Abstract
The ESAT-6 antigen from Mycobacterium tuberculosis is a dominant target for cell-mediated immunity in the early phase of tuberculosis (TB) in TB patients as well as in various animal models. The purpose of our study was to evaluate the potential of ESAT-6 in an experimental TB vaccine. We started out using dimethyl dioctadecylammonium bromide (DDA), an adjuvant which has been demonstrated to be efficient for the induction of cellular immune responses and has been used successfully before as a delivery system for TB vaccines. Here we demonstrate that, whereas immune responses to both short-term-culture filtrate and Ag85B are efficiently induced with DDA, this adjuvant was inefficient for the induction of immune responses to ESAT-6. Therefore, we investigated the modulatory effect of monophosphoryl lipid A (MPL), an immunomodulator which in different combinations has demonstrated strong adjuvant activity for both cellular and humoral immune responses. We show in the present study that vaccination with ESAT-6 delivered in a combination of MPL and DDA elicited a strong ESAT-6-specific T-cell response and protective immunity comparable to that achieved with Mycobacterium bovis BCG.
The only available vaccine against tuberculosis is the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine. This vaccine generally induces high levels of protection in animal models of tuberculosis (TB). However, in humans its efficacy is highly variable, ranging from no protection to almost complete protection, depending on the population tested (14).
The hypothesis that culture filtrate antigens may play a role as targets of protective immune responses (2, 28) has been supported by a number of studies in the mouse and guinea pig models of TB infection (2, 30, 36). The mycobacterial antigen ESAT-6 can be isolated from a highly stimulatory low-molecular-mass fraction of short-term-culture filtrate (ST-CF), and this antigen is strongly recognized in TB patients (34, 41), in cattle infected with M. bovis (32), and in several strains of TB-infected mice (10). Because ESAT-6 is such a broadly and strongly recognized antigen in several species, we have previously suggested a role for this molecule in future vaccines against tuberculosis (3, 10), and recently this antigen has shown promise when delivered as a DNA vaccine (21, 22).
The purpose of our study was to evaluate the potential of ESAT-6 given as a subunit vaccine and to compare the outcome with those of vaccines based on preparations with already demonstrated protective efficacy, such as Ag85 (18, 19) and ST-CF (2). We chose the adjuvant dimethyl dioctadecylammonium bromide (DDA) for our initial studies because this adjuvant combines low toxicity with the induction of strong cell-mediated immunity (CMI) responses (16, 23). In addition, this adjuvant has previously been used successfully for TB vaccines based on culture filtrate antigens (2, 23) and more recently for vaccines against M. bovis (9).
In the present study we show that ESAT-6 has a relatively low inherent immunogenicity and requires a stronger adjuvant than DDA to prime a specific immune response. However, if monophosphoryl lipid A (MPL) is used as a coadjuvant with DDA, ESAT-6 primes a very potent immune response which efficiently controls infection at the same level as BCG vaccination. Our data therefore emphasize the importance of the choice of adjuvant for the screening of new antigen candidates for TB vaccines and demonstrate that ESAT-6 has major potential as a component in a future TB vaccine.
MATERIALS AND METHODS
Animals.
Studies were performed with 8- to 12-week-old C57BL/6 (C57BL/6J; H-2b) female mice, purchased from Bomholtegaard, Ry, Denmark.
Infected animals were housed in cages contained within a laminar flow safety enclosure.
Bacteria.
Mycobacterium tuberculosis H37Rv and Erdman were both grown at 37°C on Löwenstein-Jensen medium or in suspension in Sauton medium enriched with 0.5% sodium pyruvate and 0.5% glucose.
Immunization.
Mice were immunized three times at 2-week intervals subcutaneously on the back with experimental vaccines containing either 50 or 100 μg of ST-CF/dose, 10 μg of Ag85B/dose, or 10 to 50 μg of ESAT-6/dose emulsified in DDA (250 μg/dose; Eastman Kodak, Inc., Rochester, N.Y.) with or without 25 μg of MPL (Ribi Immunochem, Hamilton, Mont.) in a volume of 0.2 ml. MPL was mixed into sterile water containing 0.2% triethylamine. The mixture was heated in a 70°C water bath for 30 s and then sonicated for 30 s. The heating and sonicating procedure was repeated twice. The MPL was mixed with DDA immediately before use.
