Abstract
Spinal cord injury (SCI) has been considered to cause sudden, irreversible loss of function in patients. However, developments in stem cell biology and regenerative medicine are changing this conventional notion. Here we reviewed the overview of regenerative medicine of SCI.
As a consequence of the establishment of human induced pluripotent stem cells (hiPSCs), hiPSC-based therapies for SCI, such as neural stem/progenitor cell (NS/PC) transplantation, have emerged as promising therapeutic modalities. Using several animal models, hiPSC-NS/PC transplantation into subacute injured spinal cords has been repeatedly demonstrated to improve locomotor function.
Some biological mechanisms underlying this improvement have been proposed. In particular, combined with advanced neuroscience techniques such as designer receptors exclusively activated by designer drugs (DREADDs), neuronal relay theory, in which the transplanted cell-derived neurons reconstruct disrupted neuronal circuits, was proven to be involved histologically, pharmaceutically, electrophysiologically, and via in vivo bioimaging.
Based on these findings, hiPSC-NS/PC transplantation for subacute SCI was moved ahead to a clinical study on human patients. At the same time, the search for effective treatments for chronic SCI is proceeding gradually, combining hiPSC-NS/PC transplantation with other treatment modalities such as rehabilitation, pharmaceutical interventions, or optimal scaffolds. In addition to NS/PCs, oligodendrocyte precursor cells (OPCs) are also a promising cell source for transplantation, as demyelinated axons affected by SCI can be repaired by OPCs. Therapies with OPCs derived from hiPSCs are still in preclinical studies but have shown favorable outcomes in animal models.
In the future, several therapeutic options may be available according to the pathological conditions and the time period of SCI. Moreover, the application of regenerative therapy for the spinal cord could be broadened to degenerative disorders, such as spinal canal stenosis.
Summary sentence: A historical review of human induced pluripotent stem cell (hiPSC) based cell transplantation therapy for spinal cord injury (SCI), in particular about footsteps of hiPSC-derived neural stem/progenitor cell transplantation, recent clinical study, and its future perspective.
Keywords: Spinal cord injury, Transplantation, Human induced pluripotent stem cell (iPS cell), Neural stem/progenitor cell (NS/PC), Oligodendrocyte precursor cell (OPC), Regenerative medicine, Clinical study
Introduction
Spinal cord injury (SCI) is a grueling condition in which the spinal cord parenchyma, part of the central nervous system (CNS), is damaged, resulting in the paralysis of the sensory, motor, and autonomic nerves below the level of injury. SCI occurs mainly as a result of traumatic injuries such as traffic accidents, falls, or sports accidents. Treatment for SCI consists mainly of 1) decompression and fusion surgery on the physically compressed spinal cord to prevent further damage and 2) rehabilitation to enhance residual function. However, a fundamental treatment to regenerate the damaged spinal cord and to recover lost function has not been established. This is because nerve cells do not self-renew, nor does CNS tissue regenerate once damaged [1].
In recent years, research in stem cell biology and regenerative medicine has attracted attention with positive expectations for therapeutic approaches for the tragic condition of SCI [2], [3], [4], [5]. For example, clinical studies have been conducted on therapies involving intravenous administration of bone marrow-derived mesenchymal stem cells and therapeutic agents such as granulocyte colony-stimulating factor [6], [7], [8] and hepatocyte growth factor [9,10]. Based on the belief that the injured spinal cord can be regenerated by replacing the damaged cells, the authors’ group has long been studying intraspinal transplantation of neural stem/progenitor cells (NS/PCs) derived from human induced pluripotent stem cells (hiPSCs) for SCI therapy. In this review, we present an overview of previous studies and recent progress in hiPSC-derived NS/PC transplantation therapy for SCI.
Pathophysiology of SCI
The condition of the injured spinal cord changes over time [3,11,12]. Initially, the trauma causes mechanical damage to the tissue. This mechanical damage is called the primary injury. After the primary injury, secondary injury occurs due to the self-destructive tissue process and inflammatory reaction. The primary injury disrupts the blood–spinal cord barrier, leading to edema in the white matter of the spinal cord and hemorrhage in the gray matter within a few minutes. This disruption of blood flow in the injured area results in vasoconstriction and thrombus formation in the microenvironment, resulting in localized ischemia. Accordingly, the subsequent oxidase-induced neuronal cell death and axonal demyelination further impair the normal function of the spinal cord. This period of intense inflammation at the injured site causing secondary injury is generally referred to as the acute phase [13], [14], [15], [16], [17].
