Abstract
Background
In the past two decades, three coronavirus epidemics have been reported. Coronavirus disease 2019 (COVID-19) is caused by a severe acute respiratory syndrome (SARS)-like coronavirus (SARS-CoV-2). In most patients, the disease is characterized by interstitial pneumonia, but features can affect other organs.
Purpose
To document the radiological features of the patients and to perform a narrative review of the literature.
Material and Methods
We conducted a retrospective, single-center study on 1060 consecutive hospitalized patients with COVID-19 at our institution. According to the inclusion criteria, we selected patients to be studied in more radiological detail. All images were obtained as per standard of care protocols. We performed a statistic analysis to describe radiological features. We then presented a systematic review of the main and conventional neuroimaging findings in COVID-19.
Results
Of 1060 patients hospitalized for COVID-19 disease, 15% (159) met the eligibility criteria. Of these, 16 (10%) did not undergo radiological examinations for various reasons, while 143 (90%) were examined. Of these 143 patients, 48 (33.6%) had positive neuroimaging. We found that the most frequent pathology was acute ischemic stroke (n=16, 33.3%). Much less frequent were Guillain–Barre syndrome (n=9, 18.8%), cerebral venous thrombosis (n=7, 14.6%), encephalitis or myelitis (n=6, 12.5%), intracranial hemorrhage and posterior hemorrhagic encephalopathy syndrome (n=4, 8.3%), exacerbation of multiple sclerosis (n=4, 8.3%), and Miller–Fisher syndrome (n=2, 4.2%).
Conclusion
Our data are coherent with the published literature. Knowledge of these patterns will make clinicians consider COVID-19 infection when unexplained neurological findings are encountered.
Keywords: COVID-19, neuroradiology, brain, spine, stroke
Introduction
Coronavirus disease 2019 (COVID-19) is caused by a severe acute respiratory syndrome (SARS)-like coronavirus (SARS-CoV-2). In most patients, the disease is characterized by fever, dry cough, dyspnea, and hypoxia, with interstitial pneumonia features on chest X-ray, computed tomography (CT), or spectral photon-counting CT scan (1–3). However, COVID-19 is not just a respiratory disease and can affect other organs, including the brain (4). The primary aim of the present study was to systematically characterize the neurological characteristics of patients admitted with COVID-19 at our institution. A secondary aim was to describe in a narrative review the current data by comparing it with previous papers (5,6). This is in order to validate the data published so far, with the large study population as our strength.
Material and Methods
We used a retrospective, mono-center study design. The institutional review board approved the study and waivers for informed consent were obtained at our institution. Radiological exams were performed at the request of clinicians in cases of symptoms that raised suspicion of nervous system involvement. All images were obtained as per standard of care protocols. Magnetic resonance imaging (MRI) scans of the brain and spine were obtained with 1.5-T scanners with a standardized protocol. For the brain, 3D-T1mprage, 3D-Balance, TSE-T2, and FLAIR sequences were used on the axial and coronal planes, diffusion-weighted imaging/apparent diffusion coefficient and susceptibility-weighted imaging were used on the axial planes; contrast media (Gadovist) was used only in selected cases according to clinical query. For the spinal cord, SE-T1 and STIR were used on the sagittal planes and TSE-T2 on the axial and sagittal planes; we used Gadovist and axial SE-T1 only in selected cases. The image analysis was performed at the time of the radiological exam by neuroradiologists at our institution using a structured report. Then, two neuroradiologists (AN and FS, with >20 years of experience) retrospectively performed the image analysis double-blinded. Data confidentiality was achieved using images extracted from the PACS system only after anonymization and with data reported by first and last name coding (e.g. MaRiO RoSsI = MRORSI). The inclusion criteria were as follows: (i) hospitalized patients who were positive for COVID-19 by means of real-time reverse-transcriptase polymerase chain reaction testing from March 2020 to March 2022; (ii) the presence of acute neurologic symptoms during the hospital stay; and (iii) any neurologic imaging studies. No exclusion criteria were used for this study. The reasons why some patients meeting the inclusion criteria did not undergo radiological investigations were related to internal organizational issues or extremely high-risk health conditions. We reviewed the electronic medical records, and all statistical analyses were performed using software (Stata version 15; StataCorp, College Station, TX, USA). We then conducted a systematic review of the main and conventional imaging findings in COVID-19 to help neuroradiologists in the evaluation of these patients.
