Highlights
-
•
Study methods significantly impact estimates of colorectal cancer screening rates.
-
•
Several methods overestimate colorectal cancer screening rates as >65%
-
•
Including high-risk people or a retrospective cohort design overestimates rates.
-
•
In a cross-sectional design, 51% were up to date with colorectal cancer screening.
-
•
Evaluating adherence to colorectal cancer screening guidelines requires method clarity.
Keywords: Screening, Colorectal cancer, Average-risk, Screening rates
Abstract
Colorectal cancer screening rates are important metrics for public health and quality indicators for health care systems; however, published estimates of colorectal cancer screening rates often include both high-risk and average-risk patients, and the use of different epidemiologic methods makes between-study comparisons tenuous. The objective of this study was to measure the proportion of average-risk American adults who are up to date with colorectal cancer screening guidelines and examine the impact of evaluation methods on screening rate estimates. This repeated cross-sectional study used administrative claims to identify individuals aged 50–75 years between 2015 and 2018 with ≥ 1-year of continuous health plan enrollment. Sensitivity analyses to replicate prior studies in the literature included: 1) retrospective cohort study requiring ≥ 10 years of continuous enrollment to identify the most current screening rates (2018), and 2) inclusion of individuals with higher colorectal cancer risk. A total of 2,579,898; 2,948,064; 3,312,882; and 2,752,864 individuals were included in the 2015, 2016, 2017, and 2018 populations, respectively. In the cross-sectional sample, the proportion of individuals with up-to-date colorectal cancer screening was 51.8%, 51.3%, 51.0%, and 51.1% in 2015, 2016, 2017, and 2018, respectively. The inclusion of high-risk individuals increased estimates approximately 37%. Using a retrospective cohort design, 67.5% of average-risk individuals were up to date in 2018. This study demonstrated the impact of methodological differences on rate estimates. Efforts to track screening rates require transparency in measurement methods to accurately evaluate progress in improving rates.
1. Introduction
In 2020, an estimated 147,950 new cases of colon and/or rectal cancer were diagnosed, and approximately 53,200 persons died from colorectal cancer. (American Cancer Society, 2020) Colorectal cancer risk increases with age; median age at diagnosis is 66 years in men and 69 years in women. (American Cancer Society, 2020) Early detection of colorectal cancer is an important predictor of prognosis, and healthcare costs associated with treatment are lower with earlier diagnosed colorectal cancer, (Chastek et al., 2013) providing a compelling rationale to increase participation in population-level colorectal cancer screening. Guidelines, including those from United States Preventive Services Task Force (USPSTF), the American Cancer Society (ACS) and the National Comprehensive Cancer Network (NCCN), recommend several screening strategies for people at average risk for colorectal cancer including colonoscopy; fecal occult blood tests (fecal immunochemical test [FIT] and guaiac-based fecal occult blood test [FOBT]); mt-sDNA test (Cologuard®); flexible sigmoidoscopy; and computed tomography colonography. (American Cancer Society, 2020, Network and Guidelines Colorectal Cancer, 2020, Davidson et al., 2021) Guidelines have traditionally recommended screening average-risk individuals at 50–75 years of age; however, recent guidelines updates have lowered the age of screening initiation to 45 (ACS in 2018 and USPSTF, NCCN, and United States Multi-Society Task Force of Colorectal Cancer in 2021). (Davidson et al., 2021, Patel et al., 2021, Wolf et al., 2018, Clinical Practice Guidelines, 2022) Individuals with an above-average risk of colorectal cancer (ie, those with a family or personal history of colorectal cancer or hereditary colorectal cancer syndrome, inflammatory bowel disease [IBD], or history of abdominal or pelvic radiation) may be advised to begin screening prior to age 45 and have more frequent screenings (Wolf et al., 2018).