Subsequently, reactive astrocytes migrate around the injured area to surround the inflammatory cells that have infiltrated the lesion [12]. This process prevents further spreading of the injury. This period is defined as the subacute phase, generally corresponding to one to two weeks after SCI in rodents and two to four weeks in primates [18].
Eventually, the injury gradually moves to the chronic phase. Throughout the chronic phase, glial scars and cavities are formed in the injured area [4,19]. In this condition, the spinal cord becomes firmly stable, with the disruption of the healthy and natural neural circuit. Hence, it was thought that functional disability in the patient is irreversible once the SCI reaches the chronic phase.
In this way, the pathophysiology of SCI varies greatly according to the time after the injury, so treatments for SCI should differ depending on the time after and pathophysiology of the injury. The aforementioned decompression and fusion surgeries are aimed at reducing secondary injury. Therefore, these surgeries are carried out in the acute phase for physically compressed spinal cords associated with fractures, dislocations, or swelling. Methylprednisolone has been administered in the acute phase to reduce secondary injury, but a consensus on its safety and effectiveness has not yet been reached [20], [21], [22]. In this way, these existing treatments mainly focus on preserving surviving tissue and preventing further damage. Various attempts have been made, but the recovery of lost function has been a great challenge in the treatment of SCI.
NS/PC transplantation therapy for SCI
Stem cells are somatic cells that possess both a multipotent capacity to differentiate into various cell types and a self-renewal capacity to proliferate while maintaining an undifferentiated state. Endogenous stem cells are involved in the self-repair of damaged tissues, such as lizard tail or human skin. It has been reported that intrinsic neural stem cells exist in the spinal cord, but most intrinsic neural stem cells in the spinal cord differentiate into astrocytes after SCI [23,24]. Therefore, intrinsic neural stem cells contribute little to the cellular replenishment of neurons and oligodendrocytes, so the damaged spinal cord fails to recover to a healthy intact condition. This has been thought to be one of the main causes of irreversible damage.
Therefore, it was hypothesized that spinal cord regeneration could be achieved by transplanting NS/PCs, which have the ability to differentiate into neural cells such as neurons, astrocytes, and oligodendrocytes, into the injured spinal cord. This revolutionary therapeutic approach differs fundamentally from conventional therapies that aim to reduce secondary damage in the acute phase. It aims to regenerate damaged tissue and recover functional loss by replacing nerve cells through transplantation.
Optimal time for NS/PC transplantation
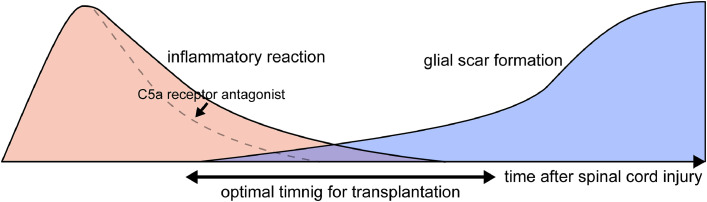
Several studies have been conducted to determine when transplanted cells can be successfully engrafted. The results of these studies indicated that the acute phase, when strong inflammation occurs, is not suitable for the successful engraftment of transplanted cells [3,18,25]. This is because the strong inflammation, especially the action of complement proteins such as C5a, in the acute phase is hostile to the transplanted NS/PCs and differentiated immature neural cells, as it is to the damaged spinal cord [26]. On the other hand, the chronic phase, when glial scar formation has completed, was also reported to be less than ideal for transplantation. This is because although the transplanted cells are viable in the glial scar, they fail to exert their therapeutic effect due to the strong intercellular adhesion of the glial scar and the axonal growth inhibitory factors released from the glial scar [27].
Therefore, the subacute phase, when inflammation has subsided but the glial scar has not completely formed, was considered to be the optimal time period for transplantation [25]. Thus far, most cell transplantation therapy studies, including ours, have mainly focused on the subacute phase (Fig. 1). Recently, a study showed that inflammation can be reduced faster by using a C5a receptor antagonist, and the optimal time window for transplantation can be broadened [26].
