Google Scholar]
- 22. Zhou BF, Cooperative Meta‐Analysis Group of the Working Group on Obesity in C . Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults‐study on optimal cut‐off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002; 15(1): 83–96. [PubMed] [Google Scholar]
- 23. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15(7): 539–553. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva: WHO Press, 2006. [Google Scholar]
- 25. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36(10): 1953–2041. [DOI] [PubMed] [Google Scholar]
- 26. Ye C, Niu J, Zhao Z, et al. Genetic susceptibility, family history of diabetes and healthy lifestyle factors in relation to diabetes: a gene‐environment interaction analysis in Chinese adults. J Diabetes Investig 2021; 12(11): 2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feldman AL, Long GH, Johansson I, et al. Change in lifestyle behaviors and diabetes risk: evidence from a population‐based cohort study with 10 year follow‐up. Int J Behav Nutr Phys Act 2017; 14(1): 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamer M, Bostock S, Hackett R, et al. Objectively assessed sedentary time and type 2 diabetes mellitus: a case‐control study. Diabetologia 2013; 56(12): 2761–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wagnild JM, Hinshaw K, Pollard TM. Associations of sedentary time and self‐reported television time during pregnancy with incident gestational diabetes and plasma glucose levels in women at risk of gestational diabetes in the UK. BMC Public Health 2019; 19(1): 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsueh MC, Liao Y, Chang SH. Associations of total and domain‐specific sedentary time with Type 2 diabetes in Taiwanese older adults. J Epidemiol 2016; 26(7): 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cooper AJ, Sharp SJ, Lentjes MA, et al. A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care 2012; 35(6): 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng JS, Sharp SJ, Imamura F, et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC‐InterAct case‐cohort study in eight European countries. BMJ 2020; 370: m2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter P, Gray LJ, Troughton J, et al. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta‐analysis. BMJ 2010; 341: c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamer M, Chida Y. Intake of Fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta‐analysis. J Hypertens 2007; 25(12): 2361–2369. [DOI] [PubMed] [Google Scholar]
- 35. Cooper AJ, Forouhi NG, Ye Z, et al. Fruit and vegetable intake and type 2 diabetes: EPIC‐InterAct prospective study and meta‐analysis. Eur J Clin Nutr 2012; 66(10): 1082–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang Z, Sun TY, He Y, et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: results from two large human cohort studies. BMC Med 2020; 18(1): 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kautzky‐Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev 2016; 37(3): 278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Group EUCCS , Regitz‐Zagrosek V, Oertelt‐Prigione S, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J 2016; 37(1): 24–34. [DOI] [PubMed] [Google Scholar]
- 39. Pan A, Wang Y, Talaei M, et al. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2015; 3(12): 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maddatu J, Anderson‐Baucum E, Evans‐Molina C. Smoking and the risk of type 2 diabetes. Transl Res 2017; 184: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oba S, Goto A, Mizoue T, et al. Passive smoking and type 2 diabetes among never‐smoking women: the Japan Public Health Center‐based Prospective Study. J Diabetes Investig 2020; 11(5): 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akter S, Goto A, Mizoue T. Smoking and the risk of type 2 diabetes in Japan: A systematic review and meta‐analysis. J Epidemiol 2017; 27(12): 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park SE, Seo MH, Cho JH, et al. Dose‐dependent effect of smoking on risk of diabetes remains after Smoking Cessation: a nationwide population‐based cohort study in Korea. Diabetes Metab J 2021; 45(4): 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Sensitivity analysis of the hazard ratios (95% confidence intervals) of incident diabetes with healthy lifestyle scores
Data Availability Statement
Application for datasets generated during and/or analyzed during the current study may be considered by the corresponding author on reasonable request.


