Abstract
In this study, eight different levels of commercial blueberry wines were studied to establish the comprehensive quality evaluation method of blueberry wine. Eleven physicochemical indexes (total carbohydrates (TC), total acids (TA), total esters (TE), total phenols (TP), total anthocyanins (ANT), color density (CD), tint (T), alcohol by volume (ABV), total soluble solids (TSS), Ph value, total dry extracts (TDE)) were used to establish the quality evaluation model and conduct the principal component analysis (PCA). Based on the results from PCA, the first three principal components accounted for 85.73% of the total quality variability. The consistent ranking of blueberry wines between quality evaluation model and sensory evaluation test verified the reliability of this model. In addition, ultrasonic-treated blueberry wine showed a higher score than the untreated group, which reflected the sensory quality changes of blueberry wine during ultrasonic treatment with high sensitivity. This study provides a theoretical basis for the comprehensive quality evaluation of blueberry wines and reasonable instruction for consumers’ choices.
Keywords: Blueberry wine, Sensory evaluation model, Principal component analysis, Ultrasonic aging
Graphical abstract
Highlights
-
•
A quality evaluation model of blueberry wine was established based on PCA.
-
•
Quality evaluation model was verified by consistency with sensory evaluation test.
-
•
This model can differentiate blueberry wines before and after ultrasonication.
1. Introduction
Commercial blueberry species used for winemaking originate from the genus Vaccinium, which contains numerous volatile organic compounds and contributes to olfactory sensation (Sater et al., 2020). As a high value-added product, blueberry wine was reported to be a rich anthocyanin source (Cho et al., 2004) and has excellent antioxidant activity (Johnson and de Mejia, 2012), exhibiting potential health-enhancing benefits (Yang et al., 2012).
The quality of blueberry wine is usually determined by intuitional sensory parameters, including color expression, aroma, taste, typicality, and other organoleptic indexes (Santos et al., 2016). First, as one of the organoleptic characteristics of blueberry wine, color primarily derived from anthocyanins plays a critical role in the comprehensive quality and overall evaluation of blueberry wine (Sun et al., 2022). According to previous studies, the total color of wines can be reflected by co-pigmentation (8–30%), total free anthocyanins (24–35%), and polymeric pigment (35–63%) (Versari et al., 2008). In addition, the color density (CD) and tint (T) of blueberry wine can directly reflect the quality of the wine (Li et al., 2020). Second, the aromatic substances of wine are closely connected to the features and qualities of blueberry wine. Different types of aromatic compounds such as alcohols, esters, aldehydes, acids, aldehyde ketones, terpenes, sulfur compounds, and lactones can constitute multifarious flavors and tastes according to the corresponding contents and proportions (Yuan et al., 2018), which can stimulate the olfactory and gustatory organs and provide tasters a specific and complex sense (Pittari et al., 2020). Third, the physicochemical parameters such as total carbohydrates (TC), total acids (TA), total esters (TE), and pH value can represent the taste quality of blueberry wine effectively (Liu et al., 2019). Fourth, total soluble solids (TSS) and total dry extracts (TDE) determine the typicality and express the mouthfeel of the blueberry wines (Caldeira et al., 2018).
Although a great deal of studies has investigated physicochemical indexes and organoleptic parameters of blueberry wines, there are not enough studies focusing on predicting and evaluating the sensory quality of blueberry wine through physicochemical indexes. Furthermore, different indexes may interact with each other and contribute a different weight to the overall sensory quality. Therefore, it is essential to propose an overall method to evaluate the quality of blueberry wines from an objective perspective. In this study, considering the limited effect of a single index, a comprehensive evaluation model of the blueberry wine sensory quality was established based on sensory-related physicochemical indexes by principal component analysis (PCA). Through dimensionality reduction of PCA, this model is able to decomplicate multivariate contributions and identify the most important contributors for blueberry wine quality evaluation. The reliability of this blueberry wine quality evaluation model was verified by the sensory evaluation test. Ultrasonication has been widely used in food industry for wine-making in order to improve taste and maintain color (Wu et al., 2022). However, the priory study concentrated on the impacts of ultrasonication on the physicochemical indexes of blueberry wine, without discussions about the relationship between ultrasonication and sensory evaluation. In addition, since the subtle difference in the quality changes of the ultrasonic-treated blueberry wine could not be evaluated by comprehensive quality sensory tests in a short period of time, the evaluation model was further applied to compare the quality levels of blueberry wines with and without ultrasonic treatment. The comprehensive scores obtained from this model were able to determine the quality level of blueberry wines.
2. Materials and methods
2.1. Materials
Eight commercial blueberry wines of different prices, grades and origin countries with different quality levels were purchased from online grocery stores, which were labeled from A to H. Laboratory-developed ultrasonic aging blueberry wine was produced according to our previous study (Li et al., 2020). All chemicals used in this study were of analytical grade unless otherwise noted.
2.2. Methods or procedures
2.2.1. Measurement of total carbohydrates, total acids, total esters, and total phenols
The TC content in blueberry wine was determined by Fehling's Reagent. TA and TE were determined by continuous potentiometric titration according to Huang et al. (2001), and calculated as tartaric acid and ethyl acetate equivalents, respectively. Total phenolic (TP) content was quantified via the Folin–Ciocalteu method based on Wang et al. (2015) and expressed in gallic acid equivalents.
2.2.2. Determination of total anthocyanins
The total anthocyanin (ANT) content in blueberry wine was determined by the pH differential test according to Mercali et al. (2015) and Teng et al. (2020) with modifications.
Briefly, two buffer dilutions of the sample were prepared, one with 0.2 mol/L potassium chloride buffer, pH 1.0, and the other with 0.2 mol/L sodium acetate buffer, pH 4.5. The sample was diluted at 1:9 (mL/mL) by each buffer solution and equilibrated for 30 min at dark. The absorbance was measured at 510 nm and 700 nm. The content of total anthocyanin is calculated based on the equation below as cyanidin-3-glucoside (cy-3-glu).
| A = (A510-A700) pH 1.0 - (A510-A700) pH 4.5 |
| (1) |
Where, MW is the molecular weight of cy-3-glu (449.2); DF is the dilution factor; ε is the molar absorptivity for cyanidin-3-glucoside (26,900); 1 indicates the 1 cm pathlength.
2.2.3. Color density and tint
The color characteristics of blueberry wine are expressed by CD and T, which were measured by the spectrophotometer (GEN10S UV–Vis, Thermo Fisher Scientific, USA) based on a previous study (Li et al., 2020).