Colorectal cancer screening rates are important metrics for public health and quality indicators for health care systems. Prior epidemiological studies have estimated that a majority (58%–65%) of average-risk individuals are up to date with colorectal cancer guideline recommendations; (Cyhaniuk and Coombes, 2016, Inadomi et al., 2012, Sinicrope et al., 2012) however, published estimates of colorectal cancer screening rates often do not exclude high-risk patients, and the use of different epidemiologic methods for observational studies makes between-study comparisons tenuous. While the USPSTF guidelines are applicable to average-risk patients, the number quoted for up-to-date screening (69%) cited a study by Joseph, et al., that did not exclude high-risk patients from their estimate (Joseph et al., 2020).
The objective of this retrospective, administrative claims study was to evaluate the proportion of the eligible, average-risk population in the United States that was up to date with colorectal cancer screening guidelines in each calendar year between 2015 and 2018. A secondary objective was to explore trends in the use of different colorectal cancer screening modalities. Finally, we sought to understand how screening estimates are impacted by colorectal cancer risk, as well as the study design and methods used to estimate the screening rate within the study population. Thus, we conducted a main analysis using a repeated cross-sectional study design, and a sensitivity analysis using a retrospective cohort study design, to estimate the proportion of average risk individuals aged 50–75 who received colorectal cancer screening.
2. Methods
2.1. Data source
This study utilized the Optum Research Database (ORD), a fully de-identified and HIPAA compliant database comprised of medical and pharmacy claims data linked to enrollment information on more than 67 million insured individuals across the United States. In 2016, approximately 19% of the United States commercially enrolled population, plus 17% of the Medicare Advantage and 23% of the Medicare Part D population, were represented in the ORD. Institutional review board approval or waiver of approval was not required for this study because the study data were secondary and de-identified in accordance with the United States Department of Health and Human Services Privacy Rule’s requirements for de-identification codified at 45C.F.R. § 164.514(b).
2.2. Main analysis study design (repeated cross-sectional study)
Eligible individuals were commercially insured or covered by Medicare Advantage with Part D and were aged 50–75 years during the calendar year of analysis (2015–2018). Study individuals were required to have continuous enrollment with medical and pharmacy benefits ≥ 12 months prior to the year of analysis through the entire year of analysis (minimum of 24 months of continuous eligibility). Additionally, individuals were required to be continuously benefit eligible for the duration of time prior to and including the study year in which criteria for colorectal cancer screening guidelines (based on age during the study analysis year) were met. A sensitivity analysis required individuals to be eligible for 10 years prior to the study analysis year for those of sufficient age to meet colorectal cancer screening guideline criteria (ie, 59–75 years of age during study analysis year). Exclusionary criteria included any of the following conditions which confer higher risk for colorectal cancer identified through International Classification of Diseases, ninth revision/tenth revision (ICD-9/ICD-10) diagnosis codes: colorectal cancer familial syndromes, prior diagnosis of precancerous polyp (adenoma or sessile serrated polyp), history of or current colorectal cancer, family history of gastrointestinal cancer, or IBD (Supplementary Table 1). (Limburg et al., 2021) Up-to-date colorectal cancer screening status for included average-risk individuals was evaluated for 2015, 2016, 2017, and 2018 within four cross-sectional study samples based on eligibility described above (Supplementary Fig. 1). Baseline demographic and clinical characteristics were evaluated for the 12-month period preceding the study analysis year. In addition, the effect of including higher-risk individuals on estimates was assessed.
2.3. Sensitivity analyses
2.3.1. Primary sensitivity analysis (retrospective cohort study)
The sensitivity of study design estimates was assessed within the sub-cohort of individuals who turned 60 years old during the calendar year 2018 and had continuous medical and pharmacy benefit eligibility for the 10-year period preceding 2018 (Supplementary Fig. 1). Individuals at higher colorectal cancer risk were excluded based on the same criteria used for the repeated cross-sectional study samples.
2.3.2. Secondary sensitivity analysis
Individuals who met continuous eligibility criteria for the repeated-cross sectional study samples during each calendar year, but who had ≥ 1 condition identified as higher risk for colorectal cancer were evaluated in a secondary sensitivity analysis within the repeated cross-sectional study to evaluate the impact of including individuals at elevated colorectal cancer risk in the sample estimates of up-to-date screening. For this analysis, individuals were classified as ‘higher risk’ or not based on the presence or absence of any these conditions during the continuous eligibility period.
