Abstract
Introduction
Early eradication of methicillin-resistant Staphylococcus aureus (MRSA) in cystic fibrosis is desirable. Prospective studies are challenging owing to the feasibility of recruiting patients with a rare event in an orphan disease. Our prior randomised study (Staph Aureus Resistance-Treat Or Observe (STAR-too)) showed improved clearance and outcomes with aggressive therapy compared to no treatment. We present a novel trial design to guide treatment for eradicating incident infection with a focus on feasibility.
Methods
Subjects with cystic fibrosis with incident MRSA infection were enrolled into the Staph Aureus Resistance-Treat Early And Repeat (STAR-ter) protocol and treated with a combination of an oral antibiotic and topical (nare and throat) decolonisation. The primary outcome was MRSA-negative respiratory culture at Day 28, i.e. 14 days after completion of oral antibiotics. What was novel about this study design was that the control/comparator group was the untreated group of the STAR-too trial. This design was developed because having a “no treatment” group would be unethical given prior findings and a superiority design would delay the time to results based on small numbers of eligible subjects. Both studies used the same inclusion and exclusion criteria and drew subjects from the same geographic regions. The main difference between the studies was the use of a single oral antibiotic, trimethoprim-sulfamethoxazole, rather than the combination with oral rifampin used in STAR-too.
Discussion
An innovative approach to address a clinical question for a rare event in an orphan disease, cystic fibrosis, is presented to enhance current clinical evidence to guide cystic fibrosis care in relation to new MRSA infection.
Short abstract
To accomplish an ethical and timely trial for incident infection in a rare disease, this novel study design uses the control group from a prior randomised study as the comparator group #MRSA #cysticfibrosis https://bit.ly/3dICuqS
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease that affects approximately 34 000 people in the USA. Those living with CF produce abnormally viscous mucus, resulting in chronic bacterial lung infections [1]. Pulmonary infection and the resultant lung disease are the leading cause of death for CF patients [2]. Several bacteria are associated with higher mortality, including Pseudomonas aeruginosa, Burkholderia cepacia and methicillin-resistant Staphylococcus aureus (MRSA) [3]. MRSA is also a risk factor for lower lung function [4] increased rates of hospitalisation and failure to recover baseline lung function after intravenous antibiotics for pulmonary exacerbations [5]. Conversely, MRSA could be a marker of worse underlying disease. Studies have reported that 2 years after MRSA acquisition there was no disease progression but MRSA was associated with more intense therapies [6, 7]. Of note, these cited studies were done before the availability of highly effective modulator therapies (HEMT).
Similar to the high rates of MRSA carriage and infection among the general population in the USA, there is high prevalence of MRSA across US CF care centres, at ∼25% [8, 9]. Chronic, but not intermittent, MRSA infection is associated with lower survival when compared with those without MRSA [3]. This highlights the need for early intervention, prior to the development of chronic MRSA infection. Current CF care guidelines in the USA do not recommend prophylaxis against S. aureus and there are no guidelines for treating new or chronic MRSA [10].
Limited clinical trials have resulted in inconsistent treatment approaches for MRSA eradication. Several uncontrolled retrospective series report variable rates of eradication with a multitude of treatment approaches [11–13]. To date, only two randomised controlled clinical trials have compared treatment of incident or early MRSA infection to no treatment [14, 15]. Both trials used complex dual antibiotic therapy, topical therapy and environmental decontamination. Each trial showed a reduction in MRSA positivity in the treatment compared to the control arm, which achieved significance for the primary end-point in the Staph Aureus Resistance-Treat Or Observe (STAR-too) study [14].
The STAR-too protocol (NCT01349192) was the first randomised trial of eradication treatment for early MRSA infection in CF [14]. The treatment arm received 2 weeks of oral trimethoprim-sulfamethoxazole (TMP-SMX) and rifampin, combined with nasal mupirocin and throat decontamination with chlorhexidine for 5 days and antiseptic skin washes for 5 days. Subjects also performed intensified environmental cleaning in their homes for 3 weeks. The comparator was a no-treatment arm rather than a placebo arm because topical therapy and environmental contamination were difficult to conceal. The primary outcome was MRSA culture negativity on Day 28. Results showed that 82% of participants in the treatment arm were MRSA negative at Day 28, compared to only 26% in the control arm. Adjusted for interim monitoring, this difference was 52% (95% CI 23–80%, p<0.001). The aggressive treatment approach used in STAR-too yielded positive results but there were concerns over the feasibility of a demanding treatment regime, and the multiple drug interactions with rifampin led to the proposal and testing of the simpler regime described here.