At the time of the first subunit vaccination, one group of mice received a single dose of BCG Danish 1331 (5 × 104 CFU) injected subcutaneously at the base of the tail. Mice were challenged 10 to 12 weeks after the first vaccination.
Experimental infections.
Mice were infected intravenously (i.v.) via the lateral tail vein with an inoculum of 5 × 104 CFU of M. tuberculosis H37Rv suspended in phosphate-buffered saline (PBS) in a volume of 0.1 ml. They were sacrificed after 2 weeks. When challenged by the aerosol route, the animals were infected with approximately 100 CFU of M. tuberculosis Erdman/mouse. These mice were sacrificed 6 weeks after challenge. Numbers of bacteria in the liver, spleen, or lung were determined by double serial threefold dilutions of individual whole-organ homogenates on 7H11 medium. Organs from the BCG-vaccinated animals were grown on medium supplemented with 2 μg of 2-thiophene-carboxylic acid hydrazide (TCH)/ml to selectively inhibit the growth of the residual BCG bacteria in the test organs. Colonies were counted after 2 to 3 weeks of incubation at 37°C. Protective efficacies are expressed as log10 reductions in bacterial counts in immunized mice compared with bacterial counts in the adjuvant controls. All results are based on groups of five animals.
Mycobacterial antigens.
ST-CF was produced as described previously (5). Briefly, M. tuberculosis bacteria (2 × 106 CFU/ml) were grown in modified Sauton medium without Tween 80 on an orbital shaker for 7 days. The culture supernatants were sterile filtered and concentrated on an Amicon (Danvers, Mass.) YM3 membrane.
Recombinant ESAT-6 was produced as described previously (17). The lipopolysaccharide (LPS) content of this preparation was measured by a Limulus amoebocyte lysate test and shown to be below 0.3 ng/μg of protein; this concentration had no influence on cellular activity. The Ag85B (MPT 59) preparation used was kindly provided by S. Nagai (Toyonaka, Osaka, Japan). All antigen preparations were kept at −80°C until use.
Lymphocyte cultures.
Lymphocytes from spleens were obtained as described previously (6). Blood lymphocytes were purified on a density gradient. Cells pooled from three to five mice in each experiment were cultured in microtiter wells (96-well plates; Nunc, Roskilde, Denmark) containing 2 × 105 cells in a volume of 200 μl of RPMI 1640 supplemented with 5 × 10−5 M 2-mercaptoethanol, 1% penicillin-streptomycin, 1 mM glutamine, and 5% (vol/vol) fetal calf serum. In some cultures, the T-cell coreceptor CD4 or CD8 was blocked by adding monoclonal antibody GK1.5 (anti-CD4) or 2.43 (anti-CD8) directly into the cultures in various dilutions at the onset of the culture period (both antibodies were kindly provided by R. Appelberg, University of Porto, Porto, Portugal). Based on previous dose-response investigations, the mycobacterial antigens were all used at 5 μg/ml. Concanavalin A at a concentration of 1 μg/ml was used in all experiments as a positive control for cell viability. All preparations were tested in cell cultures and found to be nontoxic at the concentrations used in the present study. Supernatants were harvested from cultures after 24 and 48 h of incubation for the investigation of interleukin 5 (IL-5) and after 72 h of incubation for the investigation of gamma interferon (IFN-γ).
IFN-γ enzyme-linked immunosorbent assay (ELISA).
Microtiter plates (96 wells; Maxisorb; Nunc) were coated with monoclonal hamster anti-murine IFN-γ (Genzyme, Cambridge, Mass.) in PBS at 4°C. Free binding sites were blocked with 1% (wt/vol) bovine serum albumin–0.05% Tween 20. Culture supernatants were tested in triplicate, and IFN-γ was detected with a biotin-labelled rat anti-murine monoclonal antibody (clone XMG1.2; Pharmingen, San Diego, Calif.). Recombinant IFN-γ (Pharmingen) was used as a standard.
IL-5 titers were determined by a mouse IL-5 ELISA (Endogen, Cambridge, Mass.).
ELISPOT technique.