Results
A total of 1060 consecutive hospitalized patients with COVID-19 were reviewed. Of these patients, 159 (15%) met the eligibility criteria. Of these 159 patients, 16 were not examined radiologically for various reasons, while 143 (90%) underwent radiological examinations. Among them, 96% were examined with unenhanced brain CT, 18% with head and neck CT angiography, and 20% with brain or spine MRI. Of these 143 patients, 48 (33.6%) had positive neuroimaging. Among the patients with positive neuroimaging (M/F = 29/19; mean age = 66 ± 14 years), we found that the most frequent pathology was acute ischemic stroke (n = 16, 33.3%). Much less frequent were cerebral venous thrombosis (n = 7, 14.6%) and intracranial hemorrhage (ICH) and posterior hemorrhagic encephalopathy syndrome (PRES) (n = 4, 8.3%). Guillain–Barre syndrome (GBS) (n = 9, 18.8%) was the second most frequent manifestation. Other possible manifestations were encephalitis or myelitis (n = 6, 12.5%), exacerbation of multiple sclerosis (n = 4, 8.3%), and Miller–Fisher syndrome (MFS) (n = 2, 4.2%). No significant differences were found between the prevalence of neuroradiological manifestations and the sex of patients. The most common predominant neurologic symptoms were altered mental status (n = 30, 62.5%). Other predominant symptoms were headache (n = 6, 12.5%), myalgias (n = 5, 10.4%), seizure (n = 4, 8.3%), ataxia (n = 2, 4.2%), and hyposmia (n = 1, 2.1%). There was a statistically significant association between the prevalence of altered mental status and patient age (mean age = 74 years vs. 60 years) (P = .006). About one-quarter of patients had no known past medical history while the rest had one of the following chronic disorders: hypertension (n = 25, 52.1%); diabetes mellitus (7); coronary artery disease (8); and cerebrovascular disease (9).
Discussion
The present study demonstrated varied imaging features without a specific pattern but dominated by acute ischemic infarction and ICH. We also demonstrated a broad neuroradiological spectrum of MRI different from stroke. The understanding of neurological symptoms in patients with COVID-19 remains poor even two years after the onset of the pandemic. Indeed, it is still debated whether they result from the critical illness or direct invasion of the central nervous system by the coronavirus. Our results showed a lower prevalence of central nervous system symptoms than the early Wuhan experiences (1) with a higher prevalence of ischemic stroke in our study (33% vs. 11%). On the other hand, our results are in agreement with the national reports (33% vs. 31%) (5).