2.4. Study measures
For both the main analyses and sensitivity analyses, the central outcome measure was up-to-date status (yes/no) per ACS and USPSTF guidelines for average-risk colorectal cancer screening (Davidson et al., 2021, Wolf et al., 2018), defined as having met ≥ 1 of the following conditions: 1) colonoscopy during the prior 10-year period, 2) computed tomography colonography or flexible sigmoidoscopy during the prior five-year period, 3) mt-sDNA during the prior three-year period, or 4) FIT or FOBT during the prior 15-month period. For the FIT and FOBT, while the guidelines recommend annual measurements, a 15-month period was included to allow for variability and access in scheduling. In addition to overall up-to-date status with colorectal cancer screening, specific screening types employed by individuals to qualify as up to date were evaluated using ICD-9/ICD-10 and procedural codes from administrative claims data (Supplementary Table 2). For the main analysis, screening rates were calculated, both overall and via different screening modalities, for each calendar year 2015–2018 as follows. The numerator was defined as the number of individuals with evidence of colorectal cancer screening during their eligibility period preceding the calendar year. Individuals were counted as screened if they either received screening in that year or had received screening in the previous period. For the sensitivity analysis, screening rates were calculated for the retrospective cohort based on evidence of screening codes identified during the 10-year period prior to 2018.
Baseline demographic characteristics were evaluated among both the repeated cross-sectional and retrospective cohort study samples. These characteristics included age (age during calendar year of analysis [cross-sectional sample] or during 2018 [cohort sample]), gender (ie, male, female), United States census region, race/ethnicity, and educational level.
2.5. Statistical analysis
Descriptive statistics for baseline demographic characteristics included frequencies and percentages for categorical variables and means and standard deviations for continuous variables. For the central outcome variable within the repeated cross-sectional analysis, Kaplan-Meier estimates were created to adjust for the censoring inherent in the variable baseline period based on continuous enrollment. Kaplan-Meier methods were used to look backward in time (rather than forward in time) from the year of analysis to allow estimates accounting for incomplete observation and varying age-based screening eligibility during the prior 10 years. For example, in assessing the use of colonoscopy in the 10 years prior to the year of analysis, individuals with less than 10 years of observation who did not have a colonoscopy during their observed time were counted as censored. Rates of colorectal cancer screening were calculated as proportions in the sensitivity analyses.
3. Results
3.1. Study attrition and demographic characteristics
Supplementary Fig. 2 includes study sample attrition data, based on eligibility criteria, for both the repeated cross-sectional samples and retrospective cohort sample. For the main repeated cross-sectional study samples, 2.6 million, 2.9 million, 3.3 million, and 2.8 million individuals were included in calendar years 2015, 2016, 2017, and 2018, respectively (Supplementary Fig. 2). Within the retrospective cohort sample (sensitivity analysis), 11,398 individuals met all inclusion/exclusion and 10-year continuous eligibility criteria prior to 2018 (Supplementary Fig. 2). In the secondary sensitivity analysis, inclusion of higher-risk individuals increased the sample size to 3,116,282 in 2015; 3,556,826 in 2016; 4,017,444 in 2017; and 3,544,359 in 2018.
Demographic characteristics for each cross-sectional study sample are included in Table 1. Subject samples defined by calendar year of analysis (2015–2018) were comparable relative to the distribution of demographic characteristics. Among the 2018 study sample, 18.7% were aged 50–54 years and 53.2% were female. Almost half of individuals resided in the southern United States (45.9%), while 26.1% lived in the Midwest, 13.4% in the west, and 13.0% in the northeast. Almost 3 in 4 individuals (66.0%) were White, while about 1 in 10 were African American (10.5%) or Hispanic (9.6%), and 3.1% Asian. Most individuals (48.9%) had some college or a bachelors or advanced degree. Distributions were similar in the other calendar years, with a slightly higher proportion of younger individuals in the earlier years. Demographic characteristics for patients in the retrospective cohort sample are included in Supplementary Table 3.
