Abstract
Background:
Although the standard of care is to perform surgery of primary breast cancer (BC) after neoadjuvant chemotherapy (NAC), for certain patients achieving clinical complete response (cCR) and pathologic complete response (pCR), omission of surgical treatment may be an option. Levels of circulating tumor DNA (ctDNA) during and after therapy could identify patients achieving minimal residual disease. In this study, we evaluated whether ctDNA clearance during NAC could be a correlate to effective response in human epidermal growth factor receptor 2 positive (HER2+) and triple-negative (TN) BC patients.
Methods:
A prospective study was conducted to identify patient-specific PIK3CA and TP53 mutations in tissue using next-generation sequencing, which could then be used to track the presence/absence of mutations prior to, during, and following NAC using Sysmex SafeSEQ technology. All patients underwent a surgical excision after NAC, and pCR was assessed.
Results:
A total of 29 TN and HER2+ BC patients were examined and 20 that carried mutations in the PIK3CA and/or TP53 genes were recruited. Overall, 19 of these 20 patients harbored at least one tumor-specific mutation in their plasma at baseline. After NAC, 15 patients (75.0%) achieved pCR according to the histopathologic evaluation of the surgical specimen, and 15 patients (75.0%) had a cCR; 18 of 20 patients (90.0%) had concordant pCR and cCR. The status of ‘no mutation detected’ (NMD) following NAC in cCR patients correctly identified the pCR in 14 of 15 patients (93.33%), as well as correctly ruled out pCR in three patients, with an accuracy of 89.47%. During the 12-month follow-up after surgery, 40 plasma samples collected from 15 patients all showed no detectable ctDNA (NMD), and no patient recurred.
Conclusion:
These findings prompt further research of the value of ctDNA for non-invasive prediction of clinical/pathological response, raising the possibility of sparing surgery following NAC in selected BC patients.
Keywords: breast cancer, circulating tumor DNA, liquid biopsy, neoadjuvant therapy, pathologic complete response
Introduction
Breast cancer (BC) is the most commonly diagnosed malignancy in women worldwide and accounts for almost one out of four cancer cases. It is also the second cause of cancer-related death in developed countries.1 The use of neoadjuvant chemotherapy (NAC) has increased over the years, not only in locally advanced BC, but also in early high-risk tumors. The availability of NAC in therapeutic modalities enables more immediate treatment of local and micrometastatic disease. Hence, a greater number of breast-conserving surgeries may be possible in the future by assessing the efficacy of effective NAC treatment by visualizing tumor shrinkage by imaging, as well as by other sensitive molecular techniques. In many cases, pathologic complete responses (pCR) can be achieved, both in the breast and axilla, which significantly improves disease-free and overall survival.2,3 The likelihood of achieving pCR after NAC depends on tumor molecular subtype. Triple-negative (TN) and human epidermal growth factor receptor 2 positive (HER2+) BC patients present a higher rate of pCR (up to 60%) as compared to luminal BC patients after NAC.4,5
Currently, the standard of care for primary BC after NAC is to perform surgery to completely remove any residual disease that may remain in non-pCR cases or to diagnose/confirm a pCR. However, if a pCR could be diagnosed with sufficient certainty using non-invasive procedures, a surgical intervention might hypothetically be without benefit and cause unnecessary harm for the patient.6,7 Breast imaging methods are not able to accurately estimate residual disease after NAC; hence, they cannot replace the pathologic diagnosis of the surgical excision specimen.8,9 A recent meta-analysis that assessed the diagnostic accuracy of image-guided minimally invasive biopsy techniques in predicting breast pCR after NAC concluded that they are not accurate enough to locally visualize residual disease pathology in the tissue.10
Liquid biopsy has been shown to be a very powerful non-invasive technology that provides valuable supplementary information in terms of prognosis assessment, drug resistance, and individualized treatment.11 Circulating tumor DNA (ctDNA) is fragmented genomic DNA (fgDNA) resulting from apoptosis and necrosis of tumor cells that can be detected in blood by the presence of somatic mutations.12 In BC, integrated molecular analyses revealed that TP53 and PIK3CA are the most frequently mutated genes, each with a mutation frequency >35%.13 Moreover, several forward-looking studies have provided seminal clinical evidence that levels of ctDNA in general, as well as PIK3CA and TP53 mutations in particular, detected during NAC and post-surgery can identify patients that exhibit effective responses versus those showing minimal residual disease14 –18 and can be used to predict tumor response to NAC and prognosis in early BC.19 –21
Based on these results, our working hypothesis was that the detection and quantification of ctDNA from patients receiving NAC may be a complementary method to assess pCR along with current imaging techniques. This should allow for future identification of patients without infiltrating or residual disease that could eventually avoid surgery. Thus, we performed longitudinal tracking of plasma TP53 and PIK3CA mutations in HER2+ and TN BC patients to examine ctDNA clearance during NAC as a correlate to effective response to treatment, as benchmarked by clinical complete response (cCR) and pCR. We also analyzed serial samples after surgery in a subset of patients to explore the possible re-emergence of ctDNA over time and whether continued ctDNA surveillance over time might also identify patients who would later recur radiologically.