Regarding stroke, the ischemic subtype was the most common. Compared with strokes without infection, people were younger, sometimes without cardiovascular risk factors, and the stroke was more often characterized by multiplied cerebral infarcts and caused by occlusion of a large artery. An early emergency thrombectomy is useful for such patients (8–13). On the other hand, because of the difficult management of these patients, out-of-window recanalization can be assumed, even after >12 h from the onset of symptoms (14). According to our experience, it is necessary to suspect a stroke in any patient with COVID-19 with cognitive impairment and altered mental status, even in the absence of typical manifestations such as aphasia or paresis. A COVID-19 stroke may be mainly due to cardioembolism or paradoxical embolism and less often to atherosclerosis and plaque rupture (7). Ischemic stroke due to occlusion of the internal carotid artery is a potentially devastating condition that could be embolic in nature. Tandem endovascular treatment is required in such cases (15). An important question is whether stroke in COVID-19 is causally related or represents an accidental association. COVID-19 can be a trigger or risk factor for stroke. In addition, stroke could complicate the course of COVID-19. Therefore, physicians must pay attention to the signs and symptoms of cerebral involvement to ensure appropriate clinical interventions. The mechanisms of cerebrovascular manifestations could be related to conventional mechanisms of stroke, with COVID-19 acting as a factor (16,17). Alternatively, it could be directly caused by SARS-CoV-2 infection through specific pathophysiological mechanisms leading to both ischemic stroke and hemorrhagic. Activation of the coagulation pathway with elevated D-dimer and elevated fibrinogen is a feature common to many individuals with severe COVID-19 infection. This coagulopathy, called “sepsis-induced coagulopathy” (SIC), is related to the systemic inflammatory response induced by infection and may contribute to an increased risk of thrombosis and stroke (18,19). In addition, the presence of anti-phospholipid antibodies (aPL), including IgA anticardiolipin antibodies and IgA and IgG beta 2 glycoprotein I antibodies, have been reported in severely infected patients with multiple cerebral infarcts (20). COVID-19 uses the angiotensin-converting enzyme 2 (ACE-2) receptor to enter cells (21) in the lungs, heart, kidneys, and vascular endothelium. Direct viral invasion of endothelial cells causes inflammation or “endothelitis,” which has been proposed as one of the substrates for thrombotic complications of COVID-19 (22) (Fig. 1). COVID-19-related hemorrhagic strokes are much less common than ischemic strokes. Some mechanisms may also play a role in promoting intracranial bleeding (23,24). The affinity of SARS-CoV-2 for ACE-2 receptors could allow the virus to directly damage intracranial arteries, causing the vessel wall to rupture. In addition, downregulation of the renin-angiotensin system may raise blood pressure and put patients already diagnosed with hypertension at higher risk for hemorrhagic stroke (25). In addition to ICH, rupture of the blood–brain barrier (BBB) could explain cases of reversible PRES and hemorrhagic transformation of ischemic stroke that have been reported in some patients with COVID-19 (26). Furthermore, SARS-CoV-2 infection could be associated with a consumable coagulopathy related to fibrinogen depletion (from metabolic acidosis or disseminated intravascular coagulation), which may increase the risk of ICH (16). Finally, perivascular micro-hemorrhages with cerebral micro-bleeds in the corpus callosum and subcortical and deep white matter suggest a potential role of brain hypoxia in causing brain damage in severe COVID-19 (27). An atypically high incidence of venous thromboembolism (VTE) has been reported in patients hospitalized with COVID-19 (28).
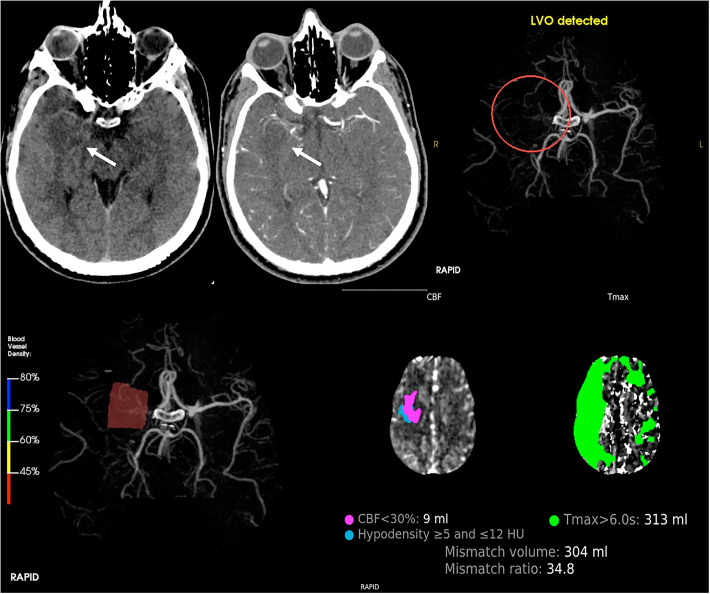
Fig. 1.