2.2.4. Other detection methods
The detection methods of percent alcohol and total dry extracts referred to the research of Qu et al. (2016) with modifications. TSS (°Brix) was measured using a portable refractometer (MANUAL, ATAGO, Tokyo, Japan), and pH was detected by the pH meter (PHS–3C, INESA, Shanghai, China).
2.2.5. Sensory test
The methods of sensory evaluation followed GB/T15038-2006 with modifications. The sensory test panel was composed of 19 trained panelists aged from 25 to 45. Eight kinds of blueberry wine were graded by evaluators according to their color expression, aroma, taste, and typicality. To prevent the individual difference among the evaluators, the sensory test questionnaire was conducted by a descriptive test based on specific standards and scores listed in Appendix Table 1. All sensory tests were conducted independently. All the samples used for the sensory test were stored at a thermostatic incubator (20 ± 2) °C for 24 h and were randomly coded with three-digit numbers. Each blueberry wine was poured equally (50 mL) into the corresponding random coded glass cup.
Table 1.
(a) Physicochemical parameters determined in blueberry wines. Note: Data were expressed as mean ± SD of triplicate tests. A-H: eight commercial blueberry wines. Untreated: blueberry wine sampled before ultrasonic aging. Ultrasonic: blueberry wine sampled one week after ultrasonic aging. (b) Standardization of physicochemical parameters in blueberry wines.
| (a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TC (g/L) | TA (g/L) | TE (g/L) | TP (μg/mL) | ANT (mg/L) | CD | T | ABV (%) | TSS (° Brix) | pH | TDE (g/L) | |
| A | 10.83 ± 0.04 | 4.60 ± 0.11 | 33.92 ± 2.56 | 39.74 ± 2.29 | 0.15 ± 0.06 | 27.71 ± 0.10 | 1.269 ± 0.001 | 11.50 ± 0.00 | 7.13 ± 0.12 | 3.26 ± 0.02 | 21.97 ± 1.13 |
| B | 11.23 ± 0.04 | 6.96 ± 0.02 | 42.99 ± 5.01 | 58.03 ± 6.08 | 0.97 ± 0.03 | 64.25 ± 0.09 | 1.066 ± 0.001 | 11.00 ± 0.00 | 10.03 ± 0.06 | 3.06 ± 0.03 | 48.83 ± 0.35 |
| C | 4.24 ± 0.03 | 4.84 ± 0.31 | 66.40 ± 2.47 | 21.67 ± 0.74 | 4.98 ± 0.04 | 57.22 ± 0.16 | 0.606 ± 0.001 | 8.00 ± 0.00 | 13.17 ± 0.06 | 2.83 ± 0.01 | 119.46 ± 0.14 |
| D | 23.17 ± 0.09 | 5.59 ± 0.10 | 86.75 ± 1.13 | 19.09 ± 1.31 | 0.81 ± 0.03 | 40.16 ± 0.10 | 1.471 ± 0.001 | 7.00 ± 0.00 | 12.93 ± 0.12 | 2.83 ± 0.02 | 96.48 ± 1.06 |
| E | 11.69 ± 0.02 | 6.25 ± 0.21 | 90.04 ± 1.18 | 86.04 ± 11.08 | 8.23 ± 0.03 | 107.05 ± 1.57 | 0.810 ± 0.002 | 11.00 ± 0.00 | 16.80 ± 0.20 | 2.75 ± 0.01 | 134.96 ± 0.21 |
| F | 10.29 ± 0.04 | 7.98 ± 0.06 | 38.15 ± 3.39 | 51.24 ± 7.16 | 0.66 ± 0.02 | 40.9 ± 0.06 | 1.132 ± 0.001 | 12.00 ± 0.00 | 8.07 ± 0.12 | 2.92 ± 0.01 | 36.81 ± 0.17 |
| G | 24.66 ± 0.05 | 4.88 ± 0.04 | 86.69 ± 1.38 | 41.66 ± 2.62 | 7.54 ± 0.02 | 70.02 ± 0.03 | 0.828 ± 0.001 | 7.00 ± 0.00 | 14.67 ± 0.12 | 2.77 ± 0.01 | 110.49 ± 0.21 |
| H | 42.86 ± 0.11 | 5.80 ± 0.11 | 103.53 ± 1.69 | 24.77 ± 1.59 | 0.73 ± 0.07 | 28.86 ± 0.17 | 1.800 ± 0.003 | 8.00 ± 0.00 | 15.00 ± 0.00 | 2.69 ± 0.01 | 94.34 ± 0.14 |
| Untreated | 23.59 ± 0.17 | 7.12 ± 0.41 | 74.04 ± 4.93 | 114.31 ± 9.26 | 29.19 ± 3.61 | 60.98 ± 8.66 | 0.984 ± 0.001 | 11.65 ± 0.21 | 11.4 ± 1.70 | 3.99 ± 0.00 | 81.60 ± 5.49 |
| Ultrasonic | 23.95 ± 0.31 | 8.18 ± 0.23 | 82.42 ± 3.34 | 103.03 ± 3.69 | 28.43 ± 0.31 | 68.73 ± 0.39 | 1.012 ± 0.002 | 10.50 ± 0.00 | 11.1 ± 0.14 | 3.98 ± 0.00 | 83.65 ± 1.72 |
| (b) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | TA | TE | TP | ANT | CD | T | ABV | TSS | pH | TDE | |
| A | −0.69572 | −1.24675 | −1.50543 | −0.47420 | −0.71096 | −1.21653 | 1.07062 | 0.85484 | −1.58878 | 0.30950 | −1.65867 |
| B | −0.66014 | 0.56950 | −1.13209 | 0.06058 | −0.63826 | 0.32277 | 0.25954 | 0.60777 | −0.64848 | −0.09774 | −0.92753 |
| C | −1.28194 | −1.06205 | −0.16848 | −1.00255 | −0.28273 | 0.02662 | −1.60593 | −0.87460 | 0.36963 | −0.56606 | 0.99719 |
| D | 0.40199 | −0.48485 | 0.66918 | −1.07799 | −0.65244 | −0.69206 | 1.88170 | −1.36873 | 0.29182 | −0.56606 | 0.37096 |
| E | −0.61922 | 0.02309 | 0.80460 | 0.87957 | 0.00541 | 2.12579 | −0.79486 | 0.60777 | 1.54663 | −0.72895 | 1.41957 |
| F | −0.74376 | 1.35450 | −1.33131 | −0.13795 | −0.66574 | −0.66088 | 0.50287 | 1.10190 | −1.28399 | −0.38280 | −1.25509 |
| G | 0.53453 | −1.03126 | 0.66671 | −0.41806 | −0.05577 | 0.56584 | −0.71375 | −1.36873 | 0.85600 | −0.68823 | 0.75275 |
| H | 2.15352 | −0.32323 | 1.35988 | −0.91191 | −0.65954 | −1.16809 | −0.51098 | −0.87460 | 0.96300 | −0.85112 | 0.31265 |
| Untreated | 0.43935 | 0.69264 | 0.14600 | 1.70616 | 1.86371 | 0.18502 | −0.10544 | 0.95366 | −0.20427 | 1.79591 | −0.03453 |
| Ultrasonic | 0.47138 | 1.50842 | 0.49094 | 1.37634 | 1.79633 | 0.51150 | 0.01622 | 0.36071 | −0.30154 | 1.77555 | 0.02270 |
Note: Data were expressed as Mean ± SD of triplicate tests. A-H: eight commercial blueberry wines. Untreated: blueberry wine sampled before ultrasonic aging. Ultrasonic: blueberry wine sampled one week after ultrasonic aging.TC: total carbohydrates, TA: total acids, TE: total esters, TP: total phenols, ANT: total anthocyanin, CD: color density, T: tint, ABV: alcohol by volume, TSS: total soluble solids, TDE: total dry extracts.
