Abstract
The evaluation of the electrochemical determination of Saccharomyces cerevisiae was carried out using a screen-printed carbon electrode (SPCE) modified with Nafion-dispersed oxidized multi-walled carbon nanotubes (OMWCNT). The morphology was studied using scanning electron microscopy (SEM), showing a complete modification of the surface with the nanotubes and yeast interaction with them instead of the graphite surface. The redox couple Fe(CN)64−/Fe(CN)63− was used to determine the electroactive area, the heterogeneous transfer constant, and the Nafion® effect. Results showed increases in electroactive area and heterogeneous transfer constant of 146% and 20.4%, respectively, due to the presence of nanotubes. Studies of the Nafion® effect showed that the polymeric membrane affects the electroactive area but not the heterogeneous transfer constant. Studies of the scan rate effect show that yeast oxidation is an irreversible mixed control process. As the concentration and scan rate increased, the anodic potential shifted toward more anodic values. The relationship between yeast concentration and the anodic current density (current/electroactive area) of yeast showed a linear range between 0.61 and 7.69 g L−1, the limit of detection (LOD) and the limit of quantification (LOQ) were 0.17 g L−1, and 0.61 g L−1, respectively, and the sensibility obtained was 0.03 μA L g−1 mm−2. These results show that with the screen-printed carbon electrodes it is possible to improve the electrochemical determination of this microorganism, enhancing the analytical parameters and quantification, allowing greater portability and decreasing measurement times and associated waste.
Graphical abstract
Highlights
-
•
Saccharomyces cerevisiae participates in different bioprocesses and the food industry. So, yeast determination is necessary.
-
•
The electroanalytical techniques can help the rapid and low-cost determination of S. cerevisiae.
-
•
The SPCE modified with carbon nanotubes improves the electrochemical determination of S. cerevisiae yeast and analytical parameters.
1. Introduction
Saccharomyces cerevisiae is a unicellular microorganism belonging to the Fungi kingdom and the Ascomycetes family. This microorganism is widely known and is a model that facilitates the study of different biological phenomena and the understanding biochemical processes in eukaryotic organisms. Nowadays, this yeast participates in many bioprocesses due to advances in genetic and metabolic engineering (Acevedo Restrepo et al., 2022; Thyab et al., 2020; Turker, 2014).
S. cerevisiae has a high content of energy, proteins, amino acids, and micronutrients also is used as food and feed additives since the polysaccharides present in the cell walls favor animal health and growth (Ostergaard et al., 2000; Walker and Stewart, 2016). S. cerevisiae does not produce toxic metabolites and is a desirable host for protein expression with medical, chemical, biochemical, and nutritional importance (Hong and Nielsen, 2012; Turker, 2014). The microorganism produces many chemical molecules (lactic acid, glycerol, malic acid, isoprenoids, and insulin, among others) with applications in medicine, perfumery, the food industry, vaccine development, and disease mechanism studies, and so on (M. Huang et al., 2014; Turker, 2014; G. Wang et al., 2017). Currently, S. cerevisiae plays a determining role in biofuel production from different substrates, even those that yeast does not metabolize (cellulose, xylose, galactose), but now it is possible thanks to the genetic engineering advances (Hong and Nielsen, 2012; Turker, 2014).
Because of S. cerevisiae's importance, the need to study the microorganism and its behavior is evident, and developments can occur from different known areas. Electroanalysis is one important electrochemistry issue. It provides techniques that facilitate decentralized measurements and the best analytical parameters and reduces costs and response times when electrochemical sensors are used. (Fernández Abedul, 2020). Different studies have investigated the yeast through electroanalytical techniques and electrochemical sensors (Acevedo Restrepo et al., 2022; Han et al., 2000; T Matsunaga et al., 1979; Matsunaga et al., 1984; Tadashi Matsunaga & Namba, 1984a; Tsukatani et al., 2003; Villalonga et al., 2019).
Electrochemical sensors are analytical tools integrated by a transducer and a recognition element, and in these aspects, there are innumerable possibilities. The recognition element can be made of different materials such as nanoparticles, biomolecules, carbon materials, polymers, and so on (Jadon et al., 2016; Stradiotto et al., 2003; J. Wang, 2006). Recognition elements improve sensitivity, selectivity, detection, and quantification limits. They can even detect signals from molecules that initially have no electroactivity.
The transducer element is a working electrode on whose surface the charge transference occurs (Ganjali et al., 2016). Although the glassy carbon electrode (GCE) is the most common transducer element, in recent decades, versatile measurement tools such as screen-printed electrodes (SPE) have been developed. The SPE is printed using a serigraphic technique with different materials on a ceramic or plastic substrate (Renedo et al., 2007). The resulting electrodes integrate all the required elements for the electrochemical measurements (Hayat and Marty, 2014).
SPEs are successful analytical devices due to their low cost, disposable nature, simplicity, and portability. SPE does not require polishing, helps reduce sample volume, waste amount, and response time; avoids sample pre-treatment; and favors sensibility, stability, decentralization, and in situ speed measurement. SPEs operate at room temperature and have a reconfigurable format (Ferrari et al., 2021; W. Huang et al., 2003; Lee et al., 2007; Lien et al., 2012; Umasankar et al., 2007; J. Wang et al., 2003; Wu and Hu, 2004; Yang et al., 2010, 2013; Yusof et al., 2012).