The enzyme-linked immunospot (ELISPOT) technique has been described before (10). Briefly, 96-well microtiter plates (Maxisorb) were coated with 2.5 μg of monoclonal hamster anti-murine IFN-γ (Genzyme)/well. Free binding sites were blocked with bovine serum albumin followed by washing with PBS–0.05% Tween 20. Analyses were always conducted on cells pooled from three to five mice. Cells were stimulated with 5 μg of ESAT-6/ml in modified RPMI 1640 for 18 to 22 h and were subsequently cultured without antigen for 7 h directly in the ELISPOT plates. The cells were removed by washing, and the site of cytokine secretion was detected with a biotin-labelled rat anti-murine IFN-γ monoclonal antibody (clone XMG1.2; Pharmingen) and phosphatase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). The enzyme reaction was developed with 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Sigma Chemical Co., St. Louis, Mo.). Blue spots were counted microscopically. The correlation between the number of cells per well and the number of spots was linear at concentrations of 2 × 105 to 2.5 × 103 cells/well. Wells with fewer than 10 spots were not used for calculations.
ESAT-6-specific IgG ELISA.
ELISA plates (Maxisorb, type 96F; Nunc) were coated with ESAT-6 (0.1 μg/well) overnight at 4°C. Free binding sites were blocked by 1% bovine serum albumin–PBS. Individual serum samples from five mice per group were analyzed in threefold dilutions. Horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG) (P260; DAKO) and horseradish peroxidase-conjugated rabbit anti-mouse IgG2b (Harlan, Sera-Lab Limited, Blackthorn, England) were diluted 1/1,000 and 1/5,000, respectively. Antibody titers are expressed as reciprocal end point titers.
Statistical methods.
The efficacies of different vaccination protocols were compared by one-way analysis of variance of the log10 CFU. A P value of <0.05 was considered significant.
RESULTS
Comparative evaluation of experimental vaccines based on ST-CF, Ag85B, and ESAT-6.
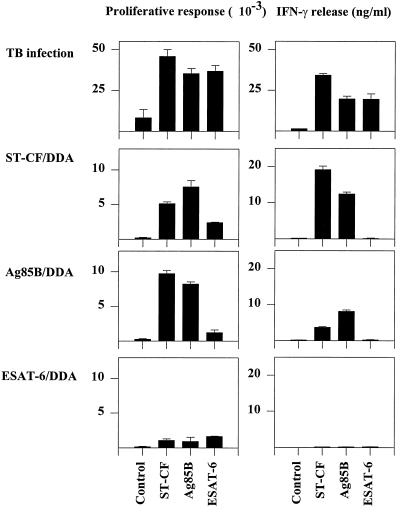
An experimental vaccine based on ESAT-6 was investigated in parallel with vaccines containing the well-known protective antigen preparations Ag85 and ST-CF, both with demonstrated efficacy against TB infection in animal models (2, 18, 19). The vaccines based on these molecules were all emulsified in DDA, an adjuvant which has previously been used successfully to induce a highly efficient Th1 response protective against TB (16, 23). All vaccines were given three times at 2-week intervals, and the immune responses induced in the regional lymph nodes were investigated 3 weeks after the last booster injection. All the antigens have previously been reported to be strong targets for the immune response during TB infection (4, 10). In the present study, we therefore included mice at day 14 of a primary infection as a positive control (Fig. 1). In confirmation of earlier reports, infected mice recognized ST-CF (∼34 ng of IFN-γ/ml), as well as recognizing the purified antigens at a very high level (∼20 ng of IFN-γ/ml). The immune responses induced by the vaccines, in contrast, differed markedly. ST-CF with DDA as an adjuvant promoted a very strong immune response to the homologous preparation (∼19 ng/ml), and the prominent culture filtrate antigen Ag85B was also recognized strongly in animals given this vaccine. Ag85 has previously been demonstrated to be strongly recognized after experimental vaccination (18, 19, 25). Mixed with DDA, this antigen also primed a strong recall response (∼7 ng/ml), and in agreement with the presence of this antigen in culture filtrate, immunization with this molecule primed cross-recognition of ST-CF. ESAT-6, in contrast, did not prime any detectable responses to either the homologous preparation or ST-CF when used for immunization together with DDA.
FIG. 1.
Evaluation of immune responses induced by TB subunit vaccines. Proliferative responses and IFN-γ release were measured 14 days postinfection (spleen cell cultures) or 3 weeks after vaccination (lymph node cell cultures) with either ST-CF–DDA, Ag85B–DDA, or ESAT-6–DDA. Cells are pooled from three to five mice per group. Each bar represents the mean of triplicate values. Error bars, standard errors of the means. This experiment was performed twice with similar results.