Table 1.
Demographic characteristics for main study population (cross-sectional population).
| Demographics | 2015 (n = 2,579,898) | 2016 (n = 2,948,064) | 2017 (n = 3,312,882) | 2018 (n = 2,752,864) |
|---|---|---|---|---|
| Age category, n (%) | ||||
| 50–54 | 597,935 (23.2) | 622,149 (21.1) | 638,779 (19.3) | 515,710 (18.7) |
| 55–59 | 534,206 (20.7) | 579,951 (19.7) | 614,424 (18.6) | 503,810 (18.3) |
| 60–64 | 424,763 (16.5) | 480,084 (16.3) | 519,173 (15.7) | 439,506 (16.0) |
| 65–69 | 520,611 (20.2) | 624,883 (21.2) | 739,186 (22.3) | 540,795 (19.6) |
| 70–74 | 446,986 (17.3) | 571,841 (19.4) | 709,578 (21.4) | 644,220 (23.4) |
| 75 | 55,397 (2.2) | 69,156 (2.4) | 91,742 (2.8) | 108,823 (4.0) |
| Gender, n (%) | ||||
| Female | 1,364,791 (52.9) | 1,568,333 (53.2) | 1,763,513 (53.2) | 1,465,315 (53.2) |
| Male | 1,215,107 (47.1) | 1,379,731 (46.8) | 1,549,369 (46.8) | 1,287,549 (46.8) |
| Region, n (%) | ||||
| Northeast | 340,098 (13.2) | 367,013 (12.5) | 431,521 (13.0) | 356,832 (13.0) |
| Midwest | 771,469 (29.9) | 844,975 (28.7) | 874,707 (26.4) | 717,317 (26.1) |
| South | 1,086,944 (42.1) | 1,291,803 (43.8) | 1,515,177 (45.7) | 1,263,785 (45.9) |
| West | 372,065 (14.4) | 413,384 (14.0) | 444,000 (13.4) | 369,117 (13.4) |
| Other | 1,144 (0.04) | 1,391 (0.1) | 1,817 (0.1) | 1,555 (0.1) |
| Missing | 8,178 (0.3) | 29,498 (1.0) | 45,660 (1.4) | 44,258 (1.6) |
| Race/ethnicity, n (%) | ||||
| White | 1,865,504 (72.3) | 2,044,677 (69.4) | 2,193,095 (66.2) | 1,816,579 (66.0) |
| African American | 251,776 (9.8) | 322,787 (11.0) | 353,392 (10.7) | 290,071 (10.5) |
| Asian | 85,314 (3.3) | 93,405 (3.2) | 100,133 (3.0) | 85,540 (3.1) |
| Hispanic | 225,053 (8.7) | 273,018 (9.3) | 317,711 (9.6) | 263,884 (9.6) |
| Other | 18,135 (0.7) | 19,628 (0.7) | 20,715 (0.6) | 17,296 (0.6) |
| Unknown or missing | 134,116 (5.2) | 194,549 (6.6) | 327,836 (9.9) | 279,494 (10.2) |
| Education, n (%) | ||||
| Less than 12th grade | 8,426 (0.3) | 11,201 (0.4) | 12,457 (0.4) | 9,740 (0.4) |
| High school diploma | 677,058 (26.2) | 847,063 (28.7) | 941,227 (28.4) | 775,459 (28.2) |
| Some college or Associate degree |
1,362,492 (52.8) | 1,488,959 (50.5) | 1,623,043 (49.0) | 1,346,199 (48.9) |
| Bachelor/graduate or professional degree |
444,534 (17.2) | 472,497 (16.0) | 514,549 (15.5) | 434,423 (15.8) |
| Unknown or missing | 87,388 (3.4) | 128,344 (4.4) | 221,606 (6.7) | 187,043 (6.8) |
3.2. Study outcome measures
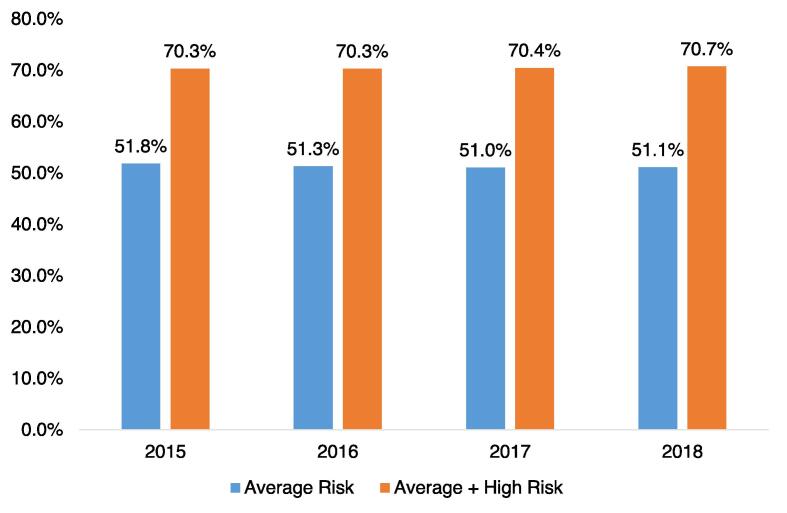