In a previous work, it was shown the possibility of determining the S. cerevisiae yeast using electrochemical sensors developed with glassy carbon electrodes modified with oxidized multi-walled carbon nanotubes dispersed in water-Nafion® (Acevedo Restrepo et al., 2022). The current study intends to assess how employing SPCE (SPCE/OMWCNT-N) improves the microbial electrochemical response obtained using GCE. The experimental conditions, pH and scan rate were established to observe the electrochemical behavior of the microorganism, which was evaluated through different electroanalytical techniques.
2. Materials and methods
2.1. Materials, chemicals, and culture media
Saccahromyces cerevisiae strain (commercial bakery yeast grown in sugarcane molasses) was purchased from Levapan S.A. (Medellín-Colombia). Yeast suspensions at different concentrations were prepared from a stock suspension of 50 g L−1. All suspensions were prepared in a phosphate-buffer solution (PBS) at pH 7.00 and incubated at 37 ◦C for 15 min.
The phosphate buffer concentration was 0.01 mol L−1 and was made up of monobasic sodium phosphate (NaH2PO4, Sigma) and dibasic sodium phosphate (Na2HPO4, Sigma); potassium chloride (KCl, Merck) 0.10 mol L−1 was used as a supporting electrolyte. Potassium hexacyanoferrate (III) trihydrate solution (K3[Fe(CN)6].3H2O, Sigma), at a concentration of 1 × 10−3 mol L−1 in KCl 0.10 mol L−1, was used to evaluate the electroactive area, heterogeneous transfer constant, and general behavior of the electrode.
Screen-printed carbon electrodes (SPCE) manufactured by Zenzor (CH Instruments Inc., Texas, USA) with a three-electrode configuration (geometric areas of 7.07 mm2) were employed. Commercial multi-walled carbon nanotubes (MWCNTs; Nano-Lab, Waltham, Massachusetts, USA) were oxidized in an acid medium assisted by microwave for 15 min according to the method proposed by Blandon et al. (Blandón-Naranjo et al., 2018). As stabilizers and surface modifiers, sodium hydroxide (NaOH, Sigma) 2.0 mol L−1 and Nafion® perfluorinated resin (Sigma-Aldrich, Missouri, USA) solution of 5% weight in water were used. The electrochemical experiments were performed in a cell using an AUTOLAB PGSTAT 101 potentiostat (Utrecht, the Netherlands) with NOVA 1.11 software.
2.2. Screen-printed electrode modification and characterization
The nanotube dispersion was prepared according to previous work (Acevedo Restrepo et al., 2022). Before the modification of the SPCEs, they were washed with Milli Q water and cycled ten times in PBS using a potential window from 0 to 1.0 V at a scan rate of 100 mV s−1, to ensure a constant capacitive current. Finally, six drops of 2.50 μL of the dispersion of nanotubes in water–Nafion® were deposited upon the electrode's surface. The prepared sensor was named SPCE/OMWCNT-N. Additionally, electrodes were modified with oxidized multi-walled carbon nanotubes dispersed in water without Nafion® to compare the electrochemical behavior of both systems. This sensor was named SPCE/OMWCNT. All modified electrodes were dried at room temperature after each drop deposition and were cycled (CV, 20 scans) in PBS at a scan rate of 100 mV s−1 in a potential window of 0–1.0 V to ensure a constant capacitive current.
Morphological characterization of SPCE and SPCE/OMWCNT-N, before and after being submerged in a 2.50 g L−1 yeast suspension, was performed by scanning electron microscopy (SEM) (thermionic) JEOL- JSM 6490LV equipped with a secondary electron detector (SEI) and backscatter (BES) using different augments. For the electrochemical characterization of the electrodes, cyclic voltammograms were recorded in potassium ferricyanide (K3[Fe (CN)6]) 1 × 10−3 mol L−1 in 0.10 mol L−1 KCl, at different scan rates (5, 20, 40, 80, 100, and 200 mV s1), and both the electroactive area and the heterogeneous transfer constant were calculated.
2.3. Nafion® and scan rate effect
The Nafion® effect was assessed by comparison between the electroactive area and heterogeneous transfer constant of both the SPCE/OMWCNT and SPCE/OMECNT-N. The scan rate effect was observed by cyclic voltammetry, through the electrochemical response of yeast suspensions in the range of 2.5–8.0 g L−1, at scan rates between 5 and 100 mV s−1.
2.4. Yeast determination
The yeast concentration effect was observed by cyclic voltammetry at a potential window of 0–1 V. Finally, chronoamperometry studies were conducted at a potential of 0.85 V for 1200 s. Initially, the electrochemical cell contained 2000 μL of PBS pH 7.00, and successive additions of 50 g L−1 yeast stock were made every 120 s. The first two additions were 100 μL and the next ones 50 μL. All measurements were made in triplicate, considering the last 60 s of every addition. At all points, the current density value of the blank was subtracted.
The detection and quantification limits were calculated as LD = (3 * SD Blank)/m and LQ = (10 * SD Blank)/m, respectively, according to the methodology proposed by D. C. Harris (2007), where SD is the standard deviation of the blank, and m is the slope of the calibration curve. Statistical analyses were performed using Statgraphics® Centurion XVI, (Statpoint Technologies Inc., USA).
3. Results and discussion
3.1. Sensors characterization and Nafion® effect
The adequate disposition of the OMWCNT-N on the SPCE was verified through SEM micrographs. Fig. 1A, B, and C show the morphology before submerging the sensors in a suspension of S. cerevisiae. Fig. 1D and E, and F, show the morphology after submerging them in a 2.5 g L−1 yeast suspension for 20 s.
Fig. 1.
