Abstract
Obesity is defined by an imbalance between energy expenditure and energy consumption. Presently, it is considered a global problem because people are consuming junk food and doing less physical activity in every country of the world. It is all due to sedentary life style. The currently available drugs for the treatment of obesity are not giving satisfactory results as they have many adverse effects along with rebound obesity complications. To evaluate new drug in pre-clinical study, we need to have better supportive animal models. Obesity can be induced by giving drugs, fat food, surgical procedures, and by genetic modifications. In the present review, various obesity induced models have been explained to evaluate new compounds. In experimental animal models, monogenic and polygenic obesity models have been reviewed, with a proper pathway to prepare new drugs being given. While in the existing models, genetic obesity models were not explained so far, here genetic engineered transgenic models were described to evaluate new anti-obesity drugs. This short review on chemically and surgically induced obesity models aimed to provide a better understanding of the experimental design of obesity.
Keywords:obesity, monogenic, polygenic, genetic engineered, surgical obesity models.
INTRODUCTION
Obesity is a term applied to excess body weight with an abnormally high proportion of body fat. Thermodynamically speaking, it is an imbalance between energy intake and energy expenditure that presents a risk to health, leading to reduced life expectancy (WHO-2022). Obesity is a global health problem and its prevalence has been increasing alarmingly in developing and developed countries. Obesity is termed as a “new world syndrome” because it causes many non-communicable diseases such as diabetes and metabolic syndrome, and adversely affects the body homeostasis (1). It is considered a disarray of energy balance and fundamentally a disorder of lipid metabolism (2). Sedentary lifestyle and consumption of junk food are the major reasons for the increase in obesity prevalence. The body mass index (BMI) is commonly used to assess overweight and obesity in adults (1, 3) and it exceeds 30 in people with obesity and overweight, which are the major risk factors for non-communicable diseases such as cardiovascular diseases, diabetes, musculoskeletal disorders and cancer. Obesity linked hyperlipidemia can increase systemic oxidative stress (4). Hence, the management of obesity is a herculean task for clinicians as it is the underlying cause of many diseases (5). According to World Health Organization (WHO) reports, more than one billion people worldwide are obese, including 650 million adults, 340 million adolescents and 39 million children; this number is still increasing, with 2.8 million deaths occurring throughout the world due to complications arising from obesity and overweight as per WHO report 2022 (6). Interestingly, the prevalence of obesity is higher in women (about 297 million) than men (205 million) (7). On the occasion of the World Obesity Day 2022, WHO is urging countries to do more to reverse this predictable and preventable health crisis.
Globally, by 2030, an estimated number of one billion people will live with obesity, which is double the number reported in 2010. The rates of obesity, an important risk factor for diabetes, continue to rise. While lack of progression on reducing obesity is a global issue, each region has its own story. In parts of Europe and North America, obesity is starting to plateau, albeit at high rate, while it is rising fastest in low and middle income countries as well as small island developing states, and adding pressure to many countries, also grappling with malnutrition. Prevention of obesity as well as early intervention and treatment through government policy is vital (8, 9).
The present available drugs can control and prevent weight gain by their pharmacological actions, e.g., inhibition on fat absorption from GIT and accelerate fat metabolism. However, the use of drug use is linked to their considerable, both tolerable and intolerable, side effects, including hypertension, heart-related complications, psychiatric problem (mental illness), and constipation (10, 11). The most commonly used drug in the management of obesity is orlistat, which reduces fat absorption by inhibiting lipase activity. However, orlistat is also associated with side effects such as gastrointestinal complications, abdominal pain, bloating, flatulence, and diarrhoea, and it may also lead to dyspepsia and liver damage (12). Hence, there is a need for developing safer alternatives to orlistat which are effective and associated with minimum or fewer side effects.
To develop a new drug for a better treatment of obesity with fewer side effects, various models should be used to evaluate the potency of upcoming drugs. The study and understanding of various mechanisms and physiological pathways in obesity are very important to establish new drug development methods. Hence, different animal models have been discussed in a systematic manner. Diet induced obesity, drug induced obesity, and various genetic models are discussed here. The overweight or obesity of an individual is determined by his/her BMI. The classification of BMI is described in Table 1 (13).
OBJECTIVES OF THE STUDY
Our study had the following objectives: to review and draw an inference from issues regarding drug induced obesity; to review and demonstrate an experimental rodent model of obesity; to review the existing genetic engineered transgenic models; to review and demonstrate surgical and chemical induced obesity in animals.