Fig. 1.
Optimal time period for cell transplantation. Neither the acute phase, when strong inflammation occurs, nor the chronic phase, when glial scar formation has completed, are suitable for cell transplantation. Therefore, the subacute phase, when inflammation has subsided but the glial scar has not completely formed, is considered to be the optimal time period for transplantation. To broaden the time window for transplantation, a C5a receptor antagonist was reported to be effective for reducing inflammation.
Transplantation of hiPSC-derived NS/PCs into injured spinal cords
First, we and other researchers transplanted NS/PCs derived from fetal rats into subacute injured adult rat spinal cords and reported improved locomotor function [25,28,29]. Then, we furthered our research by transplanting NS/PCs derived from human fetuses into subacute injured common marmoset (a nonhuman primate) spinal cords [30,31]. Improved locomotor function was observed in this research. The findings of these studies showed great promise for the clinical application of regenerative medicine for SCI. However, aborted fetal tissue was needed to obtain the NS/PCs for these studies, which raised a serious ethical problem. In some countries, including ours, clinical application of fetal tissue-derived NS/PCs is not permitted under law.
The establishment and development of hiPSCs drastically changed this difficult situation. iPSCs are established by inducing some reprogramming factor genes in somatic cells, thus bypassing the ethical problem described above [32,33]. Therefore, hiPSCs were presumed to be a promising cell source for NS/PC transplantation therapy.
We first established a method for stable induction of hiPSCs to NS/PCs [34,35]. Then, we transplanted the hiPSC-derived NS/PCs into the subacute injured spinal cords of mice [36]. In this study, hindlimb locomotor function was improved after transplantation. Pathologically, transplanted NS/PCs differentiated into three neural lineage cells: neurons, astrocytes, and oligodendrocytes. Moreover, synapse formation between grafted and host neurons was confirmed through immunoelectron microscopic examination. Next, we transplanted the hiPSC-derived NS/PCs into the subacute injured spinal cords of common marmosets [37]. Secondary injury was inhibited, and graft-derived axons were elongated into the host spinal cord. Motor function was successfully improved in the transplanted animals. On the basis of these research findings, we started a clinical study of hiPSC-derived NS/PC transplantation into human subacute injured spinal cords [38,39]. We are carefully following the clinical courses of the patients after transplantation.
Biological mechanism underlying the effectiveness of hiPSC-derived NS/PC transplantation therapy
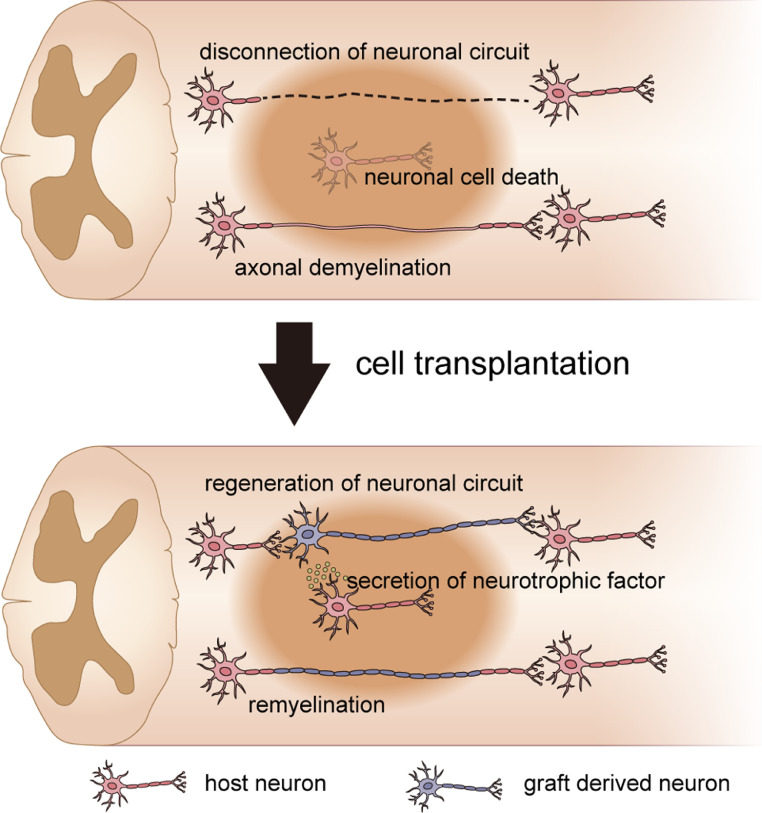
The clinical study of hiPSC-derived NS/PC transplantation therapy has already begun, but the biological mechanism underlying the effectiveness of this therapy has remained elusive. In previous studies, several factors were reported to be involved in the efficacy of this transplantation therapy [5]. Three of these factors, which are the most widely propounded, are the regeneration of the neuronal circuit between the graft and host tissue [40], [41], [42], the remyelination of demyelinated host axons [43], [44], [45], [46], and neuroprotection by neurotrophic factors secreted by the NS/PCs and host tissue [27,47] (Fig. 2).
