Abstract
Background
A number of different medications are used in combination with intrathecal bupivacaine for cesarean section anesthesia, but their relative efficacy has not been well established.
Objective
To study the relative efficacy of adding either intrathecal fentanyl, intrathecal sufentanil, or intravenous acetaminophen-morphine-fentanyl to intrathecal bupivacaine spinal anesthesia for pain control in elective cesarean section operations.
Methods
In this randomized, double-blinded, controlled trial, 105 pregnant women eligible for cesarean section received 10 mg intrathecal bupivacaine (0.5%) in combination with 2 μg intrathecal sufentanil (group 1), 10 μg intrathecal fentanyl (group 2), and an intravenous cocktail of 1 g acetaminophen, 5 mg morphine, and 100 μg fentanyl (group 3). Patients were assessed for analgesia, time to block, and adverse effects.
Results
The 3 groups were similar in terms of the time to onset of sensory block and the duration of both sensory and motor block. Groups 1 and 3 differed significantly in the time to peak sensory block, whereas group 1 differed significantly from groups 2 and 3 in the time to peak motor block. The sensory block level reached T4 in most patients. Significant differences in pain (visual analog scale) were demonstrated between groups 1 and 3 at 5 minutes after spinal injection, between groups 1 and 2 at the end of the surgery and upon arrival to the recovery room, and between all groups in the recovery room.
Conclusions
When used in addition to 10 mg intrathecal bupivacaine, an intravenous cocktail of 1 g acetaminophen-5 mg morphine-100 μg fentanyl was as efficient as either 10 μg intrathecal fentanyl or 2 μg intrathecal sufentanil in terms of sensory and motor block duration and produced a higher dermatomal level of sensory block. However, intrathecal sufentanil provided better anesthesia quality (less time to onset of motor block and peak sensory-motor block) and better pain control. (Curr Ther Res Clin Exp. 2023; 84:XXX–XXX)
Key words: acetaminophen, cesarean section, fentanyl, spinal anesthesia, sufentanil
Introduction
The cesarean section (C/S) is among the most commonly performed surgical procedures.1,2 The standard anesthetic approach for elective C/S is regional anesthesia,1,2 especially spinal anesthesia. It is commonly used to avert the side effects of general anesthesia, such as pneumonia and postoperative pain.3 Furthermore, it is popular due to its simplicity, reliability, and rapid onset of sensory analgesia.1 Other advantages of regional anesthesia over general anesthesia include less maternal mortality, blood loss, hospital stay, incision-site infections, and drug passage to the placenta, as well as appropriate postoperative pain control.2,4
Various medications are used for spinal anesthesia; the more commonly used are local anesthetics.5 Bupivacaine (0.5%) is the preferred choice for C/S on account of its lower maternal hemodynamic instability, tachyphylaxis, and passage to the fetus, as well as separated sensory and motor blocks.5,6 Low-dose diluted bupivacaine is generally used to achieve rapid recovery but may compromise the anesthetic efficacy by conferring the risk of analgesic supplementation.6,7 Thus, intrathecal opioids are often combined with local anesthetics to prolong the duration of analgesia, achieve a deeper sensory block (without increasing the sympathetic block), reduce the required local anesthetic dose, improve the hemodynamic stability and analgesia quality, and minimize side effects for the fetus and mother.5,6
Among the various opioids, fentanyl and sufentanil are more appropriate than morphine due to their rapid onset of action and fewer side effects when used as adjuvants to local anesthetics in C/S anesthesia.8 The high lipophilic characteristics of fentanyl and sufentanil and their high affinity for μ-receptors compensate for the relatively late onset of local anesthetics and rapid sensory block induction.8 Research has shown that combining fentanyl or more lipophilic drugs such as sufentanil with bupivacaine for spinal anesthesia improves intraoperative and postoperative pain control and hemodynamic stability with adequate spinal anesthesia.9,10 However, the clinical applications of these opioids are limited due to a range of side effects, such as respiratory depression, hypopnea, nausea, and dizziness.
Intravenous acetaminophen (paracetamol) has been widely utilized as a pain reliever in postoperative and perioperative settings.11 According to published guidelines, acetaminophen should be used as the first-line analgesic.12 Compared with opioids, acetaminophen causes little or no respiratory depression but is largely ineffective in controlling extreme pain.11 Therefore, it needs to be accompanied by other strategies.12
This study compared the efficacy of 3 adjuvants in combination with intrathecal bupivacaine for elective C/S anesthesia, namely intrathecal fentanyl, intrathecal sufentanil, or intravenous acetaminophen-morphine-fentanyl. The key objective was to determine the best adjuvant for intrathecal bupivacaine in terms of spinal anesthesia quality, pain control, and postoperative side effects.
Methods and Materials
This randomized, double-blinded controlled clinical trial was conducted on 105 pregnant women referred to the operation room for elective C/S in Hafez Hospital, affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. The study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences and was registered in the Iranian Registry of Clinical Trials (IRCT20141009019470N101, July 28, 2020). Written informed consent was obtained from each eligible participant.