The protective efficacies of the immunizations were compared to the protection elicited by a standard BCG vaccine. The mice were left alone for 6 to 8 weeks after the last subunit booster and then received either an i.v. or an aerosol infection with live M. tuberculosis. Bacterial loads in livers and lungs were determined and are given in Table 1 together with the protective efficacies of the vaccines expressed as log10 reductions in CFU compared to those in the control mice. Vaccinations with either BCG, ST-CF–DDA, or Ag85B–DDA all produced high levels of resistance (0.75 log10 to 1.10 log10 reductions in CFU compared to those in control mice). In agreement with the almost complete lack of immune response induced by the ESAT-6 vaccine, no major significant reduction in the bacterial load was detected after vaccination with this molecule (Table 1).
TABLE 1.
Protective efficacies of vaccines emulsified in DDA
| Vaccine groupc | Expt 1a
|
Expt 2b
|
||||
|---|---|---|---|---|---|---|
| Log10 CFUd | Log10 resistancee | Pf | Log10 CFU | Log10 resistance | P | |
| Naive | 5.06 ± 0.04 | 5.74 ± 0.12 | ||||
| DDA | 5.04 ± 0.05 | <0.05 | 5.75 ± 0.08 | <0.05 | ||
| BCG | 4.33 ± 0.06 | 0.73 | 0.008 | 4.76 ± 0.18 | 0.98 | 0.008 |
| ST-CF–DDA | 3.94 ± 0.08 | 1.10 | 0.008 | 4.94 ± 0.11 | 0.81 | 0.006 |
| Ag85B–DDA | 4.29 ± 0.05 | 0.75 | 0.007 | NDg | ND | |
| ESAT-6–DDA | 4.93 ± 0.01 | 0.11 | 5.50 ± 0.02 | 0.25 | 0.009 | |
Mice received an i.v. challenge, and bacterial loads in the liver were determined.
Mice received an aerosol TB infection, and bacterial loads in the lungs were determined.
Mice were vaccinated once with BCG or three times at 2-week intervals with the subunit vaccines.
Geometric means ± standard error of the means. Data are means based on duplicate analysis of five animals in each group.
Protective efficacies of vaccines, expressed as log10 reductions in bacterial loads compared to those in the adjuvant controls.
P values are given for bacterial loads significantly different from those in control mice.
ND, not done.
An optimized DDA-MPL adjuvant formulation efficiently promotes immune responses to ESAT-6.
MPL has strong adjuvant properties and, like DDA, skews responses in a Th1 direction (11). MPL can be used on its own, but due to its hydrophobic nature it is mostly used together with a delivery vehicle such as an oil-in-water emulsion of the oil squalene (MPL-SE) (manufacturer's insert, Ribi ImmunoChemicals Research) or the surface-active agent QS21 (39). For TB vaccines, a stable emulsion of MPL has previously been used together with ST-CF and was found to have an efficacy level similar to that of DDA (12). Because DDA on its own proved inefficient for the induction of an immune response to ESAT-6, an antigen of low inherent immunogenicity, we continued by testing the potential of a combination of MPL and DDA. MPL was dissolved in sterile water and mixed with DDA as described in Materials and Methods.
Mice were immunized three times, and 1 week after the last vaccination the ESAT-6-specific immune response of blood cells was investigated (Table 2). No IFN-γ production could be detected in mice which received DDA alone or the combination of DDA and MPL (data not shown). A very low frequency (<1:105 in experiment 1 and 1:14,800 in experiment 2) of ESAT-6-specific IFN-γ-producing T cells could be detected by the sensitive ELISPOT technique after ESAT-6–DDA vaccination. In contrast, ESAT-6 emulsified in DDA plus MPL stimulated a very high frequency (∼1:500) of IFN-γ-secreting cells in both experiments, whereas no recognition of ESAT-6 was detected after vaccination with ESAT-6 plus MPL. The T-cell subset primed by this vaccination was CD4 cells, as evidenced by the fact that the response was completely blocked by anti-CD4 antibodies whereas anti-CD8 antibodies had no influence on the level of T-cell reactivity. No IL-5 was detected in any of the supernatants tested (data not shown).
TABLE 2.