According to the sensory evaluation methods of Pittari et al. (2022), evaluators were asked to rinse their mouths with water before tasting and between samples to remove the taste remaining in their mouths. When evaluating, evaluators were asked to observe the appearance (under suitable light, hold the bottom of the cup, raise the cup to the height of eyebrow, and observe the color, transparency and clarity of the blueberry wine), smell the flavor (sniff the fragrance with nose for several times in the static state, then hold the wine cup in hand, warm the blueberry wine slightly, and shake the cup. Put the wine cup under the nostril, smell its volatile aroma, and distinguish the flavor of fruit, alcohol, or other odors), taste (drink a small amount of sample in the mouth and distribute it in the tasting area evenly, taste carefully, swallow after having a clear impression, and identify the aftertaste), and evaluate the typicality (according to the comprehensive analysis of the characteristics of color expression, aroma and taste, evaluate the type, style and typicality).
2.2.6. Development of index evaluation model
PCA was used to analyze the data, reduce the complexity of the data and find the most important features.
Assuming that there are n samples in a practical problem. Each sample has p indexes which are regarded as p random variables and recorded as X1, X2 … Xp. Through PCA, p indicators are standardized, reduced dimensions, performed linear regression and formed k principal components F1, F2 … Fk (k p) according to equation (2).
| F1 = α11ZX1 + α21ZX2 + … + αp1ZXp |
| F2 = α12ZX1 + α22ZX2 + … + αp2ZXp |
| Fk = α1kZX1 + α2kZX2 + … + αpkZXp | (2) |
Where α = , a is the factor load, λ is the eigenvalue, and Z is the standardized index. Use the variance contribution βi of each principal component as a weight factor to construct a comprehensive evaluation function.
| Y = β1F1 + β2F2 + … + βmFm | (3) |
The scores of different blueberry wine samples were calculated and ranked to evaluate the sensory quality.
2.2.7. Ultrasonic aging process of blueberry wine
The laboratory-developed blueberry wines were processed by an energy-accumulating probe-type ultrasonic cell crusher at room temperature (25 °C) with a frequency of 20 kHz and a 10 mm diameter horn. The ultrasonic probe was centered into the blueberry wine to ensure that the bottom of the head was 1.0–1.5 cm away from the liquid level during each treatment. The optimized ultrasonic conditions (180 W, 20 min, and 2 treatment cycles) from our previous study (Li et al., 2020) were applied with the processing mode of 1 s running and 1 s stop. After ultrasonic treatment, the blueberry wine was poured into bottles, sealed with an oak stopper, and stored in the dark at room temperature. The blueberry wines with and without ultrasonic treatment were evaluated by two methods mentioned above, respectively.
2.2.8. Statistical analysis
All tests were performed in triplicate. One-way analysis of variance (ANOVA) with Tukey's multiple comparisons test was used to judge the significance of the means among samples. The data were presented as the mean ± standard deviation (SD) and p < 0.05 was considered statistically significant. The statistical analysis and graph presentation were performed by GraphPad Prism 8. PCA was carried out via SPSS 22.0.
3. Results
3.1. Collection and standardization of physicochemical parameters in blueberry wine
TC, TA, TE, TP, ANT, CD, T, ABV, TSS, pH, and TDE were selected as evaluation indexes for blueberry wine based on the studies of Santos et al. (2016). It was reported that these parameters were also wildly used to evaluate other fruit wines, such as red raspberry wine (Xia et al., 2018) and Gouqi wine (Zhao et al., 2019). Table 1a shows physicochemical parameters determined in eight commercial blueberry wines and laboratory-developed blueberry wines with and without ultrasonic aging. TC, TA, TE, and ABV contents of various blueberry wines were determined as 4.24–42.86 g/L, 4.60–8.18 g/L (as tartaric acid equivalent), 33.92–103.53 g/L (as ethyl acetate equivalent), and 7–12%, respectively, which indicate different levels of flavor and taste. Similar physicochemical values were also reported in other studies (Mendes-Ferreira et al., 2019; Santos et al., 2016; Zhong et al., 2021). Table 1b shows the standardization of physicochemical parameters in blueberry wines.
3.2. Modeling of quality evaluation of blueberry wine based on PCA
3.2.1. Eigenvalue and contribution rate
PCA is the statistical tool used to explain the differentiation between samples and to obtain more information on the variables that mainly influence the sample similarities and differences, permitting us to achieve a reduction in dimensionality, data exploration for finding the relationship between objects, estimation of the correlation structure of the variables (Bro and Smilde, 2014). Thus, PCA is often applied to classify and evaluate fruit wines (Moran et al., 2021; Rodríguez-Delgado et al., 2002; Šamec et al., 2016). In this experiment, PCA was performed with 11 measured variables, and the correlations between these physicochemical indexes of the blueberry wines were represented in Table 2.
Table 2.