Fig. 2.
Biological mechanism underlying the effectiveness of cell transplantation therapy. Neuronal circuit disconnection, axonal demyelination, and neuronal cell death occur following spinal cord injury. Cell transplantation therapy is reported to regenerate neuronal circuits, remyelinate demyelinated host axons, and protect remaining neural cells by neurotrophic factors.
However, how those factors specifically yield functional improvement and how much those factors contribute to the improvement have not been sufficiently elucidated [48]. It is critically important to clarify the mechanism underlying this functional improvement for the further advancement of this therapy.
Application of designer receptors in basic research for spinal cord regeneration
Currently, with advancements in neuroscience and molecular cell biology, various new methods have been developed to control neuronal activity. One of these new techniques is designer receptors exclusively activated by designer drugs (DREADDs) [49], [50], [51], [52]. These are genetically modified G-protein-coupled receptors that are engineered to respond exclusively to synthetic ligands, such as clozapine N-oxide (CNO), and not to endogenous ligands. By using DREADDs, researchers can selectively and reversibly manipulate the neural activity of target neurons [52].
We established hiPSC-derived NS/PCs expressing hM4Di, which is a type of DREADD that inhibits neural activity when CNO is administered. Then, we transplanted these hM4Di-expressing hiPSC-derived NS/PCs into the subacute injured spinal cords of mice [53]. As we observed before, hindlimb locomotor function was significantly improved after transplantation in transplanted mice compared to sham control mice. Thereafter, we injected the mice with CNO intraperitoneally at 10 weeks after transplantation. One hour after CNO administration, part of the improved locomotor function was diminished temporally in the transplanted group. On the other hand, locomotor function in the sham control group was not affected by CNO administration. This result showed that selective inhibition of hM4Di-expressing graft-derived neurons decreased the improved function and indicated that the graft-derived neurons contributed to the reconstruction of damaged neural circuits. Moreover, the result in which a part of the motor function recovery was reduced by selective inhibition means that the rest of the functional recovery was assumed to be the contribution of other mechanisms, such as remyelination or neurotrophic factor release.
We also used hM3Dq, which is another type of DREADD that activates neural activity when CNO is administered. We transected the corticospinal tract (CST) of mice and transplanted hiPSC-derived NS/PCs into the lesion [40]. Then, we injected hM3Dq-expressing adeno-associated virus into the mouse primary motor cortex. After CNO administration, the in vivo imaging system revealed that E-SARE promoter activity was enhanced in graft-derived neural cells. E-SARE is a synthetic promoter whose activity is enhanced soon after neuronal activity. This result showed that the graft-derived neuron in the CST was activated after the stimulation of the primary motor cortex and indicated the reconstruction of neuronal connectivity from the primary motor cortex to the graft-derived neuron in the cervical CST.
Furthermore, in another study, we established hiPSC-derived NS/PCs expressing hM3Dq. Then, we transplanted these hM3Dq-expressing hiPSC-derived NS/PCs into the subacute injured spinal cords of mice [54]. After transplantation, CNO was injected peritoneally each day. Through this continuous stimulation of the graft, synaptic activity was enhanced in the host spinal cord tissue around the graft, and locomotor function was significantly improved in the stimulated group compared with the transplantation-alone group. Moreover, it was also shown that electrical activity within the host spinal cord was enhanced soon after CNO administration. This result revealed that the activation of transplanted neural cells can enhance the efficacy of NS/PC transplantation therapy and provided a basis for further advancement of this therapy to be combined with adjuvant therapy, such as rehabilitation and electrical or magnetic stimulation.