Materials and methods
Study design and patients
We prospectively recruited patients diagnosed with BC and scheduled to receive NAC in a Spanish Hospital Cancer center from 2018 to 2019. Eligible patients were enrolled upon histologically confirmed stage I/III HER2+ or TN infiltrating breast carcinoma. The pilot cohort was subsequently stratified to select those patients having specific PIK3CA and/or TP53 mutations detected in the biopsied tissue; this was determined by performing next-generation sequencing (NGS) of the patients’ primary tumor sample. TP53 and/or PIK3CA mutations identified in the tumor sample were used to generate ‘bespoke’ or tumor-informed ctDNA liquid biopsy assays customized for each patient using SafeSEQ technology, that were then used to track the presence/absence of these specific mutations in each patient’s plasma samples for longitudinal tracking of ctDNA during NAC. Radiological assessment (mammography, ultrasound, and magnetic resonance imaging), as part of the routine clinical practice, and mutation detection in plasma samples were carried out at baseline prior to NAC, at treatment mid-point, and at post-treatment immediately prior to surgery, as well as at three time-points post-surgery (10 weeks, 6 months, and 12 months). The NAC regimens were administered according to the hospital’s protocol, based on anthracyclines and taxanes. In some TN patients, the regimens also included platinum agents and, in HER2+ patients, treatments included was specific antibody therapies associated with HER2 signaling pathway blockade. All patients underwent a surgical excision after NAC, and pCR was assessed. The study was approved by the medical ethical committee of the Hospital Universitario Vall d’Hebron (PR(AG)204/2016) on 30 September 2016. All patients provided oral and written informed consent for sample acquisition for research purposes.
Targeted sequencing analysis of tissue and plasma DNA
Tumor tissue samples from all patients were initially examined for mutations in both the PIK3CA and TP53 genes using the NGS-based SureSeq technology (Oxford Gene Technology) after isolation of DNA using Qiasymphony DNA Tissue Kits (Qiagen, Hilden, Germany) following the manufacturer’s instructions. For liquid biopsy assays, blood was collected in Streck® Cell-Free DNA BCT tubes and processed to plasma using a double-spin protocol in which the blood was centrifuged twice to obtain 8–9 ml of plasma; samples were then stored at −80°C until DNA extraction. DNA was extracted with the QIAamp Circulating Nucleic Acid Kit (Qiagen) using extended lysis of 60 min and analyzed using the Plasma SafeSEQ technology (Sysmex Inostics, Inc., Hamburg, Germany). Personalized SafeSEQ liquid biopsy assays were designed custom to each patient based on the tumor mutations identified by the SureSeq NGS assay. To examine each mutation in PIK3CA and TP53 that were found in the patient’s tumor sample in liquid biopsy, patient sample specific multiplex reactions were designed at the research laboratories at Sysmex Inostics Germany to detect the specific mutations previously identified in tissue.22 For certain patients, other specific mutations detected in patients’ tumor samples were also analyzed alongside of PIK3CA or TP53. Prior to detection of ctDNA in patient plasma samples, DNA was isolated from plasma and quantified using LINE-1 qPCR as described previously.23 Patient-customized multiplexes assay was designed and QCed on fgDNA to a mean size of ~160 bp using the Covaris M220 instrument (Covaris LLC, Woburn (MA), USA). Background noise was determined for each multiplex using 2× 10,000 genomic equivalents of genomic DNA (NIST, RM8398). Each multiplex contained primers attached to unique identifiers (UIDs), which served as