The Staph Aureus Resistance-Treat Early And Repeat (STAR-ter) trial design represents a novel study design with an external, matched control group, enabling study completion in a rare disease (CF and incident MRSA) within a reasonable timeframe (NCT03489629).
Methods
Rationale and study design
A systematic study of incident events (MRSA infection) in a rare disease (CF) poses significant challenges for classical randomised controlled trials. Because antibiotic treatment for incident MRSA has been shown to lead to lower infection rates, the study team considered an untreated control group to no longer be ethical nor consistent with the Data and Safety Monitoring Board research standards, despite the use of no treatment remaining an issue of discussion [16]. Use of an active comparator arm requires either a superiority or a non-inferiority trial design. Given the STAR-too trial results, one could anticipate a much smaller effect size in any additional comparator trial. Incident MRSA continues to be a relatively rare event in an orphan disease. Thus, follow-on studies comparing potentially better (superiority) or equal (equivalence or non-inferiority) treatment approaches would likely not be feasible given the available population (see “Analytic approach”). These limitations led us to create a study design with an external comparator group, as has been done in other rare diseases [17]. Such an approach has seen increasing interest from regulatory bodies [18–20].
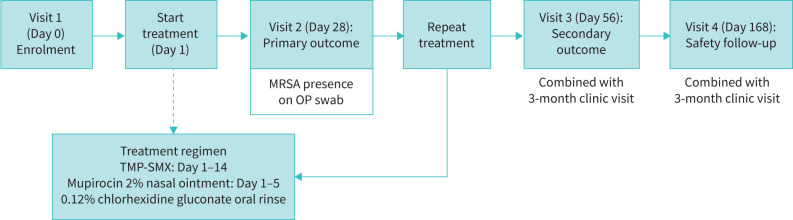
STAR-ter is an open-label multicentre interventional trial in patients with CF with new isolation of MRSA from the respiratory tract (oropharyngeal swab, sputum or bronchoscopy) who are at their clinical baseline. The study will investigate a cyclical and multifaceted treatment protocol to eradicate incident MRSA. Over the course of 6 months, subjects will have four study visits and receive two courses of antibiotic treatment (at start and repeated after a 2-week washout) targeting MRSA. Repeat oropharyngeal swabs and nasal swabs to monitor colonisation will be collected at all visits: screening, Day 28, Day 56 and Day 168 (figure 1).
FIGURE 1.
STAR-ter trial scheme. TMP-SMX: trimethoprim-sulfamethoxazole; MRSA: methicillin-resistant Staphylococcus aureus; OP: oropharyngeal.
The primary end-point will be the proportion of positive MRSA respiratory cultures at Day 28 compared to the rate of MRSA positivity in the placebo group in STAR-too as an external comparator control group. We hypothesise that the short-term microbiological efficacy of STAR-ter will be superior to an untreated control while accounting for a rare study population.
Novel trial design
Studies with an external control group are broadly defined as those in which the control group is not part of the current randomised study. External control groups are discussed as part of the US Food and Drug Administration (FDA) guidelines for industry studies [21]. Often the control subjects are selected from the wider population of the disease to be studied. In our case, the control group is from a prior trial that had the same inclusion criteria; participants are well characterised in regard to characteristics relevant to the study questions and the bias of inclusion is minimised owing to the randomisation in the STAR-too study. Further, the primary end-point is objectively defined as the proportion of subjects with MRSA-negative cultures and the 28-day observation period from the STAR-too trial provided carefully curated data regarding the short-term outcome of untreated incident MRSA in CF.
Ethical considerations strongly influenced the study design. Including a no-treatment arm would be unethical. Conducting a non-inferiority design would lead to an estimated sample size requirement of 366 incident MRSA cases assuming a non-inferiority margin of 10% [22]. Enrolment in STAR-too was around one to two patients per month across 10 CF centres. Given the rarity of such infection, a sample size of 366 was deemed unfeasible without including upwards of 69 CF centres or, at enrolment rates within the STAR-too trial, the trial would require 25 years to reach full enrolment. Such an approach would delay the answer to an important clinical question and have a negative impact on CF care. Given an increasingly crowded clinical space in CF with many competing studies for people with CF, such a large trial would also be challenging to justify. Thus, the use of a prior clinical trial observation arm as a comparator arm matches criteria recommended by the FDA and is the best method to allow for study of a rare infection in a rare disease population.