Application for chronic SCI
As mentioned above, we and other researchers have conducted many studies regarding hiPSC-derived NS/PC transplantation therapy for subacute SCI. Consequently, a clinical study on human patients was eventually started [38,39]. However, the study of SCI is just the beginning because there remain important challenges for chronic SCI [55]. A majority of patients are in the chronic phase, in which regeneration is extremely difficult because of glial scar and cavity formation [4,19,56]. Thus far, studies in which the efficacy of hiPSC-NS/PC transplantation has been reported are very limited, but researchers have been tackling this problem.
We first transplanted hiPSC-NS/PCs into chronic injured spinal cords of mice [27]. The NS/PCs survived in the lesion, but the glial scar enclosed the transplanted cells, axonal regrowth beyond the glial scar did not occur, and we could not confirm locomotor function recovery. Therefore, combined approaches in which some other strategy is added to the hiPSC-NS/PC transplantation have been attempted.
One approach is to combine rehabilitation with cell transplantation. We transplanted mouse fetus-derived NS/PCs into injured mouse spinal cords 49 days after SCI, and those mice underwent treadmill training for approximately 50 days [57]. Compared to the control group, the transplantation and rehabilitation combination group showed a significant improvement in hindlimb motor function. On the other hand, transplantation alone or rehabilitation alone did not lead to significant improvement. The recovery due to this combination therapy is considered to be triggered by the synergistic effect of neuronal differentiation of transplanted cells and neuronal pattern generator activation.
Another approach for chronic SCI is pharmaceutical administration to hiPSC-NS/PCs before transplantation. We focused on gamma-secretase inhibitor (GSI), which is reported to promote neuronal differentiation of NS/PCs by inhibiting Notch signaling [58,59]. We administered GSI to hiPSC-NS/PCs and then transplanted them into the chronic injured spinal cords of mice [60]. As a result, the transplanted cells were engrafted in the spinal cord, the graft-derived axon extended beyond the glial scar, and locomotor function was significantly improved in the transplanted group. Moreover, some researchers have focused on chondroitinase ABC because this enzyme can degrade chondroitin sulfate proteoglycans, which are one component of glial scars and act as a barrier for regeneration. They combined intrathecal chondroitinase ABC and intraspinal cell transplantation of hiPSC-derived NS/PCs or human cell-derived oligodendrocyte precursor cells (OPCs), and they reported the long-term survival, migration, and integration of transplanted cells as well as behavioral recovery [61], [62], [63], [64].
As just described, the application of hiPSC-NS/PC transplantation for chronic SCI has a long way to go, but steady progress is being made. Further advancement is expected.
Other strategies for spinal cord regeneration using hiPSCs
Because of their multipotency, hiPSCs can be differentiated into various kinds of cells under well-coordinated culture conditions. Naturally, different kinds of cells other than NS/PCs have been considered for transplantation. One promising candidate is OPCs, considering that one of the main causes of functional impairment after SCI is demyelination. Oligodendrocytes are absolutely essential for remyelination, and indeed, mouse embryonic stem cell-derived OPC transplantation into the injured spinal cords of rats can myelinate demyelinated axons and improve locomotor function [65]. The transplantation of hiPSC-NS/PCs can theoretically replenish neurons, astrocytes, and oligodendrocytes, but a major proportion of transplanted cells differentiate into neurons, whereas the proportion that differentiate into oligodendrocytes is as low as three percent [36]. Therefore, culture conditions were optimized for hiPSC-OPCs [43,66], and these hiPSC-OPCs were transplanted into the subacute injured spinal cords of mice [43,45]. Forty percent of transplanted cells differentiated into oligodendrocytes, and the remyelination of host-damaged axons was confirmed by immunoelectron microscopy. Consequently, locomotor function was improved in the transplanted group.