SEM micrographs of sensors before submerging in 2.5 g L−1 yeast suspension at different augments (A) SPCE 37x, (B) SPCE 50000x, (D) SPCE/OMWCNT-N 37x, (E) SPCE/OMWCNT-N 50000x, and SEM micrographs after submerging in 2.5 g L−1 yeast suspension (C) SPCE 2000x, (F) SPCE/OMWCNT-N 2000x.
Fig. 1A corresponding to SPCE before submerging, shows a homogeneous surface with some imperfections, possibly due to the binder used in the formulation of the ink (Cinti et al., 2015; J. Wang et al., 1998). Fig. 1D shows morphological differences compared to Fig. 1A showing well-dispersed clusters of nanotubes covering all electrode surfaces. Van der Waals forces and entanglements probably favored cluster formation (Atif and Inam, 2016; Cinti et al., 2015; Sven Pegel et al., 2008). Fig. 1B and E with higher magnification confirm the above mentioned, showing the graphite structure (Kadara et al., 2009) and the nanotubes (Asmatulu et al., 2018). So, the SPCE was covered successfully. The sensors submerged in a 2.5 g L−1 yeast suspension (micrographs of Fig. 1C and F) showed yeast cells on the SPCE/OMWCNT-N, but they were not present on the surface of SPCE without modification.
The electrochemical behavior of the system was studied using K3[Fe(CN)6.3H2O] 1.0 × 10−3 mol L−1/KCl 0.1 mol L−1 on the SPCE and SPCE/OMWCNT-N. The results are shown in Fig. 2.
Fig. 2.
Electrochemical response of K3[Fe(CN)6.3H2O] 1.0 × 10−3 mol L−1/KCl 1.0 mol L−1 on (A) SPCE (■) y (B) SPCE/OMWCNT-N ( ). Scan rate 100 mV s−1.
). Scan rate 100 mV s−1.
Fig. 2 shows that the presence of carbon nanotubes and Nafion® increases both anodic and cathodic current peaks. The changes in the capacitive current indicate that the electroactive area has increased due to the modification. This can be attributed to the nanotubes since they have a large superficial area and excellent electronic properties that improve the charge transfer (Banks and Compton, 2006; Macpherson et al., 2009; J. Wang, 2005).
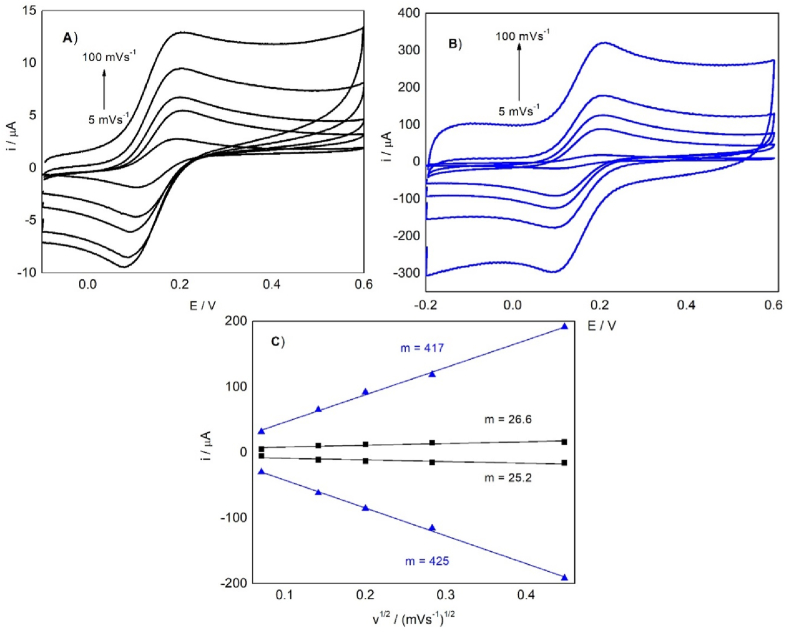
The electroactive area was calculated using the Randles–Sevcik equation, a diffusion-controlled redox process, and a linear relationship between the oxidation and reduction currents and the square root of the scan rate, with a K3[Fe(CN)6.3H2O] concentration of 1.0 × 10−3 mol L−1, a diffusion coefficient of 7.60 × 10−6 cm2 s−1, and n equal to one electron. The heterogeneous transfer constant (ok) was determined using Nicholson's methodology (Nicholson, 1965). Fig. 3 shows the cyclic voltammograms.
Fig. 3.
Cyclic voltammetry for (A) SPCE and (B) SPCE/OMWCNT-N at different scan rates ranging from 5, 20, 40, 80, to 100 mV s−1. (C) Graph of current vs. the square root of the scan rate (i vs. v1/2) for SPCE (■) and SPCE/OMWCNT-N ( ).
).
The significant augment in the slope of the plot i vs. v1/2 for the SPCE/OMWCNT-N sensor is related to the well-covered surface of SPCE by the immobilized nanomaterial and to the electroactive area increasing up to 146% of that of SPCE. The electroactive area and heterogeneous transfer constant parameters for SPCE, SPCE/OMWCNT, and SPCE/OMWCNT-N are shown in Table 1.
Table 1.
SPCE, SPCE/OMWCNT and SPCE/OMWCNT-N sensor parameters.
| Sensor | (AE)/mm2 | k0/(cm s−1) x 10−3 |
|---|---|---|
| SPCEa | 4.5 ± 0.1 | 2.347 ± 0.005 |
| SPCE/OMWCNTa | 6.8 ± 0.7 | 2.811 ± 0.004 |
| SPCE/OMWCNT-Nb | 11.1 ± 1.3 | 2.826 ± 0.001 |
n = 3 electrodes.
n = 15 electrodes.