To allow comparisons between STAR-ter and STAR-too, STAR-ter was designed to mirror STAR-too as closely as possible. The studies use the same exclusion and inclusion criteria, except for a slightly expanded age range to better address the affected patient population seen in STAR-too (table 1).
TABLE 1.
Similarities and difference between STAR-ter and STAR-too protocols
| STAR-ter | STAR-too | |
| Study sites by region | West: 2 Midwest: 6 South: 1 North East: 0 |
West: 2 Midwest: 6 South: 3 North East: 0 |
| Eligibility age | ≥2–≤45 years | ≥4–≤45 years |
| Drug cycle | 14-day oral antibiotic treatment cycle 14-day washout period Repeat treatment cycle# |
14-day oral antibiotic treatment cycle |
| Primary end-point | The proportion of subjects with MRSA eradicated from respiratory tract cultures at Day 28 compared to the placebo arm in STAR-too | The proportion of subjects with MRSA eradicated from respiratory tract cultures at Day 28 compared to the placebo arm |
| Inclusion | Incident or ≤2 positive MRSA cultures | Incident or ≤2 positive MRSA cultures |
| Exclusion | Receiving oral or i.v. anti-MRSA antibiotics within 28 days MRSA resistant to study drug Patient allergic to any of the study medications Abnormal renal of liver function or FEV1 <25% predicted Pregnancy, lactation or not using barrier contraception Post transplant (any solid organ) |
Receiving oral or i.v. anti-MRSA antibiotics within 28 days MRSA resistant to study drug Patient allergic to any of the study medications Abnormal renal of liver function or FEV1 <25% predicted Pregnancy, lactation or not using barrier contraception Post transplant (any solid organ) |
Text in bold highlights differences between studies. MRSA: methicillin-resistant Staphylococcus aureus; FEV1: forced expiratory volume in 1 s. #: this repeat cycle occurs past the primary end-point thus no direct comparison to STAR-too placebo will be done.
The two trials recruited subjects from as many of the prior study sites as possible to minimise bias. Study design and procedures differ only in the medication of interest to the study, with STAR-ter not administering rifampin and not employing chlorhexidine body wipes for 5 days (see below for rational). Clinically stable subjects with new MRSA infection will be enrolled and receive oral TMP-SMX for 2 weeks, and nasal mupirocin for 5 days (table 2).
TABLE 2.
Study intervention in STAR-ter and STAR-too
| Drug | STAR-ter | STAR-too |
| Oral TMP-SMX | BW <40 kg: 8 mg·kg−1 TMP and 40 mg·kg−1 SMX twice daily for 14 days BW ≥40 kg: 320 mg TMP and 1600 mg SMX twice daily for 14 days |
BW <40 kg: 8 mg·kg−1 TMP and 40 mg·kg−1 SMX twice daily for 14 days BW ≥40 kg: 320 mg TMP and 1600 mg SMX twice daily for 14 days |
| Oral minocycline (if TMP-SMX allergy)# | BW <50 kg: 2 mg·kg−1 orally twice daily for 14 days BW ≥50 kg: 100 mg twice daily for 14 days |
BW <50 kg: 2 mg·kg−1 orally twice daily for 14 days BW ≥50 kg: 100 mg twice daily for 14 days |
| Oral rifampin | None | BW <40 kg: 7 mg·kg−1 (range 5–10 mg) orally twice daily for 14 days BW ≥40 kg: 300 mg twice daily |
| Nasal mupirocin 2% | Apply into each nostril twice daily for the first 5 days | Apply into each nostril twice daily for the first 5 days |
| Oral gargle (0.12% chlorhexidine gluconate wash)¶ | Oral rinse twice daily for 14 days | Oral rinse twice daily for 14 days |
| Chlorhexidine 2% body wipes | None | Once daily for initial 5 days |
TMP-SMX: trimethoprim-sulfamethoxazole; BW: body weight. #: for subjects ≥8 years; ¶: for subjects old enough to gargle.