In the cross-sectional study sample for 2018, overall, approximately half (51.1%) of those aged 50–75 years who were at average risk for colorectal cancer were up to date with colorectal cancer screening recommendations during the prior 10 years (Fig. 1). Estimates of up-to-date colorectal cancer screening were consistent across time periods: 51.8% in 2015, 51.3% in 2016, and 51.0% in 2017 and 51.1% in 2018. Inclusion of individuals with ≥ 1 condition defined as higher risk for colorectal cancer increased estimates to about 2 in 3 individuals, and these proportions were similar across all time periods: 70.3% in 2015, 70.3% in 2016, 70.4% in 2017, and 70.7% in 2018 (Fig. 1).
Fig. 1.
Kaplan-Meier rates of adherence with colorectal cancer screening guidelines based on population risk of colorectal cancer, 2015–2018: main study and secondary sensitivity analysis (cross-sectional population).
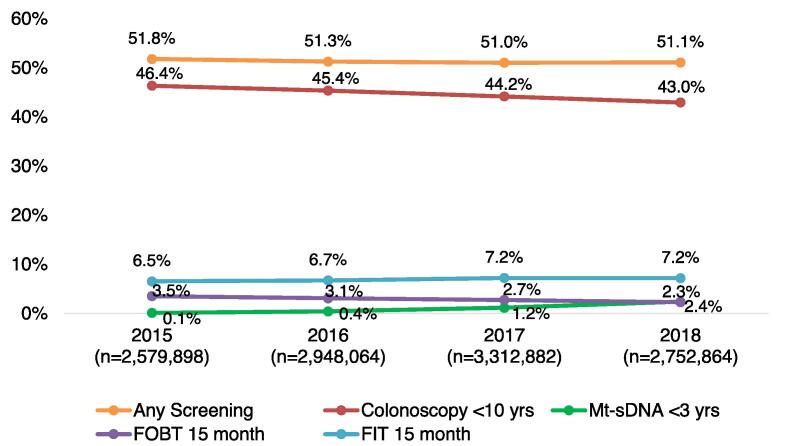
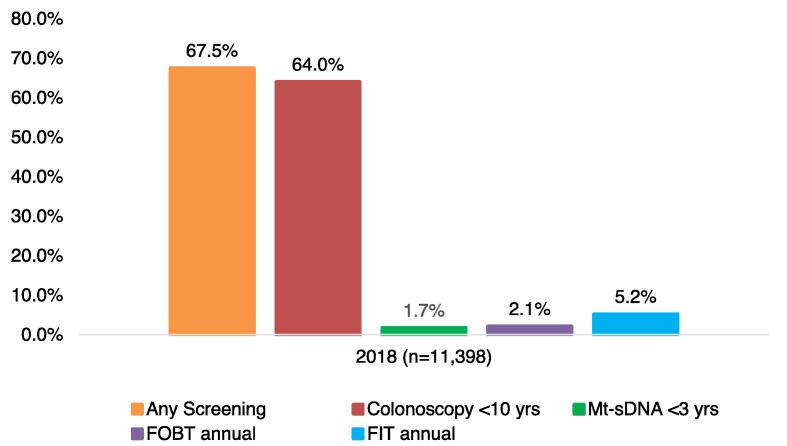
In the cross-sectional study, about half of average-risk individuals aged 50–75 did not have a colonoscopy or other screening within the prior 10 years (Fig. 2). In 2018, 43.0% of the eligible average-risk population had a colonoscopy within the prior 10 years, 2.4% had mt-sDNA every-three years, 7.2% had FIT annually, and 2.3% had FOBT annually. Results of the sensitivity analysis using the retrospective cohort sample suggest that estimates of population-level colorectal cancer screening compliance are highly sensitive to the study design used: among the cohort of 60-year-old individuals, 67.5% were up to date with colorectal cancer screening, 64.0% had colonoscopy over a 10 year-year period, 1.7% had mt-sDNA every-three years, 5.2% had FIT annually, and 2.1% had FOBT annually (Fig. 3).
Fig. 2.
Kaplan Meier adherence with colorectal cancer screening tools among average-risk colorectal cancer population, 2015–2018: main analysis (cross-sectional population).
Fig. 3.
Compliance with screening among average-risk colorectal cancer population, 2018: primary sensitivity analysis (retrospective cohort).
4. Discussion
Colorectal cancer is among the most common cancers occurring in the United States, and early detection has important implications for public health, improved survival, and healthcare costs. Thus, colorectal cancer screening rates are an important public health and healthcare system quality metric; however, the results of this measure are highly driven by the specific characteristics of the population included in the measurement and the specific design of the measure. Prior research has estimated that 58–65% of the average-risk American population are up to date with recommended colorectal cancer screenings (Cyhaniuk and Coombes, 2016, Inadomi et al., 2012, Sinicrope et al., 2012), and since individuals at high risk of colorectal cancer may be screened more frequently, studies that have included individuals with one or more conditions associated with increased colorectal cancer risk (eg, polyps, family gastrointestinal cancer history) may not