As just described, neurons and oligodendrocytes are both promising cell targets for spinal cord regeneration. On the other hand, astrocytes, the other type of neural cell, have also been considered graft targets. Hayashi et al. differentiated mouse iPSCs into astrocytes and transplanted them into the injured spinal cords of rat [67]. In this case, locomotor function was not recovered, and neuropathic pain worsened after transplantation. This result indicates that astrocyte transplantation is apparently harmful to SCI [12]. In early studies, reactive astrocytes were characterized into the A1 neurotoxic phenotype and A2 neuroprotective phenotype [68,69], but binary characterization is currently insufficient to describe diverse astrocyte heterogeneity [70]. There remains much to be revealed regarding the role of reactive astrocytes after SCI, but if neuroprotective astrocytes can be distinguished and phenotype conversion can be controlled, astrocytes or astrocyte precursor cells might be prospective options for spinal cord regeneration.
As mentioned above, hiPSCs can differentiate into various cell types, and each cell type can induce different reactions after transplantation. Therefore, future strategies should be investigated to select optimal cell types depending on the pathological condition of SCI, for example, the transplantation of hiPSC-NS/PCs for SCI with severe damage in gray matter and/or hiPSC-OPCs for SCI with demyelination. Moreover, researchers have mainly focused on motor functional recovery after SCI, but future studies are also necessary to focus on pain or sensory function considering the fact that this treatment is starting to be applied in human patients, as sensory disturbance and neuropathic pain are also major complaints after SCI [71,72]. As research topics in spinal cord regeneration, astrocytes and microglia are less well investigated, and further studies are awaited.
Conclusions
We reviewed progress thus far and future perspectives in spinal cord regeneration using hiPSCs for the treatment of SCI. A clinical study has just begun on hiPSC-NS/PC transplantation for the treatment of subacute SCI, and the results are now being validated. At the same time, basic research is actively being conducted on the mechanism underlying the efficacy of this transplantation therapy, the development of combined therapy for chronic SCI, and the induction of other iPSC-derived cell types that are distinct from NS/PCs. Although many challenges remain to be solved, we and other researchers around the world have been working on these issues and making steady progress. A therapeutic approach for patients with impaired function due to not only SCI but also degenerative diseases such as spinal canal stenosis is expected in the near future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (B) (22H03205) to N.N..
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: MK: Nothing to disclose. NN: Nothing to disclose. HO: Nothing to disclose. MN: Nothing to disclose.
References
- 1.Okano H, Kaneko S, Okada S, Iwanami A, Nakamura M, Toyama Y. Regeneration-based therapies for spinal cord injuries. Neurochem Int. 2007;51:68–73. doi: 10.1016/j.neuint.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Tsuji O, Miura K, Okada Y, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci USA. 2010;107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okano H. Stem cell biology of the central nervous system. J Neurosci Res. 2002;69:698–707. doi: 10.1002/jnr.10343. [DOI] [PubMed] [Google Scholar]
- 4.Nagoshi N, Okano H. iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury. Cell Mol Life Sci. 2018;75:989–1000. doi: 10.1007/s00018-017-2676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnabe-Heider F, Frisen J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Inada T, Takahashi H, Yamazaki M, et al. Multicenter prospective nonrandomized controlled clinical trial to prove neurotherapeutic effects of granulocyte colony-stimulating factor for acute spinal cord injury: analyses of follow-up cases after at least 1 year. Spine (Phila Pa 1976) 2014;39:213–219. doi: 10.1097/BRS.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Yamazaki M, Okawa A, et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: a phase I/IIa clinical trial. Eur Spine J. 2012;21:2580–2587. doi: 10.1007/s00586-012-2213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koda M, Nishio Y, Kamada T, et al. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007;1149:223–231. doi: 10.1016/j.brainres.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura K, Fujiyoshi K, Yamane J, et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One. 2011;6:e27706. doi: 10.1371/journal.pone.0027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura K, Iwanami A, Nakamura M, et al. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J Neurosci Res. 2007;85:2332–2342. doi: 10.1002/jnr.21372. [DOI] [PubMed] [Google Scholar]
