Abstract
We report a case of a 56-year-old man diagnosed with non-ischemic cardiomyopathy in 2017 with progressive decline in left ventricular systolic function and frequent hospitalisations for heart failure in the context of a presumed atrial fibrillation with high ventricular rate. Electrocardiographic changes after adenosine administration raised the suspicion of dual atrioventricular nodal non-reentrant tachycardia, a diagnosis that was confirmed by electrophysiological study, making adenosine a potential diagnostic aid in such cases. The ablation of the slow pathway terminated the tachycardia and led to a marked improvement in symptomatology and echocardiographic parameters.
Keywords:supraventricular tachycardia, dual atrioventricular nodal non-reentrant tachycardia, adenosine, tachycardia-induced cardiomyopathy, radiofrequency ablation.
INTRODUCTION
Dual atrioventricular nodal non-reentrant tachycardia (DAVNNT) is a rare and often misdiagnosed node-dependent arrhythmia in which one atrial impulse generates two ventricular complexes via concomitant fast and slow pathway conduction (1). Incessant cases invariably lead to tachycardia-induced cardiomyopathy, which is rapidly reversible after ablation of the slow pathway (2, 3). We present such a case, describing the particular use of adenosine as a complementary tool in the diagnosis of DAVNNT.
CASE PRESENTATION
A 56-year-old Caucasian male, former professional athlete, was referred to our clinic for worsening signs and symptoms of biventricular heart failure in the context of presumed atrial fibrillation with high ventricular rate.
The patient was diagnosed with non-ischemic cardiomyopathy (NICM) with moderately reduced ejection fraction (EF 45%) in 2017, when heart failure pharmacological treatment with betablockers (nebivolol 5 mg/day) and angiotensin converting enzyme inhibitor (ACEI; perindopril 5 mg/day) was initiated.
The patient did not seek medical attention until late 2019, when he started complaining of frequent episodes of palpitations. Holter electrocardiography monitoring for 24 hours detected episodes of ventricular bigeminy, frequent premature supraventricular complexes and paroxysmal atrial fibrillation, leading to initiation of anticoagulant (dabigatran 150 mg twice a day) and antiarrhythmic treatment (amiodarone 200 mg once a day), discontinued after few months due to poor tolerance and development of amiodarone- induced hypothyroidism. Cardiac magnetic resonance imaging (MRI) depicted an enlarged left ventricle [indexed left ventricular end-diastolic volume LVEDVi 168 mL/m²] with a severely reduced ejection fraction (EF 22%), moderate mitral and tricuspid regurgitation and the presence of late gadolinium enhancement with septal mid-wall striae pattern.
Over time, despite an adequate medical treatment in maximal tolerated doses for heart failure, including a betablocker (bisoprolol 10 mg/day), angiotensin receptor-neprilysin inhibitor (sacubitril/ valsartan 26/24 mg twice a day), mineralocorticoid receptor antagonist (eplerenone 50 mg/day), digoxin (0,25 mg/day), and loop diuretics (furosemide in adjusted dosages for congestion), the patient showed a gradual enlargement of the left ventricle and a declining systolic function with frequent ho- spitalizations for heart failure decompensations. In 2020 he was evaluated and included on the waiting list for heart transplantation.
In April 2021, the patient was referred to our clinic for worsening exertional dyspnea and moderate pitting edema with a diagnosis of persistent atrial fibrillation with high ventricular rate and presumed tachycardia-induced cardiomyopathy.
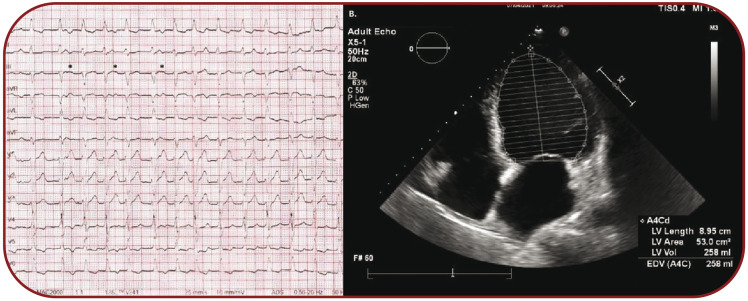
The electrocardiography (ECG) revealed a regularly irregular fast supraventricular tachycardia with intermittent visible P waves with a constant, yet unclear relationship with wide ventricular complexes with left bundle branch block (LBBB) morphology (Figure 1A).
Echocardiography showed an enlarged left ventricle (LVEDVi 136 mL/m², indexed left ventricular end-systolic volume LVESVi 111mL/m²), with an EF of 18.3%, moderate mitral and tricuspid regurgitation, with elevated pulmonary artery pressures (Figure 1B).