accurately reflect screening rates among a ‘true’ average-risk population. The National Committee for Quality Assurance as part of the Healthcare Effectiveness Data and Information Set (HEDIS) has estimated that in 2018, 64.1% of commercial health maintenance organizations and 60.3% of commercial preferred provider-insured individuals aged 50–75 years were up to date with recommended colorectal cancer screenings (National Committee for Quality Assurance, 2020). However, HEDIS estimates are derived using medical exclusionary criteria for identifying the at-risk population that only specifies a history of colorectal cancer or total colectomy, and thus HEDIS estimates include other conditions that predispose individuals to higher colorectal cancer risk. In the current study, using a cross-sectional study design, we estimated that slightly fewer than half of insured individuals at average colorectal cancer risk were up to date with ACS-recommended colorectal cancer screening in 2018, and that this estimate was consistent between 2015 and 2018, despite the improved availability of newer screening procedures. With the administrative burdens of the United States healthcare system, even if we (as a healthcare system) are unable to generate numbers at the health plan level, the average risk measure is useful at a national or regional level to monitor the average-risk in claims data to measure improvements, or lack thereof, in public health.
The purpose of our analyses was driven in part by the uncertainty around the discussion of screening rates in both the popular press and the literature, including discussions of screening guidelines. In the current study, the cohort study design consisted of a population who were both eligible for and observed for a full 10 years of follow-up regarding their colorectal cancer screening (information was available for a full 10-year history of screening procedures). As all patients in the cohort were of the same age and had the same length of “exposure” to screening eligibility, the calculated numbers for this cohort represent the cumulative screening within that population after 10 years of being eligible for screening. By contrast, the study population in the cross-sectional analysis reflects the full population eligible for screening in a calendar year, just as health plans and public health measures assess at a population level all eligible patients for screening. This includes patients who have only been eligible for screening for any number of years less than 10 (eg, 55-year-olds with only five years of screening eligibility). Our analyses demonstrate that we can both replicate the results found in previously published literature and estimate a more applicable measure for the average-risk population.