All data for SPCE/OMWCNT-N electroactive areas and heterogeneous transfer constant fit a normal distribution. The Grubbs test does not identify outliers, indicating the reproducibility of the results. The electroactive areas showed significant differences, with the nanotubes dispersed in water - Nafion® modification achieving the greatest increase. The ko values for SPCE/OMWCNT and SPCE/OMWCNT-N did not present statistically significant differences between them, but compared to SPCE, they showed statistically significant differences (p < 0.05), indicating that the electron transfer is due to nanotubes exclusively by increasing ko by 20%. When compared with k0 values of GCE (2.26 × 10−3 cm s−1) and GCE/OMWCNT-N (2.82 × 10−3 cm s−1) obtained by Acevedo et al. (Acevedo Restrepo et al., 2022) differences were not found. We can conclude that the polymeric membrane does not contribute to the heterogeneous transfer constant change, but it is associated with an increase in the electroactive area.
3.2. Nafion® and scan rate effect in the yeast electrochemical response
Fig. 4A and B shows the cyclic voltammograms obtained for solutions of 2.0 g L−1 and different concentrations on different sensors and SPCE/OMWCNT-N, respectively.
Fig. 4.
(A) Electrochemical response of S. cerevisiae 2.0 g L−1 on SPCE (■), SPCE/OMWCNT ( ), and SPCE/OMWCNT-N (
), and SPCE/OMWCNT-N ( ). (B) Cyclic voltammetry of S. cerevisiae on SPCE/OMWCNT-N at different concentrations. Scan rate 100 mV s−1.
). (B) Cyclic voltammetry of S. cerevisiae on SPCE/OMWCNT-N at different concentrations. Scan rate 100 mV s−1.
S. cerevisiae does not show signals on SPCE. The yeast response on the SPCE/OMWCNT sensor is perceptible at an anodic peak located at 0.70 V, approximately and a current density of 0.122 μA mm−2. The yeast response on the SPCE/OMWCNT-N was more intense, with an anodic peak located at 0.65 V, approximately, and a current density of 0.425 μA mm−2. These results indicate that the nanotubes favor the electrochemical response of yeast, and Nafion® contributes to improving the signal intensity. Fig. 4B shows that an increase in yeast concentration causes a current density increase and a shift in the peak potential toward the anodic direction.
Fig. 5 shows the potential peak variation evaluated using cyclic voltammetry at yeast concentrations and scan rates between 2.5 and 8.0 g L−1 and 5–100 mV s−1, respectively.
Fig. 5.
S. cerevisiae electrochemical response at (A) 2.5 g L−1 and (B) 8.0 g L−1. The scan rates of 5, 20, 40 80, and 100 mV s−1. Inserts show logarithm of current density vs. logarithm of scan rate.
Under the conditions of the assay, it can be noted that up to a scan rate of 100 mV s−1, at 2.5 g L−1 (Fig. 5A), the anodic peak potential does not appear to depend on the scan rate. The slope of the plot (J) vs. log (v) is close to the expected theoretical value for a controlled diffusion process (Shayani-jam, 2019). For the 8.0 g L−1 yeast concentration (Fig. 5B), it is evident that the scan rate caused a shift in the anodic peak potential toward the anodic position, and the slope of the graph log (J) vs. (v) is far from the expected theoretical value for a diffusion-controlled process, so an adsorptive–diffusion control can be considered (Acevedo Restrepo et al., 2022).
To determine how much the potential shifts with increasing yeast concentration and scan rate, Table 2 shows the anodic peak potential difference between the scan rates of 100 and 5 mV s−1 at 2.5, 4.0, and 8.0 g L−1 yeast concentrations.
Table 2.
Subtraction between anodic peak potential at 100 and 5 mV s−1 at different concentrations.
| S. cerevisiae concentration/g L−1 | E100 – E5/mV |
|---|---|
| 2.5 | 0.049 |
| 4.0 | 0.059 |
| 8.0 | 0.095 |
The results of Fig. 5 and Table 2 indicate that the system is a mixed-controlled process. The scan rate and the yeast concentration increases caused a noticeable shift of the anodic potential toward more anodic values.
3.3. Yeast determination
Assays were carried out using the chronoamperometry technique and the SPCE/OMWCNT-N sensor to evaluate whether it was possible to improve the yeast electrochemical response in terms of analytical parameters. Fig. 6 shows the current density vs. yeast concentration calibration curve. The insert presents the electrochemical response.
Fig. 6.
Calibration curve for S. cerevisiae using the SPCE/OMWCNT-N and electroanalytical technique chronoamperometry at 0.850 V.
The calibration curve presented a linear relationship statistically significant (p < 0.05), with an R2 = 99.87 that explains 99.5% of the current density variability adjusted to a linear model (J = −3.67 × 10−3 + 0.0343*C). The model's linearity was confirmed by a residual analysis. A multiple range test revealed statistically significant differences in the current densities obtained for the various yeast concentrations. Table 3 shows the analytical methods for yeast determination and some analytical parameters. Also shown are the calibration curves for analytical parameters obtained using the GCE/OMWCNT-N (Acevedo Restrepo et al., 2022) and SPCE/OMWCNT-N sensors.
Table 3.