Pregnant women in their first or second pregnancy aged 20 to 30 years with American Society of Anesthesiologists score I or II, 50 to 100 kg weight, and 150 to 180 cm height were included in this study. The exclusion criteria were spinal anesthesia contraindications, including patients’ refusal, localized infection in the lumbar region (the entry site of the spinal anesthesia needle), intake of antiplatelet or anticoagulation drugs, allergic reactions to local anesthetics, dependent on narcotics or nicotine, history of hypovolemic shock, and comorbidities such as diabetes mellitus, renal disease (serum creatinine >1.5 mg/dL), coagulation disorders, liver disease (alanine transaminase >60 U/L), heart disease, history of seizures or any neurological disease, systolic blood pressures above 150 or below 100 mm Hg, preeclampsia, fetal anomalies, intrauterine growth restriction, or hemoglobin levels below 8 mg/dL. The principal investigator questioned potential patients about the inclusion/exclusion criteria. If the patient was deemed to be eligible, the principal investigator explained the study procedures. Adequate time was provided to allow patients to decide about participation and ask questions. Written informed consent was then obtained if they volunteered to enroll in the study. The sample size was calculated at 33 in each group, based on a similar study with SD1 = 2.3, SD2 = 4.2, d = 2.4, α = 5%, and power = 90%. Considering a dropout rate of 10%, a minimum of 35 patients was required in each group.13
A total of 120 patients were screened, 105 of whom were eligible (according to the inclusion and exclusion criteria) and provided informed consent to participate in this trial. Patients were divided into 3 groups using a random number sequence based on a computer-generated chart (https://commentpicker.com/) with a block size of 3 (sequence of 35 blocks) by an individual external to the study. The randomization sequence was enclosed within a sealed envelope.
The patient participants were divided into 3 study groups:
-
•
Group 1 (BS group): Received intrathecal bupivacaine 0.5% (10 mg), sufentanil (2 μg), and normal saline (0.1 mL) (total volume was 2.5 mL + distilled water), with 200 mL normal saline intravenously infused after umbilical cord removal as a placebo.
-
•
Group 2 (BF group): Received intrathecal bupivacaine 0.5% (10 mg), fentanyl (10 μg), and normal saline (0.3 mL) (total volume was 2.5 mL + distilled water), with 200 mL normal saline intravenously infused after umbilical cord removal as a placebo.
-
•
Group 3 (BC group): Received intrathecal bupivacaine 0.5% (10 mg) (total volume was 2.5 mL + distilled water) and an intravenous cocktail comprising 1 g acetaminophen, 5 mg morphine, and 100 μg fentanyl diluted in 200 mL normal saline. The intravenous infusion was commenced through a peripheral vein after the cord was crossclamped.
Patients, physicians, and research staff were blinded to the medications provided to each individual patient. To blind the investigators, an anesthesiologist external to the study prepared all medications and coded them according to the respective groups. A nurse passed the medications to the anesthesiologist who performed the procedure. Also, generating the random allocation sequence, making measurements, and assigning participants to interventions were done by individuals who were blinded regarding the identity of the study groups.
Patients were placed in the supine, left lateral position to reduce the incidence of early-onset hypotension. After obtaining venous access using a No. 18 angiocatheter, all patients were hydrated with ringer lactate solution (10 mL/kg). Supplemental oxygen was administered via a face mask at a rate of 5 to 8 L/min.
Before spinal anesthesia, an electrocardiogram was obtained, and the heart rate; peripheral oxygen saturation (pulse oximetry); and systolic, diastolic, and mean arterial blood pressure (MAP) levels were recorded.
Spinal anesthesia was performed in the sitting position via the midline approach with a 25-gauge Quinke needle in the L3–L4 intervertebral space by an anesthesiologist who was blind to the study groups.
If the blood pressure decrement was >20% of baseline after induction of anesthesia, fluid therapy and ephedrine (5 mg to a maximum of 30 mg) were prescribed. In case of a heart rate drop ≥20% of baseline, an initial dose of 0.6 mg atropine was prescribed. Alternative therapies in case of pain in the recovery room (visual analog scale [VAS] >4) included a 100 mg suppository of diclofenac, with 20 mg intravenous meperidine being administered if the pain did not subside. Ondansetron (0.15 mg/kg) was administered in the case of nausea and vomiting, with promethazine being reserved for severe cases unresponsive to ondansetron. For respiratory depression (respiratory rate <8), 0.4 mg naloxone was administered every 3 to 5 minutes up to a maximum dose of 10 mg (0.01 mg/kg). Patients with tremors benefited from heating and warm fluids. After spinal anesthesia, heart rate and systolic blood pressure were recorded every 2 minutes until 20 minutes and then every 5 minutes until the end of the operation and also in the recovery room.
The sensory block was measured by the pinprick method by the blinded anesthesiologist.14 This method was fulfilled with gentle skin stimulation using a size-10 needle or safety pin, primarily activating Aδ fibers.15 The time of sensory block onset (the interval between the time of intrathecal injection and the loss of pinprick sensation up to the dermatomal level of T10), the maximum dermatomal level of sensory block (checked every 5 minutes for the first 20 minutes and then every 10 minutes in the following 60 minutes), the time to reach the maximum dermatomal level of sensory block, and the duration of the sensory block (when the sensory block dermatomal level returned below T10) were measured.