Choice of study medications and optimisation
TMP-SMX was chosen owing to low rates of antibiotic resistance (∼3–12% in CF depending on strain type), its oral availability and its approval for children as young as 3 months of age with a good safety and tolerability profile [23]. The most common side-effects are rash and nausea occurring in 2–4% of patients taking TMP-SMX. Further, TMP-SMX has good penetration into lung tissue with sputum levels close to those in serum and is dose-dependent between 2 µg·mL−1 and 4.5 µg·mL−1 [24]. Dosage was based on recommendations and pharmacokinetic/pharmacodynamic studies in CF [25]. In cases of known TMP-SMX or sulfa allergy, minocycline is used in both trials for patients aged ≥8 years.
Additionally, topical decontamination of nares and throat is included in each trial (table 2). An exception will be children aged 2–4 years who may be too young to reliably gargle and are not receiving throat decolonisation. The number of subjects enrolled into the trial in this age range is unclear and sensitivity analyses will be done with and without inclusion of this age group. The initial study (STAR-too) also included skin decontamination with chlorhexidine based on the high rates of skin and soft tissue infections in non-CF patients. Yet, there was only a single occurrence of a MRSA-positive axilla/groin swab out of 44 subjects in the STAR-too study, leading us to not include this procedure in STAR-ter. Because MRSA skin colonisation was very low in the prior trial, with the difference between study groups at enrolment being 4% (95% CI −12–20%, p=1.00) and all subsequent axilla/groin cultures being MRSA negative in both arms, the colonisation status was deemed unlikely to affect outcomes. In contrast, nasal colonisation was present in 14 subjects (32%) at enrolment and decolonisation is included in this protocol and analysis.
The key difference between studies is the additional use of rifampin in the STAR-too study; rifampin was initially included based on its excellent penetration into respiratory secretions and the pharynx, and reports of it effectively reducing nasal carriage in asymptomatic persons [26]. Yet, given high rates of gastrointestinal side-effects and drug–drug interactions with agents often used in CF, this trial tests a more tolerable regimen.
Optimisation in relation to recurrence
Although STAR-too showed significant rates of eradication at Day 28, the differences in culture status decreased over time, with 62% in the treatment arm and 41% in the placebo arm remaining MRSA negative on Day 84 (difference −21%, 95% CI −47–10%, p=0.328). Such apparent recurrence could be persistence in other body sites [27] or true reinfection either from environmental sources in the household or other household members. The repeat cycle of eradication treatment using the same regimen as the initial one after a 2-week washout period will assess this as an exploratory end-point. Microbiologically, the MRSA at incident and any recurrent isolates will be sequenced to establish relatedness of the MRSA isolates.
Contemporaneous Clinical Care Comparison Arm
A descriptive study component of STAR-ter is the inclusion of subjects who meet inclusion criteria but decline to participate in the intervention. This study arm is termed the Contemporaneous Clinical Care Comparison Arm (CCCCA). Data collected from these patients include if and which treatment regimens are chosen, and complications and outcomes for the same duration as for the interventional arm. This design allows clinical practice evaluation and data collection on early interventions without influencing the patient's treatment plan to capture additional detailed data in this rare disease population.
Analytic approach
The primary end-point, the proportion of subjects with a negative culture for MRSA at Day 28, will be descriptively summarised with a corresponding 95% confidence interval. The primary analysis population will be composed of all subjects with culture results available at baseline and Day 28 as defined by the intention-to-treat efficacy (ITT-E) population. The primary efficacy analyses will also be performed on a per-protocol efficacy (PPE) population consisting of protocol-defined compliant subjects. The proportion of subjects with a negative respiratory culture for MRSA at Day 28 will be compared to the treatment arm of the STAR-too trial using Fisher's exact test, with corresponding 95% confidence intervals derived using the Newcombe–Wilson method. Event rate comparisons will be performed using Poisson regression.
To evaluate the robustness of primary study results, multiple sensitivity analyses for the primary end-point will be conducted, e.g. restricting the analysis to patients who were positive for MRSA at the screening visit. Because several subjects in the prior observational control group were treated with TMP-SMX prior to Day 28, sensitivity analyses will assess the robustness of study results by restricting the analysis to patients who do not require treatment for MRSA prior to Day 28. We will also compare the eradication proportion achieved in the STAR-ter trial to our CCCCA arm at follow-up, assessing patients both treated and not treated for incident MRSA. However, no sample size estimate was conducted because the goal is to primarily enrol into the interventional arm and heterogeneity of therapeutic approach may exist.