Analytical methods for yeast determination.
| Objective | Method | Observations | Ref. |
|---|---|---|---|
| Viability determination | Methylene Blue dye Reduction Test (MBRT) | Non-viable cells are dyed blue. High variability. Response times of 48 h. | (Bapat et al., 2006; Dale, 1941) |
| A commercial kit was reduced by cellular dehydrogenases to an orange formazan product soluble in a buffer. | The amount of formazan produced was directly proportional to the number of living cells. Response time 3 h. Maximum determination 1 × 107 cell mL−1. | Kwolek-mirek & Zadrag-tecza (2014) | |
| Cell count hemacytometer method | Inexpensive analysis, easy to carry out, but introduces high variability and low reproducibility. Linear ranges de 5.0 × 104–1.0 × 107 cell mL−1. R2 = 0.9992. | (Fiorino et al., 2004; Lawrence, 2002) | |
| Suspension inoculated on solid YPD medium. | Response time 48 h, possible errors due to dilution and final count. | Kwolek-Mirek & Zadrag-Tecza (2014) | |
| Vitality determination | Acidification power test. | The change in pH caused by the yeast as it degrades a substrate (glucose) is measured. R2 = 0.9430. | Kara et al. (1988) |
| The spectrophotometric method is based on the reaction of luciferin with ATP in the presence of luciferase, Mg2+ ions, and oxygen, resulting in the emission of light. | The analysis must be carried out very quickly, since the physiological conditions of the sample change drastically once taken, affecting the reaction. | Kwolek-Mirek & Zadrag-Tecza (2014) | |
| Estimation of microbial biomass | Gravimetric method. Dry weights |
The drying process may take time, and the preparation difficult. The presence of adsorbed non-microbial substances can interfere. | Harris & Kell (1985) |
| Spectrophotometric method | It determines the turbidity associated with the microorganism's presence. The number and size of cells influences the measure. Response time 7 h. Linear range (0.0–1.0) g L−1. Sensibility 0.63 g L−1. R2 = 0.9994. Error 1.22%. | Hernández & Marín (2002) | |
| Cell count | Flow cytometric method | It analyzes the scattered and fluorescent signals produced by a cell passing through a light beam. It provides automated data but is expensive and requires specialized personnel and high aseptic conditions. It is more directed to the medical field. | (Bochner et al., 1989; Lawrence, 2002; Michelson AL, 1996) |
| Electrochemistry, chronoamperometric method (CA). Cell count of S. cerevisiae and Lactobacillus fermentum. | Measurement of the current generated by microorganisms on a platinum anode covered with a cellulose dialysis membrane. A linear relationship between current and cell number, below 4.0 × 108 cells mL−1. Error 5.0%. | (T Matsunaga et al., 1979) | |
| Electrochemistry, differential pulse voltammetry (DPV). | The current measurement employed a graphite electrode modified with 4,4′-bipyridine. Linear range (0.03–2.00) x 108 cells mL−1. | (Matsunaga and Namba, 1984b) | |
| Measurement of microorganism populations | Electrochemistry cyclic voltammetry (CV). | GCE modified with tetracycline. Linear range (2.0–5.0) x104 cells mL−1. | Han et al. (2000) |
| Electrochemistry cyclic voltammetry (CV). | Graphite working electrode vs. SCE. Linear range (0.1–1.9) x108 cell mL−1. Peak currents were reproducible, error of 4%. | (Matsunaga and Namba, 1984a) | |
| Electrochemistry chronoamperometry method (CA). | GCE modified with 2,3,5 - TBQ. Range lineal (6.4 × 103–1.6 × 106) cells mL−1. | Tsukatani et al. (2003) | |
| Electrochemistry chronoamperometry method (CA). | Screen-printed electrodes modified with magnetic nanoparticles. Linear range 1.0–1.0 × 104 CFU mL−1. LOD 5.0 UFC mL−1 | Villalonga et al. (2019) | |
| Cellular density determination | Electrochemistry chronoamperometry method (CA). | GCE modified with oxidized multi-wall carbon nanotubes dispersed in water – Nafion® (GCE/OMWCNT-N). Range lineal 3.36–6.52 g L−1. LOD 0.98 g L−1, LOQ 3.36 g L−1. R 0.99. | Acevedo Restrepo et al. (2022) |
| Electrochemistry chronoamperometry method (CA). | SPCE modified with oxidized multi-wall carbon nanotubes dispersed in water – Nafion® (SPCE/OMWCNT-N). Range lineal 0.61–7.69 g L−1. LOD 0.17 g L−1, LOQ 0.61 g L−1. R 0.99. | This work |
1YPD: Culture medium of yeast extract, peptone, and dextrose.
22,3,5-TBQ: 2,3,5-trimetil-1,4-benzoquinona. Redox mediator.
Table 3 shows that there are four ways to express the yeast amount; the cellular viability (% of live cells in a population), the cellular vitality (physiological cell capacity), the colony forming units (CFU, cells mL−1), and the biomass quantitation as cellular density (g L−1). Of particular interest is that some of the yeast quantification methodologies employ electrochemical techniques (CV, DPV, CA). Electrochemical techniques compared to conventional methods favor linear ranges, relative error, detection, and quantification limits; reduction costs, response time, reagents, and waste. So, the electroanalytic techniques are attractive alternatives for yeast study.
Using the SPCE/OMWCNT-N, the analytical parameters were improved with respect to the GCE/OMWCNT-N, achieving a minor dispersion, better detection and quantification limits, linearity, and linear range. Considering that reports of microbiological counting are generally in colony-forming units or cells by milliliter, the possibility of realizing it in terms of grams per liter adds versatility to the electrochemical tool. These results prove that it is possible to improve the electrochemical determination of Saccharomyces cerevisiae using SPCE modified with carbon nanotubes dispersed in water–Nafion®.