Correlation of physicochemical parameters in blueberry wines.
| TC | TA | TE | TP | ANT | CD | T | ABV | TSS | pH | TDE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | 1 | 0.045 | 0.706 | −0.01 | 0.191 | −0.266 | 0.055 | −0.405 | 0.386 | 0.057 | 0.196 |
| TA | 0.045 | 1 | −0.109 | 0.67 | 0.501 | 0.206 | 0.125 | 0.586 | −0.268 | 0.534 | −0.248 |
| TE | 0.706 | −0.109 | 1 | 0.043 | 0.279 | 0.295 | −0.318 | −0.612 | 0.879 | −0.133 | 0.819 |
| TP | −0.01 | 0.67 | 0.043 | 1 | 0.851 | 0.569 | −0.119 | 0.651 | −0.048 | 0.779 | 0.014 |
| ANT | 0.191 | 0.501 | 0.279 | 0.851 | 1 | 0.395 | −0.203 | 0.277 | 0.056 | 0.862 | 0.202 |
| CD | −0.266 | 0.206 | 0.295 | 0.569 | 0.395 | 1 | −0.489 | 0.139 | 0.537 | 0.057 | 0.6 |
| T | 0.055 | 0.125 | −0.318 | −0.119 | −0.203 | −0.489 | 1 | 0.145 | −0.542 | 0.145 | −0.597 |
| ABV | −0.405 | 0.586 | −0.612 | 0.651 | 0.277 | 0.139 | 0.145 | 1 | −0.585 | 0.517 | −0.579 |
| TSS | 0.386 | −0.268 | 0.879 | −0.048 | 0.056 | 0.537 | −0.542 | −0.585 | 1 | −0.417 | 0.941 |
| pH | 0.057 | 0.534 | −0.133 | 0.779 | 0.862 | 0.057 | 0.145 | 0.517 | −0.417 | 1 | −0.284 |
| TDE | 0.196 | −0.248 | 0.819 | 0.014 | 0.202 | 0.6 | −0.597 | −0.579 | 0.941 | −0.284 | 1 |
Note: TC: total carbohydrates, TA: total acids, TE: total esters, TP: total phenols, ANT: total anthocyanin, CD: color density, T: tint, ABV: alcohol by volume, TSS: total soluble solids, TDE: total dry extracts.
The eigenvalue and contribution rate of the principal component (PC) are the basis of selecting the PCs. The larger the eigenvalue, the more information the PC contains. First, as shown in Fig. 1a, a significant inflection point appeared at PC3, indicating that the first three PCs can summarize most of the information of the original 11 indexes. Second, according to Table 3, the first three PCs give eigenvalues greater than 1.0 and explain 85.73% of the total variance. In detail, PCA was carried out with 38.40%, 32.09%, and 15.24% in PC1, PC2, and PC3, respectively. Thus, the first three PCs were selected for further analysis.
Fig. 1.
(a) Scree plot of principal component analysis (PCA). (b) Loading plot of PCA. The parameters with correlation were indicated in the same color (i.e., total soluble solids and total dry extracts were indicated in white, total phenols and total anthocyanin were indicated in purple, pH and total acids were indicated in blue). (c) Distribution of PCA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Eigen values and contribution rates of principal components.
| Component | Initial eigenvalues | Contribution rate of variance (%) | Cumulative variance contribution rate (%) |
|---|---|---|---|
| 1 | 4.224 | 38.402 | 38.402 |
| 2 | 3.529 | 32.085 | 70.487 |
| 3 | 1.676 | 15.238 | 85.725 |
| 4 | 0.66 | 6.002 | 91.728 |
| 5 | 0.504 | 4.582 | 96.31 |
| 6 | 0.289 | 2.624 | 98.934 |
| 7 | 0.097 | 0.881 | 99.815 |
| 8 | 0.013 | 0.123 | 99.938 |
| 9 | 0.007 | 0.062 | 100 |
| 10 | −3.21E-17 | −2.92E-16 | 100 |
| 11 | −3.72E-16 | −3.38E-15 | 100 |
3.2.2. Principal component matrix
The contribution rate of each index to PC was expressed as a component matrix (Table 4). According to equation (2) in section 2.2.6, PCs can be expressed as:
| F1 = 0.186X1 - 0.264X2 + 0.387X3 - 0.207X4 - 0.104X5 + 0.118X6 - 0.232X7 - 0.404X8+ 0.444X9 - 0.301X10 + 0.416X11; |
| F2 = 0.090X1 + 0.291X2 + 0.233X3 + 0.472X4 + 0.475X5 + 0.374X6 - 0.214X7 + 0.018X8+ 0.205X9 - 0.101X10 + 0.247X11; |
| F3 = 0.654X1 + 0.107X2 + 0.301X3 - 0.022X4 + 0.174X5 - 0.441X6 + 0.358X7 - 0.205X8- 0.045X9 + 0.239X10 - 0.127X11 | (4) |
Table 4.
Component matrix.
| Parameters | Contribution rate |
||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| TC (X1) | 0.382 | 0.17 | 0.847 |
| TA (X2) | −0.542 | 0.546 | 0.139 |
| TE (X3) | 0.796 | 0.437 | 0.39 |
| TP (X4) | −0.426 | 0.886 | −0.029 |
| ANT (X5) | −0.213 | 0.892 | 0.225 |
| CD (X6) | 0.242 | 0.703 | −0.571 |
| T (X7) | −0.477 | −0.402 | 0.463 |
| ABV (X8) | −0.831 | 0.278 | −0.266 |
| TSS (X9) | 0.912 | 0.356 | −0.058 |
| pH (X10) | −0.619 | 0.615 | 0.309 |
| TDE (X11) | 0.855 | 0.439 | −0.165 |
Note: PC: principal component, TC: total carbohydrates, TA: total acids, TE: total esters, TP: total phenols, ANT: total anthocyanin, CD: color density, T: tint, ABV: alcohol by volume, TSS: total soluble solids, TDE: total dry extracts.
In the principal component load matrix, the absolute value of the loading value reflects the importance of each variable to the principal component. The larger the absolute value, the closer the relationship, and vice versa.
As shown in Table 4, the contribution rate of the first PC accounted for 38.40% of the total variance. Among all the parameters, TSS (X9) and TDE (X11) mainly reflected PC1 with contribution rates of 0.912 and 0.855, respectively. Both variables were important quality indexes of blueberry wine. The second PC explained 32.09% of the total variability, most correlating with TP (X4), ANT (X5) as well as CD (X6) with contribution rates of 0.886, 0.892, and 0.703, respectively. All three parameters were color indexes of blueberry wine, suggesting that the color and luster of blueberry wine were the main sources of information in PC2. TC (X1) with a contribution rate of 0.847 dominated the third PC, representing 15.24% of the total variation.
3.2.3. Analysis of PCA distribution
The loading plot was shown in Fig. 1b, the content of TSS highly correlated with TDE, since TDE (or sugar-free extract) was a general term for the content of non-volatile soluble solids other than sugar, including various non-volatile acids, polysaccharides, proteins, glycerol, nitrogenous substances, tannins, minerals, etc. (Yoncheva, 2022). TDE determines the main skeleton of the taste, which is directly related to the typicality of the wine (Silva et al., 2018). TSS and TDE can represent the quality of the blueberry wines directly. In addition, TP and ANT had a close relationship. It was reported that the content of total phenolics and anthocyanins was strongly correlated with antioxidant capacity in strawberries, raspberries, highbush blueberries, and lowbush blueberries (Kalt et al., 1999). Besides, pH is negatively correlated with total acids since lower pH indicates that there are more acids in blueberry wine.