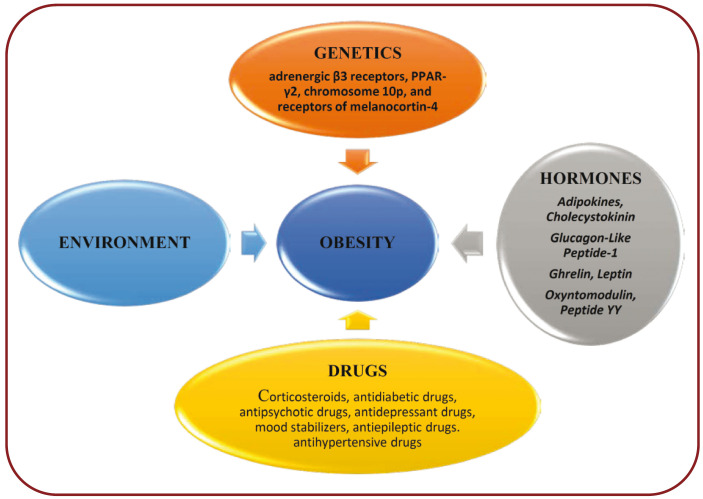
Several etiological factors of overweight or obesity have been reported, including hormonal dysregulation, genetic polymorphism and environmental factors. It may be due to either a single factor or multiple factors or even to complex interactions between the above stated.
DRUG INDUCED OBESITY
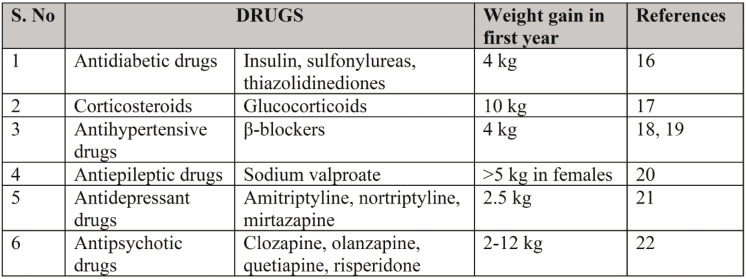
Drug induced obesity is seen in patients treated with corticosteroids, antidiabetic drugs, antipsychotic drugs, antidepressant drugs, mood stabilizers, antiepileptic drugs, and antihypertensive drugs. The mechanism of induction by glucocorticoids includes up-regulation of phosphokinases activated by adenosine monophosphate and exaggerate HPA axis progressively. This in turn causes dysregulation of endocannabinoid receptor activity resulting in obesity (14, 15). Antidiabetic drugs like thiazolidinediones and sulfonylureas lead to weight gain as a result of anabolic effects of insulin. β-blockers will also induce weight gain due to their genetic variations in β receptor genes. The other drugs are shown in Table 2.
EXPERIMENTAL MODELS OF OBESITY IN ANIMALS
Mostly rodent models are used for obesity studies. The common ones are described below.
Eligibility criteria
Only case reports that described perforation of the cervix by the strings or threads of IUDs were included in the present review. Studies reporting perforation of the cervix or uterus and any other pelvic organ by IUDs, and articles not written in English as well as those that appeared as grey literature were all excluded from our review.
1. Monogenic models
Leptin-deficient model
Leptin-deficient ob/ob mouse model has been used by many studies. Ku S K et al (2016) conducted a study using five C57BL/6JJms mice aged five weeks and 25 male (C57BL/6JHam-ob/ob) genetically-obese mice aged five weeks after acclimatization of 28 days. Equal quantities of food (150 g) were provided per cage and residual food was measured after 24 hours with an automated electronic balancing system. Body weight measurements were done weekly for three weeks after baseline. During the fourth week, body-weights were measured three times. The ob/ob-mice exhibited a significantly elevated body weight in comparison to wild-type mice prior to the start of study product administration. Also, in all ob/obmice, a significant rise in mean food intake per day was observed relative to wild-type mice (23).
Koffi C et al (2019) research comprised 3-4-month-old ob/ob or ob/+ mice with one week acclimatization of both sexes. Mice were fed daily for four weeks and their body weight increased tremendously. In contrast with lean mice, ob/ob mice showed a higher body weight and lower food consumption (24). Bruno R S et al (2008) contrasted male leptin-deficient-mice (ob/ob) with their lean-littermates of C57BL/6J. In line with their phenotypic tendency to be obese at young ages, five-week-old ob/ob mice weighed 34% much as their lean-mates at the start of the study. As predicted, obese-mice (ob/ob) showed increased body-weight than lean-mice at the end of the experiment (25).
Leptin-receptor deficient mouse
A study by Zhao R et al (2014) used wild-type and db/db mice, which were fed with chow, and reported an increased body weight in db/db mice in comparison to wild-type mice (26). Another study also reported increased body weight along with the incidence of hepatic steatosis in db/db mice (27). The increased body weight was linked to up-regulated AMPK signalling and down-regulation of mTOR in the liver (28).