ESAT-6-specific immune responses in mice vaccinated with different ESAT-b subunits vaccines
| Vaccinec | IFN-γ responsea
|
Humoral responseb
|
||
|---|---|---|---|---|
| Concn (ng/ml) | Frequency by ELISPOT | IgG | IgG2b | |
| Expt 1 | ||||
| ESAT-6 + DDA | <0.05 | <1:105 | ND | ND |
| ESAT-6 + MPL | <0.05 | <1:105 | ND | ND |
| ESAT-6 + DDA/MPL | 7.13 ± 0.2 | 1:690 | ND | ND |
| Expt 2 | ||||
| ESAT-6 + DDA | <0.05 | 1:14,800 | 32,768 | 128 |
| ESAT-6 + DDA/MPL | 12.24 ± 0.5 | 1:470 | 262,144 | 4,096 |
One week after the last booster injection, the IFN-γ responses were measured in peripheral blood mononuclear cell cultures pooled from five animals in each group. ELISPOT results are means of duplicate values; the difference between duplicate cultures was always <12% of the mean. No IFN-γ responses were detectable in any of the adjuvant controls.
Monitored 4 weeks after the last vaccination. Sera from five mice were pooled, and the antibodies were determined by duplicate ELISA analysis. Data are reciprocal end point titers. ND, not done.
Mice were vaccinated three times at 2-week intervals with the subunit vaccines.
In experiment 2 we also monitored the development of an ESAT-6-specific antibody response in mice 4 weeks after the last vaccination (Table 2). High titers of ESAT-6-specific IgG were present in sera from mice vaccinated with ESAT-6–DDA–MPL, and compared with titers found in sera after ESAT-6–DDA vaccination, the optimized adjuvant mixture induced 8 times more specific antibody. In C57BL/6 mice the gene coding for IgG2a is deleted (26). Therefore, in the absence of a functional IgG2a gene, we used the IgG2b isotype as an indicator of a Th1 response. Compared with total IgG, an even more pronounced increase in the IgG2b subclass titer was observed. The use of the DDA-MPL adjuvant formulation, compared to DDA alone as an adjuvant, resulted in a 32-fold increase in the titer of this antibody subclass, indicating that the overall immune response had been skewed in a Th1 direction.
Protective efficacies of vaccines with DDA–MPL as an adjuvant.
We continued by analyzing if the amplified immune response obtained with DDA-MPL would be reflected in increased protective efficacies of the vaccines. A series of experiments with different antigen-adjuvant combinations was conducted (Table 3). In the first experiment mice were immunized with ST-CF emulsified in MPL, DDA, or the combination. MPL used on its own together with ST-CF did not stimulate significant levels of protective immunity to a subsequent TB challenge, whereas ST-CF with DDA as an adjuvant gave substantial levels of protection, similar to the protection observed in the first experiment (Table 1). The addition of MPL to the DDA adjuvant resulted in higher levels of protective immunity (log10 reductions in bacterial load, 0.93 versus 0.69 in the spleen and 1.30 versus 1.09 in the liver). The difference was statistically significant only in the liver (Table 3). In the next two experiments the efficacy of ESAT-6 in different adjuvant combinations was evaluated. In these experiments mice were challenged with M. tuberculosis Erdman by the aerosol route, and 6 weeks postinfection, spleens and lungs were harvested and bacteria were enumerated. As shown in Table 3, experiment 2, neither ESAT-6 emulsified in MPL alone nor ESAT-6 emulsified in DDA alone promoted any significant protection. ESAT-6 in combination with DDA-MPL induced high levels of immunity which protected the lung at the same level as BCG (P = 0.60). In the spleen BCG still gave significantly higher levels of protection than the subunit vaccine (P = 0.017). The next experiment confirmed these findings and demonstrated that ESAT-6 with the DDA-MPL combination can induce protective efficacies up to the same level as BCG.
TABLE 3.