4. Conclusions
Screen-printed electrodes modified with oxidized multi-walled carbon nanotubes dispersed in water-Nafion®, enable the detection of an electrochemical signal for the yeast and a greater increase in the electroactive area compared with nanotubes modified electrode in the absence of a polymeric membrane. S. cerevisiae electrochemical response was controlled by diffusion at low concentrations and by diffusion-adsorption mixed-control at high concentrations. There is a linear relationship between the electrochemical response of the yeast S. cerevisiae, expressed as current density, and its concentration. The analytical parameters obtained using the SPCE/OMWCNT-N are better than those obtained using GCE/OMWCNT-N electrodes, proving that it is possible to improve the electrochemical determination of Saccharomyces cerevisiae yeast using screen-printed electrodes.
CRediT authorship contribution statement
Isabel Acevedo-Restrepo: Methodology, Conceptualization, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Funding acquisition. Lucas Blandón-Naranjo: Methodology, Conceptualization, Formal analysis, Funding acquisition. Mario Víctor Vázquez: Supervision, Project administration, Funding acquisition. Nora Restrepo-Sánchez: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
I.A.R wishes to thank the Colombian Ministry of Science and Technology Call 785–2017 for the doctoral scholarship.
I.A.R, L.B.N, and M.V.V wish to thank the Committee for the Development of Research – CODI -of the University of Antioquia for the support of the project 2015–7523.
Data availability
Data will be made available on request.
References
- Acevedo Restrepo I., Blandón Naranjo L., Hoyos-Arbeláez J., Víctor Vázquez M., Gutiérrez Granados S., Palacio J. Electrochemical determination of Saccharomyces cerevisiae sp using glassy carbon electrodes modified with oxidized multi-walled carbon nanotubes dispersed in water –Nafion®. Curr. Res. Food Sci. 2022;5:351–359. doi: 10.1016/j.crfs.2022.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmatulu R., Khan A., Adigoppula V., Hwang G. Enhanced transport properties of graphene ‐ based , thin Nafion ® membrane for polymer electrolyte membrane fuel cells. Energy Res. 2018;42(July 2017):508–519. doi: 10.1002/er.3834. [DOI] [Google Scholar]
- Atif R., Inam F. Reasons and remedies for the agglomeration of multilayered graphene and carbon nanotubes in polymers. Beilstein J. Nanotechnol. 2016;7:1174–1196. doi: 10.3762/bjnano.7.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks C.E., Compton R.G. New electrodes for old : from carbon nanotubes to edge plane pyrolytic graphite. Analyst. 2006;131(i):15–21. doi: 10.1039/b512688f. [DOI] [PubMed] [Google Scholar]
- Bapat P., Nandy S.K., Wangikar P., Venkatesh K.V. Quantification of metabolically active biomass using Methylene Blue dye Reduction Test (MBRT): measurement of CFU in about 200 s. J. Microbiol. Methods. 2006;65(1):107–116. doi: 10.1016/j.mimet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Blandón-Naranjo L., Hoyos-Arbeláez J., Vázquez M.V., Della Pelle F., Compagnone D. NADH Oxidation onto different carbon-based sensors: effect of structure and surface-oxygenated groups. J. Sens. 2018;2018:1–9. doi: 10.1155/2018/6525919. [DOI] [Google Scholar]
- Bochner B.S., McKelvey A.A., Schleimer R.P., Hildreth J.E.K., MacGlashan D.W. Flow cytometric methods for the analysis of human basophil surface antigens and viability. J. Immunol. Methods. 1989;125(1–2):265–271. doi: 10.1016/0022-1759(89)90102-6. [DOI] [PubMed] [Google Scholar]
- Cinti S., Arduini F., Carbone M., Sansone L., Cacciotti I. Screen-printed electrodes modified with carbon nanomaterials : a comparison among carbon black , carbon nanotubes and graphene. Electroanalysis. 2015;27:2230–2238. doi: 10.1002/elan.201500168. [DOI] [Google Scholar]
- Dale M. Differential staining of living and dead yeast cells. Food Sci. (N. Y.) 1941;6:361–371. [Google Scholar]
- Fernández Abedul M.T. Dynamic electroanalysis. Lab. Methods Dynam. Electroanal. 2020;1959:1–10. doi: 10.1016/b978-0-12-815932-3.00001-2. [DOI] [Google Scholar]
- Ferrari A.G., Rowley-neale S.J., Banks C.E. Screen-printed electrodes : transitioning the laboratory in-to-the field. Talanta Open. 2021;3(January):1–10. doi: 10.1016/j.talo.2021.100032. [DOI] [Google Scholar]
- Fiorino S., Szabo S.E., Monroe S.L., Bitzan J., Loper K. Evaluation of an automated instrument for viability and concentration measurements of cryopreserved hematopoietic cells. Lab. Hematol. 2004;10(2):109–111. doi: 10.1532/lh96.04020. [DOI] [PubMed] [Google Scholar]
- Ganjali M.R., Gupta V.K., Faridbod F., Norouzi P. Lanthanides Series Determination by Various Analytical Methods. 2016. Electrochemical determination of lanthanides series; pp. 91–208. [DOI] [Google Scholar]