There are no major variations in the lipid profile in T2DM human subjects with regard to sex, besides a higher level of HDL-cholesterol in females. This lipid profile is distinct to the above-mentioned animal models. Drawn collectively, the obesogenic phenotype found in T2DM leptin-based rodent- models does not properly mimic human causal factors, history or pathology (29).
Zucker rat
Various mechanisms of obesity and therapeutic mechanisms have been demonstrated using Zucker rats in anti-obesity drug screening. Y Li et al (2005) also demonstrated up-regulation of á-glucosidase activity in Zucker rats (30). Derailed gastric lipase activity was shown by Vaquero M R et al (2012) in Zucker rats, which were compared with lean (fa/+) rats after a feeding period of 64 days (31). Another six-week study demonstrated the activity of uncoupling-protein- 1, PP-ARá, and PP-ARã-coactivator-1á in obese Zucker rats. The role of peroxisome proliferators- activated receptor-á in obesity was stressed in a study conducted by Kim Y J et al (2006) using Zucker rats (32). Lemaure B et al (2007) suggested the role of â adrenoceptor activity in obese Zucker rats (33). Zucker rats exhibited increased levels of lipids, glucose, insulin, nitric-oxide synthase, TNF-á and adiponectins, which partly resembles obesity in humans (34). The disadvantages with this strain include expensive and sophisticated maintenance, high mortality, insulin requirements after certain ages, which are differing from obesity in humans (35).
Wistar Kyoto fatty rat
This strain was used by many studies to screen anti-obesity properties of various phytochemicals, plant extracts and drugs. Apart from acquiring obesity, this strain also exhibited a binge-eating behaviour (36). Zawistowski J et al (2009) demonstrated the occurrence of hypercholesterolemia in Wistar Kyoto fatty rats fed with experimental diets for 10 weeks (37). Hepatic and renal injuries along with raised oxidative markers were shown in these rats by the study of JM Jaffri et al (2011) (38). Reduced gastric and increased small intestinal transit along with altered intestinal flora was found by Dalziel JE et al (2017), with high esters of cholesterol and low glycerophospholipids in this rat strain (39). Wistar Kyoto rats were used as controls in few studies evaluating spontaneous-hypertensive rats, which showed a significant rise in the body-weight of Kyoto rats (40, 41).
Otsuka Long-Evans Tokushima fatty rat
This strain was bred from Long-Evans rats and showed features of obesity and insulin resistance, which exhibited characteristic abdominal fat, as well as development of diabetes at the age of one year (42). It has also shown up-regulation of neuropeptide- Y and discrepancy of cholecystokinin-1 receptor gene (43).
2. Polygenic models
Diet-induced obese (DIO) and diet-resistant (DR) Sprague-Dawley rats
Sprague-Dawley rats are used to produce diet-induced obese rats and about 33% of them acquire diet-resistant obesity, which may be considered as a drawback of this model. But if the state is established, it continues with the same. The behaviour of hyperphagia is unique as they have large meals rather than more frequent meals (44). The DIO rats develop glucose-intolerance and insulin resistance. They exhibit raised NPY protein and leptin levels. These models resemble to humans when they develop resistance to leptin and reduced abdominal fat in restricted food intake (45).
Cafeteria diet-induced obesity (CIO)
Compared to DIO rats, CIO rats were considered as robust obesity model. The weight-gain in CIO rats was double to that observed with DIO rats. Food intake was greater (30%) in CIO rats than DIO rats, which implied that CIO rats were more hyperphagic. These rats exhibited obesity with chronic low-grade inflammation, visceral fat, macrophage infiltrated-adipose, increased TNF-á, and insulin resistance. Insulin resistance was high in CIO rats compared to DIO rats. Lipid accumulation in droplets was observed profoundly in structures closely related to vascular components in comparison to DIO rats. Fatty liver associated with hepatosteatosis including microvesicular events (portal-triad and central vein) was observed only in CIO rats, which resembles obesity in humans. Characteristics of Kupffer cells in CIO rats resembled human pathology of obesity, which was not seen DIO rats.
Gil-Cardoso K et al (2017) compared CIO rats with genetic rat models in their extensive study. They inferred increased gut inflammation in CIO rats compared to genetic obese-rats, which showed the severity of obesity and metabolic derailments. They also evaluated gut flora of both models, but could not obtain any significant events. Their study was different in approach to indicate the necessity of drugs altering the gut permeability and inflammation in treatment of obesity, as many studies were focusing on the inflammatory events in the liver (46).
Barber T et al (1985) revealed an increased body weight of CIO rats associated with a reduction in uptake of amino-acids by the liver and other enzymes of the cell-cycle. They also showed a decreased excretion of urea in urine samples of CIO rats, which was a peculiar aspect of this model in comparison to other models.