molecular barcodes and used for error correction according to the Safe-SeqS principle.22 Specifically, UID PCR was performed with 13 cycles followed by bead-based purification of patient-specific amplicons using AMPure XP beads (Beckman Coulter, Brea (CA), USA) and dilution. A second PCR in which sample-specific index sequences were attached served to generate and then discriminate UID families. Index PCR products were purified in a pooled format using QIAquick (Qiagen) followed by one round of AMPure XP bead purification (Beckman Coulter). DNA samples were then quantified using a Bioanalyzer (Agilent, Santa Clara (CA), USA) and sequenced on NextSeq 550 instrument (Illumina, San Diego (CA), USA). Sequencing data were analyzed using proprietary software packages developed by Sysmex Inostics. Mutation analyses and individual mutation calls were performed with a positive mutation call [mutation detected, MD9 or negative mutation call (no mutation detected, NMD)] for each patient sample time point. Positive mutation calls for each tumor informed mutation that were reported in mutant molecules [i.e. copies)/ml of plasma (MM/ml)]. The positive call thresholds (cutoffs) for each tumor-informed mutation detected in plasma were determined individually; these were set as mutant molecule values at 3× the value of the background established for each mutation position (Limit of Blank) and above a minimum threshold of 3 MM (copies) detected in a patient’s sample. Any values falling below both mutations-specific and sample level calling thresholds failed ‘Mutation Detected’ call criteria by the software program and were thus designated as ‘No Mutations Detected (NMD).
Statistical analysis
The primary outcome measure was pCR, as defined by the absence of invasive residual disease both in the breast and axillary lymph nodes following NAC. The ctDNA mutation results (undetectable; NMD/detectable; MD) from each patient’s plasma sample obtained at the baseline/pre-surgery were then compared to the sample(s) obtained after NAC. The accuracy of the test as well as positive predictive value and negative predictive value (NPV) were calculated for the combination of ctDNA positive and cCR using the Diagnostic test evaluation calculator (MedCalc Statistical Software Version 20.027, MedCalc Software bv, Ostend, Belgium).
Results
Patient and tumor characteristics
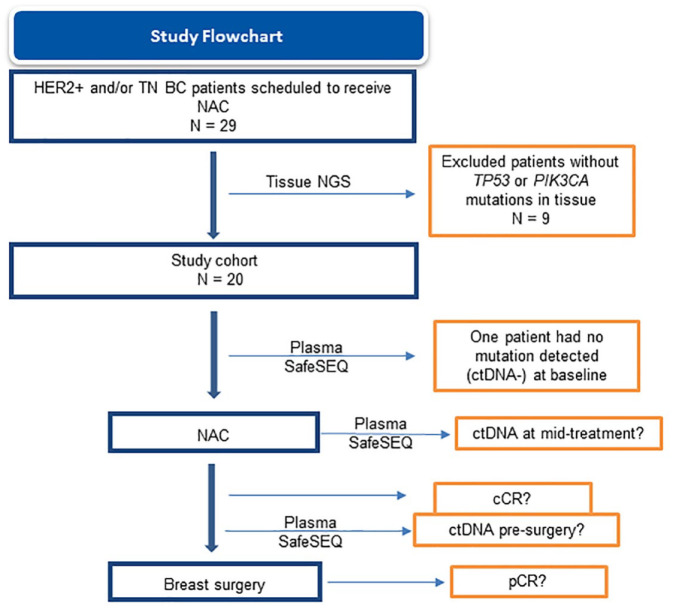
A total of 29 BC patients scheduled to receive NAC were prospectively enrolled and histologically examined; nine patients were excluded from further study because of the absence of TP53 or PIK3CA mutations in tissue, as assessed by NGS (Figure 1). Within the study cohort (N = 20), all patients had at least one TP53 mutation detected and five patients also had a PIK3CA mutation. Patient-specific SafeSEQ assays were generated for all of the TP53 and PIK3CA mutations that were detected in tissue to detect these mutations in plasma during NAC. In addition, three patients (32, 33, and 34) – each having a single TP53 mutation detected in their tumors – also had accompanying mutations in the following genes (NTRK1, NTRK3, HER2/ERBB2, GRM3, AKT3, FGF10, and FANCF); patient-specific SafeSEQ plasma assays were also prepared to detect the presence of these additional mutations in plasma. The clinical and pathological characteristics, tumor mutations identified, and plasma mutations detected in baseline plasma samples prior to NAC are all shown in Table 1. Overall, there were 12 TN and 8 HER2+ BC patients enrolled in the pilot cohort. The median (range) age of patients was 57 (38–88) years. Overall, 19 of these 20 patients (overall percent agreement = 95%) showed at least one tumor-specific mutation detected in their plasma (ctDNA positive) at baseline.