Top, from left to right: CT axial sections without and with contrast enhancement depict a focal area of ipo-density correlated with ischemic site (white arrow) due to occlusion of right middle cerebral artery, confirmed with RAPID software – LVO detected (red circle). Bottom, from left to right: 3D CT angiography shows flow reduction with blood vessel density <45% in right M1; then, RAPID software calculates CBF and Tmax for depiction of mismatch volume and ratio. These evaluations are fundamental for our endovascular treatment planning. CBF, cerebral blood flow; CT, computed tomography; LVO, large vessel occlusion; M1, first tract of middle cerebral artery; Tmax, time-to-maximum.
Studies have demonstrated that COVID-19 infection is associated with an increase in pro-thrombotic markers such as fibrinogen and D-dimer, as well as inflammatory markers such as C-reactive protein and interleukin-6, which are associated with a hypercoagulable state. Cerebral venous sinus thrombosis (cVST) is an uncommon subtype of stroke with a predilection for younger women. Interestingly, there appears to be a roughly equal incidence in men and women with COVID-19-associated cVST (29) (Fig. 2). For patients with ICH or cVST, it is necessary to not underestimate headaches, especially of the transfixed type. Encephalitis is an inflammation of the brain parenchyma, usually caused by an infection. Detection of the SARS-Cov-2 virus in the cerebrospinal fluid (CSF) on its own does not provide a diagnosis of encephalitis if there is no evidence (EEG or neuroimaging abnormalities) of brain inflammation (30) (Fig. 3). No specific treatment exists for SARS-CoV-2 encephalitis. Acute disseminated encephalomyelitis and myelitis are syndromes of multifocal demyelination, typically occurring weeks after an infection, which generally presents with focal neurological symptoms (31). Patients could have normal CSF and high signal intensities on MRI (Fig. 4a). An examination showed hyporeflexia and a sensory level. Acute disseminated encephalomyelitis and myelitis, usually considered post-infectious diseases, are treated typically with corticosteroids or other immunotherapies. In patients with COVID-19, clinicians might need to be more cautious, especially if the virus is detected in the CSF, because such treatment might diminish the patient's immune response (32). cytotoxic lesions of the corpus callosum (CLOCCs) are non-specific findings on brain MRI associated with reversible neurological signs, such as behavior changes and multiple etiologies, including viral illness, drug toxicity, seizures, malignancy, subarachnoid hemorrhage, and metabolic disturbances (33). The physiopathological hypothesis is that an inflammatory process involving cytokines such as IL-6 triggers the accumulation of glutamate in the extra-cellular space, resulting in cytotoxic edema, particularly of astrocytes. The selective vulnerability of the corpus callosum could be explained by its high density of cytokine and glutamate receptors. CLOCCs has been reported in association with SARS-CoV-2 infection. Interestingly, immune-mediated post-infectious mechanisms associated with SARS-CoV-2 are suggested. Other mechanisms are direct invasion of the brain via the olfactory bulb, carriage across the BBB after viremia or entry through infected leucocytes (34). Although intraVenous immunoglobulin and methylprednisolone are the first-choice treatments, therapeutic plasma exchange may be an option for the treatment of unresponsive cases (35) (Fig. 5).
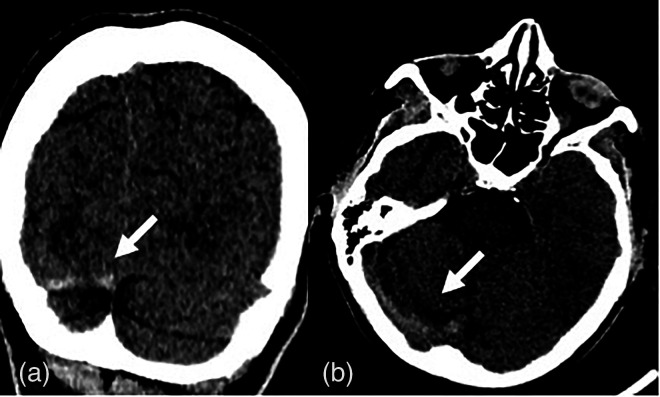
Fig. 2.
(a) Computed tomography coronal section shows an area of hyper-density due to thrombotic occlusion (white arrows) of right transverse sinus, better seen in (b) the axial section.
