Abstract
Background
It is undetermined how effective superior capsule/capsular reconstruction (SCR) is, and which factors influence clinical outcomes.
Questions/purposes
(1) To identify which factors influence outcomes in SCR, (2) to evaluate the effect of graft integrity on clinical outcomes, and (3) to compare SCR to other procedures for irreparable rotator cuff tears.
Methods
PubMed and EMBASE databases were searched for clinical SCR studies. Data on specific factors that influenced outcomes, that compared outcomes between intact/torn graft groups, or compared SCR to alternative treatments for irreparable tears were extracted by two investigators. Random-effects meta-analysis was performed to compare outcomes between intact vs torn SCR grafts.
Results
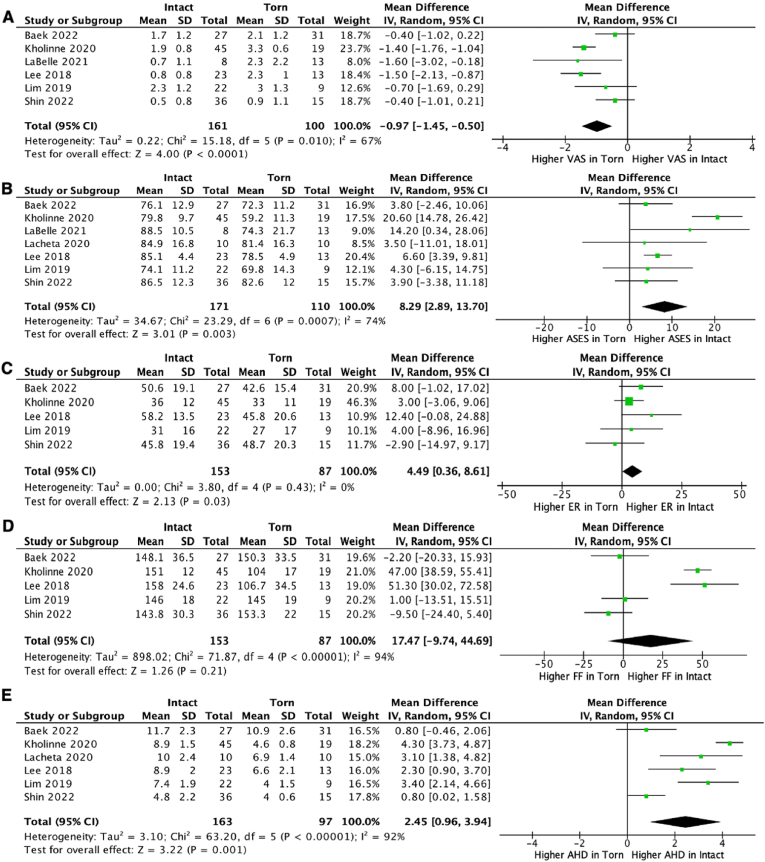
394 articles were identified. 100 full-text articles were screened. 13 studies were included for scoping review. Eight studies were meta-analyzed. Better clinical scores were found in younger patients, with intact/repairable subscapularis, without acetabulization/arthritis, who played sports. In patients with irreparable tears without arthritis, SCR produced similar clinical scores at 2 years as shoulder arthroplasty and partial infraspinatus repair, and greater improvements in ASES and Constant scores than latissimus dorsi tendon transfer. Intact grafts produced better VAS (mean difference [MD] = 0.97, 95% confidence interval [-1.45–0.50], P < 0.0001, I2 = 67%, n [patients] = 261), ASES (MD = 8.29, [2.89–13.70], P = 0.003, I2 = 74%, n = 281), external rotation (MD = 4.49, [0.36–8.61], P = 0.03, I2 = 0%, n = 240), and acromiohumeral distance (MD = 2.45, [0.96–3.94], P = 0.001, I2 = 92%, n = 260) than torn grafts.
Conclusions
Patients who underwent SCR for irreparable rotator cuff tears were more likely to have better clinical outcomes if they were younger, had intact/repairable subscapularis, without acetabulization/arthritis, played sports and had intact grafts.
Keywords: Superior capsular reconstruction, Massive rotator cuff tear, Irreparable rotator cuff tear, Arthroscopy, SCR
1. Introduction
Massive, irreparable rotator cuff tears are difficult injuries to treat, and there are various approaches to their management. These have included debridement, tendon transfers, interposition grafts, shoulder arthroplasty and superior capsular reconstruction (SCR).1, 44
Mihata et al.2 first described SCR in 2012, utilizing a tensor fascia lata autograft that was anchored to the glenoid medially, and to the greater tuberosity laterally in eight human cadaveric shoulders. SCR effectively restored superior humeral head stability and reduced subacromial contact pressure when compared to a torn state.
Several studies have shown that patients who underwent SCR experienced improved clinical and radiological outcomes from the preoperative to 2-year postoperative period, with one study reporting that 90% of postoperative survey respondents were satisfied, whilst another study reported that 65% of patients experienced persistent pain and/or lack of shoulder function.3, 4, 5, 6, 7 Mihata et al.6 reported on 30 patients with 5-year follow-up, where 90% of patients had intact grafts, and all of whom were satisfied. In contrast, all patients with failed grafts developed severe cuff arthropathy. The association between graft failure and worse outcomes was also reported by Denard et al.8 and Woodmass et al.5
How SCR compares to other treatments for irreparable tears amongst patients with the same inclusion criteria is undetermined. The aims of this study were to review SCR clinical studies to determine (1) which specific factors influence clinical outcomes in SCR patients, (2) to investigate the association between graft integrity and clinical outcomes in SCR, and (3) how SCR compared to alternative treatments for irreparable rotator cuff tears in studies where both the SCR and alternative treatment groups had the same inclusion criteria.
2. Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines (Supplement File 1).9, 15 PubMed and EMBASE databases were searched in April 2022 using the following terms: (superior capsule reconstruction OR superior capsular reconstruction) AND (clinical studies OR clinical study OR follow-up OR follow up). Duplicates were identified with Endnote X9 (Ver 3.3, Clarivate Analytics, London, UK). The remaining records were screened by two independent investigators based on their titles and abstracts for relevance. Reference lists of reviews returned by the search were screened for studies that were not captured during title/abstract screening. The full texts of selected papers were obtained and evaluated by the investigators. The results were compared and discussed.