The degree of motor block was evaluated using the Modified Bromage Scale, which assesses the ability to move the lower extremities using a 0 to 3 score (0 = no motor block, 1 = unable to raise extended legs but able to move knees and feet, 2 = unable to raise extended legs and move knees but able to move feet, and 3 = complete motor block of the lower limbs).16 The time of motor block onset (checked every 1 minute in the first 5 minutes and then every 5 minutes in the following 20 minutes; equal to reaching a score of 1 on the Modified Bromage Scale), the duration of motor block (return of the score to 0), and maximum time to motor block were evaluated. The neonatal Apgar score was recorded at 1 and 5 minutes.
Pain intensity was rated by the participants using a VAS ranging from 0 (pain-free) to 10 (worst pain imaginable). The scores were measured 9 times, including 5, 10, 15, 20, 30, and 40 minutes after injection; end of the surgery; upon arrival at the recovery room; and inside the recovery room. The end of analgesia was defined as the time when the VAS score was recorded as <4.
Itching, shivering, nausea, and vomiting during and after surgery were checked every 10 minutes from the beginning of anesthesia. The occurrence of bradycardia (heart rate <50 beats per minute) and hypotension (systolic blood pressure <90 mm Hg or 20% drop from baseline) was also checked. Postoperative nausea and vomiting were evaluated by asking the patients to grade their symptoms on a 3-point scale: 0 = no nausea or vomiting, 1 = nausea only, and 2 = retching or vomiting. Shivering was measured by the following criteria: 0 = no tremor; 1 = vasoconstriction, piloerection, but no clear tremor; 2 = muscle activity in only 1 muscle; 3 = muscle activity in more than 1 muscle; and 4 = body tremor.
The primary outcomes were sensory block characteristics, motor block characteristics, and pain; secondary outcomes included Apgar score and complications (eg, shivering, nausea/vomiting, bradycardia, and hypotension).
Statistical analysis
Data were analyzed using SPSS (version 22, IBM-SPSS Inc, Armonk, New York). Continuous variables are reported as mean (SEM) or median and interquartile range. Categorical variables are summarized using numbers and percentages. The differences between groups were examined by ANOVA with the Tukey post hoc test to analyze parametric variables and the Kruskal–Wallis test to analyze nonparametric variables. Also, the χ2 test or Fisher exact test was used to test differences in categorical variables. The repeated measures ANOVA test was also used because time and group had an interaction effect. P values < 0.05 were considered statistically significant.
Result
The study was performed from June to October 2020. Among 120 screened patients, 105 were enrolled and divided equally into 3 study groups. Spinal anesthesia was achieved in all 105 patients, all of whom were included in the statistical analysis (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials flow diagram of the study.
No significant differences were demonstrated between the groups in demographic characteristics and baseline data, including age, height, weight, body mass index, blood pressure values, heart rate, and oxygen saturation (Table 1).
Table 1.
Demographic and baseline data.*
| Group 1 | Group 2 | Group 3 | P value | |
|---|---|---|---|---|
| Age, y | 31.28 (0.99) | 31.60 (0.89) | 32.02 (0.92) | 0.854 |
| Height, cm | 160.57 (0.82) | 160.18 (0.82) | 160.88 (0.98) | 0.860 |
| Weight, kg | 77.542 (1.50) | 81.22 (1.62) | 77.65 (2.50) | 0.311 |
| BMI | 30.11 (0.60) | 31.95 (0.70) | 30.13 (0.89) | 0.143 |
| SBP, mm Hg | 131.86 (2.20) | 125.08 (2.24) | 124.85 (2.48) | 0.055 |
| DBP, mm Hg | 81.66 (1.61) | 81.31 (1.81) | 80.88 (2.24) | 0.959 |
| MAP, mm Hg | 98.39 (1.63) | 95.90 (1.84) | 95.54 (2.20) | 0.513 |
| HR, bpm | 103.75 (2.80) | 97.11 (3.27) | 94.28 (2.55) | 0.063 |
| Spao2 (%) | 96.55 (0.25) | 96.61 (0.23) | 97.22 (0.24) | 0.107 |
BMI = body mass index; DBP = diastolic blood pressure; HR = heart rate; MAP = mean arterial pressure; SBP = systolic blood pressure; Spao2 = arterial oxygen saturation.
Group 1= Intrathecal bupivacaine-sufentanil, group 2: Intrathecal bupivacaine-fentanyl, and group 3: Intrathecal bupivacaine + intravenous acetaminophen-morphine-fentanyl. Values are presented as mean (SEM).
As demonstrated in Table 2, the groups were similar in the onset and duration of sensory block. However, the time to peak sensory block differed between the BS and BF groups (P = 0.049).
Table 2.