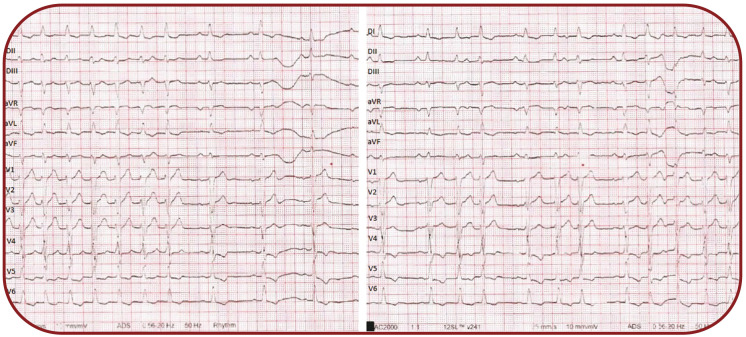
In order to reveal and better identify the underlying atrial activity on ECG, we decided to administer 20 mg of adenosine followed by a rapid saline flush and an ECG was recorded immediately afterwards (Figure 2).
A sinus rhythm with 1:1 conduction was noted, followed by a distinctive ECG pattern: each P wave was followed alternatively by two or one QRS complexes, suggestive of a dual nodal pathway conduction with 2:1 block over the slow pathway and subsequently each P wave was followed constantly by two QRS complexes with identical morphology.
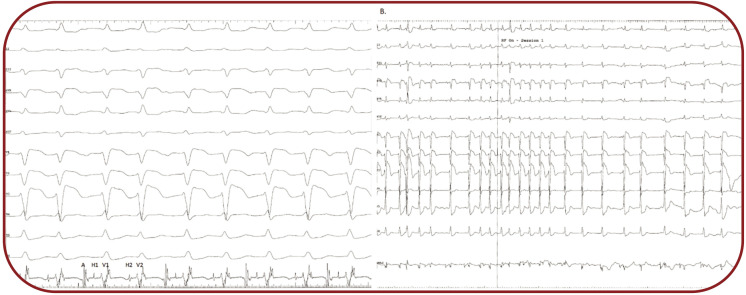
We performed an electrophysiological study which confirmed dual AV node conduction (each P wave was conducted through both fast and slow pathway), resulting in a A-H1V1-H2V2 pattern, with an AH1 interval of 140 ms, an AH2 interval of 570 ms and a HV interval of 65 ms. (Figure 3A). After temperature-controlled radiofrequency ablation of the slow pathway (30-40 W, 55¢ªC), sinus rhythm resumed, with constant 1:1 conduction through the fast pathway (Figure 3B) with a shortening of the AH interval to 125 ms and with a Wenckebach point of the fast pathway of 490 ms. Programmed atrial stimulation with extrastimuli demonstrated the disappearance of the slow pathway, with an effective refractory period of the fast pathway of 290 ms. Ventricular stimulation after ablation showed normal retrograde decremental conduction through the AV node.
There were no periprocedural complications, the patient was discharged the next day, on medical treatment with betablockers (bisoprolol 5 mg once daily), angiotensin receptor/neprilysin inhibitor (sacubitril/valsartan 24/26 mg twice daily), mineralocorticoid receptor antagonist (eplerenone 50 mg once daily), loop diuretics (furosemide 40 mg once daily) and oral anticoagulation with apixaban (5 mg twice daily) because paroxysmal atrial fibrillation could not be excluded.
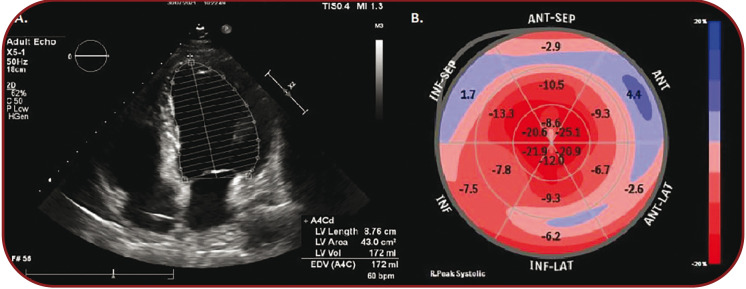
At the three-month follow-up, the patient was asymptomatic, in New York Heart Association (NYHA) class I. The ECG showed normal sinus rhythm with 62 bpm. The control echocardiography showed an EF of 45.7%, global longitudinal strain (GLS) of -10.0%, with a significant decrease in left ventricular volumes (LVEDVi 92 mL/m², LVESVi 49 mL/m²) (Figure 4 A and B).