- 11.Ahuja CS, Mothe A, Khazaei M, et al. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9:1509–1530. doi: 10.1002/sctm.19-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res. 2018;126:39–43. doi: 10.1016/j.neures.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp Neurol. 2003;184:313–325. doi: 10.1016/s0014-4886(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 15.Ulndreaj A, Chio JC, Ahuja CS, Fehlings MG. Modulating the immune response in spinal cord injury. Expert Rev Neurother. 2016;16:1127–1129. doi: 10.1080/14737175.2016.1207532. [DOI] [PubMed] [Google Scholar]
- 16.Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 17.Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013;23:70–80. doi: 10.1038/cr.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 20.Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(Suppl 1):36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 21.Hurlbert RJ. Methylprednisolone for the treatment of acute spinal cord injury: point. Neurosurgery. 2014;61(Suppl 1):32–35. doi: 10.1227/NEU.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 22.Ahuja CS, Schroeder GD, Vaccaro AR, Fehlings MG. Spinal cord injury-what are the controversies? J Orthop Trauma. 2017;31(Suppl 4):S7–S13. doi: 10.1097/BOT.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 23.Frisen J, Johansson CB, Torok C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakakibara S, Imai T, Hamaguchi K, et al. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol. 1996;176:230–242. doi: 10.1006/dbio.1996.0130. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa Y, Sawamoto K, Miyata T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 26.Shibata R, Nagoshi N, Kajikawa K, et al. Administration of C5a receptor antagonist improves the efficacy of human induced pluripotent stem cell-derived neural stem/progenitor cell transplantation in the acute phase of spinal cord injury. J Neurotrauma. 2022;39:667–682. doi: 10.1089/neu.2021.0225. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura S, Yasuda A, Iwai H, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumagai G, Okada Y, Yamane J, et al. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS One. 2009;4:e7706. doi: 10.1371/journal.pone.0007706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald JW, Liu XZ, Qu Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 30.Iwanami A, Yamane J, Katoh H, et al. Establishment of graded spinal cord injury model in a nonhuman primate: the common marmoset. J Neurosci Res. 2005;80:172–181. doi: 10.1002/jnr.20435. [DOI] [PubMed] [Google Scholar]
- 31.Iwanami A, Kaneko S, Nakamura M, et al. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80:182–190. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 35.Okada Y, Matsumoto A, Shimazaki T, et al. Spatiotemporal recapitulation of central nervous system development by murine embryonic stem cell-derived neural stem/progenitor cells. Stem Cells. 2008;26:3086–3098. doi: 10.1634/stemcells.2008-0293. [DOI] [PubMed] [Google Scholar]
- 36.Nori S, Okada Y, Yasuda A, et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi Y, Okada Y, Itakura G, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugai K, Sumida M, Shofuda T, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen Ther. 2021;18:321–333. doi: 10.1016/j.reth.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuji O, Sugai K, Yamaguchi R, et al. Concise review: laying the groundwork for a first-in-human study of an induced pluripotent stem cell-based intervention for spinal cord injury. Stem Cells. 2019;37:6–13. doi: 10.1002/stem.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abematsu M, Tsujimura K, Yamano M, et al. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu P, Wang Y, Graham L, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamata Y, Isoda M, Sanosaka T, et al. A robust culture system to generate neural progenitors with gliogenic competence from clinically relevant induced pluripotent stem cells for treatment of spinal cord injury. Stem Cells Transl Med. 2021;10:398–413. doi: 10.1002/sctm.20-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawabata S, Takano M, Numasawa-Kuroiwa Y, et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2016;6:1–8. doi: 10.1016/j.stemcr.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuda A, Tsuji O, Shibata S, et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells. 2011;29:1983–1994. doi: 10.1002/stem.767. [DOI] [PubMed] [Google Scholar]