Lopez IP et al (2003) analysed the genetic aberrations in CIO rats. They reported that the genes related to macronutrient metabolism (6-phosphofructo- 2-kinase/fructose-2, 6-bisphosphatase, alanine-aminotransferase, aldose reductase, apolipoprotein B, beta-3-adrenergic receptor, fatty-acid transporter, glycerol 3-phosphate dehydrogenase, leptin ob, low-molecular-weight FABP, lysosomal acid-lipase, uncoupling protein-3), transcription factors (C/EBP-related transcription- factor, DNA-binding protein-C/EBP, NF-1-like DNA-binding protein, nuclear receptor-binding factor-1, PPAR-gamma protein, small nucle- ar-RING-finger-protein), hormone receptor and signal transduction (adenylyl cyclase-type-V, cyclic- GMP-stimulated-phosphodiesterase, GM2 activator-protein, growth hormone-receptor, insulin receptor substrate-3 (IRS-3), insulin-induced growth-response-protein, insulin-like growth factor- I (IGF-I), neuroendocrine-specific-protein, oxytocin receptor, scavenger receptor-class-B, and cellular cytoskeleton (alpha B-crystallin, secreted protein acidic rich in cisteine (SPARC), synaptic density protein PSD-93) were all up-regulated.
The down regulated genes related to macronutrient metabolism (acyl-CoA hydrolase, aldehyde dehydrogenase (ALDH), apolipoprotein E, aspartate aminotransferase, GLUT1, glycogen storage disease type 1b protein, phosphofructokinase C, squalene epoxidase), redox proteins (glutathione S-transferase-P-subunit, gluta-thione- S-transferase-subunit-8, metallothionein- 1), transcription factor (Homeobox-protein (Hox-1.11), Krox20-6-EGR-2 (early growth response protein 2), hormonal and signal transductions (acidic calcium-independent phospholipase A2, estrogen-responsive uterine, FGF receptor activating protein FRAG1, fibroblast growth factor receptor subtype 4, MAP kinase kinase kinase 1 (MEKK1), vitamin-D binding-protein), and cellular cytoskeleton (beta-A4 crystallin, connexin-31) were also demonstrated (47).
Macedo IC et al (2012) showed that CIO rats were non-inferior to other rats in gaining body weight associated with increased leptin levels and hyperlipidemia (48). A study by Jindal V et al (2011) showed that CIO rats significantly differed from sulpiride-induced obese (SIO) rats in weight gain when fed for 40 days. The CIO rats exhibited increased levels of cholesterol, triglycerides, glucose and HDL-cholesterol (49).
New Zealand obese mice
These mice were developed from agouti mice. They present the polygenic syndrome with manifestations of hyperphagia, hyperglycemia, increased body weight, and insulin-resistance. Igel M et al (1997) demonstrated the existence of polymorphic leptin-receptors and leptin resistance in these mice models (50). The limitations with this model include fertility problems, and more susceptibility to diabetes (51).
Age-related obesity model
This model was studied using both mice and rats. The rat models exhibited leptin derailments. Scarpace PJ et al (2000) showed impaired leptin-signal transduction in aged rats with obesity (52). Scarpace PJ et al (2001) demonstrated the occurrence of leptin resistance by evaluating leptin levels in aged obese rats (53). Reduced oxidation was also observed in association with obesity in age-related obesity models (54). Increased IL6 levels and hyperlipidemia were reported in another study (55).
3. Genetic engineered transgenic models
Transgenic corticotropin-releasing factor (CRF)-overexpressing animals
With the metallothionein-1 enhancer, which truly mirrors chronic modulation of brain CRF mechanisms, CRF-overexpressing mice were established (56). Corticotropin-releasing factor modifies the behaviour of food intake-regulators like neuropeptides Y (NPY) and leptin (57). Stengel A et al (2009) demonstrated the dysfunction of NPY pathway due to CRF overexpression (58).
Glucose transporter subtype 4
The up-regulated insulin-mediated glucose distribution of adipocytes in young-obese Zucker rats is associated with an upsurge in cytochalasin-B binding as opposed to lean-rat adipocytes. The fa gene imposes differential regulation over the distribution of GLUT 4 in adipose-tissue and supports the idea that the genotype is a key metabolic factor of GLUT 4 expression in this tissue, autonomous of hyperinsulinemia in obesity (59).
Beta-3 adrenergic receptor knockout
ADR-B3 KO mice confirmed that by facilitating adrenergic activation of white-adipose-tissue lipolysis, especially once the adipocytes are packed with triglycerides, ADR-B3 is the key regulator of body weight. This model resembles the DIO one as ADR-B3 KO increases predisposition to DIO (60).
Serotonin 5-HT-2c receptor knockout, neuropeptide- Y 1 receptor (NPY1R) knockout mouse, NPY2R knockout mice, bombesin 3 receptor knockout mice, neuronal insulin receptor knockout models target specific pathway and may not resemble human obesity as it is multifactorial. Apart from this limitation, they are more expensive to use in research.