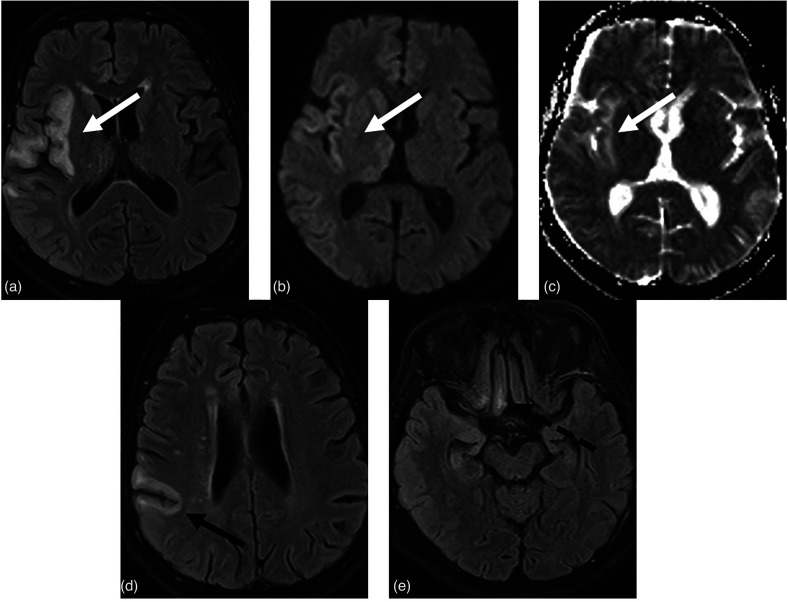
Fig. 3.
(a, b) MR-FLAIR sequences show a diffuse cortical-subcortical hyper-intensity signal in the right temporo-parietal insular region (white arrows) that correlates with diffusivity restriction in (c) the ADC map (white arrow). (d) Another area of similar meaning (encephalitis) is showed at the central cortex (black arrow). (e) At the same time, hyper-intensity in FLAIR images in the right rectal and orbital gyrus, near the olfactory groove (dotted black arrow). This patient manifests anosmia so we could think about an olfactory involvement. ADC, apparent diffusion coefficient; FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance.
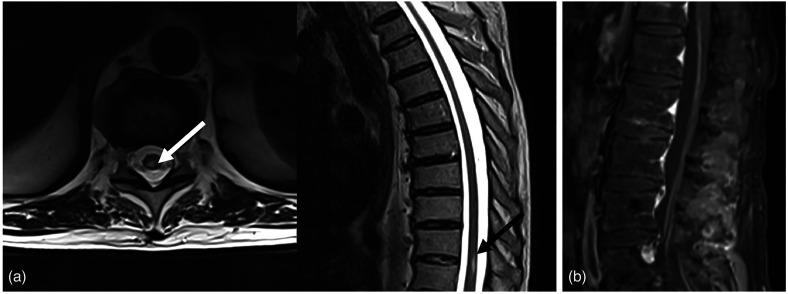
Fig. 4.
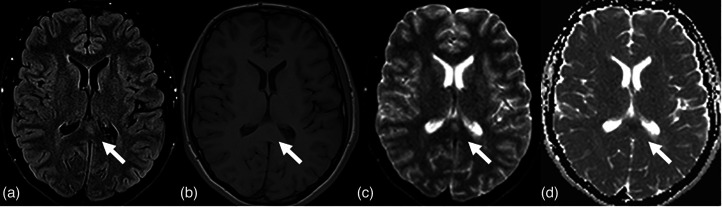
(a) MR T2-weighted sequences depict the myelitis process as an area of hyper-intensity that involved the right posterior portion of the spinal cord on the axial (white arrow) and sagittal (black arrow) planes at the D9-D10 level. (b) MR T1-weighted contrast enhancement sequence on the sagittal plane shows linear enhancement at cauda equina roots as a typical feature of Guillain–Barre syndrome.
Fig. 5.