2.1. Inclusion and exclusion criteria
The inclusion criteria for this study were primary clinical studies involving SCR patients treated with human dermal allograft and/or fascia lata graft. The results were limited to articles with full texts in the English language, published from 2012 onwards, when Mihata et al.2 first described SCR. Studies needed to have reported at least one clinical score or functional outcome between torn and intact SCR groups to be eligible for inclusion into quantitative review. Laboratory studies, book chapters, commentaries, discussions, letters, technical notes, and case series or cohort studies where there were <5 SCR patients were excluded.
2.2. Scoping review protocol
For a scoping review of factors influencing clinical outcomes in SCR, the full texts of studies that identified patient or operative factors that improved/worsened outcomes following SCR were independently summarized by two investigators to identify factors that influenced clinical or functional outcomes. Studies that compared SCR to other treatments for irreparable tears were included if inclusion criteria for SCR and non-SCR treatment groups were the same, and if there was a minimum of 2-years follow-up.
2.3. Meta-analysis protocol
For quantitative review of the clinical effect of intact versus torn SCR grafts, studies that reported clinical or functional outcomes in torn and intact graft groups with >5 patients per cohort, with at least 12 months follow-up were included. Data reported in torn and intact groups were extracted without assumption or simplification during full text review. Random-effects meta-analysis was performed to compare ASES, VAS, forward flexion, external rotation and acromiohumeral distance (mean ± SD) between intact and torn SCR graft groups using Review Manager (Ver 5.4, The Cochrane Collaboration, London, UK). The pooled outcomes were reported as raw mean difference (95% CI).
Where a study reported an outcome of interest in subgroups of either intact or torn groups, the data was then combined using the formulae for combining summary statistics across groups from Section 6.5.2.10 of the Cochrane Handbook for Systematic Reviews of Interventions.10
2.4. Quality appraisal
Levels of evidence were appraised per Oxford Center for Evidence-based Medicine (OCEBM) guidelines.11 Respective Joanna Briggs Institute (JBI) checklists were used to assess the methodological quality and risk of bias in each of the included studies (Table 1).12, 13, 14 Quality appraisal was performed by an independent investigator, and verified by the senior investigator.
Table 1.
Conversion table for JBI checklists for each respective study design. I: High methodological quality and low risk of bias; II: moderate methodological quality and risk of bias, III: low methodological quality and high risk of bias.
| Study Design | JBI Checklist Score Range | Grade |
|---|---|---|
| Randomized Controlled Trial | 0–13 |
I: 11-13 II: 8-10 III: 0-7 |
| Quasi-Experimental Studies | 0–9 |
I: 8-9 II: 6-7 III: 0-5 |
| Cohort Studies | 0–11 |
I: 10-11 II: 8-9 III: 0-7 |
| Case Series | 0–10 |
I: 9-10 II: 7-8 III: 0-6 |
3. Results
3.1. Study selection
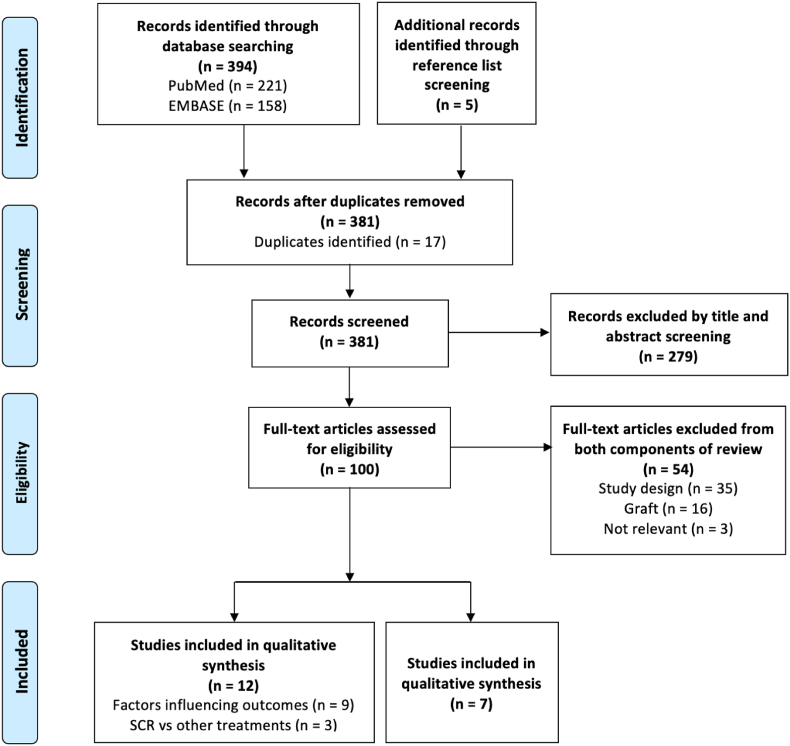
Our PubMed and EMBASE database searches returned 394 scientific articles. Five articles were identified through reference list screening. 381 articles remained after 17 duplicates were removed. 279 articles were excluded by title and abstract screening for relevance, leaving 100 articles for full-text screening. 54 articles were excluded based on exclusion criteria (Fig. 1), leaving 46 eligible articles.
Fig. 1.
PRISMA study selection flowchart.1
For qualitative review, 33 articles were excluded because no specific factors that significantly influenced clinical outcomes in SCR were identified, two articles because the outcomes assessed were non-clinical (histological and radiological studies, respectively), one article because it investigated the same factor as another included study, but did not have a control group and had a smaller cohort, and one study because it was underpowered, leaving nine studies for inclusion for review of factors that influenced SCR clinical outcomes. Five articles (from the eligible 46 articles) compared SCR to alternative treatments for irreparable rotator cuff tears, one article was excluded as the minimum follow-up was <2 years, and one article because there were different inclusion criteria between SCR and alternative treatment groups, leaving three studies for inclusion. Therefore, a total of 12 studies were included in scoping review.
For quantitative review, 14 studies reported outcomes in intact and torn SCR graft groups. Four studies were excluded because they had an intact or torn SCR graft group with ≤5 patients, and two studies because they had overlapping enrolment periods with other included studies that were conducted at the same institutions, but had smaller cohort sizes, and one study because the minimum follow-up period was <2 years. Thus, seven studies were included for meta-analysis.
The reasons for inclusion and exclusion of all studies based on full-text review are outlined in Supplement File 2.