3.2.4. Model evaluation and principal component scores
Based on the standardized physicochemical indicators in equation (4), the scores of three PCs were calculated and indicated in Table 5a. The ratio of the single PC contribution rate to the cumulative contribution rate was used as the weight to calculate the comprehensive evaluation scores of different blueberry wines. According to equation (3) in section 2.2.6, the comprehensive evaluation model scores were calculated and expressed as equation (5):
| Y = 38.402/85.725 × F1 + 32.085/85.725 × F2 + 15.238/85.725 × F3 = 0.448F1 + 0.374F2 + 0.178F3 |
| = 0.233X1 + 0.010X2 + 0.314X3 + 0.080X4 + 0.162X5 + 0.114X6 - 0.120X7 - 0.162X8+ 0.262X9 + 0.030X10 + 0.251X11 | (5) |
Table 5.
(a) Principal component scores after standardization. (b) Ranking comparison between model evaluation and sensory test of blueberry wines. (c) Evaluation scores for blueberry wines.
| (a) | ||||
|---|---|---|---|---|
| Sample | Model Evaluation |
Sensory Test |
||
| Score | Rank | Score | Rank | |
| A(Y1) | −2.13906 | 8 | 56.53 | 8 |
| B(Y2) | −1.12350 | 6 | 60.20 | 6 |
| C(Y3) | 0.32048 | 5 | 69.00 | 5 |
| D(Y4) | 0.33667 | 4 | 69.42 | 4 |
| E(Y5) | 1.26322 | 2 | 78.28 | 1 |
| F(Y6) | −1.68121 | 7 | 59.56 | 7 |
| G(Y7) | 1.23221 | 3 | 73.74 | 3 |
| H(Y8) | 1.30711 | 1 | 75.73 | 2 |
| (b) | |||
|---|---|---|---|
| Sample | F1 | F2 | F3 |
| A(Y1) | −2.43664 | −2.77307 | −0.05417 |
| B(Y2) | −1.56944 | −0.70810 | −0.87435 |
| C(Y3) | 1.69206 | −0.35405 | −1.71581 |
| D(Y4) | 1.24176 | −1.24524 | 1.38654 |
| E(Y5) | 1.69385 | 2.27359 | −1.94945 |
| F(Y6) | −2.53016 | −1.23382 | −0.48374 |
| G(Y7) | 2.40772 | 0.45019 | −0.08366 |
| H(Y8) | 2.41739 | −0.30975 | 1.91347 |
| Untreated(Y9) | −1.57625 | 1.84282 | 0.82010 |
| Ultrasonic(Y10) | −1.34029 | 2.05744 | 1.04107 |
| (c) | |
|---|---|
| Sample | Score |
| Untreated | 0.12940 |
| Ultrasonic | 0.35470 |
Through the established comprehensive evaluation model of blueberry wine, i.e., equation (5), the final model evaluation scores of the eight commercial blueberry wines were ranked in Table 5b.
In Fig. 1c, when the scores of every blueberry wine sample were examined in a three-dimensional plot, all the samples were distributed into two groups according to different quality levels. The first group, shown as the blue shade in Fig. 1c, includes H, E, G, and D, with better comprehensive quality. The second group was presented as the purple shade part in Fig. 1c, including C, B, F, and A. Combined with Fig. 1b, it should be mentioned that the first two PCs (including TSS, TDE, TP, and TA) made a greater contribution to the comprehensive quality of blueberry wine.
3.2.5. Sensory test verification
The scores and ranking of the sensory test were also listed in Table 5b. The results of the model evaluation were consistent with those of the sensory test except for the order of Y5 and Y8, that is, the first two blueberry wines. Both evaluation orders were followed by Y7, Y4, Y3, Y2, Y6, Y1, which verified the reliability of this evaluation model. Besides, the result of model evaluation was in accord with the presupposed high and low levels of commercial blueberry wines.
3.3. Application of blueberry wine evaluation model to ultrasonic-treated blueberry wine
3.3.1. Physicochemical parameters before and after ultrasonication
The optimal ultrasonic condition (180 W, 20 min, and 2 times) was selected based on our previous study by Li et al. (2020). The physicochemical indexes of untreated and treated blueberry wines were compared to analyze the effect of ultrasonic aging.
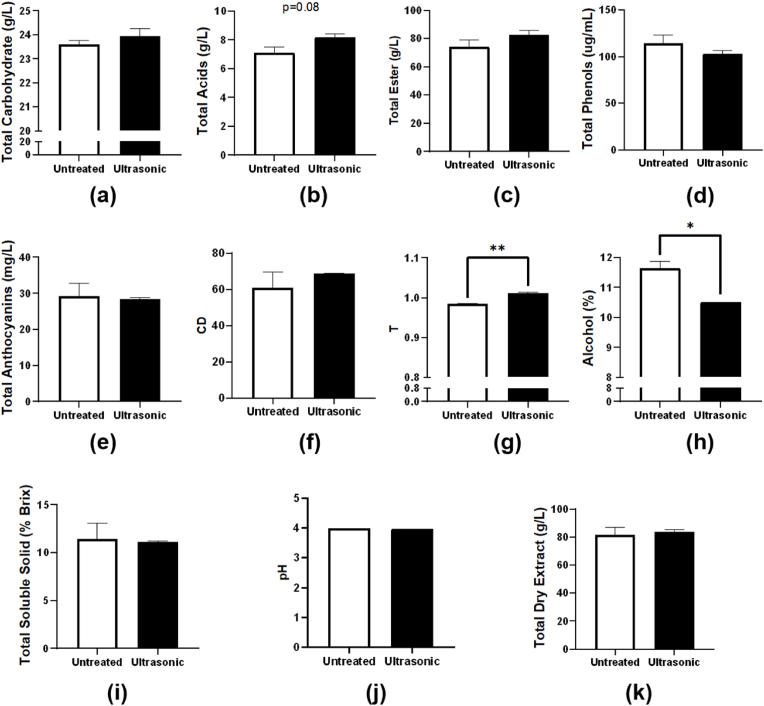
T is an indicator of maturity, which is closely related to the chemical age of the wine. Higher T represents the greater proportions of yellowness in the wine color, while lower T indicates the greater proportions of redness. This is because the color of the newly brewed wine is mainly derived from the free anthocyanin in the peel, that is, purple or ruby red. As the aging extends, the free anthocyanins gradually combine with other substances, such as tannin and form polymerized anthocyanins (Li et al., 2020), which decrease the proportions of redness and increase the proportions of yellowness. The color of wine gradually changes into brick red with the increasing aging time (Wang et al., 2002). As shown in Fig. 2g, T value of blueberry wine increased significantly from 0.984 ± 0.001 to 1.012 ± 0.002 through ultrasonic treatment (p < 0.01), which proved that the color of blueberry wine became more stable. Therefore, ultrasonic aging had positive effects on the color stability of blueberry wine.