Our sensitivity analyses suggest that estimates of population-level colorectal cancer screening rates are impacted by inclusion and exclusion criteria and epidemiological methods. Inclusion of individuals with a higher-risk condition increased our estimates of up-to-date screening status by about 37%; a similar increase in up-to-date colorectal cancer screening estimates was observed by using a retrospective cohort design (versus cross-sectional). A cohort design may produce higher estimates since the inclusion criteria requires individuals to be continuously enrolled in a healthcare plan for a long period (10 years in the current study) and represents a cumulative rate. This could influence population screening estimates in several ways. For example, selection bias may play a role, as individuals who are continuously enrolled in a health plan for a long time period may differ systematically from those who are not; they may have more stable employment or higher socioeconomic status than those who enrolled for a shorter time period, which may also influence the likelihood of receiving screening. Regardless, our findings suggest that prior studies may have overestimated the proportion of insured average-risk individuals over 50 years of age who are up to date with recommended colorectal cancer screenings in the United States.
Our study did not find an increase in colorectal cancer screening rates with more recently available screening tests. Mt-sDNA, a non-invasive screening method that detects DNA markers and blood in stool, was made available in the United States in 2014, which marked the beginning of our study. We observed an increase in use of mt-sDNA in our repeated cross-sectional study, from 0.1% of average-risk persons in 2014 to 2.4% in 2018. This finding warrants further investigation on whether mt-sDNA may have been used among those who would have otherwise undergone another screening method or among individuals who would have otherwise remained unscreened.
While our study has several strengths, including the large sample size afforded by the administrative claims database and the use of different epidemiological methods to explore our results, our findings should be interpreted in the context of the study limitations. Our findings represent insured individuals and may be specific to the database used for the research, although the ORD represents about 19% of the United States commercially insured and 17% of the Medicare Advantage population and has a similar age distribution as the overall United States insured population. The presence of a diagnosis code on a medical claim is not definitive confirmation of disease status. In addition, comprehensive information on individuals was unavailable in the claims data, and some of those unmeasured characteristics may have been associated with our outcome measures. For example, patients with high-risk conditions who did not use health care may not have shown up in the claims data. Additionally, there may have been average-risk individuals included in the denominator with limited life expectancy, such as those with advanced cancer (other than colorectal cancer), who may have been ineligible for colorectal cancer screening due to their condition. The presence of certain conditions in the claims data were used to identify high-risk patients. It is possible that some high-risk patients were misclassified, which likely would have artificially increased the screening rates estimated in this study for the average-risk population. Lastly, the list of codes used to identify screening tests may vary by study, further complicating the ability to make comparisons between studies.
4.1. Conclusions
Results suggest that most of the United States population at average risk of colorectal cancer are not adherent with current ACS and USPSTF guidelines. Previous studies may have overestimated the true screening rate for colorectal cancer in the United States among insured, average-risk individuals. The populations studied and analysis methods used likely contribute to the variation in reported estimates of colorectal cancer screening adherence. Inclusion of individuals with a condition that has an increased risk of colorectal cancer has a substantial impact on population estimates of colorectal cancer screening. Efforts to track screening rates need transparency in their measurement methods to accurately evaluate progress in improving screening rates.
5. Disclosures
NMEN is currently employed by Optum and holds stock in UnitedHealth Group and AbbVie. LAMW is an employee of Exact Sciences and holds stock in Sanofi and Exact Sciences. LL is currently employed by Optum and holds stock in UnitedHealth Group. PL served as the Chief Medical Officer for Screening at Exact Sciences through a contracted services agreement with Mayo Clinic at the time the study was conducted and is currently employed by Exact Sciences. DAF was employed by Duke University at the time the study was conducted and is currently employed at Eli Lilly and Company and has served as a consultant for Exact Sciences and Guardant Health.