3.2. Characteristics of included studies
Included studies were published between 2018 and 2022. There was one Level II randomized controlled trial,16 nine Level III cohort studies,17, 18, 19, 20, 21, 22, 23, 24, 25 and eight Level IV case series.8,26, 27, 28, 29, 30, 31, 32 All included studies had I: high methodological quality and low risk of bias, in context of their level(s) of evidence (Table 2).
Table 2.
Quality appraisal results of included studies.
| Study ID | Country | Level of Evidence | JBI Checklist | JBI Grade (Score) | Review Component |
|---|---|---|---|---|---|
| Ozturk 202116 | Turkey | II | RCT | I (11/13) | Qualitative |
| Greiner 202117 | Germany | III | Quasi-Experimental | I (9/9) | Qualitative |
| Kholinne 202018 | South Korea | III | Quasi-Experimental | I (8/9) | Qualitative & Quantitative |
| Kholinne 202119 | South Korea | III | Quasi-Experimental | I (9/9) | Qualitative |
| Lacheta 2020b20 | USA | III | Quasi-Experimental | I (8/9) | Qualitative |
| Mihata 201821 | Japan | III | Cohort Study | I (11/11) | Qualitative |
| Mihata 202022 | Japan | III | Cohort Study | I (11/11) | Qualitative |
| Takayama 202023 | Japan | III | Cohort Study | I (11/11) | Qualitative |
| Takayama 202124 | Japan | III | Cohort Study | I (9/11) | Qualitative |
| Denard 20188 | USA | IV | Case Series | I (9/10) | Qualitative |
| Evuarherhe 202226 | USA | IV | Case Series | I (10/10) | Qualitative |
| Gilat 202127 | USA | IV | Case Series | I (9/10) | Qualitative |
| LaBelle 202125 | USA | III | Cohort Study | I (10/11) | Quantitative |
| Baek 202128 | South Korea | IV | Case Series | I (9/10) | Quantitative |
| Lacheta 2020a29 | USA | IV | Case Series | I (9/10) | Quantitative |
| Lee 201830 | South Korea | IV | Case Series | I (9/10) | Quantitative |
| Lim 201931 | South Korea | IV | Case Series | I (9/10) | Quantitative |
| Shin 202232 | South Korea | IV | Case Series | I (10/10) | Quantitative |
3.3. SCOPING REVIEW: factors contributing to superior outcomes in SCR
3.3.1. Patient factors
3.3.1.1. Age
A cohort study by Kholinne et al.19 grouped fascia lata SCR patients by age, followed-up 36 patients aged <65 years (mean 59.2 years) and 37 patients aged >65 years (mean 70.5) for minimum 2-years. Significant differences between the younger and older groups were found in graft thickness (6.5 mm vs 5.9 mm) (p = 0.04), graft healing rate (81% vs 65%) (p = 0.049) and defect size in the event of graft failure (133 mm2 vs 198 mm2) (p = 0.032) respectively at final follow-up.
A retrospective cohort study of 58 SCR patients with mean 23 months follow-up by Evuarherhe et al.26 utilized a distribution-based approach to define thresholds for clinically significant outcomes, based off preoperatively collected clinical scores (ASES, SANE, and Constant scores). They found that increased age reduced odds of achieving SANE scores that met the ‘substantial clinical benefit’ threshold postoperatively.
3.3.1.2. Subscapularis
A cohort study by Mihata et al.22 investigated the effect of subscapularis on SCR by allocating patients to one of the following groups: SCR with no subscapularis tear (n = 160), SCR with repaired subscapularis (n = 26), or SCR with irreparable subscapularis tear (n = 7). The SCR with no subscapularis tear and SCR with repaired subscapularis groups experienced significant improvements in JOA (54–93; 49–93), ASES (36–94; 33–94), VAS (7.0–0.7; 6.6–0.5) scores, active elevation (97°-155°; 88°-157°), active external rotation (27°-42°; 27°-46°), active internal rotation (L4-L1; S-L2) and manual muscle test strength scores in abduction (3+ to 5-; 3+ to 5-), external rotation (4- to 5-; 3+ to 5-) and internal rotation (4+ to 5-; 4+ to 5) from preoperative to final follow-up respectively, with no difference between groups. The SCR with irreparable subscapularis tear group only experienced improvements in JOA (54–93), ASES (36–19) and VAS (7.0–0.7) scores, without any improvement in range of motion or strength.
Rates of graft tear were significantly higher in both repaired subscapularis (12%; 3/26 patients) (p < 0.01) and irreparable subscapularis (43%; 3/7 patients) (p < 0.001) groups than in the no subscapularis tear group (2%; 3/160 patients). The infection rate was significantly higher in the irreparable subscapularis group (57%; 4/7 patients) than in the no subscapularis tear (1%; 2/160 patients) (p < 0.001), or reparable subscapularis (0%; 0/26 patients) tear groups (p < 0.001).
A cohort study by Takayama et al.23 divided patients with irreparable supraspinatus and infraspinatus tears into groups with either intact subscapularis (n = 12), repairable subscapularis (n = 11), or irreparable subscapularis tear (n = 4) for minimum 2-years follow-up. No patients with irreparable subscapularis recovered from pseudoparalysis, whilst all pseudoparalytic patients with intact or repairable subscapularis recovered. Postoperative flexion (70° vs 153°, 155°) (p = 0.003 for both), abduction (68° vs 148°, 140°) (p = 0.003, p = 0.004 respectively) and internal rotation (L4 vs T10, T11) (p = 0.003, p = 0.004 respectively) were significantly lower in patients with irreparable subscapularis than in patients with intact or repairable subscapularis respectively.
Gilat et al.27 compared demographic, clinical and radiographic factors between successful (n = 43) and clinically-failed SCR (n = 11) in a series of 54 patients with mean 24 months follow-up. Clinical failure was defined by requiring conversion to RTSA, a decrease in shoulder-specific patient-ranked outcomes at 1 year versus preoperative scores, or patient reporting that their shoulder was in a worse condition than it was preoperatively. They found that a significantly greater proportion of patients with clinical failures had subscapularis tears (46% vs 16%) (p = 0.029) and were female (64% vs 26%) (p = 0.023) compared to successful SCR patients.
The study by Evuarherhe et al.26 that defined clinically significant outcomes in 58 SCR patients also found that patients with subscapularis tears were less likely to achieve a minimum clinically important difference (MCID) in Constant and SANE scores on univariate analysis.