Fig. 2.
The physicochemical parameters of blueberry wine before and after ultrasonic aging. * indicates a significant difference between ultrasonic and untreated groups. *p<0.05, **p<0.01.
ABV is also an essential index of blueberry wine. Fig. 2h revealed that the alcohol content decreased significantly after ultrasonic aging from 11.65 ± 0.21 to 10.50 ± 0.00% (p < 0.05). TSS mainly refers to soluble sugars (including monosaccharides, disaccharides, polysaccharides), acids, vitamins, minerals, etc. Ultrasonication has insignificant effects on TSS (p > 0.05), as shown in Fig. 2i. TSS of blueberry wine before and after ultrasonic aging were 11.4 ± 1.70 and 11.1 ± 0.14 °Brix, respectively.
Other parameters showed no significant difference before and after ultrasonic aging treatment. TC is an important index in the sensory evaluation of blueberry wine. As shown in Fig. 2a, TC increased slightly after ultrasonic aging (from 23.59 ± 0.17 to 23.95 ± 0.31 g/L) with no significant difference (p > 0.05). The type and quantity of sour ingredients in fruit wine have a great influence on the flavor, taste, and storage properties of wine (Fujita et al., 2010). Fig. 2b showed that ultrasonic aging caused a slight increase in total acids (from 7.12 ± 0.41 to 8.18 ± 0.23 g/L), but the effect was not significant (p = 0.08). TE is a principal taste compound that can reflect the general quality of blueberry wine. In Fig. 2c, ultrasonic treatment did not make a significant increase on TE content (from 74.04 ± 4.93 to 82.42 ± 3.34 g/L) (p > 0.05).
As shown in Fig. 2d, TP content of the untreated group was 114.31 ± 9.26 μg/mL, and that in the ultrasonic group was 103.03 ± 3.69 μg/mL, which showed a not significant decrease (p > 0.05). This is because, under the effect of ultrasonic cavitation, cavitated bubbles collapse with instantaneous high temperature and high pressure, and make water molecules crack into hydroxyl radicals and hydrogen ion free radicals, leading to the degradation of phenolic substances and flavonoids in wine (Shen et al., 2015). It was reported that ultrasonication may degrade phenolic substances in ethanol solutions (Zhang et al., 2015). Similarly, in Fig. 2e, ANT also showed an insignificant decrease (p > 0.05) from 29.19 ± 3.61 to 28.43 ± 0.31 mg/L. Ultrasonic waves utilize ultrasonic vibration energy and generate localized high temperatures in the food medium. With the extension of ultrasonic time, the temperature generated by the ultrasonic wave will also increase, which accelerates the reactions of anthocyanins with other substances in the wine, and results in the degradation of the anthocyanins (Mori et al., 2005).
CD is the sum of absorbance at 420 nm, 520 nm, and 620 nm, which can reflect the color shades of blueberry wine. The change in CD is related to the formation of anthocyanin derivatives (Atanasova et al., 2002). Fig. 2f indicated that ultrasonic aging increased CD value from 60.98 ± 8.66 to 68.73 ± 0.39 insignificantly (p > 0.05).
Sourness is an important sensory index in wine, which is mainly determined by pH value (Fischer and Noble, 1994). The pH values of the untreated and ultrasonic groups are displayed in Fig. 2j. After ultrasonic aging, the pH value decreased from 3.99 to 3.98. TDE is a direct and intuitional indicator of the quality of blueberry wine and is related to the denseness of taste. It can be observed from Fig. 2k that ultrasonic aging can enhance TDE of blueberry wine insignificantly (p > 0.05) from 81.60 ± 5.49 to 83.65 ± 1.72 g/L.
3.3.2. Sensory evaluation model scores before and after ultrasonication
Because of the subtle difference in the quality change of blueberry wine before and after ultrasonication, it was difficult to evaluate the comprehensive quality of blueberry wine by sensory test in a short period of time. Considering that the model evaluation scores were basically the same as the ranking of the sensory test, as displayed in Table 5b, it is reliable to evaluate the sensory quality of blueberry wines based on this sensory evaluation model. Thus, the established sensory evaluation model was applied to the blueberry wines before and after ultrasonication and the scores of the evaluation model were shown in Table 5c.
It indicated that after ultrasonic aging, the evaluated scores of blueberry wine increased from 0.12940 to 0.35470, which meant the quality of blueberry wine had been enhanced by ultrasonication. Different from the single physicochemical index and sensory test, the scores of the sensory evaluation model can achieve a more comprehensive and objective reflection of the overall quality of blueberry wine.
4. Conclusions
This research utilized eight commercial blueberry wines with different quality levels to establish a sensory evaluation model, which was applied to the evaluation of ultrasonic blueberry wine.
Standardized physicochemical parameters were treated with PCA to achieve data dimension reduction. PCA showed that the first three PCs (based on the eigenvalues) accounted for 85.73% of the total variance. PC1 (38.40% of the total variance) correlated with TSS and TDE. PC2 (32.09%) was described with TP and ANT. PC3 (15.24%) reflected the TC.
In addition, eleven indexes were labeled respectively to establish a sensory evaluation model of blueberry wine. The model is constructed as Y = 0.233X1 + 0.010X2 + 0.314X3 + 0.080X4 + 0.162X5 + 0.114X6 - 0.120X7 - 0.162X8 + 0.262X9 + 0.030X10 + 0.251X11. Eight commercial blueberry wines were evaluated by this model and ranked, besides, this sensory evaluation model was verified to be reliable.
The application of the sensory evaluation model to the ultrasonic blueberry wine could reflect the changes in the sensory quality of blueberry wines before and after ultrasonic treatment. Ultrasonic treatment had a significant promoting aging effect on blueberry wine, especially on T and ABV. Besides, the results of the sensory evaluation model showed that the score of the ultrasonic group (0.35470) was higher than the untreated group (0.12940).