Characteristics of the spinal block in the different study groups.*
| Group 1 | Group 2 | Group 3 | P value | |
|---|---|---|---|---|
| Time to onset of sensory block | 2 (1–2) | 2 (1–3) | 2 (1–3) | 0.181 |
| Time to onset of motor block | 1.50 (1–2) | 5 (2–8) | 2 (1–6) | < 0.0001†‡ |
| Time to peak sensory block | 3 (1.25–4) | 4 (3–6) | 3 (2–5) | 0.049† |
| Time to peak motor block | 4 (3–6) | 7 (5–10.25) | 7 (4–11) | < 0.0001†‡ |
| Duration of sensory block | 107 (93–125) | 113.50 (83.50–128) | 114 (95–150) | 0.401 |
| Duration of motor block | 110 (88–133) | 113.50 (83.50–89.50) | 114 (95–60.31) | 0.597 |
| Maximum dermatomal level of sensory block | ||||
| T2 | 0 (0) | 1 (2.9) | 0 (0) | 0.364 |
| T4 | 21 (60) | 28 (80) | 30 (85.72) | 0.032‡ |
| T6 | 14 (40) | 6 (17.1) | 5 (14.28) | 0.022‡ |
Group 1: Intrathecal bupivacaine-sufentanil, group 2: Intrathecal bupivacaine-fentanyl, group 3: Intrathecal bupivacaine + intravenous acetaminophen-morphine-fentanyl. Values are presented as median (interquartile range). Values are presented as median(interquartile) and n(%).
P < 0.05 between groups 1 and 2.
P < 0.05: between groups 1 and 3.
The duration of the motor block was similar between the groups, yet the onset of motor block was earlier in the BS group (median = 1.50 minutes) compared with the BF (median = 5 minutes) and BC groups (median = 2 minutes) (P < 0.0001). Also, the time to peak motor block was significantly different between the groups. These differences were between the BS and BF groups as well as the BS and BC groups (P < 0.0001) (Table 2).
In all groups, the spinal block was successful. The block level in most patients was T4 (60% of patients in the BS group, 80% in the BF group, and 86% in the BC group). A less-effective neuraxial block (ie, T6) was observed in 40% of the patients in the BS group, 17% in the BF group, and 14% in the BC group. None of the patients in groups BS and BC reached the T2 block level, and just 2.9% of patients in the BF group reached this level (Table 2). There was no significant difference between the groups in terms of neonatal Apgar scores at 1 and 5 minutes (P = 0.279 and P = 0.138, respectively).
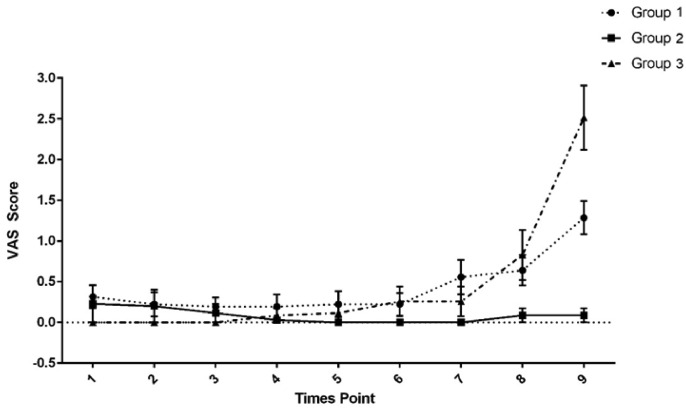
Regarding the VAS score of pain, a significant time–group interaction was found. The pain intensity differed significantly between groups BS and BC 5 minutes after spinal injection (P = 0.034), between groups BS and BF at the end of the surgery (P = 0.012) and upon arrival to the recovery room (P = 0.014), and among all groups in the recovery room (P < 0.001). At other time points (10–45 minutes after injection), the pain scores were similar across the 3 groups (Figure 2).
Figure 2.
Visual analog scale (VAS) scores at 0 different time points. Group 1: Intrathecal bupivacaine-sufentanil. Group 2: Intrathecal bupivacaine-fentanyl. Group 3: Intrathecal bupivacaine + intravenous acetaminophen-morphine-fentanyl. Data are reported as mean (SEM). Time points: 1 = 5 minutes after injection, 2 = 10 minutes after injection, 3 = 15 minutes after injection, 4 = 20 minutes after injection, 5 = 30 minutes after injection, 6 = 40 minutes after injection, 7 = end of surgery, 8 = arrival at the recovery room, and 9 =during recovery.
Complications such as itching, respiratory complications, hypotension, bradycardia, and atropine requirement were similar among the 3 groups. However, there were significant differences in nausea/vomiting and vasopressor requirement (P ≤ 0.0001 and P = 0.0004, respectively). Nausea and vomiting were more common in the BS group (80% of patients) and less common in the BF group (22.85% of patients). Vasopressors (ephedrine or epinephrine) were administered to 51.42% of patients in the BF group, 20% in the BC group, and 11.42% in the BS group (Table 3).
Table 3.