3.3.1.3. Acetabulization and/or arthritis
A prospective study of 59 patients with minimum 1-year follow-up by Denard et al.8 found that 75% of patients with Hamada Grade 1 and 2 tears, characterized by acromiohumeral distances of ≥6 mm and ≤5 mm respectively, had a successful SCR. This was defined as a postoperative ASES >50 with ≥17 points improvement, without requiring revision surgery or reverse total shoulder arthroplasty (RTSA). The success rate fell to 44% in patients with Hamada Grade 3 and 4 patients, characterized by acromiohumeral distances of ≤5 mm with acetabulization, and glenohumeral arthritis respectively.33
3.3.1.4. Prior sports participation
A cohort study in 100 patients with mean final follow-up of 48 months by Mihata et al.21 divided patients into sports (n = 26) versus non-sports (n = 74) and physical work (n = 34) versus non-physical work (n = 66) groups. Significant differences were found between the sports group and the non-sports group in active elevation (160° vs 146°) (p = 0.04) and ASES scores (97 vs 91) (p = 0.02) at final follow-up. There were no significant differences between the physical work and non-physical work groups. 26/26 sports patients fully returned to sports, and 32/34 physical work patients returned fully to work, whilst 2/34 physical work patients returned with reduced hours or workloads.
3.3.2. Operative factors
3.3.2.1. Mesh augmentation
A retrospective cohort study by Kholinne et al.18 compared patients treated with fascia lata SCRs with polypropylene mesh augmentation (n = 30) to patients treated with fascia lata SCRs without mesh augmentation (n = 34), with a mean 31 and 24 months follow-up respectively. Mesh augmentation was performed by inserting a single sheet of polypropylene mesh (Prolene Mesh, Ethicon Inc., Raritan, NJ, USA) between the layers of the folded fascia lata graft, which was closed in a sandwich fashion and secured around the three open margins using a running 2–0 polyester suture (Ethibond, Ethicon Inc., Raritan, NJ, USA). They found significantly greater improvements in mean ASES (29 vs 18) (p = 0.006), active forward flexion (40° vs 28°) (p = 0.003), active external rotation (11° vs 6°) (p = 0.004), graft healing rate (83% vs 59%) (p = 0.039) and acromiohumeral distance (9 mm vs 6 mm) (p = 0.001) at final follow-up in the mesh augmented group than in the non-mesh augmented group. Rates of graft failure were significantly lower in the mesh augmented group than in the non-mesh augmented group (17% vs 41%).
3.3.2.2. Mini-open technique
A retrospective cohort study by Takayama et al.24 reported outcomes of 46 consecutive patients treated with fascia lata autograft SCR using a mini-open technique for mean 37 months follow-up. They found that operative times were significantly longer in the arthroscopic SCR group at 175 ± 48 min, versus the mini-open SCR group at 133 ± 25 min (p < 0.001). There were no significant differences in length of skin incision, graft size, ASES scores, range of motion, and graft retear rates.
3.3.3. SCR vs other treatments for massive, irreparable tears
3.3.3.1. Reverse total shoulder arthroplasty (RTSA)
Lacheta et al.20 compared patients with irreparable posterosuperior tears aged <70 years without glenohumeral osteoarthritis who were treated with either SCR (n = 22) or RTSA (n = 29) with minimum 2 years follow-up. The SCR patients were significantly younger (57 vs 63) (p < 0.001). There were no significant differences between the two groups in preoperative or postoperative clinical outcome scores (ASES, SANE, QuickDASH, SF-12), or return-to-sport at baseline or postoperatively.
3.3.3.2. Latissimus dorsi transfer (LDT)
A randomized controlled trial by Ozturk et al.16 treated patients with massive, irreparable tears, whose symptoms were unresponsive to minimum 6 months conservative management, with either SCR (n = 21) or LDT (n = 21) for mean 31 months follow-up. Patients with advanced arthritis, irreparable subscapularis tears, deltoid dysfunction or failed rotator cuff surgeries were excluded. Both interventions resulted in significant improvements in ASES, WORC, Constant and VAS scores at final follow-up. LDT resulted in significantly greater acromiohumeral distance (1.8 mm vs 0.4 mm) (p = 0.006), whilst SCR resulted in significantly greater improvements in ASES (58 vs 46) (p = 0.007) and Constant (45 vs 33) (p = 0.008) scores, and reversal of pseudoparalysis (92% vs 45%) (p = 0.011).
3.3.3.3. Partial infraspinatus repair
A cohort study by Greiner et al.17 matched 20/21 SCR patients to 20/60 partial infraspinatus repair patients for mean 29 months follow-up in a 1:1 ratio based on sex, age, tear configuration, all treated for irreparable posterosuperior tears. At final follow-up, there were no differences between SCR and partial infraspinatus repair in Constant (77 vs 83), age- and sex-adapted Constant (86% vs 91%), DASH (16 vs 8) or WORC Index (81 vs 90) scores respectively. The reoperation rate was 5% (1/21) in the SCR group, and 15% (9/60) in the partial infraspinatus repair group.
3.4. Meta-analysis: intact vs torn SCR graft
Clinical scores, functional outcomes and acromiohumeral distances were compared between intact and torn SCR graft groups using random-effects meta-analysis.
3.4.1. Characteristics of included studies
Seven studies were included for meta-analysis. Five studies were conducted in South Korea, and two in the USA. Two were Level III cohort studies and five were Level IV case series. The total population in these studies was 281 patients, of whom 171 had intact grafts, and 110 had torn grafts.