- Han S., Li X., Guo G., Sun Y., Yuan Z. Voltammetric measurement of microorganism populations. Anal. Chim. Acta. 2000;405(1–2):115–121. doi: 10.1016/S0003-2670(99)00753-9. [DOI] [Google Scholar]
- Harris C.M., Kell D.B. The estimation of microbial biomass. Biosensors. 1985;1(1):17–84. doi: 10.1016/0265-928X(85)85005-7. [DOI] [PubMed] [Google Scholar]
- Hayat A., Marty J.L. Disposable screen printed electrochemical sensors: tools for environmental monitoring. Sensors (Switzerland) 2014;14(6):10432–10453. doi: 10.3390/s140610432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A., Marín M. New turbidimetric method for estimating bacterial growth in heterogeneous media. Process Biochem. 2002;37(10):1125–1128. doi: 10.1016/S0032-9592(01)00328-4. [DOI] [Google Scholar]
- Hong K.K., Nielsen J. Metabolic engineering of Saccharomyces cerevisiae: a key cell factory platform for future biorefineries. Cell. Mol. Life Sci. 2012;69(16):2671–2690. doi: 10.1007/s00018-012-0945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Bao J., Nielsen J. Biopharmaceutical protein production by Saccharomyces cerevisiae : current state and future prospects. Pharmaceut. Bioprocess. 2014;2(2):167–182. doi: 10.4155/pbp.14.8. [DOI] [Google Scholar]
- Huang W., Yang C., Zhang S. Simultaneous determination of 2-nitrophenol and 4-nitrophenol based on the multi-wall carbon nanotubes Nafion-modified electrode. Anal. Bioanal. Chem. 2003;375(5):703–707. doi: 10.1007/s00216-002-1745-5. [DOI] [PubMed] [Google Scholar]
- Jadon N., Jain R., Sharma S., Singh K. Recent trends in electrochemical sensors for multianalyte detection ??? A review. Talanta. 2016;161(September):894–916. doi: 10.1016/j.talanta.2016.08.084. [DOI] [PubMed] [Google Scholar]
- Kadara R.O., Jenkinson N., Banks C.E. Sensors and Actuators B : chemical Characterisation of commercially available electrochemical sensing platforms. Sensor. Actuator. B Chem. 2009;138:556–562. doi: 10.1016/j.snb.2009.01.044. [DOI] [Google Scholar]
- Kara B.V., Simpson W.J., Hammond J.R.M. Prediction of the fermentation performance of brewing yeast with the acidification power test. J. Inst. Brew. 1988;94(3):153–158. doi: 10.1002/j.2050-0416.1988.tb04573.x. [DOI] [Google Scholar]
- Kwolek-mirek M., Zadrag-tecza R. Comparison of methods used for assessing the viability and vitality of yeast cells. Fermentation Eur. Microbiol. Soc. 2014;14:1068–1079. doi: 10.1111/1567-1364.12202. [DOI] [PubMed] [Google Scholar]
- Kwolek-Mirek M., Zadrag-Tecza R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014;14(7):1068–1079. doi: 10.1111/1567-1364.12202. [DOI] [PubMed] [Google Scholar]
- Lawrence D. International Journal of Food Science and Technology (Vol. 37, Issue 3) 2002. Brewing yeast fermentation performance second edition. [DOI] [Google Scholar]
- Lee C.H., Wang S.C., Yuan C.J., Wen M.F., Chang K.S. Comparison of amperometric biosensors fabricated by palladium sputtering, palladium electrodeposition and Nafion/carbon nanotube casting on screen-printed carbon electrodes. Biosens. Bioelectron. 2007;22(6):877–884. doi: 10.1016/j.bios.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Lien C.H., Chang K.H., Hu C.C., Wang D.S.H. Green electrode for Pb2+ sensing based on the Nafion-graphene/CNT composite. Proc. IEEE Sensors. 2012:12–14. doi: 10.1109/ICSENS.2012.6411548. [DOI] [Google Scholar]
- Macpherson J.V., Unwin P.R., Liu Z., Dumitrescu I., Unwin P.R., Macpherson J.V. Electrochemistry at carbon nanotubes : perspective and issues. Chem. Commun. 2009;7345(45):6865–7052. doi: 10.1039/b909734a. [DOI] [PubMed] [Google Scholar]
- Matsunaga T., Karube I., Suzuki S. Electrode system for the determination of microbial populations. Appl. Environ. Microbiol. 1979;37(1):117–121. doi: 10.1128/AEM.37.1.117-121.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Tadashi, Namba Y. Detection of microbial cells by cyclic voltammetry. Anal. Chem. 1984;56(1):798–801. doi: 10.1021/ac00268a047. [DOI] [PubMed] [Google Scholar]
- Matsunaga Tadashi, Namba Y. Selective determination of microbial cells by graphite electrode modified with adsorbed 4,4-bipyridine. Anal. Chim. Acta. 1984;159:87–94. doi: 10.1016/S0003-2670(00)84284-1. [DOI] [Google Scholar]
- Matsunaga Tadashi, Namba Y., Nakajima T. Electrochemical sterilization of microbial cells. Bioelectrochem. Bioenerg. 1984;13(4–6):393–400. doi: 10.1016/0302-4598(84)87040-3. [DOI] [Google Scholar]
- Michelson A.L. Flow cytometry: a clinical test of platemel function. J. Am. Soc. Hematol. 1996;87(12):4925–4936. doi: 10.1136/bmj.s4-1.148.880-a. [DOI] [PubMed] [Google Scholar]
- Nicholson R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965;37(11):1351–1355. doi: 10.1021/ac60230a016. [DOI] [Google Scholar]