6. Ethics statement
Institutional Review Board approval or waiver of authorization was not required as no identifiable protected health information was accessed during this study.
CRediT authorship contribution statement
Nicole M. Engel-Nitz: Conceptualization, Methodology, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing. Lesley-Ann Miller-Wilson: Conceptualization, Writing – review & editing. Lisa Le: Formal analysis, Validation, Data curation, Software, Writing – review & editing. Paul Limburg: Conceptualization, Writing – review & editing. Deborah A. Fisher: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: This work was supported by Exact Sciences Corporation.
Acknowledgements
Authors would like to acknowledge Felix Cao, Lynn Wacha, Priyanka S. Koka, Yiyu Fang, Jaycee L. Karl, and Damon L. Van Voorhis for their assistance with programming and construction of the analytic datasets; Timothy Bancroft and Lee Brekke for consultation on the analyses; and Deja Scott-Shemon and Jenifer Wogen for medical writing assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.102082.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- American Cancer Society. Colorectal Cancer Facts & Figures. Atlanta, GA2020.
- Chastek B., Kulakodlu M., Valluri S., Seal B. Impact of metastatic colorectal cancer stage and number of treatment courses on patient health care costs and utilization. Postgrad. Med. 2013;125(2):73–82. doi: 10.3810/pgm.2013.03.2642. [DOI] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): colorectal cancer screening version 2.2021. National Comprehensive Cancer Network. https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf. Accessed January 28, 2022.
- Cyhaniuk A., Coombes M.E. Longitudinal adherence to colorectal cancer screening guidelines. Am. J. Manag. Care. 2016;22(2):105–111. (https://www.ncbi.nlm.nih.gov/pubmed/26885670 [PubMed] [Google Scholar]
- Davidson K.W., Barry M.J., Mangione C.M., Cabana M., Caughey A.B., Davis E.M., Donahue K.E., Doubeni C.A., Krist A.H., Kubik M., Li L.i., Ogedegbe G., Owens D.K., Pbert L., Silverstein M., Stevermer J., Tseng C.-W., Wong J.B. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325(19):1965. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- Inadomi J.M., Vijan S., Janz N.K., et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch. Intern. Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D.A., King J.B., Dowling N.F., Thomas C.C., Richardson L.C. Vital signs: Colorectal cancer screening test use - United States, 2018. MMWR Morb. Mortal Wkly. Rep. 2020;69(10):253–259. doi: 10.15585/mmwr.mm6910a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limburg P.J., Finney Rutten L.J., Ozbay A.B., Kisiel J., Parton M. Recent trends in colorectal cancer screening methods based on Medicare claims data. Curr. Med. Res. Opin. 2021;37(4):605–607. doi: 10.1080/03007995.2021.1879754. [DOI] [PubMed] [Google Scholar]
- National Committee for Quality Assurance. Colorectal Cancer Screening. 2020.
- National Comprehensive Cancer Network. NCCN Guidelines Colorectal Cancer Screening. 2020.
- Patel S.G., May F.P., Anderson J.C., Burke C.A., Dominitz J.A., Gross S.A., Jacobson B.C., Shaukat A., Robertson D.J. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. multi-society task force on colorectal cancer. Am. J. Gastroenterol. 2021 doi: 10.14309/ajg.0000000000001548. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Sinicrope P.S., Goode E.L., Limburg P.J., et al. A population-based study of prevalence and adherence trends in average risk colorectal cancer screening, 1997 to 2008. Cancer Epidemiol. Biomarkers Prev. 2012;21(2):347–350. doi: 10.1158/1055-9965.EPI-11-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A.M.D., Fontham E.T.H, Church T.R., Flowers C.R., Guerra C.E., LaMonte S.J., Etzioni R., McKenna M.T., Oeffinger K.C., Shih Y-C.T., Walter L.C., Andrews K.S., Brawley O.W., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Siegel R.L., Wender R.C., Smith R.A. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018;68:250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.