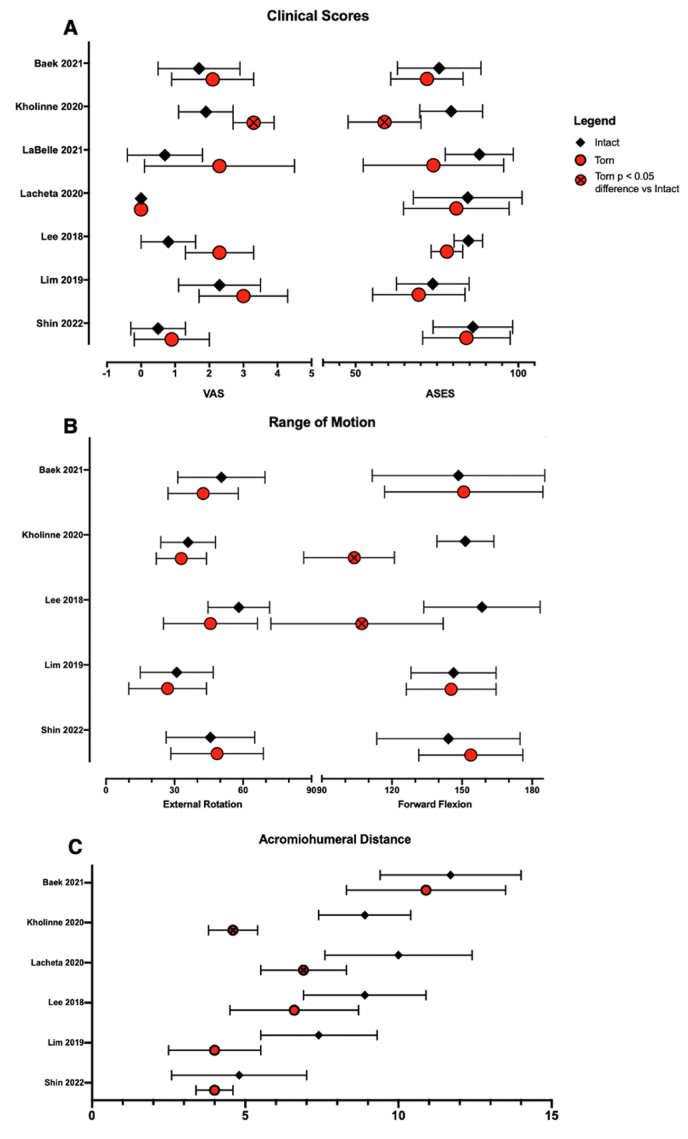
Summaries of the included studies and their reported outcomes of interest are outlined in Table 3, Table 4. Amongst included studies, VAS and ASES scores were the most reported clinical scores (Fig. 2A), and range of motion in forward flexion and external rotation were the most reported functional outcomes (Fig. 2B). Summary of acromiohumeral distances between groups of included studies are shown in Fig. 2C.
Table 3.
Summary of demographic and imaging follow-up data amongst included studies. i, incomplete SCR graft tear group; c, complete SCR graft tear group; MRI, magnetic resonance imaging; US, ultrasound.
| Study ID | Groups = n | Age (y) | Males | BMI | Radiological Assessment | Clinical Follow-Up |
|---|---|---|---|---|---|---|
| Baek 202128 | Total = 58 | 65 | 59% | 25 | MRI at mean 31 ± 7 (24–37) months | mean 30.5 (24–37) months |
| Intact = 27 | 66 | 52% | – | |||
| Torn = 31 | 64 | 65% | – | |||
| Kholinne 202018 | Total = 64 | – | 47% | – | MRI at 3, 6, 12 months and annually thereafter | mean 31.3 ± 8.2 months in mesh augment group mean 24.1 ± 4.6 months in non-mesh augment group |
| Intact = 45 | 65 | – | – | |||
| Torn = 19 | 63 | – | – | |||
| LaBelle 202125 | Total = 21 | 63 | 68% | – | MRI at final follow-up, range 24–41 months | 24–41 months |
| Intact = 8 | – | – | – | |||
| Torn = 13 | – | – | – | |||
| Lacheta 202029 | Total = 20 | 56 | 57% | – | MRI at mean 2.5 (0.3–10.2) months | mean 25 (24–36) months |
| Intact = 10 | 56 | – | ||||
| Torn = 10 | 58 | – | ||||
| Lee 201830 | Total = 36 | 61 | – | 25 | US at 3 months, MRI at 6 and 12 months | 24.8 ± 6.9 months |
| Intact = 23 | ||||||
| Torn = 13 | ||||||
| Lim 201931 | Total = 31 | 65 | 29% | – | MRI at mean 12.8 (12–24) months (intact) or mean 5.7 (3–12) months (torn) | mean 15 (12–24) months |
| Intact = 22 | 64 | 44% | ||||
| Torn = 9 | 67 | 27% | ||||
| Shin 202232 | Total = 51 | 63 | 61% | 25 | MRI at mean 9 ± 4 (6–22) months | mean 18 ± 5.4 (12–30) months |
| Intact = 36 | 64 | 58% | 26 | |||
| Torn = 15 | ||||||
| i = 9 | i = 65 | i = 44% | i = 23 | |||
| c = 6 | c = 57 | c = 100% | c = 25 |
Table 4.
Outcomes of interest at final follow up post-SCR. Data represented as means ± SD unless otherwise as stated. ∗, study reported significant difference between intact and torn group (p < 0.05);C, combination of summary statistics performed.
| Study ID | VAS |
ASES |
External Rotation (degrees) |
Forward Flexion (degrees) |
Acromiohumeral distance (mm) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intact | Torn | Intact | Torn | Intact | Torn | Intact | Torn | Intact | Torn | |
| Baek 202128 | 1.7 ± 1.2 | 2.1 ± 1.2 | 76.1 ± 12.9 | 72.3 ± 11.2 | 50.6 ± 19.1 | 42.6 ± 15.4 | 148.1 ± 36.5 | 150.3 ± 33.5 | 11.7 ± 2.3 | 10.9 ± 2.6 |
| Kholinne 202018 | 1.9 ± 0.8 | 3.3 ± 0.6∗ | 79.8 ± 9.7 | 59.2 ± 11.3∗ | 36 ± 12 | 33 ± 11∗ | 151 ± 12 | 104 ± 17∗ | 8.9 ± 1.5 | 4.6 ± 0.8∗ |
| LaBelle 202125 | 0.7 ± 1.1 | 2.3 ± 2.2 | 88.5 ± 10.5 | 74.3 ± 21.7 | – | – | – | – | – | – |
| Lacheta 202029 | Median 0 (0–3) | Median 0 (0–3) | 84.9 ± 16.8 | 81.4 ± 16.3 | – | – | – | – | 10.0 ± 2.4 | 6.9 ± 1.4∗ |
| Lee 201830 | 0.8 ± 0.8 | 2.3 ± 1.0 | 85.1 ± 4.4 | 78.5 ± 4.9 | 58.2 ± 13.5 | 45.8 ± 20.6 | 158.0 ± 24.6 | 106.7 ± 34.5∗ | 8.9 ± 2.0 | 6.6 ± 2.1 |
| Lim 201931 | 2.3 ± 1.2 | 3.0 ± 1.3 | 74.1 ± 11.2 | 69.8 ± 14.3 | 31 ± 16 | 27 ± 17 | 146 ± 18 | 145 ± 19 | 7.4 | 4.0∗ |
| Shin 202232 | 0.5 ± 0.8 | 0.9 ± 1.1C | 86.5 ± 12.3 | 84.5 ± 13.5C | 45.8 ± 19.4 | 48.7 ± 20.3C | 143.8 ± 30.3 | 153.3 ± 22.0C | 4.8 ± 2.2 | 4 ± 0.6C |
Fig. 2.