Protective efficacies of different ST-CF and ESAT-6 subunit vaccines
| Vaccinea | Log10 resistanceb
|
||
|---|---|---|---|
| Spleen | Liver | Lung | |
| Expt 1 | |||
| ST-CF + MPL | 0.10 (±0.08) | 0.07 (±0.10) | |
| ST-CF + DDA | 0.69 (±0.10)* | 1.09 (±0.04)* | |
| ST-CF + DDA–MPL | 0.93 (±0.11)* | 1.30 (±0.11)* | |
| BCG | 1.29 (±0.09)* | 1.33 (±0.10)* | |
| Expt 2 | |||
| MPL | <0.05 | <0.05 | |
| DDA | <0.05 | <0.05 | |
| DDA + MPL | <0.05 | 0.10 (±0.11) | |
| ESAT-6 + MPL | <0.05 | 0.21 (±0.12) | |
| ESAT-6 + DDA | 0.16 (±0.15) | 0.14 (±0.10) | |
| ESAT-6 + DDA–MPL | 0.67 (±0.17)* | 0.85 (±0.13)* | |
| BCG | 1.25 (±0.19)* | 0.70 (±0.15)* | |
| Expt 3 | |||
| DDA + MPL | <0.05 | <0.05 | |
| ESAT-6 + DDA–MPL | 0.89 (±0.32)* | 0.50 (±0.09)* | |
| BCG | 0.94 (±0.24)* | 0.46 (±0.09)* | |
Mice were vaccinated once with BCG or three times at 2-week intervals with the subunit vaccines.
Expressed as log10 reductions in the bacterial loads measured in the spleen and lung 6 weeks after aerosol challenge (experiments 2 and 3) or in the spleen and liver 2 weeks after i.v. (experiment 1). TB infection and compared to those in the adjuvant controls. Data are means based on duplicate analysis of five animals in each group. Asterisks, bacterial loads significantly different from those in control mice.
DISCUSSION
The data presented herein demonstrate for the first time that protective immunity at the same level as BCG can be obtained by subunit vaccination with a single purified antigen from M. tuberculosis. The TB vaccine currently in use is BCG, a live attenuated strain of M. bovis. As with all the classical whole-cell killed or attenuated vaccines, BCG presents the immune system with a very complex protein repertoire. The complexity of these vaccines provides both advantages and drawbacks from an immunization point of view. When multiple antigens are presented for the immune system, they will compete for presentation, and the antigens dominating the response are not necessarily those most relevant for protection (29) or continuously expressed by the pathogen (15). In addition, there is always concern that such complex mixtures may hold immunosuppressive elements or molecules modulating the immune system in an undesired direction (20). On the other hand, the numerous epitopes represented ensure broad coverage when a genetically heterogeneous population is vaccinated. In addition, and of particular relevance for the present study, whole cells or complex mixtures are often very immunogenic and contain their own built-in immunostimulating molecules embedded in the outer cell wall (35).
In subunit vaccines the problems are reversed. The antigen components can be a few narrowly selected molecules relevant for protection and consistently expressed by the pathogen. However, selecting a few desired antigens and excluding the rest of the complex protein repertoire has some obvious implications: (i) as a consequence of their simplified defined structure, protein subunits often lack immunogenicity and therefore require a powerful immunostimulant or adjuvant to elicit appropriate immunity; (ii) the number of potential T-cell epitopes is limited. Therefore, the components need to be carefully selected to ensure that a heterogeneous population representing a broad spectrum of major histocompatibility complex molecules responds to the vaccine.
ESAT-6 selected for the present study fulfills one of these criteria, as this antigen contains numerous epitopes (34) recognized by a very high percentage of individuals (up to >90%, depending on the sensitivity of the assay used) (31, 34). However, it is also clear from the present study that highly purified recombinant ESAT-6 has a very low inherent immunogenicity and therefore requires an adjuvant that is more effective than DDA. This problem was not found with either ST-CF or Ag85B, to which strong and sustained immune responses were obtained after vaccination with the respective vaccines given with DDA as an adjuvant. That an immune response to ST-CF was easily elicited is probably not surprising, because this mixture, in addition to truly extracellular proteins, contains components shed from the outer cell wall (5), which in mycobacteria is recognized for its many inflammatory components, such as muramyl dipeptide and trehalose dimycolate (27). In fact, these products have actually been suggested as adjuvant components for many modern vaccines (13). What is more surprising, however, and difficult to explain is the difference in immunogenicity between ESAT-6 and Ag85B. Both molecules are strongly recognized during the natural infection, but when they were administered as vaccines with DDA as an adjuvant, Ag85B elicited a strong response whereas almost no priming of ESAT-6-specific immune responses was observed. This observation is in agreement with the finding that antibodies against the Ag85 complex are a dominant part of the humoral response found after immunization with culture filtrate antigens, a fact that the numerous monoclonal antibodies against this molecule generated in different laboratories also clearly demonstrate (24, 42).