DISCUSSION
Dual atrioventricular nodal non-reentrant tachycardia is a supraventricular tachycardia, with less than 100 cases described worldwide, mostly as clinical cases or reports of small series (4). It usually goes undiagnosed for a mean period of over one year (2-31 months), being misinterpreted as atrial fibrillation, supraventricular bigeminy, frequent premature complexes, etc, leading to inappropriate and ineffective treatment (1, 4, 5). The age of patients with this arrhythmia ranges between 16 and 84 years, with a five year-old boy being the youngest reported patient (4).
Regardless of whether the patients have other structural heart disease or not, there is no evident relationship between DAVNNT and other cardiac pathologies, apart from atrioventricular nodal reentrant tachycardia, an arrhythmia requiring dual nodal pathways (4, 6).
We suspect that in our patient it went undiagnosed for at least two years (during this period of time being misinterpreted as atrial fibrillation), inducing a cardiomyopathy with a progressive decrease in systolic function and enlargement of the left ventricle.
The pathophysiology behind this arrhythmia is determined by the unique and complex anatomical and functional structure of the atrioventricular junction. It encompasses a compact atrioventricular node connected to the atrial tissue through atrio-nodal junction, an area formed from transitional cells with mainly two functional types of inputs – superior-left fast input and inferior- right slow input – the mainstay of dual pathway physiology (7). Apart from nodal reentrant tachycardias, dual nodal pathways can lead to what was firstly described as “double-fire” tachycardia, nowadays known as dual atrioventricular nodal non-reentrant tachycardia, when a single atrial impulse in conducted simultaneously through fast and slow pathway (1, 7, 8).
In our case, hidden P waves, fast ventricular rate and consequent conduction abnormalities (aberrancy and intermittent blockage in the slow pathway) led to misinterpretation of the arrhythmia as atrial fibrillation or frequent premature ventricular and supraventricular complexes.
There are few conditions to be fulfilled for the tachycardia to occur. The conduction via the slow pathway should be slow enough and the refractory period of the subjacent tissue short enough to allow recovery of the excitability and propagation of the impulse. Also, there should be no retrograde conduction through the slow pathway, blocking the antegrade impulse (1, 7).
The diagnosis is mainly electrocardiographic, with a characteristic appearance of P-QRS1-QRS2. Apart from this commonly seen pattern, different grades and levels of block in the atrioventricular junction make the diagnosis of the tachycardia more challenging.
Dual atrioventricular nodal non-reentrant tachycardia is an AV node dependent tachycardia, thus making nodal blocking agents (like adenosine) potential tools for unmasking dual nodal pathway conduction.
Adenosine is known for its chronotropic and dromotropic on human hearts since 1930, with a different effect on fast versus slow pathway and antegrade versus retrograde conduction (8-11). Although the use of adenosine in nodal re-entering tachycardias is well known, its utility in diagnosis of dual atrioventricular non-reentrant tachycardia has not been previously described.
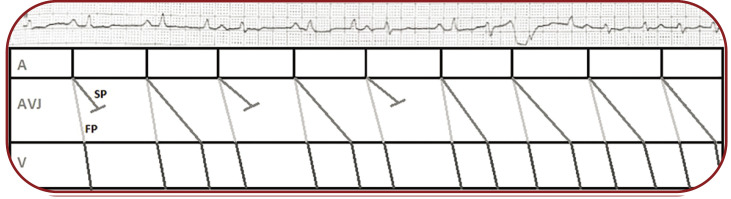
Because of an intermittent atrial activity on our patient's ECG, but without an identifiable relationship with QRS complexes, we decided to use adenosine. Shortly after administration, a normal sinus rhythm was revealed by an adenosine induced complete block in the slow pathway. With progressive clearance of adenosine, a 2:1 block in the slow pathway developed, followed by the restoration of the initial rhythm after the complete clearance of the agent (Figure 5).
The electrophysiological study usually confirms the diagnosis, depicting a typical A-H1V1-H2V2 sequence of the endocardial electrogram. The treatment for DAVNNT is catheter ablation of the slow pathway, being highly effective and safe, similarly to slow pathway ablation/modulation in AVNRT (7). The incessant nature of tachycardia and the ineffectiveness of antiarrhythmic drugs (including amiodarone) lead to tachycardia-induced cardiomyopathy, which is rapidly reversible after ablation (2). Our patient had a significant improvement in his clinical status as well as echocardiographic parameters at the three-month follow-up visit.
CONCLUSION
In conclusion, DAVNNT requires a high index of suspicion and thorough analysis of the ECG recordings for a timely diagnosis and adequate treatment. Electrocardiographic clues that raise the suspicion are visible atrial activity and a regularly irregular rhythm, with a typical 1:2 atrioventricular conduction. We suggest that adenosine, as a short acting nodal blocking agent, might be used as a safe and easily available diagnostic aid, unmasking dual pathway physiology, thus clearing the differential diagnosis with other supraventricular fast tachycardias.