- 47.Kumamaru H, Ohkawa Y, Saiwai H, et al. Direct isolation and RNA-seq reveal environment-dependent properties of engrafted neural stem/progenitor cells. Nat Commun. 2012;3:1140. doi: 10.1038/ncomms2132. [DOI] [PubMed] [Google Scholar]
- 48.Shinozaki M, Nagoshi N, Nakamura M, Okano H. Mechanisms of stem cell therapy in spinal cord injuries. Cells. 2021;10 doi: 10.3390/cells10102676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth BL. DREADDs for neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichols CD, Roth BL. Engineered G-protein coupled receptors are powerful tools to investigate biological processes and behaviors. Front Mol Neurosci. 2009;2:16. doi: 10.3389/neuro.02.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okano H. Transplantation of neural progenitor cells into the human CNS. Trends Mol Med. 2022 doi: 10.1016/j.molmed.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Kitagawa T, Nagoshi N, Kamata Y, et al. Modulation by DREADD reveals the therapeutic effect of human iPSC-derived neuronal activity on functional recovery after spinal cord injury. Stem Cell Rep. 2022;17:127–142. doi: 10.1016/j.stemcr.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawai M, Imaizumi K, Ishikawa M, et al. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.110019. [DOI] [PubMed] [Google Scholar]
- 55.Nagoshi N, Okano H, Nakamura M. Regenerative therapy for spinal cord injury using iPSC technology. Inflamm Regen. 2020;40:40. doi: 10.1186/s41232-020-00149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumamaru H, Saiwai H, Kubota K, et al. Therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells. 2013;31:1535–1547. doi: 10.1002/stem.1404. [DOI] [PubMed] [Google Scholar]
- 57.Tashiro S, Nishimura S, Iwai H, et al. Functional recovery from neural stem/progenitor cell transplantation combined with treadmill training in mice with chronic spinal cord injury. Sci Rep. 2016;6:30898. doi: 10.1038/srep30898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okubo T, Iwanami A, Kohyama J, et al. Pretreatment with a gamma-secretase inhibitor prevents tumor-like overgrowth in human iPSC-derived transplants for spinal cord injury. Stem Cell Rep. 2016;7:649–663. doi: 10.1016/j.stemcr.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogura A, Morizane A, Nakajima Y, Miyamoto S, Takahashi J. gamma-secretase inhibitors prevent overgrowth of transplanted neural progenitors derived from human-induced pluripotent stem cells. Stem Cells Dev. 2013;22:374–382. doi: 10.1089/scd.2012.0198. [DOI] [PubMed] [Google Scholar]
- 60.Okubo T, Nagoshi N, Kohyama J, et al. Treatment with a gamma-secretase inhibitor promotes functional recovery in human iPSC- derived transplants for chronic spinal cord injury. Stem Cell Rep. 2018;11:1416–1432. doi: 10.1016/j.stemcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nori S, Khazaei M, Ahuja CS, et al. Human Oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Rep. 2018;11:1433–1448. doi: 10.1016/j.stemcr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuhrmann T, Anandakumaran PN, Payne SL, et al. Combined delivery of chondroitinase ABC and human induced pluripotent stem cell-derived neuroepithelial cells promote tissue repair in an animal model of spinal cord injury. Biomed Mater. 2018;13 doi: 10.1088/1748-605X/aa96dc. [DOI] [PubMed] [Google Scholar]
- 63.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki H, Ahuja CS, Salewski RP, et al. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brustle O, Jones KN, Learish RD, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 66.Numasawa-Kuroiwa Y, Okada Y, Shibata S, et al. Involvement of ER stress in dysmyelination of Pelizaeus-Merzbacher Disease with PLP1 missense mutations shown by iPSC-derived oligodendrocytes. Stem Cell Rep. 2014;2:648–661. doi: 10.1016/j.stemcr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayashi K, Hashimoto M, Koda M, et al. Increase of sensitivity to mechanical stimulus after transplantation of murine induced pluripotent stem cell-derived astrocytes in a rat spinal cord injury model. J Neurosurg Spine. 2011;15:582–593. doi: 10.3171/2011.7.SPINE10775. [DOI] [PubMed] [Google Scholar]
- 68.Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagoshi N, Kaneko S, Fujiyoshi K, et al. Characteristics of neuropathic pain and its relationship with quality of life in 72 patients with spinal cord injury. Spinal Cord. 2016;54:656–661. doi: 10.1038/sc.2015.210. [DOI] [PubMed] [Google Scholar]
- 72.Tashiro S, Nishimura S, Shinozaki M, et al. The Amelioration of pain-related behavior in mice with chronic spinal cord injury treated with neural stem/progenitor cell transplantation combined with treadmill training. J Neurotrauma. 2018;35:2561–2571. doi: 10.1089/neu.2017.5537. [DOI] [PubMed] [Google Scholar]