Secondary clinical efficacy end-points are identical in both STAR-too and STAR-ter with the addition of monitoring for small-colony variant S. aureus as a potential effect of repeat use of TMP-SMX. Table 3 lists all secondary end-points (clinical and microbiological).
TABLE 3.
Secondary clinical end-points
| End-point | Descriptive details |
| PEx | Proportion of subjects with protocol-defined PEx# overall and between Day 0 and Day 28 Number of PEx Proportions of subjects receiving any additional antibiotics Time to first PEx |
| Patient-reported outcomes | CFQ-R, CFRSD, CRISS |
| Other clinical end-points | Change in FEV1 from baseline to end of study Change in weight and percentile from baseline to end of study |
| Microbiology end-points | Proportion of subjects who are negative at Day 28 and remain MRSA negative and/or are MRSA negative at 3 months and 6 months Proportion of MRSA isolates developing resistance to any study medication Emergence of Pseudomonas aeruginosa infection |
| Adverse events | Frequency, by body system and severity |
| Microbiological adverse events | Emergence of small-colony variant MRSA¶ |
PEx: pulmonary exacerbation; CFQ-R: Cystic Fibrosis Questionnaire Revised quality of life respiratory domain symptom score; CFRSD: Cystic Fibrosis Respiratory Symptom Diary; CRISS: Chronic Respiratory Infection Symptom Score; FEV1: forced expiratory volume in 1 s; MRSA: methicillin-resistant Staphylococcus aureus. #: per modified Fuchs criteria [28]; ¶: differs between STAR-too and STAR-ter.
Adverse events will be monitored per standard research protocols [29]. Compliance and adherence will be defined as the proportion of subjects with >80% compliance for the study drug during the first 28 days.
Analyses of secondary end-points will be descriptively summarised by study arm with corresponding 95% confidence intervals and tested using a two-sided 0.05 level chi-squared test. Time to first exacerbation over the 6-month study will be graphically displayed for each treatment group using Kaplan–Meier estimates, and a corresponding hazard ratio will be estimated using Cox proportional hazards regression. Longitudinal analyses for changes in lung function and weight will be done using longitudinal linear regression models to estimate differences between study arms. We will also do a stratified analysis by HEMT (ivacaftor or elexacaftor/tezacaftor/ivacaftor) to address changes in CF therapeutics. Although this analysis is not part of the a priori statistical analysis plan, it will be of interest to the community.
All descriptive statistics will be completed as done in STAR-too, with two additions. STAR-ter will also collect and analyse data on household member culture results and small-colony variant rates. A two-sided p-value ≤0.05 will be considered significant.
Sample size estimate
In STAR-too, 82% of subjects with incident MRSA were MRSA negative at Day 28 compared to 26% of those who receive standard of care (observation only) (56% treatment difference, 95% CI 23–80%). The primary aim of STAR-ter is to determine whether a streamlined eradication protocol will produce comparable microbiological efficacy at Day 28. Assuming that the streamlined treatment will be comparable at 80%, a sample size of 42 subjects leading to at least 38 evaluable patients will provide an estimated proportion of subjects negative for MRSA at Day 28 of 82% (31 of 38) with precision quantified by a confidence interval ranging from 66% to 92%. If we observe an 82% rate of success (31 of 38), then compared to the rate in STAR-too (82%, 95% CI 61–93%), the 95% CI for the difference would be −22–19%. Based on an estimated 10% attrition rate, the study aims to recruit 42 subjects.
The lower bound of this confidence interval (66%) does not rule out non-inferiority to the aggressive MRSA eradication protocol but would demonstrate superiority to the STAR-too observational control; the lower bound includes a range of values to be considered efficacious for administration of the treatment protocol.
Discussion
Research on early MRSA infection treatment for the CF population is limited, yet data support the importance of early intervention [30, 31]. The lack of any guidance in the USA leads to uncertainty and variation in treatment, especially in patients who are asymptomatic at the time of MRSA-positive culture results [32–34]. Therefore, further data are necessary to inform practice. Our initial MRSA eradication trial (STAR-too) provided data to support the need for a standardised approach to early MRSA intervention. However, STAR-too used a complex treatment and raised concerns for tolerance and drug interactions with rifampin. Case series report successful eradication of MRSA in CF with combination therapies of rifampin and fusidic acid [12, 13]. However, given the side-effect profile and the fact that incident infection may require less intense therapy, and to simplify the regimen, we now test a single oral agent while keeping mucosal topical therapies the same. This simpler approach would increase patient adherence and allows a repeat cycle of therapy. However, research into rare disease populations can have reduced enrolment rates that affect the feasibility of randomised clinical controlled trials. Our prior trial required 10 study sites and 3.5 years for randomising 47 participants. This is a known challenge in rare diseases. A search of ClinicalTrials.gov showed that 30% of trials in rare diseases conducted between 2010 and 2012 were discontinued, with insufficient patient accrual being cited as the most frequent reason [35].