(a) MR-FLAIR axial sequence shows a rounded area of hyper-intensity at the splenium of corpus callosum (white arrow). (b) This lesion is hypo-intense on T1-weighted imaging (white arrow) and involves a diffusivity restriction on (c, d) DWI/ADC maps (white arrows). This lesion is reversible and is due to an (ex)citotoxic edema mediated by COVID-19. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance imaging.
The published literature on COVID-19-related GBS commonly report a classic sensorimotor variant of GBS, often with facial palsy and a demyelinating electrophysiological subtype (36,37). The disease course is frequently severe (38). The time elapsed between infection and neurologic manifestations, and a negative PCR in spinal fluid might suggest that there is a post-infectious mechanism implicated in the etiology of COVID-19-related GBS (39,40). According to our data, men might be more prone to COVID-19-related GBS (37). Interestingly, because most of the cases of COVID-19-related GBS reported a demyelinating variant of GBS, it can be anticipated that the presence of antiganglioside antibodies would be low. Thus, the spectrum of immune cascade in COVID-19-related GBS should be expanded by studying other different antibodies (41,42). One case was reported with positive NF-155 and NF-186 antibodies, which are structural proteins in the node of Ranvier (43). Interestingly, human leukocyte antigen (HLA) analysis showed several HLA alleles that are known to be associated with GBS, such as: HLA-A33 and DQB1 * 05:01 (44). Cases of GBS are treated typically with corticosteroids or other immunotherapies. In addition, for these cases, clinicians might need to be more cautious (Fig. 4b).
Last, but not least, the association between COVID-19 and MFS is largely documented. Moreover, if COVID-19 really increases the risk for MFS, it is crucial to understand the underlying mechanism. MFS is a rare neurological disorder that is considered to be a variant of GBS (45,46). Several infectious diseases have shown an epidemiological linkage. Pathologically, it is plausible that SARS- CoV-2 might directly induce neuro-pathogenic effect due to the widespread expression of ACE-2 (host receptor for SARS-CoV- 2) in the nervous system. Alternatively, deregulated immune response upon SARS-CoV-2 infection might underlie COVID-19-associated MFS. In particular, an increasing amount of evidence has illustrated that SARS-CoV-2 can induce a severe immune and inflammatory reaction that leads to tissue damage. Thus, targeting the inflammatory cascade, for example, with corticosteroids, might be effective against COVID-19-associated MFS (47).
Finally, COVID-19 infection was shown to increase the risk of relapse in patients with multiple sclerosis (MS) (48–51). One of the putative mechanisms underlying the observed association between COVID-19 and MS attacks could be the expression of peripheral pro-inflammatory mediators, such as interleukin (IL)-6, IL-7, IL-10, IL- 17, granulocyte-colony stimulating factor, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α in COVID-19 infection (52). High amounts of these factors can lead to BBB dysfunction and facilitate the migration of monocytes, macrophages, and CD4 + and CD8 + T cells into the central nervous system, which consequently can cause neurological worsening and exacerbation of MS. Another possible mechanism is a direct invasion of the central nervous system (CNS) by SARS-COV-2 (53). In addition, the activation and accumulation of immune cells in the perivascular spaces of the brain are highlighted as a central event, leading to the activation of glial cells resulting in neuro-axonal damage. In this regard, we sensitize neuroradiologists in the search for a “central vein sign” as a specific marker of MS (54). COVID-19 can exacerbate MS. According to these data, it would be appropriate to submit such patients to follow-up MRI protocols using intravenous contrast medium in order to identify new active lesions (55). Conclusive information on therapy modification is not yet accessible.
The present study has some limitations, even with a large patient cohort (1060 people). These are due to the still poor understanding about the correlations between pathophysiology and COVID-19 clinic manifestations. In addition, stroke-related manifestations are still at only a few dozen (21/1060), so it is difficult to make generalizations.