Figure 1.
Study flowchart.
Table 1.
Baseline clinical and tumor characteristics and ctDNA status.
| Patient ID | Tumor subtype | Tissue mutation | Plasma mutation |
|---|---|---|---|
| 1 | TN | TP53 p.R273P | TP53 p.R273P (351.8 MM; 58.6 MM/mL) |
| 4 | TN | TP53 p.R110Pfs*39, TP53 p.R290Pfs*55, TP53 p.R175H and PIK3CA p.R310H | TP53 p.R110Pfs*39 (70.9 MM; 12.9 MM/mL), TP53 p.R290Pfs*55 (NMD), TP53 p.R175H (11.2 MM; 2.0 MM/mL) |
| 5 | HER2+ | TP53 p.R290Pfs*55 | TP53 p.R290Pfs*55 (259.5 MM; 89.9 MM/mL) |
| 6 | TN | TP53 p.Q144* | TP53 p.Q144* (90.9 MM; 15.1 MM/mL) |
| 7 | TN | TP53 p.R110P | TP53 p.R110P (1850.1 MM; 308.4 MM/mL) |
| 9 | TN | TP53 p.R273L | TP53 p.R273L (112.7 MM; 18.8 MM/mL) |
| 10 | HER2+ | TP53 p.V173L and PIK3CA p.H1047R | TP53 p.V173L (NMD) and PIK3CA p.H1047R (4.6 MM; 0.8 MM/mL) |
| 11 | TN | TP53 p.R175H | TP53 p.R175H (15.3 MM; 7.7 MM/mL) |
| 12 | HER2+ | TP53 p.M237I and PIK3CA p.E542K | TP53 p.M237I (13.7 MM; 6.9 MM/mL) and PIK3CA p.E542K (39.2 MM; 19.6 MM/mL) |
| 14 | TN | TP53 p.C176F | TP53 p.C176F (67.2 MM; 11.2 MM/mL) |
| 17 | TN | TP53 p.N131Tfs*39 | TP53 p.N131Tfs*39 (1168.5 MM; 194.8 MM/mL) |
| 19 | TN | TP53 p.Y163* | TP53 p.Y163* (58.5 MM; 9.7 MM/mL) |
| 26 | HER2+ | TP53 p.D281V and PIK3CA p.H1047R | TP53 p.D281V (39.4 MM; 8.8 MM/mL) and PIK3CA p.H1047R (47.7 MM; 10.6 MM/mL) |
| 28 | TN | TP53 p.R273H | TP53 p.R273H (31.7 MM; 8.1 MM/mL) |
| 29 | HER2+ | TP53 p.K139_V143del | TP53 p.K139_V143del (1.0 MM; 0.2 MM/mL) |
| 30 | HER2+ | TP53 p.R213*, TP53 p.N131Tfs*39 and PIK3CA p.H1047R | TP53 p.R213* (9.9 MM; 1.7 MM/mL), TP53 p.N131Tfs*39 (NMD) and PIK3CA p.H1047R (13.3 MM; 2.2 MM/mL) |
| 31 | TN | TP53 p.S127F | TP53 p.S127F (2157.4 MM; 365.7 MM/mL) |
| 32 | HER2+ | NTRK1 p.Ser433Cys, AKT3 p.Leu262Val, NTRK3 p.Glu474Lys, TP53 p.Glu285Lys, ERBB2 p.Ile989Met, GRM3 p.Asp97His and GRM3 p.Arg101Thr | NTRK1 p.Ser433Cys (NMD), AKT3 L262V (1.3 MM; 0.2 MM/mL), NTRK3 E474K (1.5 MM; 0.3 MM/mL), TP53 E285K (2.7 MM; 0.5 MM/mL), GRM3 D97H (2.3 MM; 0.4 MM/mL) and GRM3 D101T (2.3 MM; 0.4 MM/mL) |
| 33 | TN | TP53 A74fs and FGF10 F146fs | TP53 A74fs (12.2 MM; 3.4 MM/mL) and FGF10 F146fs (8.1 MM; 2.3 MM/mL) |
| 34 | HER2+* | FANCF p.Leu241Arg and TP53 c.375 + 1dupG | NMD |
Patient ID 34 had 90% of estrogen receptor-positive cells.