Supplemental medication use and complications in the different study groups.*
| Group 1 | Group 2 | Group 3 | P value | |
|---|---|---|---|---|
| Nausea/vomiting | 28 (80) | 8 (22.85) | 20 (57.14) | < 0.0001 |
| Irritation | 7 (20) | 3 (8.57) | 2 (5.71) | 0.155 |
| Respiratory complications | 5 (14.28) | 3 (8.57) | 4 (11.42) | 0.754 |
| Vasopressor requirement | 4 (11.42) | 18 (51.42) | 7 (20) | 0.0004 |
| Atropine requirement | 0 (0) | 0 (0) | 0 (0) | |
| Hypotension | 25 (69.4) | 24 (68.6) | 24 (68.6) | 0.996 |
| Bradycardia | 3 (8.57) | 3 (8.57) | 0 (0) | 0.203 |
Group 1: Intrathecal bupivacaine-sufentanil, Group 2: Intrathecal bupivacaine-fentanyl, and group 3: Intrathecal bupivacaine + intravenous acetaminophen-morphine-fentanyl. Values are presented as n (%).
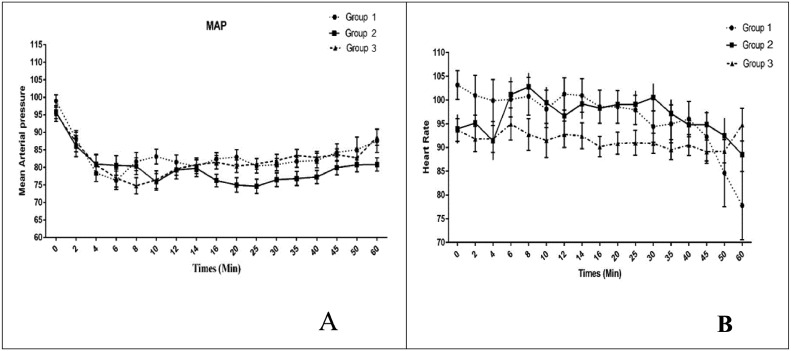
The time–group interaction had a significant effect on the MAP (P = 0.037) but not on the heart rate (P = 0.441). The MAP trend was significant over time (P < 0.001); however, for heart rate, this trend was insignificant (P = 0.063). The significant differences in MAP were demonstrated at the following postinjection time points: 20 minutes (between BS and BF, P = 0.016), 25 minutes (between BF and BC, P = 0.042), 35 minutes (between BF and BC, P = 0.031), and 60 minutes (between BF and BC, P = 0.026) (Figure 3).
Figure 3.
(A) Systolic and diastolic blood pressure and (B) heart rate at 17 time points. Group 1: Intrathecal bupivacaine-sufentanil. Group 2: Intrathecal bupivacaine-fentanyl. Group 3: Intrathecal bupivacaine + intravenous acetaminophen-morphine-fentanyl. Data are reported as mean (SEM).
Discussion
Implementing 1 local anesthetic in spinal anesthesia is insufficient to attenuate pain during surgery.17 Adjuvants have been recommended to increase anesthesia quality and decrease the dose of local anesthetics.18 The present study compared 3 adjuvants to intrathecal bupivacaine anesthesia (ie, intrathecal sufentanil; intrathecal fentanyl; or an intravenous cocktail of acetaminophen, morphine, and fentanyl) for spinal anesthesia in patients undergoing C/S. Among these adjuvants, although the intravenous cocktail was more desirable for reaching the proper dermatomal level of sensory block (ie, T4), the pain control and quality of spinal anesthesia (time to onset of motor block and peak sensory-motor block) were superior with intrathecal sufentanil.
The present study showed that the 3 groups’ onset and length of sensory block were similar. However, the BS group had the least time to reach the peak dermatomal level of sensory block, significantly differing from the BF group. We were unable to find any published study in which these 3 adjuvant regimens are compared. Gupta et al.19 reported that the time required to reach the peak dermatomal level of sensory block was longer in the groups with an adjuvant (10 mg sufentanil or 25 mg fentanyl) compared with bupivacaine alone in the control group. In line with the present study, Motiani et al.20 revealed that sufentanil is preeminent in terms of time to reach sensory blockade. Some other studies comparing the effects of sufentanil and fentanyl when combined with bupivacaine showed a significant difference in length of sensory blockade5,19, 20, 21 and time to onset of the sensory blockade, with sufentanil achieving the minimum time.20,21 The present work demonstrated that among the different adjuvants, an intravenous cocktail of acetaminophen, fentanyl, and morphine could induce a similar duration of sensory blockade as intrathecal sufentanil or intrathecal fentanyl.
Intrathecal opioids are attractive for use with bupivacaine in spinal anesthesia to achieve a better sensory blockade since lipid-soluble opioids, especially sufentanil, induce only a weak local anesthetic effect when injected intrathecally.22,23 Animal studies indicate the synergistic effect of intrathecal local anesthetics and opioids in terms of the time of their peak effect. A possible mechanism behind this synergistic effect is that each drug acts on a different ionic channel, inhibiting overall neuronal impulsiveness.24 Sufentanil, a gamma receptor agonist, can hinder the release of the transmitter by opening positive potassium ion channels and decreasing the influx of calcium cation. In addition, it can suppress neuronal activities through its hyperpolarization effects. On the other hand, bupivacaine can disrupt nerve conduction and interfere with synaptic transmission by blocking voltage-gated sodium ion channels and inhibiting presynaptic calcium cation channels. Therefore, different mechanisms can explain the synergistic effects of bupivacaine and sufentanil to provide a better sensory blockade.24,25 It is also possible that these agents' pharmacokinetic parameters are altered when injected intrathecally, giving rise to increased sufentanil concentrations in the spinal cord.24 However, it should be noted that fentanyl cannot reinforce the influence of bupivacaine on sympathetic pathways.