Summary data between intact and torn SCR graft groups in terms of (A) clinical scores: VAS and ASES, (B) range of motion: external rotation and forward flexion and (C) acromiohumeral distances. Data are means ± standard deviation. Error bars are not shown if standard deviations were not reported.
Clinical scores were better in intact than torn groups amongst all included studies, except for VAS, which Lacheta et al.29 reported as identical medians (IQR) between groups. Intact groups demonstrated greater external rotation (4/5 studies) and forward flexion (3/5 studies) than torn groups. Acromiohumeral distance was greater in intact than torn groups across all included studies.
3.4.2. Data synthesis
The study by Shin et al.32 reported postoperative outcome scores in the following groups: intact graft, incomplete tear, complete tear. The incomplete tear and complete tear groups were combined. This was performed using the formula from Section 6.5.2.10 of the Cochrane Handbook for Systematic Reviews of Interventions.10 The VAS scores reported by Lacheta et al.29 were not analyzed as they were reported as median (IQR). Results of meta-analysis are shown in Fig. 3.
Fig. 3.
Forest plot of comparison for (A) VAS, (B) ASES, (C) external rotation, (D) forward flexion and (E) acromiohumeral distance between Intact and Torn SCR graft groups. VAS, visual analog scale for pain; ASES, American Shoulder & Elbow Surgeons score; ER, external rotation; FF, forward flexion; AHD, acromiohumeral distance.
3.4.3. Forest plots
4. Discussion
Superior capsular reconstruction (SCR) is indicated in patients with massive, surgically irreparable rotator cuff tears. This review showed that younger patients with an intact subscapularis, who participated in sports pre-injury, who did not have arthritis or acetabulization, had better clinical outcomes following SCR for irreparable rotator cuff tears than patients with irreparable subscapularis tears, who did not participate in sports pre-injury or who had arthritis or acetabulization. As the literature has shown, SCR should be avoided in patients with irreparable subscapularis tears, osteoarthritis and/or acetabulization.34
Patients with irreparable rotator cuff tears without arthritis managed with SCR experienced greater improvements in clinical scores than patients who were treated with latissimus dorsi tendon transfer,16 and similar clinical scores at 2 years as those managed with reverse total shoulder arthroplasty20 or partial infraspinatus repair.17 Nonetheless, attempts at repair remain the mainstay of treatment of massive rotator cuff tears, which per the DeOrio and Cofield classification is a tear >5 cm from anterior-posterior.35,36 This is despite the retear rates for massive cuff tears being reported as high as 78%.36,37 Furthermore, patients with massive, chronic rotator cuff tears, which are associated with pathological changes that implicate repair success, such as fatty infiltration, atrophy, adhesions and tendon retraction, should be considered for an SCR.36,38
The drawback of performing an SCR instead of repairing the rotator cuff tendon is that an SCR acts as a static spacer between the humerus and acromion, unlike the supraspinatus tendon which in addition contributes to shoulder abduction.34,37 Furthermore, insertion of a graft introduces the risk of a foreign body reaction.34
The major finding of our meta-analysis was that patients with intact grafts post-SCR had significantly better VAS, ASES, external rotation and acromiohumeral distances than those with torn SCR grafts. The mean difference in VAS scores was 0.97, less than the MCID for rotator cuff disease VAS of 1.4.39 The mean difference in ASES scores was 8 points, greater than the validated minimal clinically important difference (MCID) for ASES score of 6.4 points.40 Whilst an intact SCR was associated with an 8 point improvement in ASES scores for rotator cuff insufficient patients at 2 years; the beneficial effect of this procedure was relatively modest when compared to the improvements in ASES scores following reverse total shoulder arthroplasty, which has been shown to increase ASES scores by 51 points over 2 years.41
Surgical center volumes and surgeon experience may influence clinical outcomes following SCR. The percentage of SCR grafts that remained intact from studies included in meta-analysis ranged from 47 to 71%. The studies with the highest percentages of intact SCR grafts had the largest sample sizes, and studies with the lowest percentages of intact grafts had the smallest sample sizes.18,25,29,32 The study by Kholinne et al.18 was the largest study that was included in meta-analysis. All 64 patients, of whom 70% had intact grafts, were operated on by a single surgeon. In contrast, Woodmass et al.5 followed 34 SCR patients who were treated by one of five surgeons for mean 12 months. The reoperation rate was significantly higher for cases that were performed in a surgeons first 10 cases (or less, if < 10 cases were performed) than for cases where a surgeon had performed >10 cases. Mihata et al.42 reported that 17% (4/24) of grafts had torn in their initial SCR case series, a rate that fell to 5% (9/188) in a subsequent case series.43
Another factor associated with graft failure is graft strength.44 Kholinne et al.18 found that 41% (14/34) of the non-mesh augmented SCR grafts failed, whereas only 17% (5/30) of the mesh augmented SCR grafts failed. The group with mesh augmented SCR grafts experienced greater improvements in ASES, forward flexion and external rotation than the group with non-mesh augmented SCR grafts.
The weakness of this review was that constituent studies were mostly retrospective case series, or cohort studies with small sample sizes, with only one randomized study. Whilst these studies were high in quality in context of their respective levels of evidence, the studies available for the meta-analysis were underpowered. A limitation of our meta-analysis was the variability in time to radiological assessment of graft integrity (mean 3–31 months) and short clinical follow-up (mean 25–31 months). A strength of our meta-analysis was that patients from each study population were similarly aged (mean 56–65 years), and that each gender was well represented in each study population (29%–68% males). A consideration of our meta-analysis was that included studies were conducted only in South Korea (5) or the USA (2).