In an attempt to stimulate an ESAT-6-specific T-cell response, we therefore explored the possibility of combining DDA with the well-described immunostimulant MPL. This nontoxic derivative of LPS has retained its stimulatory ability and is a promising new adjuvant for human vaccines (40). In the present study we amplified responses to ESAT-6 by mixing DDA with MPL and demonstrated that this combined adjuvant formulation can elicit strong immune reactions even to molecules with low “built-in” immunogenicity like ESAT-6. MPL stimulates macrophages to release cytokines and enhances antigen uptake, processing, and presentation (37), but in our study this component had only low efficacy on its own and needed to be combined with DDA and possibly incorporated into the DDA micelles. The absence of protection by immunization with culture filtrate proteins in MPL alone has also been seen by others (8). Of relevance in this context, MPL is very hydrophobic and has in fact worked most efficiently together with lipid-containing delivery vehicles such as squalene-in-water emulsions (13), liposomes (1), or surface-active adjuvants like QS21 (39).
The recent mapping and complete sequencing of the TB genome has paved the road for intensified discovery of novel antigens in the years to come. Our study demonstrates that with the future need for extensive comparative evaluation of these new antigens as experimental vaccines, the choice of adjuvant becomes a crucial parameter. The combination of DDA and MPL may be one such future adjuvant formulation which would allow the evaluation even of molecules with low inherent immunogenicity. Since both of these components have been tested successfully in human clinical trials (7, 38), the combination should also be considered for human use, e.g., delivery of a TB vaccine in the future. Recently ESAT-6 has been shown to have major potential as a diagnostic tool to differentiate between TB infection and BCG vaccination (33, 34). The present documentation of its high activity in vaccines demonstrates the many partially overlapping applications of strong T-cell antigens like ESAT-6 in the future control and prevention of TB.
ACKNOWLEDGMENTS
This study has been supported by the Faculty of Health Science, University of Copenhagen; the Danish National Association against Lung Diseases; and the European Commission DRXII, contract TS3*CT94-0313.
REFERENCES
- 1.Alving C R. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–446. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–131. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Askgaard D, Gottschau A, Bennedsen J, Nagai S, Heron I. Identification of immunodominant antigens during infection with Mycobacterium tuberculosis. Scand J Immunol. 1992;36:823–831. doi: 10.1111/j.1365-3083.1992.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen P, Askgaard D, Ljungqvist L, Bentzon M W, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991;59:1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astiz M E, Rackow E C, Still J G, Howell S T, Cato A, Von-Eschen K B, Ulrich J T, Rudbach J A, McMahon G, Vargas R, et al. Pretreatment of normal humans with monophosphoryl lipid A induces tolerance to endotoxin: a prospective, double-blind, randomized, controlled trial. Crit Care Med. 1995;23:9–17. doi: 10.1097/00003246-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin S L, D'Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D N, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosio C M, Orme I M. Effective, nonsensitizing vaccination with culture filtrate proteins against virulent Mycobacterium bovis infections in mice. Infect Immun. 1998;66:5048–5051. doi: 10.1128/iai.66.10.5048-5051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandt L, Oettinger T, Holm A, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 11.Carozzi S, Salit M, Cantaluppi A, Nasini M G, Barocci S, Cantarella S, Lamperi S. Effect of monophosphoryl lipid A on the in vitro function of peritoneal leukocytes from uremic patients on continuous ambulatory peritoneal dialysis. J Clin Microbiol. 1989;27:1748–1753. doi: 10.1128/jcm.27.8.1748-1753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhay M J, Andersen P. Immunological requirements for a subunit vaccine against tuberculosis. Immunol Cell Biol. 1997;75:595–603. doi: 10.1038/icb.1997.94. [DOI] [PubMed] [Google Scholar]
- 13.Ewasyshyn M, Caplan B, Bonneau A M, Scollard N, Graham S, Usman S, Klein M. Comparative analysis of the immunostimulatory properties of different adjuvants on the immunogenicity of a prototype parainfluenza virus type 3 subunit vaccine. Vaccine. 1992;10:412–420. doi: 10.1016/0264-410x(92)90072-r. [DOI] [PubMed] [Google Scholar]