Funding sources
This work was supported by the National Natural Science Foundation of China (NSFC, No.32172220, U22A20546), the Youth Science and Technology Innovation Talent of Guangdong TeZhi Plan (No. 2019TQ05N770). The authors also thank the Guangdong Key Area Research and Development Program (No. 2022B0202040001), and the Science and Technology Program of Guangzhou (No. 202206010121).
CRediT authorship contribution statement
Yaqi Zhao: Writing – original draft, Investigation, Formal analysis, Data curation. Yingyu Zeng: Writing – review & editing, Software, Visualization, Data curation. Xusheng Li: Methodology, Investigation, Validation. Kailan Yuan: Visualization, Data curation. Yue Li: Writing – review & editing. Lingmin Tian: Conceptualization, Project administration. Jianxia Sun: Conceptualization, Project administration. Weibin Bai: Conceptualization, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Nan Zhou, Kaiyun Wu, and Ying Yan for providing guidance during the experiment.
Appendix.
Table 1.
Sensory Evaluation Standards and Scores of Blueberry Wines
| Index | Standards | Scores | Index | Standards | Scores |
|---|---|---|---|---|---|
| Color | Ruby red | 7 | Harmony (fruit and wine flavor) | Very harmonious | 15 |
| Purplish red | 7 | Harmonious | 12 | ||
| Brick red | 5 | Average harmonious | 9 | ||
| Dark purplish red | 3 | Not harmonious | 5 | ||
| Faint red | 1 | Contain other flavors | 2 | ||
| Luster | Rich luster | 7 | Sourness and sweetness | Too sour, too sweet | 9 |
| Good luster | 6 | Moderate sour and sweet | 15 | ||
| Average luster | 4 | Slightly sour | 6 | ||
| Poor luster | 2 | Slightly sweet | 12 | ||
| No luster | 1 | Bitter | 4 | ||
| Clarity | clear and transparent | 6 | Alcoholic taste | Too alcoholic taste | 9 |
| Good clarity | 5 | Rich and balanced | 15 | ||
| Average clarity | 4 | Average taste | 12 | ||
| Poor clarity | 3 | Poor taste | 6 | ||
| Very poor clarity | 2 | No taste | 1 | ||
| Aroma | Rich aroma | 15 | Characteristics | Typical and unique blueberry flavor | 20 |
| Good aroma | 12 | Good and unique blueberry flavor | 16 | ||
| Average aroma | 9 | Average blueberry flavor | 12 | ||
| Poor aroma | 6 | Poor blueberry flavor | 8 | ||
| No aroma | 3 | No blueberry flavor | 4 |
References
- Atanasova V., Fulcrand H., Cheynier V., Moutounet M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta. 2002;458(1):15–27. doi: 10.1016/S0003-2670(01)01617-8. [DOI] [Google Scholar]
- Bro R., Smilde A.K. Principal component analysis. Anal. Methods. 2014;6(9):2812–2831. doi: 10.1039/C3AY41907J. [DOI] [Google Scholar]
- Caldeira I., Lopes D., Delgado T., Canas S., Anjos O. Development of blueberry liquor: influence of distillate, sweetener and fruit quantity. J. Sci. Food Agric. 2018;98(3):1088–1094. doi: 10.1002/jsfa.8559. [DOI] [PubMed] [Google Scholar]
- Cho M.J., Howard L.R., Prior R.L., Clark J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004;84(13):1771–1782. doi: 10.1002/jsfa.1885. [DOI] [Google Scholar]
- Fischer U., Noble A.C. The effect of ethanol, catechin concentration, and pH on sourness and bitterness of wine. Am. J. Enol. Vitic. 1994;45(1):6–10. http://www.ajevonline.org/content/45/1/6.abstract [Google Scholar]
- Fujita A., Isogai A., Endo M., Utsunomiya H., Nakano S., Iwata H. Effects of Sulfur Dioxide on Formation of Fishy Off-Odor and Undesirable Taste in Wine Consumed with Seafood. J. Agric. Food Chem. 2010;58(7):4414–4420. doi: 10.1021/jf9041547. [DOI] [PubMed] [Google Scholar]
- Huang C., Yin H., Zhou J. Continuous determination of the content of total acid and ester in vinegar. Food Ferment. Ind. 2001;27(12):41–43. http://sf1970.cnif.cn/CN/Y2001/V27/I12/41 [Google Scholar]
- Johnson M.H., de Mejia E.G. Comparison of chemical composition and antioxidant capacity of commercially available blueberry and blackberry wines in Illinois. J. Food Sci. 2012;77(1):C141–C148. doi: 10.1111/j.1750-3841.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- Kalt W., Forney C.F., Martin A., Prior R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999;47(11):4638–4644. doi: 10.1021/jf990266t. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang L., Peng Z., Zhao Y., Wu K., Zhou N.…Bai W. The impact of ultrasonic treatment on blueberry wine anthocyanin color and its In-vitro anti-oxidant capacity. Food Chem. 2020;333 doi: 10.1016/j.foodchem.2020.127455. [DOI] [PubMed] [Google Scholar]
- Liu F., Li S., Gao J., Cheng K., Yuan F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. Lebensm. Wiss. Technol. 2019;109:233–240. doi: 10.1016/j.lwt.2019.03.100. [DOI] [Google Scholar]
- Mendes-Ferreira A., Coelho E., Barbosa C., Oliveira J.M., Mendes-Faia A. Production of blueberry wine and volatile characterization of young and bottle-aging beverages. Food Sci. Nutr. 2019;7(2):617–627. doi: 10.1002/fsn3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercali G.D., Gurak P.D., Schmitz F., Marczak L.D.F. Evaluation of non-thermal effects of electricity on anthocyanin degradation during ohmic heating of jaboticaba (Myrciaria cauliflora) juice. Food Chem. 2015;171:200–205. doi: 10.1016/j.foodchem.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Moran M.A., Bastian S.E., Petrie P.R., Sadras V.O. Impact of late pruning and elevated ambient temperature on Shiraz wine chemical and sensory attributes. Aust. J. Grape Wine Res. 2021;27(1):42–51. doi: 10.1111/ajgw.12470. [DOI] [Google Scholar]
- Mori K., Sugaya S., Gemma H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005;105(3):319–330. doi: 10.1016/j.scienta.2005.01.032. [DOI] [Google Scholar]