The present study also revealed that whereas the duration of the motor block was similar across the groups, the onset was earlier in the BS group (median = 1.50 minutes) compared with the BF group (median = 5 minutes) and BC group (median = 2 minutes). The time to peak motor block also differed significantly between the groups, with BS requiring the minimum time to reach its peak effect (median = 4 minutes vs 7 minutes in the BF and BC groups). In a similar study, Gupta et al.19 indicated a shorter time to motor block onset in the sufentanil and fentanyl groups (as adjuvants to bupivacaine) compared with isolated bupivacaine.19 Kim et al.26 reported an insignificant difference in motor block duration between fentanyl and sufentanil as adjuvants to bupivacaine. Other studies demonstrated that sufentanil had a longer time to onset of motor block20 and a longer duration of motor block.5,27
In all groups of the present study, the spinal block was successful. Sensory block levels ranged from T2 to T6, with the dominance of T4. Most patients reaching T4 were in the BC group, whereas the fewest patients reaching T4 were in the BS group. None of the patients in the BC and BS groups and just 2.9% of patients in the BF group reached the T2 block level. A less-effective neuraxial block (ie, T6) was observed in 40% of patients in the BS group, 17.1% in the BF group, and 26.4% in the BC group. Thus, these results indicate that an intravenous cocktail of acetaminophen, morphine, and fentanyl outperformed intrathecal sufentanil or fentanyl in achieving a proper dermatomal sensory block level in combination with intrathecal bupivacaine. Compatible with these findings, similar studies showed that the maximal block height achieved was T4,5,21 with more patients in the fentanyl adjuvant group reaching this level relative to the sufentanil adjuvant group.21 On the other hand, Hasani et al.28 showed that the sensory block level in most patients in the fentanyl group was T8, compared with T4 or T5 in most patients in the sufentanil group.
A previous study found that administering 6.25 to 50 pg fentanyl as an adjuvant to 10.5 mg (on average) of hyperbaric bupivacaine can lower the requirement of intraoperative intravenous analgesics from 67% to 0% during C/S operations; similarly, adding l0 to 20 pg sufentanil to 10.5 mg hyperbaric bupivacaine diminished the analgesic requirement from 70% to 0%.29 Regarding the VAS score, the present study did not observe any significant differences in VAS from 10 minutes after injection to 45 minutes after injection. However, significant differences were demonstrated 5 minutes after spinal injection, at the end of the surgery, upon arriving at the recovery room, and inside the recovery room. Motiani et al.20 showed that maximum VAS scores were reached within 2 hours in the control group, compared with 6 hours in the fentanyl group and 8 hours in the sufentanil group. Also, the VAS scores in the sufentanil group were insignificantly lower than the fentanyl group throughout the 24 hours postoperative period.20 The analgesic effect of opioids may be more marked during pregnancy due to higher progesterone levels, leading to greater sensitivity to local anesthetics. An animal study confirmed that the antinociceptive effect of intrathecal sufentanil was enhanced by administering progesterone. In addition, the synergistic inhibitory action of opioids and bupivacaine on A8 and C-fiber conduction, and the blocking response to high and low frequency stimulations can contribute to their remarkable analgesic effects.25
Regarding complications, there were no significant differences between the groups in itching, respiratory complications, hypotension, bradycardia, and atropine requirement. However, the patients who received sufentanil as the adjuvant experienced more nausea and vomiting (80% of patients). Fewer patients who received intrathecal sufentanil required vasopressors (ephedrine or epinephrine; 11.42% of patients) compared with the patients receiving the intravenous cocktail (20%) or intrathecal fentanyl (51.42%). Other studies showed no differences in adverse effects between groups that received fentanyl or sufentanil as adjuvants to bupivacaine.26,27 On the other hand, some studies demonstrated a higher incidence of pruritus when using sufentanil as an adjuvant compared with the controls.20,21
Intrathecal opioids decrease maternal plasma catecholamines, leading to hypotension. In addition, opioids can directly induce a sympatholytic effect in the spinal cord.30 The present study demonstrated no significant difference between groups in systolic and diastolic blood pressure and heart rate. Similarly, another study showed that none of the patients developed significant heart rate or blood pressure changes during the intraoperative period.20 However, Neeta et al.21 reported statistically significant drops in the systolic blood pressure after a subarachnoid block in their sufentanil group during the sixth and eighth minutes. However, these drops did not require treatment with vasopressors.21
Limitations of the study
One study limitation is the use of single doses of fentanyl and sufentanil for neuraxial blocking; future studies should evaluate and compare other intrathecal dosages of opioids as well as nonopioid medications as adjuvants to local anesthetics to determine the optimal doses. Additionally, patient-controlled analgesia pumps should be used.