4. Surgical (or) chemical induced obesity models
Obesity can be induced by surgical procedures as well as by giving some chemicals which can cause obesity. In rats, obesity can be induced by making ventromedial hypothalamus (VMH) lesions, hypothalamic paraventricular nucleus lesions, and by arcuate nucleus lesions. Ovaries can be removed (ovariectomy) by surgical procedures in female rats to induce obesity. Ovariectomy rat models of obesity resemble human obesity, but the drawback of these models is that they require surgical interventions and have a high mortality.
In 2014, Gaur A et al demonstrated that after VMH lesion, serum cholesterol in rats was considerably greater, suggesting that VMH had a lipid lowering effect on DIO. Food intake increased even more after VMH-lesions, perhaps in the experimental rodents. Plasma glucose and total cholesterol also increased (61).
Leibowitz SF et al (1981) demonstrated that, in both gender rats, small lesions basically confined to the PVN were reported to produce binging and elevated body weight (62). Tokunaga Katsuto et al (1986) compared the VMH and PVN models, and concluded that PVN was less effective than VMH. The features of obesity caused by PVN lesions vary from all due to damage to VMH. In rats feeding ad libitum, nocturnal food consumption was smaller in the VMH-lesioned rats. The presence of an independent pathway in hypothalamic NPY gene expression that regulates the regular rhythm and suggests that leptin reinforcement action requires a preserved VMH (63, 64). Arcuate nucleus lesioned rodent model showed an increased body weight and reduced NPY-mRNA (64).
Ovariectomy rat models exhibit obesity due to estrogen deficiency, and not leptin resistance. In ovx-rats, the concurrence of bone calcium dysregulation and obesity was found related to a significant rise in adiposity, bone loss, reduction in the normalized muscle-mass to body weight ratio and a significant decrease in motor activity (65). In OVX-rats, high weight gain and BMI have been inversely related with serum estradiol (66). OVX-rats also exhibited hyperphagia (67).
The cafeteria induced obese mimics diet of people living in Western countries, which is influencing the Indian diet too. Cafeteria diet results in hyperphagia, thermogenesis and also affects meal size inducing obesity (68). This model has the highest similarity to human eating patterns (69). Genetic models have contributed for underlying gene mechanisms controlling energy balance in the body, but other mechanisms were revealed using diet induced models. Obesity, a true pandemic now, is mainly due to cafeteria diet. It reveals the physiological, biochemical and pathological including genetic mechanisms of obesity. This is the reason why this model holds more significance than others (70).
CONCLUSIONS
Evaluation of various reasons and consequences of obesity may be helpful in discovering new drugs for the treatment of obesity. The best model to induce a disease is the one which best replicates its pathophysiological characteristics. The multifactorial causes of obesity provide several options for the development of experimental models to induce obesity. Several animal studies promoting neuroendocrine, dietary and genetic alterations have been performed. It is necessary to choose the model which is best adapted to the characteristics to be researched, be they environmental or genetic. The best results were obtained based on choosing the appropriate obesity models to prepare the best drugs for obesity treatment.
Conflict of interests: none declared.
Financial support: none declared.
Aknowledgments: I am extremely thankful to my research guide Dr. Anusha, Professor of pharmacology and the management at Santhiram Medical College and General Hospital.
TABLE 1.
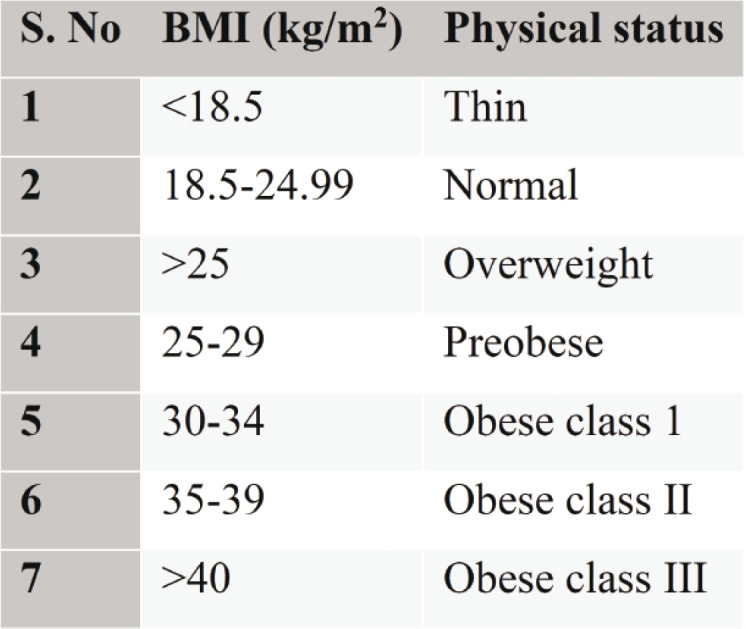
Classification of BMI
FIGURE 1.