- Pittari E., Piombino P., Andriot I., Cheynier V., Cordelle S., Feron G.…Canon F. Effects of oenological tannins on aroma release and perception of oxidized and non-oxidized red wine: A dynamic real-time in-vivo study coupling sensory evaluation and analytical chemistry. 2022;372 doi: 10.1016/j.foodchem.2021.131229. [DOI] [PubMed] [Google Scholar]
- Pittari E., Moio L., Arapitsas P., Curioni A., Gerbi V., Parpinello G.P.…Piombino P. Exploring olfactory–oral cross-modal interactions through sensory and chemical characteristics of Italian red wines. Foods. 2020;9(11):1530. doi: 10.3390/foods9111530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H., Xu D., Xu L., Yang S., Deng J. Quality and antioxidant activity of dry-red and rose wines made simultaneously by saignee technique. Food Sci. (N. Y.) 2016;37(15):179–184. https://www.cnki.com.cn/Article/CJFDTotal-SPKX201615033.htm [Google Scholar]
- Rodríguez-Delgado M.-Á., González-Hernández G., Conde-González J.-E. a., Pérez-Trujillo J.-P. Principal component analysis of the polyphenol content in young red wines. Food Chem. 2002;78(4):523–532. doi: 10.1016/S0308-8146(02)00206-6. [DOI] [Google Scholar]
- Šamec D., Maretić M., Lugarić I., Mešić A., Salopek-Sondi B., Duralija B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2016;194:828–834. doi: 10.1016/j.foodchem.2015.08.095. [DOI] [PubMed] [Google Scholar]
- Santos R.O., Trindade S.C., Maurer L.H., Bersch A.M., Sautter C.K., Penna N.G. Physicochemical, antioxidant and sensory quality of Brazilian blueberry wine. Agrar. Sci. 2016;88(3):1557–1568. doi: 10.1590/0001-3765201620140491. [DOI] [PubMed] [Google Scholar]
- Sater H.M., Bizzio L.N., Tieman D.M., Muñoz P.D. A review of the fruit volatiles found in blueberry and other Vaccinium species. J. Agric. Food Chem. 2020;68(21):5777–5786. doi: 10.1021/acs.jafc.0c01445. [DOI] [PubMed] [Google Scholar]
- Shen Y., Zhang Q., Yi X., Zhang X., Yan Y. Effect of ultrasound on total phenolic, flavonoid content and free radical scavenging actibity of red wine. Food Ind. Technol. 2015;23:111–115. doi: 10.13386/j.issn1002-0306.2015.23.014. [DOI] [Google Scholar]
- Silva A., Voss H., Van Zyl H., Hogg T., De Graaf C., Pintado M., Jager G. Temporal dominance of sensations, emotions, and temporal liking measured in a bar for two similar wines using a multi-sip approach. J. Sensory Stud. 2018;33(5) doi: 10.1111/joss.12459. [DOI] [Google Scholar]
- Sun X., Shokri S., Gao B., Xu Z., Li B., zhu T.…Zhu J. Improving effects of three selected co-pigments on fermentation, color stability, and anthocyanins content of blueberry wine. Lebensm. Wiss. Technol. 2022;156 doi: 10.1016/j.lwt.2022.113070. [DOI] [Google Scholar]
- Teng Z., Jiang X., He F., Bai W. Qualitative and quantitative methods to evaluate anthocyanins. eFood. 2020;1(5):339–346. doi: 10.2991/efood.k.200909.001. [DOI] [Google Scholar]
- Versari A., Boulton B., R, Parpinello P., G A comparison of analytical methods for measuring the color components of red wines. Food Chem. 2008;106(1):397–402. doi: 10.1016/j.foodchem.2007.05.073. [DOI] [Google Scholar]
- Wang H., Ding G., Cui F. The review of anthocyanins research in wine. Sino-Overseas Grapewine Wine. 2002;2:25–29. http://www.alljournals.cn/view_abstract.aspx?pcid=5B3AB970F71A803DEACDC0559115BFCF0A068CD97DD29835&cid=F547DC657067DBAE&jid=12DB53415F53BD31352C4A2861E512D8&aid=E03ABD761BCC78EC&yid=C3ACC247184A22C1 [Google Scholar]
- Wang X., Zhang H., Ma Y., Ye H., Zhang L., Li J., Yu H. Dynamic changes in phenolics and their antioxidant activities during the fermentation of blueberry wine. Mod. Food Sci. Technol. 2015;31(1):90–95. doi: 10.13982/j.mfst.1673-9078.2015.1.017. [DOI] [Google Scholar]
- Wu Z., Li X., Zeng Y., Cai D., Teng Z., Wu Q., Sun J., Bai W. Color stability enhancement and antioxidation improvement of sanhua plum wine under circulating ultrasound. Foods. 2022;11(16):2435. doi: 10.3390/foods11162435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T., Gao X., Liu X., Wang Q., Wang J. Optimization of clarification process and determination of physical and chemical indexes of red raspberry fruit wine. China Brew. 2018;37(8):138–142. http://110.185.172.89:8777/KCMS/detail/detail.aspx?filename=ZNGZ201808030&dbcode=CJFQ&dbname=CJFD2018 [Google Scholar]
- Yang W., Guner S., Rock C., Anugu A., Sims C., Gu L. Prospecting antioxidant capacities and health-enhancing phytonutrient contents of Southern highbush blueberry wine compared to grape wines and fruit liquors. Sustain. Agric. Res. 2012;1(1) doi: 10.5539/sar.v1n1p26. [DOI] [Google Scholar]
- Yoncheva T. Influence of meteorological conditions on the quality of grapes and aroma-releasing enzyme addition on the chemical composition, aromatic complex and organoleptic profile of red wines. Carpathian J. Food Sci. Technol. 2022;14(1):72–88. doi: 10.34302/crpjfst/2022.14.1.6. [DOI] [Google Scholar]
- Yuan F., Cheng K., Gao J., Pan S. Characterization of cultivar differences of blueberry wines using GC-QTOF-MS and metabolic profiling methods. Foods. 2018;23(9):2376. doi: 10.3390/molecules23092376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.-A., Shen H., Fan X.-H., Shen Y., Wang X., Song Y. Changes of gallic acid mediated by ultrasound in a model extraction solution. Ultrason. Sonochem. 2015;22:149–154. doi: 10.1016/j.ultsonch.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Zhao L., Ren J., Wang L., Li J., Wang M., Wang L.…Zhang B. Evolution of sensory attributes and physicochemical indexes of Gouqi fermented wine under different aging treatments and their correlations. J. Food Process. Preserv. 2019;43(3) doi: 10.1111/jfpp.13873. [DOI] [Google Scholar]
- Zhong W., Liu S., Yang H., Li E. Effect of selected yeast on physicochemical and oenological properties of blueberry wine fermented with citrate-degrading Pichia fermentans. Lebensm. Wiss. Technol. 2021;145 doi: 10.1016/j.lwt.2021.111261. [DOI] [Google Scholar]