Conclusions
When used in addition to 10 mg intrathecal bupivacaine, an intravenous cocktail of 1 g acetaminophen, 5 mg morphine, and 100 μg fentanyl was as efficient as either 10 μg intrathecal fentanyl or 2 μg intrathecal sufentanil in terms of sensory block onset and sensory-motor block duration. Furthermore, the intravenous cocktail excelled in reaching the appropriate dermatomal level of sensory block (ie, T4) and led to less nausea and vomiting than the sufentanil group and less vasopressor requirement than the fentanyl group. However, intrathecal sufentanil provided better anesthesia quality (less time to motor block onset and peak sensory-motor block) and better pain control.
Acknowledgments
Acknowledgments
This study was supported by Shiraz University of Medical Sciences and was extracted from the thesis written by Dr Sahar Chehelgerdi Samani. The authors thank the patients who participated in this trial. The manuscript was reviewed in terms of language and grammar by a native English-speaking language editor, Dr Seyed Ali Hosseini (Native Editor Co, Shiraz, Iran).
R. Jouybar was responsible for study conception, proposal writing, data analysis, and article drafting and revising. Z. Fattahi-Saravi was responsible for proposal preparation, data collection, and article drafting. S. Sadeghi and N. Dehghani were responsible for study conception, proposal writing, and article writing. S. Chehelgerdi-Samani and F. Masihi were responsible for proposal writing, data collection, and article revision. Z. Esmaeilinezhad was responsible for data analysis, manuscript preparation, and article writing and editing. N. Asmarian was responsible for study design, data analysis, and article writing.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
References
- 1.Pakniat H, Lalooha F, Movahed F, Boostan A, Khezri MB, Hedberg C, et al. The effect of ginger and metoclopramide in the prevention of nausea and vomiting during and after surgery in cesarean section under spinal anesthesia. Obstet Gynecol Sci. 2020;63(2):173–180. doi: 10.5468/ogs.2020.63.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mawson AL, Bumrungphuet S, Manonai J. A randomized controlled trial comparing early versus late oral feeding after cesarean section under regional anesthesia. Int J Womens Health. 2019;11:519–525. doi: 10.2147/IJWH.S222922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortazavi MMT, Parish M, Dorosti A, Mohammadipour HJh. Comparison of General Anesthesia With Spinal Anesthesia on the Quality of Recovery of Patients With Selective Abdominal Hysterectomy in Patients Vising the Largest Women's Disease Hospital in Northwestern Iran.11:12.
- 4.Saygı Aİ, Özdamar Ö, Gün İ, Emirkadı H, Müngen E, Akpak YKJSPMJ. Comparison of maternal and fetal outcomes among patients undergoing cesarean section under general and spinal anesthesia: a randomized clinical trial. 2015;133:227–234. doi: 10.1590/1516-3180.2014.8901012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farzi F, Mirmansouri A, Naderi Nabi B, Atrkar Roushan Z, Ghazanfar Tehran S, Nematollahi Sani M, et al. Comparing the Effect of Adding Fentanyl, Sufentanil, and Placebo with Intrathecal Bupivacaine on Duration of Analgesia and Complications of Spinal Anesthesia in Patients Undergoing Cesarean Section. Anesth Pain Med. 2017;7(5) doi: 10.5812/aapm.12738. e12738-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasr IA, Elokda SA. Safety and efficacy of intrathecal adjuvants for cesarean section: bupivacaine, sufentanil, or dexmedetomidine. Ain-Shams Journal of Anaesthesiology. 2015;8(3):388. [Google Scholar]
- 7.Arzola C, Wieczorek PM. Efficacy of low-dose bupivacaine in spinal anaesthesia for Caesarean delivery: systematic review and meta-analysis. Br J Anaesth. 2011;107(3):308–318. doi: 10.1093/bja/aer200. [DOI] [PubMed] [Google Scholar]
- 8.Braga AA, Frias JAF, Braga FS, Potério GB, Hirata ES, Torres NA. Spinal Anesthesia for Cesarean Section. Use of Hyperbaric Bupivacaine (10mg) Combined with Different Adjuvants. Brazilian Journal of Anesthesiology. 2012;62(6):775–787. doi: 10.1016/S0034-7094(12)70178-2. [DOI] [PubMed] [Google Scholar]
- 9.Vyas N, Sahu DK, Parampill R. Comparative study of intrathecal sufentanil bupivacaine versus intrathecal bupivacaine in patients undergoing elective cesarean section. Journal of anaesthesiology, clinical pharmacology. 2010;26(4):488. [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, Cho J-E, Hong J, Koo B, Kim J, Kil H. Comparison of intrathecal fentanyl and sufentanil in low-dose dilute bupivacaine spinal anaesthesia for transurethral prostatectomy. British journal of anaesthesia. 2009;103(5):750–754. doi: 10.1093/bja/aep263. [DOI] [PubMed] [Google Scholar]