Conflict of interests: none declared.
Financial support: none declared.
FIGURE 1.
A. Wide complex tachycardia with LBBB morphology with intermittent visible P waves (*) at a heart rate of 117 beats per minute. B. Apical four-chamber view depicting dilated left ventricle with LVEDV of 258 mL
FIGURE 2.
Electrocardiogram recorded immediately after administration of 20 mg of adenosine, unmasking the relationship between P waves and QRS complexes
FIGURE 3.
Endocardial electrogram depicting the A-H1V1-H2V2 pattern, 49 mm/s. B. Endocardial electrogram depicting the restoration of the sinus rhythm with 1:1 atrio-ventricular conduction through the fast pathway after ablation of the slow pathway, 12 mm/s
FIGURE 4.
A. Apical four-chamber view showing a LVEDV of 172 mL. B. Bullseye map of the left ventricular longitudinal strain showing a GLS of -10,0%
FIGURE 5.
Electrocardiogram recorded after administration of 20 mg of adenosine – DII lead, unmasking the relationship between P waves and QRS complexes and a corresponding Lewis diagram (A=atria, AVJ=atrioventricular junction, V=ventricles, FP=fast pathway, SP=slow pathway)
Contributor Information
Ecaterina CICALA, Interventional Cardiology, Brasov County Clinical Emergency Hospital, 500326, Brasov, Romania.
Alina Cristina VELICU, Acute Cardiology Unit, “Dr Benedek Geza” Hospital of Rehabilitation in Cardiovascular Diseases, 525200, Covasna, Romania.
Alexandra GHERGHINA, Intensive Coronary Care Unit, Brasov County Clinical Emergency Hospital, 500326, Brasov, Romania.
Catalin PESTREA, Interventional Cardiology, Brasov County Clinical Emergency Hospital, 500326, Brasov, Romania.
References
- 1.Wang NC. Dual atrioventricular nodal nonreentrant tachycardia: a systematic review. Pacing Clin Electrophysiol. 2011;34:1671–1681. doi: 10.1111/j.1540-8159.2011.03218.x. [DOI] [PubMed] [Google Scholar]
- 2.Clementy N, Casset-Senon D, Giraudeau C, Cosnay P. Tachycardiomyopathy secondary to nonreentrant atrioventricular nodal tachycardia: recovery after slow pathway ablation. Pacing Clin Electrophysiol. 2007;30:925–928. doi: 10.1111/j.1540-8159.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbato G, Carinci V, Badhwar N. Tachycardia-Induced Cardiomyopathy. Card Electrophysiol Clin. 2010;2:209–212. doi: 10.1016/j.ccep.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Peiker C, Pott C, Eckardt L, et al. Dual atrioventricular nodal non-re-entrant tachycardia, EP Europace. 2016;18:332–339. doi: 10.1093/europace/euv056. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann J, Jungen C, Stec S, et al. Outcomes in patients with dual antegrade conduction in the atrioventricular node: insights from a multicentre observational study. Clin Res Cardiol. 2002;109:1025–1034. doi: 10.1007/s00392-020-01596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pott C, Wegner F, Bögeholz N, et al. A patient series of dual atrioventricular nodal nonreentrant tachycardia (DAVNNT) — an often overlooked diagnosis? Int J Cardiol. 2014;172:e9. doi: 10.1016/j.ijcard.2013.12.109. [DOI] [PubMed] [Google Scholar]
- 7.Mani BC, Pavri BB. Dual atrioventricular nodal pathways physiology: a review of relevant anatomy, electrophysiology, and electrocardiographic manifestations. Indian Pacing Electrophysiol J. 2014;14:12–25. doi: 10.1016/s0972-6292(16)30711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah RL, Badhwar N. Approach to narrow complex tachycardia: non-invasive guide to interpretation and management. Heart. 2020;106:772–783. doi: 10.1136/heartjnl-2019-315304. [DOI] [PubMed] [Google Scholar]
- 9.Honey RM, Ritchie WT, Thomson WAR. The action of adenosine upon the human heart. Q J Med. 1930;23:485–489. [Google Scholar]
- 10.Rankin AC, Oldroyd KG, Chong E, et al. Value and limitations of adenosine in the diagnosis and treatment of narrow and broad complex tachycardias. Br Heart J. 1989;62:195–203. doi: 10.1136/hrt.62.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CF, Cheung JW, Ip JE, et al. Unifying Algorithm for Mechanistic Diagnosis of Atrial Tachycardia. Circ Arrhythm Electrophysiol. 2016;9:e004028. doi: 10.1161/CIRCEP.116.004028. [DOI] [PubMed] [Google Scholar]