Factors precipitating obesity
TABLE 2.
Drug induced obesity
Contributor Information
Yakaiah VANGOORI, Santhiram Medical College, Nandyal (AP), India.
Babu Sayana SURESH, Govt. Medical College, Suryapet, Telangana, India.
Madhavi Latha MIDDE, Santhiram Medical College, Nandyal (AP), India.
D ANUSHA, Sri Ramachandra Institute of Higher Education and Research, Chennai, India.
Praveen Kumar UPPALA, AMC-SRMC & GH, India.
References
- 1. . . www.who.int.news.facts sheets. 16 February 2018(WHO). 2011;18:795–801. [Google Scholar]
- 2.Birari RB, Gupta S, Mohan CG. Anti-obesity and lipid-lowering effects of Glycyrrhiza chalcones. Experimental and computational studies. Phytomedicine. 2011;18:795–801. doi: 10.1016/j.phymed.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Shiva Kumar A, et al. Antiobesity, antioxidant and hepatoprotective effect of Diallyl trisulphide (DATS) alone or in combination with Orlistat on HFD induced obese rats. Biomed Pharmacother. 2017;93:81–87. doi: 10.1016/j.biopha.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 4.Brahma Naidu P, Nemani H, Meriga B. Mitigating efficacy of piperine in the physiological derangements of high fat diet-induced obesity in Sprague Dawley rats. Chem Biol Interact. 2014;221:42–51. doi: 10.1016/j.cbi.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discovery. 2006;5:919–931. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- 8.Behla S, Misra A. Management of obesity in adult Asian Indians. Indian Heart Journal. 2017;69:539–544. doi: 10.1016/j.ihj.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg C, Khan SA, Ansari SH, Garg M. Prevalence of obesity in Indian women. Obes Rev. 2010;11:105–108. doi: 10.1111/j.1467-789X.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers R.J, Tschop M.H. Anti-obesity drugs: past, present and future. Dis Model Mech. 2012;5:621–662. doi: 10.1242/dmm.009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discov. 2006;5:919–993. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- 12.Ballinger A, Peikin SR. Orlistat: its current status as an anti-obesity drug. Eur J Pharmacol. 2002;440:109–117. doi: 10.1016/s0014-2999(02)01422-x. [DOI] [PubMed] [Google Scholar]
- 13.Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. (Updated 2020 Jul 10). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing, 2020 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541070/ [PubMed]
- 14.Christ‐Crain M, Kola B, Lolli F, et al. AMP‐activated protein kinase mediates glucocorticoid‐induced metabolic changes: a novel mechanism in Cushing's syndrome. The FASEB Journal. 2008;22:1672–1683. doi: 10.1096/fj.07-094144. [DOI] [PubMed] [Google Scholar]
- 15.Bowles NP, Karatsoreos IN, Li X, et al. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc Nat Acad Sci, USA 2015. [DOI] [PMC free article] [PubMed]
- 16.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with Metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). The Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 17.Wung PK, Anderson T, Fontaine KR, et al. Effects of glucocorticoids on weight change during the treatment of Wegener's granulomatosis. Arthritis Care Res. 2008;59:746–753. doi: 10.1002/art.23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma AM, Pischon T, Hardt S, et al. Hypothesis: βadrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250–254. doi: 10.1161/01.hyp.37.2.250. [DOI] [PubMed] [Google Scholar]
- 19.Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with β-blocker use: results from GEMINI. Am J Med. 2007;120:610–615. doi: 10.1016/j.amjmed.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Verrotti A, D'Egidio C, Mohn A, et al. Weight gain following treatment with valproic acid: pathogenetic mechanisms and clinical implications. Obes Rev. 2011;12:e32–e43. doi: 10.1111/j.1467-789X.2010.00800.x. [DOI] [PubMed] [Google Scholar]
- 21.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 22.Verma RK, Paraidathathu T. Herbal medicines used in the traditional Indian medicinal system as a therapeutic treatment option for overweight and obesity management: A review. Int J Pharm Pharm Sci. 2014;6:40–47. [Google Scholar]