- 11.Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S. Paracetamol: new vistas of an old drug. CNS Drug Rev. 2006;12(3-4):250–275. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin C-WC, Day RO, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350 doi: 10.1136/bmj.h1225. h1225-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farzi F, Mirmansouri A, Nabi BN, Roushan ZA, Sani MN, Azad SM, et al. Comparing the effect of adding fentanyl, sufentanil, and placebo with intrathecal bupivacaine on duration of analgesia and complications of spinal anesthesia in patients undergoing cesarean section. Anesth Pain Med. 2017;7(5) doi: 10.5812/aapm.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ousley R, Egan C, Dowling K, Cyna AM. Assessment of block height for satisfactory spinal anaesthesia for caesarean section. Anaesthesia. 2012;67(12):1356–1363. doi: 10.1111/anae.12034. [DOI] [PubMed] [Google Scholar]
- 15.Curatolo M, Petersen-Felix S, Arendt-Nielsen L. Sensory assessment of regional analgesia in humansa review of methods and applications. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2000;93(6):1517–1530. doi: 10.1097/00000542-200012000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Chung CJ, Yun SH, Hwang GB, Park JS, Chin YJ. Intrathecal fentanyl added to hyperbaric ropivacaine for cesarean delivery. Regional anesthesia and pain medicine. 2002;27(6):600–603. doi: 10.1053/rapm.2002.36455. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Chung KH, Lee JY, Chun DH, Yang HJ, Ko TK, et al. Comparison of fentanyl and sufentanil added to 0.5% hyperbaric bupivacaine for spinal anesthesia in patients undergoing cesarean section. Korean journal of anesthesiology. 2011;60(2):103. doi: 10.4097/kjae.2011.60.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poma S, Bossi C, Scudeller L, Broglia F, Baldi C, Ciceri M, et al. Hyperbaric bupivacaine and sufentanil for spinal anaesthesia in caesarean section: A cohort study. Journal of clinical anesthesia. 2020;62 doi: 10.1016/j.jclinane.2020.109706. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Sampley S, Kathuria S, Katyal S. Intrathecal sufentanil or fentanyl as adjuvants to low dose bupivacaine in endoscopic urological procedures. Journal of anaesthesiology, clinical pharmacology. 2013;29(4):509–515. doi: 10.4103/0970-9185.119158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motiani P, Chaudhary S, Bahl N, Sethi AK. Intrathecal sufentanil versus fentanyl for lower limb surgeries - a randomized controlled trial. Journal of anaesthesiology, clinical pharmacology. 2011;27(1):67–73. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Neeta S, Upadya M, Gosain A, Manissery JJ. A prospective randomized controlled study comparing intrathecal bupivacaine combined with fentanyl and sufentanil in abdominal and lower limb surgeries. Anesth Essays Res. 2015;9(2):149–154. doi: 10.4103/0259-1162.156287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whizar-Lugo VM. Topics in spinal anaesthesia: BoD–Books on Demand. 2014 [Google Scholar]
- 23.Meylan N, Elia N, Lysakowski C, Tramèr MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. British Journal of Anaesthesia. 2009;102(2):156–167. doi: 10.1093/bja/aen368. [DOI] [PubMed] [Google Scholar]
- 24.Campbell DC, Camann WR, Datta S. The Addition of Bupivacaine to Intrathecal Sufentanil for Labor Analgesia. 1995;81(2):305–309. doi: 10.1097/00000539-199508000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Singh H, Yang J, Thornton K, AHJCjoa Giesecke. Intrathecal fentanyl prolongs sensory bupivacaine spinal block. 1995;42(11):987–991. doi: 10.1007/BF03011070. [DOI] [PubMed] [Google Scholar]
- 26.Kim SY, Cho JE, Hong JY, Koo BN, Kim JM, Kil HK. Comparison of intrathecal fentanyl and sufentanil in low-dose dilute bupivacaine spinal anaesthesia for transurethral prostatectomy. BJA: British Journal of Anaesthesia. 2009;103(5):750–754. doi: 10.1093/bja/aep263. [DOI] [PubMed] [Google Scholar]
- 27.AdFdA Braga, FSdS Braga, Hirata ES, Pereira RIC, Frias JA. Antunes IF. Association of lipophilic opioids and hyperbaric bupivacaine in spinal anesthesia for elective cesarean section. Randomized controlled study. Acta Cirurgica Brasileira. 2014;29:752–758. doi: 10.1590/s0102-86502014001800010. [DOI] [PubMed] [Google Scholar]
- 28.Hassani V, Movassaghi G, Safaian R, Safari S, Zamani MM, Hajiashrafi M, et al. Bupivacaine-sufentanil versus bupivacaine-fentanyl in spinal anesthesia of patients undergoing lower extremity surgery. Anesth Pain Med. 2014;4(2) doi: 10.5812/aapm.12091. e12091-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahlgren G, Hultstrand C, Jakobsson J, Norman M, Eriksson EW, Martin H. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesthesia & Analgesia. 1997;85(6):1288–1293. doi: 10.1097/00000539-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Cohen SE, Cherry CM, Holbrook RH, Jr, El-Sayed YY, Gibson RN, Jaffe RAJA, et al. Intrathecal sufentanil for labor analgesia–sensory changes, side effects, and fetal heart rate changes. 1993;77(6):1155–1160. doi: 10.1213/00000539-199312000-00013. [DOI] [PubMed] [Google Scholar]