5. Conclusions
Superior capsular reconstruction produced modest clinical benefits in patients with massive, surgically irreparable rotator cuff tears, with patients who were younger, who participated in sports, and who had intact grafts experienced better clinical outcomes than patients who were older, who did not play sports, and who had torn grafts. Superior capsular reconstruction should be avoided in patients with irreparable subscapularis tears, glenohumeral osteoarthritis and/or acetabulization. An intact graft was associated with increased experience with the technique, and when augmented by a synthetic patch. Patients who underwent SCR experienced 26% greater improvements in ASES, and 36% greater improvements in Constant scores than patients who underwent latissimus dorsi transfers. The effect size of superior capsular reconstruction for irreparable rotator cuff tears was modest, with patients who had intact grafts experiencing a mean difference of 1 less point on the VAS pain scale, and 8 more ASES score points than patients who had torn grafts.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
George A.C. Murrell is a paid consultant and has research funding from Smith and Nephew and is also on the Editorial Board of the following publications: Journal of Shoulder and Elbow Surgery; Shoulder and Elbow. The other authors, Ryan S. Ting, Ron Rosenthal, Hilal S.A. Al-Housni and Patrick H. Lam, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Appendix A.
References
- 1.St Pierre P., Millett P.J., Abboud J.A., et al. Consensus statement on the treatment of massive irreparable rotator cuff tears: a Delphi approach by the Neer Circle of the American Shoulder and Elbow Surgeons. J Shoulder Elbow Surg. 2021;30:1977–1989. doi: 10.1016/j.jse.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Mihata T., McGarry M.H., Pirolo J.M., Kinoshita M., Lee T.Q. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40:2248–2255. doi: 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 3.Werthel J.D., Vigan M., Schoch B., Lädermann A., Nourissat G., Conso C. Superior capsular reconstruction - a systematic review and meta-analysis. Orthop Traumatol Surg Res. 2021;107 doi: 10.1016/j.otsr.2021.103072. [DOI] [PubMed] [Google Scholar]
- 4.Burkhart S.S., Pranckun J.J., Hartzler R.U. Superior capsular reconstruction for the operatively irreparable rotator cuff tear: clinical outcomes are maintained 2 Years after surgery. Arthroscopy. 2020;36:373–380. doi: 10.1016/j.arthro.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 5.Woodmass J.M., Wagner E.R., Borque K.A., Chang M.J., Welp K.M., Warner J.J.P. Superior capsule reconstruction using dermal allograft: early outcomes and survival. J Shoulder Elbow Surg. 2019;28:S100–s109. doi: 10.1016/j.jse.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Mihata T., Lee T.Q., Hasegawa A., et al. Five-year follow-up of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. J Bone Joint Surg Am. 2019;101:1921–1930. doi: 10.2106/JBJS.19.00135. [DOI] [PubMed] [Google Scholar]
- 7.Pennington W.T., Bartz B.A., Pauli J.M., Walker C.E., Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34:1764–1773. doi: 10.1016/j.arthro.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Denard P.J., Brady P.C., Adams C.R., Tokish J.M., Burkhart S.S. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34:93–99. doi: 10.1016/j.arthro.2017.08.265. [DOI] [PubMed] [Google Scholar]
- 9.PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J.P., Thomas J., Chandler J., et al. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 11.Howick J., Chalmers I., Glasziou P., Greenhalgh T., Heneghan C., Liberati A. OCEBM Levels Evid Work Gr; 2011. Oxford Centre for Evidence-Based Medicine Levels of Evidence. [Google Scholar]
- 12.Munn Z., Barker T.H., Moola S., et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18:2127–2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 13.Moola S. In: Joanna Briggs Institute Reviewer's Manual. Aromataris E., Munn Z., editors. The Joanna Briggs Institute; 2017. Chapter 7: systematic reviews of etiology and risk. [Google Scholar]
- 14.Tufanaru C., Munn Z., Aromataris E., Campbell J., Hopp L. Joanna Briggs Institute; 2017. Systematic Reviews of Effectiveness. Joanna Briggs Institute Reviewer's Manual. Adelaide. [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., The P.G. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 16.Ozturk B.Y., Ak S., Gultekin O., Baykus A., Kulduk A. Prospective, randomized evaluation of latissimus dorsi transfer and superior capsular reconstruction in massive, irreparable rotator cuff tears. J Shoulder Elbow Surg. 2021;30:1561–1571. doi: 10.1016/j.jse.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Greiner S., Kaeaeb M., Voss A., Lawton R., Bhide P., Achenbach L. Comparison of superior capsular reconstruction and partial infraspinatus repair: a matched-pair analysis of irreparable rotator cuff tears. Orthop J Sports Med. 2021;9 doi: 10.1177/2325967120984264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kholinne E., Kwak J.-M., Kim H., Koh K.H., Jeon I.-H. Arthroscopic superior capsular reconstruction with mesh augmentation for the treatment of irreparable rotator cuff tears: a comparative study of surgical outcomes. Am J Sports Med. 2020;48:3328–3338. doi: 10.1177/0363546520958708. [DOI] [PubMed] [Google Scholar]
- 19.Kholinne E., Kwak J.M., Cho C.H., et al. Arthroscopic superior capsular reconstruction for older patients with irreparable rotator cuff tears: a comparative study with younger patients. Am J Sports Med. 2021;49:2751–2759. doi: 10.1177/03635465211024652. [DOI] [PubMed] [Google Scholar]
- 20.Lacheta L., Horan M.P., Goldenberg B.T., Dornan G.J., Higgins B., Millett P.J. Minimum 2-year clinical outcomes after superior capsule reconstruction compared with reverse total shoulder arthroplasty for the treatment of irreparable posterosuperior rotator cuff tears in patients younger than 70 years. J Shoulder Elbow Surg. 2020;29:2514–2522. doi: 10.1016/j.jse.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Mihata T., Lee T.Q., Fukunishi K., et al. Return to sports and physical work after arthroscopic superior capsule reconstruction Among patients with irreparable rotator cuff tears. Am J Sports Med. 2018;46:1077–1083. doi: 10.1177/0363546517753387. [DOI] [PubMed] [Google Scholar]