- Ostergaard S., Olsson L., Nielsen J. Metabolic Engineering of Saccharomyces cerevisiae. 2000;64(1):34–50. doi: 10.1128/mmbr.64.1.34-50.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renedo O.D., Alonso-Lomillo M.A., Martínez M.J.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta. 2007;73(2):202–219. doi: 10.1016/j.talanta.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Shayani-jam H. Electrochemical study of adsorption and electrooxidation of 4,4′-biphenol on the glassy carbon electrode: determination of the orientation of adsorbed molecules. Monatshefte Fur Chemie. 2019;150(2):183–192. doi: 10.1007/s00706-018-2318-4. [DOI] [Google Scholar]
- Stradiotto N.R., Yamanaka H., Zanoni M.V.B. Electrochemical sensors : a powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003;14(2):159–173. [Google Scholar]
- Sven Pegel a, Petra Potschke a, Petzold Gudrun, Alig Ingo, Sergej M., Dudkin D.L. Dispersion , agglomeration , and network formation of multiwalled carbon nanotubes in polycarbonate melts. Polymer. 2008;49:974–984. doi: 10.1016/j.polymer.2007.12.024. [DOI] [Google Scholar]
- Thyab S., Al G., Altemimi A.B., Jabbar A., Al A., Niamah A.K., Lakhssassi N., Ibrahim S.A. Purification of bioactive peptide with antimicrobial properties produced by Saccharomyces cerevisiae. Foods. 2020;9 doi: 10.3390/foods9030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukatani T.T., De S.I., Ba T.O., Keda H.U., Atsumoto K.M. Electrochemical measurement of yeast cell density and vitality using 2 , 3 , 5-Trimethyl- 1 , 4-benzoquinone and effect of ethanol on the metabolism of quinone by yeast cells. Food Sci. Technol. 2003;9(3):271–275. doi: 10.3136/fstr.9.271. [DOI] [Google Scholar]
- Turker M. 27th VH Yeast Conference. Advances in Science and Industrial Productionss of Baker's Yeast, December. 2014. Yeast biotechnology: diversity and applications; pp. 1–26. [DOI] [Google Scholar]
- Umasankar Y., Thiagarajan S., Chen S.M. Nanocomposite of functionalized multiwall carbon nanotubes with nafion, nano platinum, and nano gold biosensing film for simultaneous determination of ascorbic acid, epinephrine, and uric acid. Anal. Biochem. 2007;365(1):122–131. doi: 10.1016/j.ab.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Villalonga M.L., Borisova B., Arenas C.B., Villalonga A., Arévalo-Villena M., Sánchez A., Pingarrón J.M., Briones-Pérez A., Villalonga R. Disposable electrochemical biosensors for Brettanomyces bruxellensis and total yeast content in wine based on core-shell magnetic nanoparticles. Sens. Actuators, B. 2019;279(April 2018):15–21. doi: 10.1016/j.snb.2018.09.092. [DOI] [Google Scholar]
- Walker G., Stewart G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages. 2016;2(4):1–12. doi: 10.3390/beverages2040030. [DOI] [Google Scholar]
- Wang G., Huang M., Nielsen J. Exploring the potential of Saccharomyces cerevisiae for biopharmaceutical protein production. Curr. Opin. Biotechnol. 2017;48(Figure 1):77–84. doi: 10.1016/j.copbio.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Wang J. Carbon-nanotube based electrochemical biosensors : a review. Electroanalysis. 2005;17(1):7–14. doi: 10.1002/elan.200403113. [DOI] [Google Scholar]
- Wang J. In: Analytical Electrochemistry. second ed. VCH W.-, editor. 2006. Electrochemical sensors; pp. 201–243. [Google Scholar]
- Wang J., Musameh M., Lin Y. Solubilization of carbon nanotubes by nafion toward the preparation of amperometric biosensors Joseph. JACS Communications. 2003;125:2408–2409. doi: 10.1021/ja028951v. [DOI] [PubMed] [Google Scholar]
- Wang J., Tian B., Nascimento V.B., Angnes L. Performance of screen-printed carbon electrodes fabricated from different carbon inks. Electrochim. Acta. 1998;43(23):3459–3465. doi: 10.1016/S0013-4686(98)00092-9. [DOI] [Google Scholar]
- Wu K., Hu S. Electrochemical study and selective determination of dopamine at a multi-wall carbon nanotube-nafion film coated glassy carbon electrode. Microchim. Acta. 2004;144(1–3):131–137. doi: 10.1007/s00604-003-0103-4. [DOI] [Google Scholar]
- Yang S., Li G., Yin Y., Yang R., Li J., Qu L. Nano-sized copper oxide/multi-wall carbon nanotube/Nafion modified electrode for sensitive detection of dopamine. J. Electroanal. Chem. 2013;703:45–51. doi: 10.1016/j.jelechem.2013.04.020. [DOI] [Google Scholar]
- Yang S., Yang R., Li G., Qu L., Li J., Yu L. Nafion/multi-wall carbon nanotubes composite film coated glassy carbon electrode for sensitive determination of caffeine. J. Electroanal. Chem. 2010;639(1–2):77–82. doi: 10.1016/j.jelechem.2009.11.025. [DOI] [Google Scholar]
- Yusof N.A., Daud N., Saat S.Z.M., Tee T.W., Abdullah A.H. Electrochemical characterization of carbon nanotubes/nafion/aspartic acid modified screen printed electrode in development of sensor for determination of Pb(II) Int. J. Electrochem. Sci. 2012;7(11):10358–10364. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.