- 14.Fine P E. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 15.Good M F, Zevering Y, Currier J, Bilsborough J. ‘Original antigenic sin’, T cell memory, and malaria sporozoite immunity: an hypothesis for immune evasion. Parasite Immunol. 1993;15:187–193. doi: 10.1111/j.1365-3024.1993.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 16.Grun J L, Maurer P H. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 17.Harboe M, Wiker H G, Ulvund G, Malin A S, Dockrell H, Holm A, Jørgensen M C, Andersen P. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect Immun. 1998;66:717–723. doi: 10.1128/iai.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van-Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs D M. Immunomodulatory effects of bacterial lipopolysaccharide. J Immunopharmacol. 1981;3:119–132. doi: 10.3109/08923978109026423. [DOI] [PubMed] [Google Scholar]
- 21.Kamath A T, Feng C G, Macdonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Howard A, Kelley C, Delogu G, Collins F, Morris S. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect Immun. 1999;67:4780–4786. doi: 10.1128/iai.67.9.4780-4786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindblad E B, Elhay M J, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune response to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–629. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljungqvist L, Worsaae A, Heron I. Antibody responses against Mycobacterium tuberculosis in 11 strains of inbred mice: novel monoclonal antibody specificities generated by fusions, using spleens from BALB.B10 and CBA/J mice. Infect Immun. 1988;56:1994–1998. doi: 10.1128/iai.56.8.1994-1998.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozes E, Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Vandenbussche P, Van-Vooren J P, Drowart A, Ulmer J B, Liu M A. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997;15:830–833. doi: 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 26.Martin R M, Lew A M. Is IgG2a a good Th1 marker in mice? Immunol Today. 1998;19:49. doi: 10.1016/s0167-5699(97)87499-3. [DOI] [PubMed] [Google Scholar]
- 27.Masihi K N, Lange W, Brehmer W, Ribi E. Immunobiological activities of nontoxic lipid A: enhancement of nonspecific resistance in combination with trehalose dimycolate against viral infection and adjuvant effects. Int J Immunopharmacol. 1986;8:339–345. doi: 10.1016/0192-0561(86)90116-5. [DOI] [PubMed] [Google Scholar]
- 28.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 29.Oukka M, Manuguerra J C, Livaditis N, Tourdot S, Riche N, Vergnon I, Cordopatis P, Kosmatopoulos K. Protection against lethal viral infection by vaccination with nonimmunodominant peptides. J Immunol. 1996;157:3039–3045. [PubMed] [Google Scholar]
- 30.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathan A, Brookes R, Pritchard H, Wilkinson R, Pasvol G, Hill A, Lalvani A. Human T cell responses to the antigen ESAT-6 characterize a vaccine candidate and potential diagnostic test for tuberculosis. Immunology. 1998;95:90. [Google Scholar]
- 32.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock J M, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis. 1997;175:1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 34.Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy H, Mustafa A S, Jensen A K, Holm A, Rosenkrands I, Oftung F, Olobo J, von-Reyn C F, Andersen P. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 35.Ribi E, Meyer T J, Azuma I, Parker R, Brehmer W. Biologically active components from mycobacterial cell walls. IV. Protection of mice against aerosol infection with virulent Mycobacterium tuberculosis. Cell Immunol. 1975;16:1–10. doi: 10.1016/0008-8749(75)90180-x. [DOI] [PubMed] [Google Scholar]
- 36.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 37.Rudbach J A, Johnson D A, Ulrich J T. Ribi adjuvants: chemistry, biology and utility in vaccines for human and veterinary medicine. In: Stewart-Tull D E S, editor. The theory and practical application of adjuvants. New York, N.Y: John Wiley & Sons Ltd.; 1995. pp. 287–313. [Google Scholar]
- 38.Stanfield J P, Gall D, Bracken P M. Single-dose antenatal tetanus immunisation. Lancet. 1973;i:215–219. doi: 10.1016/s0140-6736(73)90062-7. [DOI] [PubMed] [Google Scholar]
- 39.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M, Ballou W R, Cohen J D. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 40.Ulrich J T, Myers K R. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 41.Ulrichs T, Munk M E, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro M L, Kaufmann S H. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–3958. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Worsaae A, Ljungqvist L, Heron I. Monoclonal antibodies produced in BALB.B10 mice define new antigenic determinants in culture filtrate preparations of Mycobacterium tuberculosis. J Clin Microbiol. 1988;26:2608–2614. doi: 10.1128/jcm.26.12.2608-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]