In conclusion, it was not possible to correlate the frequency of neurological manifestations with the degree of pulmonary involvement. Our future goals are long-term evaluation of the outcomes of patients with neurological involvement and correlation with the degree of pulmonary involvement by retrospective evaluation of available CT findings. The aim of this paper was to evaluate organically the association between SARS-CoV-2 infection and neurological manifestations. Knowledge of these patterns will make clinicians consider COVID-19 infection when unexplained or atypical neurological findings are encountered.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mario Tortora https://orcid.org/0000-0002-4745-3061
References
- 1.Mao L, Jin H, Wang Met al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect 2020;80:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tortora M, Gemini L, D'Iglio I, et al. Spectral photon-counting computed tomography: a review on technical principles and clinical applications. J Imaging 2022;8:112. [DOI] [PMC free article] [PubMed]
- 4.Nannoni S, de Groot R, Bell S, et al. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke 2021;16:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahammedi A, Saba L, Vagal Aet al. Imaging of neurologic disease in hospitalized patients with COVID-19: an Italian multicenter retrospective observational study. Radiology 2020;297:E270–E273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aghagoli G, Gallo Marin B, Katchur NJet al. et al. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit Care 2021;34:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spence JD, de Freitas GR, Pettigrew LC, et al. Mechanisms of stroke in COVID-19. Cerebrovasc Dis 2020;49:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escalard S, Chalumeau V, Escalard C, et al. Early brain imaging shows increased severity of acute ischemic strokes with large vessel occlusion in COVID-19 patients. Stroke 2020;51:3366–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 2020;5:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan S, Xiao M, Han F, et al. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front Neurol 2020;11:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol 2020;77:1366–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang A, Mandigo GK, Yim PD, et al. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerv Surg 2020;12:648–653. [DOI] [PubMed] [Google Scholar]
- 14. Tortora M, Tortora F, Guida A, et al. Basilar Artery Occlusion (BAO) revascularization after more than 12 hours from the onset of symptoms with excellent outcome: report of a case. Radiol Case Rep 2022;17:1300–1304. [DOI] [PMC free article] [PubMed]
- 15. Guida A, Tortora F, Tortora M, et al. Dissective tandem stroke: an endovascular approach. Radiol Case Rep 2022;17:2170–2174. [DOI] [PMC free article] [PubMed]
- 16.Valderrama EV, Humbert K, Lord A, et al. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke 2020;51:e124–e127. [DOI] [PubMed] [Google Scholar]
- 17.South K, McCulloch L, McColl BW, et al. Preceding infection and risk of stroke: an old concept revived by the COVID-19 pandemic. Int J Stroke 2020;15:722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess DC, Eldahshan W, Rutkowski E. COVID-19-Related stroke. Transl Stroke Res 2020;11:322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost 2019;17:1989–1994. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godeau D, Petit A, Richard I, et al. Return-to-work, disabilities and occupational health in the age of COVID-19. Scand J Work Environ Health 2021;47:408–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect 2020;35:100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhammad S, Petridis A, Cornelius JF, et al. Letter to editor: severe brain haemorrhage and concomitant COVID-19 infection: a neurovascular complication of COVID-19. Brain Behav Immun 2020;87:150–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Tang X, Fan H, et al. Potential mechanisms of hemorrhagic stroke in elderly COVID-19 patients. Aging (Albany NY) 2020;12:10022–10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschi AM, Ahmed O, Giliberto L, et al. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. AJNR 2020;41:1173–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conklin J, Frosch MP, Mukerji S, et al. Cerebral microvascular injury in severe COVID-19. J Neurol Sci 2021;421:117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke 2018;13:612–632. [DOI] [PubMed] [Google Scholar]