- 22.Mihata T., Lee T.Q., Hasegawa A., et al. Arthroscopic superior capsule reconstruction for irreparable rotator cuff tears: comparison of clinical outcomes with and without subscapularis tear. Am J Sports Med. 2020;48:3429–3438. doi: 10.1177/0363546520965993. [DOI] [PubMed] [Google Scholar]
- 23.Takayama K., Yamada S., Kobori Y., Shiode H. Association between the postoperative condition of the subscapularis tendon and clinical outcomes after superior capsular reconstruction using autologous tensor fascia lata in patients with pseudoparalytic shoulder. Am J Sports Med. 2020;48:1812–1817. doi: 10.1177/0363546520919956. [DOI] [PubMed] [Google Scholar]
- 24.Takayama K., Yamada S., Kobori Y. Clinical effectiveness of mini-open superior capsular reconstruction using autologous tensor fascia lata graft. J Shoulder Elbow Surg. 2021;30:1344–1355. doi: 10.1016/j.jse.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 25.LaBelle M.W., Mengers S., Strony J., et al. Evaluating the role of graft integrity on outcomes: clinical and imaging results following superior capsular reconstruction. J Shoulder Elbow Surg. 2021;30:2041–2047. doi: 10.1016/j.jse.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Evuarherhe A., Jr., Condron N.B., Gilat R., et al. Defining clinically significant outcomes following superior capsular reconstruction with acellular dermal allograft. Arthroscopy. 2022;38:1444–1453. doi: 10.1016/j.arthro.2021.11.039. [DOI] [PubMed] [Google Scholar]
- 27.Gilat R., Haunschild E.D., Williams B.T., et al. Patient factors associated with clinical failure following arthroscopic superior capsular reconstruction. Arthroscopy. 2021;37:460–467. doi: 10.1016/j.arthro.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Baek C.H., Kim J.G. Impact of graft thickness on graft retear following superior capsular reconstruction using a Hybrid graft: minimum 2-year follow-up. Arthroscopy. 2022;38:1784–1792. doi: 10.1016/j.arthro.2021.11.046. [DOI] [PubMed] [Google Scholar]
- 29.Lacheta L., Horan M.P., Schairer W.W., et al. Clinical and imaging outcomes after arthroscopic superior capsule reconstruction with human dermal allograft for irreparable posterosuperior rotator cuff tears: a minimum 2-year follow-up. Arthroscopy. 2020;36:1011–1019. doi: 10.1016/j.arthro.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.-J., Min Y.-K. Can inadequate acromiohumeral distance improvement and poor posterior remnant tissue be the predictive factors of re-tear? Preliminary outcomes of arthroscopic superior capsular reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26:2205–2213. doi: 10.1007/s00167-018-4912-8. [DOI] [PubMed] [Google Scholar]
- 31.Lim S., AlRamadhan H., Kwak J.M., Hong H., Jeon I.H. Graft tears after arthroscopic superior capsule reconstruction (ASCR): pattern of failure and its correlation with clinical outcome. Arch Orthop Trauma Surg. 2019;139:231–239. doi: 10.1007/s00402-018-3025-7. [DOI] [PubMed] [Google Scholar]
- 32.Shin S.J., Lee S., Hwang J.Y., Lee W., Koh K.H. Tear pattern after superior capsular reconstruction using an acellular dermal matrix allograft. J Shoulder Elbow Surg. 2022;31:e279–e288. doi: 10.1016/j.jse.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Hamada K., Yamanaka K., Uchiyama Y., Mikasa T., Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469:2452–2460. doi: 10.1007/s11999-011-1896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werthel J.-D., Vigan M., Schoch B., Lädermann A., Nourissat G., Conso C. Superior capsular reconstruction – a systematic review and meta-analysis. J Orthop Traumatol: Surgery & Research. 2021;107 doi: 10.1016/j.otsr.2021.103072. [DOI] [PubMed] [Google Scholar]
- 35.DeOrio J.K., Cofield R.H. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. JBJS. 1984;66:563–567. [PubMed] [Google Scholar]
- 36.Burkhart S.S., Barth J.R.H., Richards D.P., Zlatkin M.B., Larsen M. Arthroscopic repair of massive rotator cuff tears with stage 3 and 4 fatty degeneration. Arthrosc J Arthrosc Relat Surg. 2007;23:347–354. doi: 10.1016/j.arthro.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Kovacevic D., Suriani R.J., Jr., Grawe B.M., et al. Management of irreparable massive rotator cuff tears: a systematic review and meta-analysis of patient-reported outcomes, reoperation rates, and treatment response. J Shoulder Elbow Surg. 2020;29:2459–2475. doi: 10.1016/j.jse.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuzel B.R., Grindel S., Papandrea R., Ziegler D. Fatty infiltration and rotator cuff atrophy. JAAOS - Journal of the American Academy of Orthopaedic Surgeons. 2013;21:613–623. doi: 10.5435/JAAOS-21-10-613. [DOI] [PubMed] [Google Scholar]
- 39.Tashjian R.Z., Deloach J., Porucznik C.A., Powell A.P. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18:927–932. doi: 10.1016/j.jse.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Michener L.A., McClure P.W., Sennett B.J. American shoulder and Elbow surgeons standardized shoulder assessment form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11:587–594. doi: 10.1067/mse.2002.127096. [DOI] [PubMed] [Google Scholar]
- 41.Simovitch R.W., Friedman R.J., Cheung E.V., et al. Rate of improvement in clinical outcomes with anatomic and reverse total shoulder arthroplasty. JBJS. 2017;99 doi: 10.2106/JBJS.16.01387. [DOI] [PubMed] [Google Scholar]
- 42.Mihata T., Lee T.Q., Watanabe C., et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29:459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Mihata T., Lee T.Q., Hasegawa A., et al. Arthroscopic superior capsule reconstruction can eliminate pseudoparalysis in patients with irreparable rotator cuff tears. Am J Sports Med. 2018;46:2707–2716. doi: 10.1177/0363546518786489. [DOI] [PubMed] [Google Scholar]
- 44.Ting R.S., Guo A.A., Rosenthal R., Al-Housni H.S.A., Lam P.H., Murrell G.A.C. Biomechanical Comparison of Synthetic Polytetrafluoroethylene (PTFE) vs Human Dermal Allograft (HDA), 2 vs 3 Glenoid Anchors, and Suture vs Minitape in Superior Capsule Reconstruction. HSS Journal®. 2022;OnlineFirst doi: 10.1177/15563316221114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.