- 23.Ku SK, Sung SH, Choung JJ, et al. Anti-obesity and anti-diabetic effects of a standardized potato extract in ob/ob mice. Exp Ther Med. 2012;12:354–364. doi: 10.3892/etm.2016.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koffi C, Soleti R, Nitiema M, et al. Ethanol extract of leaves of cassia siamea lam protects against diabetes-induced insulin resistance, hepatic, and endothelial dysfunctions in ob/ob mice. Oxid Med Cell Longev. 2019;24:1341. doi: 10.1155/2019/6560498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruno RS, Dugan CE, Smyth JA, et al. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J Nutr. 2008;138:323–331. doi: 10.1093/jn/138.2.323. [DOI] [PubMed] [Google Scholar]
- 26.Zhao R, Le K, Li W, et al. Effects of Saskatoon berry powder on monocyte adhesion to vascular wall of leptin receptor-deficient diabetic mice. J Nutr Biochem. 2014;25:851–857. doi: 10.1016/j.jnutbio.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Pang D, You L, Zhou L, et al. Averrhoa carambola free phenolic extract ameliorates nonalcoholic hepatic steatosis by modulating mircoRNA-34a, mircoRNA-33 and AMPK pathways in leptin receptor-deficient db/db mice. Food & Function. 2017;8:4496–4507. doi: 10.1039/c7fo00833c. [DOI] [PubMed] [Google Scholar]
- 28.Luo L, Fang K, Dan X, Gu M. Crocin ameliorates hepatic steatosis through activation of AMPK signaling in db/db mice. Lipids Health Dis. 2019;18:11. doi: 10.1186/s12944-018-0955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131–145. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Wen S, Kota BP, et al. Punica granatum flower extract, a potent αglucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J Ethnopharmacol. 2005;99:239–244. doi: 10.1016/j.jep.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Vaquero MR, Yáñez-Gascón MJ, Villalba RG, et al. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a rosemary extract rich in carnosic acid. PloS One. 2012;7:e39773. doi: 10.1371/journal.pone.0039773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YJ, Shin YO, Ha YW, et al. Anti-obesity effect of Pinellia ternata extract in Zucker rats. Biol Pharm Bull. 2006;29:1278–1281. doi: 10.1248/bpb.29.1278. [DOI] [PubMed] [Google Scholar]
- 33.Lemaure B, Touché A, Zbinden I, et al. Administration of Cyperus rotundus tubers extract prevents weight gain in obese Zucker rats. Phytother Res. 2007;21:724–730. doi: 10.1002/ptr.2147. [DOI] [PubMed] [Google Scholar]
- 34.Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008;16:2081–2087. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 35.Capcarova M, Kalafova A. Zucker Diabetic Fatty Rats for Research in Diabetes. In: Animal Models in Medicine and Biology, 2019.
- 36.Papacostas-Quintanilla H, Ortiz-Ortega VM, LópezRubalcava C. Wistar-Kyoto female rats are more susceptible to develop sugar binging: a comparison with wistar rats. Front Nutr. 2007;4:15. doi: 10.3389/fnut.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zawistowski J, Kopec A, Kitts DD. Effects of black rice extract (Oryza sativa L. indica) on cholesterol levels and plasma lipid parameters in Wistar Kyoto rats. J Funct Foods. 2009;1:50–56. [Google Scholar]
- 38.Jaffri JM, Mohamed S, Ahmad IN, et al. Effects of catechin-rich oil palm leaf extract on normal and hypertensive rats’ kidney and liver. Food Chem. 2011;128:433–441. doi: 10.1016/j.foodchem.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 39.Dalziel JE, Fraser K, Young W, et al. Gastroparesis and lipid metabolism-associated dysbiosis in Wistar-Kyoto rats. Am J Physiol Gastrointest Liver Physiol. 2017;313:G62–G72. doi: 10.1152/ajpgi.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez A, Sanchez-Rodriguez E, Vargas F, et al. Cardioprotective effect of a virgin olive oil enriched with bioactive compounds in spontaneously hypertensive rats. Nutrients. 2019;11:1728. doi: 10.3390/nu11081728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero M, Toral M, Gómez-Guzmán M, et al. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016;7:584–593. doi: 10.1039/c5fo01101a. [DOI] [PubMed] [Google Scholar]
- 42.Polonsky KS, Burant CF. Type 2 diabetes mellitus. In: Williams textbook of endocrinology, 2016.