- 30.Abenza Abildúa MJ, Atienza S, Carvalho Monteiro G, et al. Encephalopathy and encephalitis during acute SARS-CoV-2 infection. Spanish Society of Neurology COVID-19 registry. Encefalopatías y encefalitis durante la infección aguda por SARS-CoV2. Registro de la Sociedad Española de Neurología SEN COVID-19. Neurologia (Engl Ed) 2021;36:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pohl D, Alper G, Van Haren K, et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology 2016;87:S38–S45. [DOI] [PubMed] [Google Scholar]
- 32.Harapan BN, Yoo HJ. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). J Neurol 2021;268:3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tetsuka S. Reversible lesion in the splenium of the corpus callosum. Brain Behav 2019;9:e01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau A, Ego A, Vandergheynst F, et al. Cytotoxic lesions of the corpus callosum (CLOCCs) associated with SARS-CoV-2 infection. J Neurol 2021;268:1592–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varol F, Ergul N, Sahin EG, et al. Can plasma exchange therapy be an option for the treatment of SARS-CoV-2 related splenial lesion syndrome: two cases from the pediatric intensive care unit. Transfus Apher Sci 2022;61:103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aladawi M, Elfil M, Abu-Esheh B, et al. Guillain Barre syndrome as a complication of COVID-19: a systematic review. Can J Neurol Sci 2022;49:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Manzanedo S, López de la Oliva Calvo L, Ruiz Álvarez L. Guillain-Barré syndrome after COVID-19 infection. Síndrome de Guillain-Barré tras infección por COVID-19. Med Clin (Barc) 2020;155:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang P, Zhu M, Baker M, et al. Mechanical ventilation in Guillain-Barré syndrome. Expert Rev Clin Immunol 2020;16:1053–1064. [DOI] [PubMed] [Google Scholar]
- 39.Leonhard SE, Bresani-Salvi CC, Lyra Batista JD, et al. Guillain-Barré syndrome related to Zika virus infection: a systematic review and meta-analysis of the clinical and electrophysiological phenotype. PLoS Negl Trop Dis 2020;14:e0008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rees JH, Soudain SE, Gregson NA, et al. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med 1995;333:1374–1379. [DOI] [PubMed] [Google Scholar]
- 41.Soliven B. Animal models of autoimmune neuropathy. ILAR J 2014;54:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stathopoulos P, Alexopoulos H, Dalakas MC. Autoimmune antigenic targets at the node of Ranvier in demyelinating disorders. Nat Rev Neurol 2015;11:143–156. [DOI] [PubMed] [Google Scholar]
- 43.Tard C, Maurage CA, de Paula AM, et al. Anti-pan-neurofascin IgM in COVID-19-related Guillain-Barré syndrome: evidence for a nodo-paranodopathy. Neurophysiol Clin 2020;50:397–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schirmer L, Worthington V, Solloch U, et al. Higher frequencies of HLA DQB1*05:01 and anti-glycosphingolipid antibodies in a cluster of severe Guillain-Barré syndrome. J Neurol 2016;263:2105–2113. [DOI] [PubMed] [Google Scholar]
- 45.Abu-Rumeileh S, Abdelhak A, Foschi M, et al. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol 2021;268:1133–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayer JE, McNamara CA, Mayer J. Miller Fisher syndrome and Guillain-Barré syndrome: dual intervention rehabilitation of a complex patient case. Physiother Theory Pract 2022;38:245–254. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Li X, Shen J, et al. Miller Fisher syndrome associated with COVID-19: an up-to-date systematic review. Environ Sci Pollut Res Int 2021;28:20939–20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barzegar M, Vaheb S, Mirmosayyeb O, et al. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult Scler Relat Disord 2021;52:102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards S, Zvartau M, Clarke H, et al. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J Neurol Neurosurg Psychiatry 1998;64:736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kriesel JD, White A, Hayden FG, et al. Multiple sclerosis attacks are associated with picornavirus infections. Mult Scler 2004;10:145–148. [DOI] [PubMed] [Google Scholar]
- 51.Paules CI, Marston HD, Fauci AS. Coronavirus infections-More than just the common cold. JAMA 2020;323:707–708. [DOI] [PubMed] [Google Scholar]
- 52.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current State of the Science. Immunity 2020;52:910–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 2021;218:e20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tranfa M, Tortora M, Pontillo G, et al. The central vein sign helps in differentiating multiple sclerosis from its mimickers: lessons from Fabry disease. Eur Radiol 2022;32:3846–3854. [DOI] [PubMed]
- 55. Tortora M, Tranfa M, D'Elia AC, et al. Walk your talk: real-world adherence to guidelines on the use of MRI in multiple sclerosis. Diagnostics (Basel) 2021;11:1310. [DOI] [PMC free article] [PubMed]