We thus take a novel approach to address low enrolment by using the placebo group from a previous randomised trial, STAR-too, as a comparator group, thus allowing for lower enrolment rates while maintaining a clinical trial approach that includes a control arm. The schedule of events, study sites and inclusion and exclusion criteria are highly similar between the studies. The difference between the studies is that STAR-ter has an expanded age range (youngest age 2 instead of 4 years) to increase generalisability. The unique trial design of STAR-ter allows us to further develop a treatment protocol for incident MRSA infection for CF patients. In recent years, regulatory agencies have shown increased interest in novel study designs aiming to minimise subject numbers yet provide rigorous results [36, 37]. Such trial designs may include n=1 design, crossover trials or adaptive randomisations. None of these would be well suited to our outcome for which the goal is to achieve continued eradication. These trial designs also emphasise the focus on continuous outcomes, which is at odds with our dichotomous primary outcome.
The CCCCA further increases the size of the population to compare potential changes in baseline characteristics over time. This pragmatic, observational approach additionally contributes to an understanding of current clinical practices, which may be informative with regards to attitudes towards early treatment and preferences in medications. Results will need to be interpreted cautiously because those subjects are drawn from the same study sites, limiting the generalisability.
The FDA is cautiously discussing the use of external controls, with emphasis on the external control being as closely matched to the study population as possible, while minimising bias and having objective outcomes. We maximised these conditions as much as possible here. One limitation that was not foreseen at the time of study design was the introduction of novel, better-tolerated and highly effective cystic fibrosis transmembrane conductance regulatory (CFTR) modulators. This will potentially affect underlying disease severity in our population. However, STAR-too subjects were children with preserved lung function (3% had a forced expiratory volume in 1 s <75%). The effect of CFTR modulators on microbiology and infections is still under investigation, with several studies showing persistence of the patients’ prior organisms [38, 39].
There are several key strengths and weaknesses to our approach. The strengths include markedly improved feasibility to complete the study owing to lower enrolment requirements. Another key strength is that our primary end-point is objective and easy to assay. S. aureus is not a fastidious organism and does not require a lower airway specimen to understand its clinical implications. As in this study, our prior study accepted any respiratory source of S. aureus. In addition, a central laboratory will ensure confirmation of methicillin resistance using CF culture guidelines. The weakness of our approach is that it cannot fully account for temporal changes in MRSA spontaneous clearance rates, e.g. with increased use of HEMT. The advent of HEMT may have unanticipated effects on the study. This could be in part due to reduced sputum production in those with established CF lung disease. Recent data, however, indicate that changes in microbiology may be more limited with HEMT than initially anticipated [40]. Our sensitivity analysis will be able to explore these effects. Our CCCCA analysis could provide data to assess enhanced clearance as a potential confounder and will also provide a comparator to use of anti-MRSA antibiotics prior to Day 28 as had occurred in several subjects previously. We will also not be able to fully address selection bias if the STAR-ter trial population is different in any meaningful way (disease severity or age). We can create age and disease severity strata but this will not fully address this weakness.
In conclusion, we describe here an innovative approach to addressing a clinical question in a rare infection in an orphan disease, CF. Our study will support the current clinical evidence guiding CF care in relation to new MRSA infection and is designed with a focus on feasibility.
Footnotes
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov with identifier number NCT03489629. Findings will be made publically available on ClinicalTrials.gov and by publication once results are available.
Conflicts of interest: The authors report no relevant conflicts of interest.