- 43.Bi S, Moran TH. Obesity in the Otsuka Long Evans Tokushima Fatty Rat: Mechanisms and Discoveries. Front Nutr. 2016;3:21. doi: 10.3389/fnut.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farley C, Cook JA, Spar BD, et al. Meal pattern analysis of diet‐induced obesity in susceptible and resistant rats. Obesity Res. 2003;11:845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- 45.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- 46.Gil-Cardoso K, Ginés I, Pinent M, et al. A cafeteria diet triggers intestinal inflammation and oxidative stress in obese rats. Br J Nutr. 2017;117:218–229. doi: 10.1017/S0007114516004608. [DOI] [PubMed] [Google Scholar]
- 47.López IP, Marti A, Milagro FI, et al. DNA microarray analysis of genes differentially expressed in diet‐induced (cafeteria) obese rats. Obesity Res. 2003;11:188–194. doi: 10.1038/oby.2003.30. [DOI] [PubMed] [Google Scholar]
- 48.Macedo IC, Medeiros LF, Oliveira C, et al. Cafeteria diet-induced obesity plus chronic stress alter serum leptin levels. Peptides. 2012;38:189–196. doi: 10.1016/j.peptides.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Jindal V, Dhingra D, Sharma S, et al. Hypolipidemic and weight reducing activity of the ethanolic extract of Tamarindus indica fruit pulp in cafeteria diet- and sulpiride-induced obese rats. J Pharmacol Pharmacother. 2011;2:80–84. doi: 10.4103/0976-500X.81896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Igel M, Becker W, Herberg L, Joost HG. Hyperleptinemia, leptin resistance, and polymorphic leptin receptor in the New Zealand obese mouse. Endocrinology. 1997;138:4234–4239. doi: 10.1210/endo.138.10.5428. [DOI] [PubMed] [Google Scholar]
- 51.Weiser A, Giesbertz P, Daniel H, Spanier B. Acylcarnitine profiles in plasma and tissues of hyperglycemic NZO mice correlate with metabolite changes of human diabetes. J Diabetes Res. 2018;2:114. doi: 10.1155/2018/1864865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology. 2000;39:1872–1879. doi: 10.1016/s0028-3908(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 53.Scarpace PJ, Tümer N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol Behav. 2001;74:721–727. doi: 10.1016/s0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 54.Jeong HJ, Lee HJ, Vuong TA, et al. Prmt7 deficiency causes reduced skeletal muscle oxidative metabolism and age-related obesity. Diabetes. 2016;65:1868–1882. doi: 10.2337/db15-1500. [DOI] [PubMed] [Google Scholar]
- 55.Di Gregorio GB, Hensley L, Lu T, et al. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab. 2004;287:E182–E187. doi: 10.1152/ajpendo.00189.2003. [DOI] [PubMed] [Google Scholar]
- 56.Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- 57.Mastorakos G, Zapanti E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: the role of corticotropin releasing hormone. Nutr Neurosci. 2004;7:271–280. doi: 10.1080/10284150400020516. [DOI] [PubMed] [Google Scholar]
- 58.Stengel A, Goebel M, Million M, et al. Corticotropin-releasing factoroverexpressing mice exhibit reduced neuronal activation in the arcuate nucleus and food intake in response to fasting. Endocrinology. 2009;150:153–160. doi: 10.1210/en.2008-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hainault I, Guerre-Millo M, Guichard C, Lavau M. Differential regulation of adipose tissue glucose transporters in genetic obesity (fatty rat). Selective increase in the adipose cell/muscle glucose transporter (GLUT 4) expression. J Clin Invest. 1991;87:1127–1131. doi: 10.1172/JCI115077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preite NZ, do Nascimento BP, Muller CR, et al. Disruption of beta3 adrenergic receptor increases susceptibility to DIO in mouse. J Endocrinol. 2016;231:259. doi: 10.1530/JOE-16-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27:1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- 63.Tokunaga Katsuto, Fukushima Masataka, Kemnitz JW, Bray GA. Comparison of ventromedial and paraventricular lesions in rats that become obese. Am J Physiol. 1986;251:R1221–R1227. doi: 10.1152/ajpregu.1986.251.6.R1221. [DOI] [PubMed] [Google Scholar]
- 64.Dube MG, Xu B, Kalra PS, et al. Disruption in neuropeptide Y and leptin signaling in obese ventromedial hypothalamic-lesioned rats. Brain Res. 1999;816:38–46. doi: 10.1016/s0006-8993(98)00985-8. [DOI] [PubMed] [Google Scholar]
- 65.Ezzat-Zadeh Z, Kim JS, Chase PB, Arjmandi BH. The cooccurrence of obesity, osteoporosis, and sarcopenia in the ovariectomized rat: a study for modeling osteosarcopenic obesity in rodents. J Aging Res. 2017;5:112. doi: 10.1155/2017/1454103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma B, Zhang Q, Wang GJ, et al. GC-TOF/MS-based metabolomic profiling of estrogen deficiency-induced obesity in ovariectomized rats. Acta Pharmacologica Sinica. 2011;32:270–278. doi: 10.1038/aps.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Physiol Endocrinol Metab. 2001;280:E315–E322. doi: 10.1152/ajpendo.2001.280.2.E315. [DOI] [PubMed] [Google Scholar]
- 68.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;58:5. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lalanza JF, Snoeren EMS. The cafeteria diet: A standardized protocol and its effects on behavior. Neurosci Biobehav Rev. 2020;122:92–119. doi: 10.1016/j.neubiorev.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Sampey BP, Vanhoose AM, Winfield HM, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity, (Silver Spring, Md.) 2001. [DOI] [PMC free article] [PubMed]