Support statement: N. Mayer-Hamblett was supported a grant from the National Institutes of Health (NIH) (UL1 TR002319). C.H. Gross was supported by grants from the Cystic Fibrosis Foundation (CFF), the NIH (UM1 HL119073, P30 DK089507, U01 HL114589 and UL1TR000423), and the US Food and Drug Administration (R01 FD003704 and R01 FD006848). M.S. Muhlebach was supported by grants from the CFF (MUHLEB17A0) and NIH (UL1TR002489). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and methods of a national observational disease registry. Ann Am Thorac Soc 2016; 13: 1173–1179. doi: 10.1513/AnnalsATS.201511-781OC [DOI] [PubMed] [Google Scholar]
- 2.Blanchard AC, Waters VJ. Microbiology of cystic fibrosis airway disease. Semin Respir Crit Care Med 2019; 40: 727–736. doi: 10.1055/s-0039-1698464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasenbrook EC, Checkley W, Merlo CA, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010; 303: 2386–2392. doi: 10.1001/jama.2010.791 [DOI] [PubMed] [Google Scholar]
- 4.Ren CL, Morgan WJ, Konstan MW. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol 2007; 42: 513–518. 10.1002/ppul.20604. [DOI] [PubMed] [Google Scholar]
- 5.Sanders DB, Bittner RC, Rosenfeld M, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010; 182: 627–632. doi: 10.1164/rccm.200909-1421OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawicki GS, Rasouliyan L, Pasta DJ, et al. The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatr Pulmonol 2008; 43: 1117–1123. doi: 10.1002/ppul.20914 [DOI] [PubMed] [Google Scholar]
- 7.Muhlebach MS, Miller M, LaVange LM, et al. Treatment intensity and characteristics of MRSA infection in CF. J Cyst Fibros 2011; 10: 201–206. doi: 10.1016/j.jcf.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 8.Kourtis AP, Hatfield K, Baggs J, et al. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections - United States. MMWR Morb Mortal Wkly Rep 2019; 68: 214–219. doi: 10.15585/mmwr.mm6809e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry. Bethesda, MD, Cystic Fibrosis Foundation, 2018. www.cff.org/medical-professionals/patient-registry [Google Scholar]
- 10.Mogayzel PJ Jr, Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013; 187: 680–689. doi: 10.1164/rccm.201207-1160OE [DOI] [PubMed] [Google Scholar]
- 11.Vanderhelst E, De Wachter E, Willekens J, et al. Eradication of chronic methicillin-resistant Staphylococcus aureus infection in cystic fibrosis patients. An observational prospective cohort study of 11 patients. J Cyst Fibros 2013: 12: 662–666. doi: 10.1016/j.jcf.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 12.Garske LA, Kidd TJ, Gan R, et al. Rifampicin and sodium fusidate reduces the frequency of methicillin-resistant Staphylococcus aureus (MRSA) isolation in adults with cystic fibrosis and chronic MRSA infection. J Hosp Infect 2004; 56: 208–214. doi: 10.1016/j.jhin.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Macfarlane M, Leavy A, McCaughan J, et al. Successful decolonization of methicillin-resistant Staphylococcus aureus in paediatric patients with cystic fibrosis (CF) using a three-step protocol. J Hosp Infect 2007; 65: 231–236. doi: 10.1016/j.jhin.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 14.Muhlebach MS, Beckett V, Popowitch E, et al. Microbiological efficacy of early MRSA treatment in cystic fibrosis in a randomised controlled trial. Thorax 2017; 72: 318–326. doi: 10.1136/thoraxjnl-2016-208949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolce D, Neri S, Grisotto L, et al. Methicillin-resistant Staphylococcus aureus eradication in cystic fibrosis patients: a randomized multicenter study. PLoS One 2019; 14: e0213497. doi: 10.1371/journal.pone.0213497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis JA, Jonsson B, Kreutz G, et al. Placebo-controlled trials and the Declaration of Helsinki. Lancet 2002; 359: 1337–1340. doi: 10.1016/S0140-6736(02)08277-6 [DOI] [PubMed] [Google Scholar]
- 17.Philip PA, Chansky K, LeBlanc M, et al. Historical controls for metastatic pancreatic cancer: benchmarks for planning and analyzing single-arm phase II trials. Clin Cancer Res 2014; 20: 4176–4185. doi: 10.1158/1078-0432.CCR-13-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray CM, Grimson F, Layton D, et al. A framework for methodological choice and evidence assessment for studies using external comparators from real-world data. Drug Saf 2020; 43: 623–633. doi: 10.1007/s40264-020-00944-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemkens LG. How routinely collected data for randomized trials provide long-term randomized real-world evidence. JAMA Netw Open 2018; 1: e186014. doi: 10.1001/jamanetworkopen.2018.6014 [DOI] [PubMed] [Google Scholar]
- 20.Hunter NL, Rao GR, Sherman RE. Flexibility in the FDA approach to orphan drug development. Nat Rev Drug Discov 2017; 16: 737–738. doi: 10.1038/nrd.2017.151 [DOI] [PubMed] [Google Scholar]
- 21.Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research . Guidance for Industry: E 10 Choice of Control Group and Related Issues in Clinical Trials. Rockville, MD, Food and Drug Administration, 2001. [Google Scholar]
- 22.Blackwelder WC. “Proving the null hypothesis” in clinical trials. Control Clin Trials 1982; 3: 345–353. doi: 10.1016/0197-2456(82)90024-1 [DOI] [PubMed] [Google Scholar]
- 23.Champion EA, Miller MB, Popowitch EB, et al. Antimicrobial susceptibility and molecular typing of MRSA in cystic fibrosis. Pediatr Pulmonol 2014; 49: 230–237. doi: 10.1002/ppul.22815 [DOI] [PubMed] [Google Scholar]
- 24.Wilfert CM, Gutman LT. Pharmacokinetics of trimethoprim-sulfamethoxazole in children. Can Med Assoc J 1975; 112: 73–76. [PMC free article] [PubMed] [Google Scholar]
- 25.Reed MD, Stern RC, Bertino JS Jr, et al. Dosing implications of rapid elimination of trimethoprim-sulfamethoxazole in patients with cystic fibrosis. J Pediatr 1984; 104: 303–307. doi: 10.1016/S0022-3476(84)81019-7 [DOI] [PubMed] [Google Scholar]
- 26.Ellison RIJ, Peterson FN, LCet al. Oral rifampin and trimethoprim/sulfamethoxazole therapy in asymptomatic carriers of methicillin-resistant Staphylococcus aureus infections. West J Med 1984; 140: 735–740. [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrich JS, Sutton-Shields TN, Kerr A, et al. Prevalence of community-associated methicillin-resistant Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol 2009; 47: 1231–1233. doi: 10.1128/JCM.00255-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994; 331: 637–642. doi: 10.1056/NEJM199409083311003 [DOI] [PubMed] [Google Scholar]
- 29.Common Terminology Criteria For Adverse Events (CTCAE): version 4.0. Washington, DC, US Department of Health and Human Services, 2009. [Google Scholar]
- 30.Lo DK, Hurley MN, Muhlebach MS, et al. Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst Rev 2015; 2: CD009650. [DOI] [PubMed] [Google Scholar]
- 31.Lo DK, Muhlebach MS, Smyth AR. Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst Rev 2018; 7: CD009650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chmiel JF, Aksamit TR, Chotirmall SH, et al. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann Am Thorac Soc 2014; 11: 1120–1129. doi: 10.1513/AnnalsATS.201402-050AS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goss CH, Muhlebach MS. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros 2011; 10: 298–306. doi: 10.1016/j.jcf.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 34.Zobell JT, Epps KL, Young DC, et al. Utilization of antibiotics for methicillin-resistant Staphylococcus aureus infection in cystic fibrosis. Pediatr Pulmonol 2015; 50: 552–559. doi: 10.1002/ppul.23132 [DOI] [PubMed] [Google Scholar]
- 35.Rees CA, Pica N, Monuteaux MC, et al. Noncompletion and nonpublication of trials studying rare diseases: a cross-sectional analysis. PLoS Med 2019; 16: e1002966. doi: 10.1371/journal.pmed.1002966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Food and DrugAdministration . Complex Innovative Trial Designs Pilot Program. 2019. www.fda.gov/drugs/development-resources/complex-innovative-trial-design-meeting-program Date last accessed: March 2022.
- 37.European Medicines Agency . EMA Regulatory Science to 2025. 2018. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ema-regulatory-science-2025-strategic-reflection_en.pdf Date last accessed: March 30, 2022.
- 38.Middleton PG, Mall MA, Drevinek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809–1819. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durfey SL, Pipavath S, Li A, et al. Combining ivacaftor and intensive antibiotics achieves limited clearance of cystic fibrosis infections. mBio 2021; 12: e0314821. doi: 10.1128/mbio.03148-21 [DOI] [PMC free article] [PubMed] [Google Scholar]