ctDNA, circulating tumor DNA; HER2+, human epidermal growth factor receptor 2 positive; MM, mutant molecules; NMD, no mutation detected; TN, triple negative.
Response to NAC
In all, 15 patients (75.0%) had a pCR after NAC according to the histopathologic evaluation of surgical specimen. A pCR occurred in 10 out of 12 patients (83.3%) with TN tumors and 5 out of 8 patients (62.5%) with HER2+ tumors (Table 2). Based on clinical examination and imaging assessment, 15 patients (75.0%) had a cCR; 18 out of 20 patients (90.0%) had concordant pCR and cCR. At mid-treatment, 15 (75.0%) plasma samples had non-detectable ctDNA (12 cCR patients versus 3 non-cCR patients). Immediately prior to surgery, 17 patients (85.0%) had non-detectable ctDNA (15 cCR patients versus 2 non-cCR patients). Patients showing a good response to NAC had ctDNA drops in the middle of the treatment (examples in Figure 2).
Table 2.
Responses to NAC and results of the ctDNA analyses performed in the middle of and after the treatment.
| Patient ID | cCR | Mid-NAC plasma | Pre-surgery plasma | pCR |
|---|---|---|---|---|
| 1 | Yes | NMD | NMD | Yes |
| 4 | Yes | NMD | NMD | Yes |
| 5 | Yes | TP53 p.R290Pfs*55 (201.9 MM; 33.6 MM/mL) | NMD | Yes |
| 6 | Yes | NMD | NMD | Yes |
| 7 | No | NMD | TP53 p.R110P (1 MM; 0.2 MM/mL) | Yes |
| 9 | Yes | NMD | NMD | Yes |
| 10 | No | NMD | NMD | No |
| 11 | Yes | TP53 p.R175H (43.7 MM; 7.3 MM/mL) | NMD | Yes |
| 12 | Yes | NMD | NMD | Yes |
| 14 | Yes | NMD | NMD | Yes |
| 17 | Yes | NMD | NMD | Yes |
| 19 | No | NMD | NMD | No |
| 26 | No | TP53 p.D281V (17.7 MM; 3.0 MM/mL) and PIK3CA p.H1047R (19.3 MM; 3.2 MM/mL) | TP53 p.D281V (1.2 MM; 0.2 MM/mL) and PIK3CA p.H1047R (0.6 MM; 0.1 MM/mL) | No |
| 28 | Yes | NMD | NMD | Yes |
| 29 | Yes | TP53 p.K139_V143del (0.6 MM; 0.1 MM/mL) | NMD | Yes |
| 30 | Yes | NMD | NMD | Yes |
| 31 | No | TP53 p.S127F (2.0 MM; 0.3 MM/mL) | TP53 p.S127F (5.5 MM; 1.0 MM/mL) | No |
| 32 | Yes | NMD | NMD | No |
| 33 | Yes | NMD | NMD | Yes |
| 34 | Yes | NMD | NMD | Yes |
Patients that did not achieve cCR are highlighted in boldface type.
cCR, clinical complete response; ctDNA, circulating tumor DNA; MM, mutant molecules; NAC, neoadjuvant chemotherapy; NMD, no mutation detected; pCR, pathologic complete response.
Figure 2.
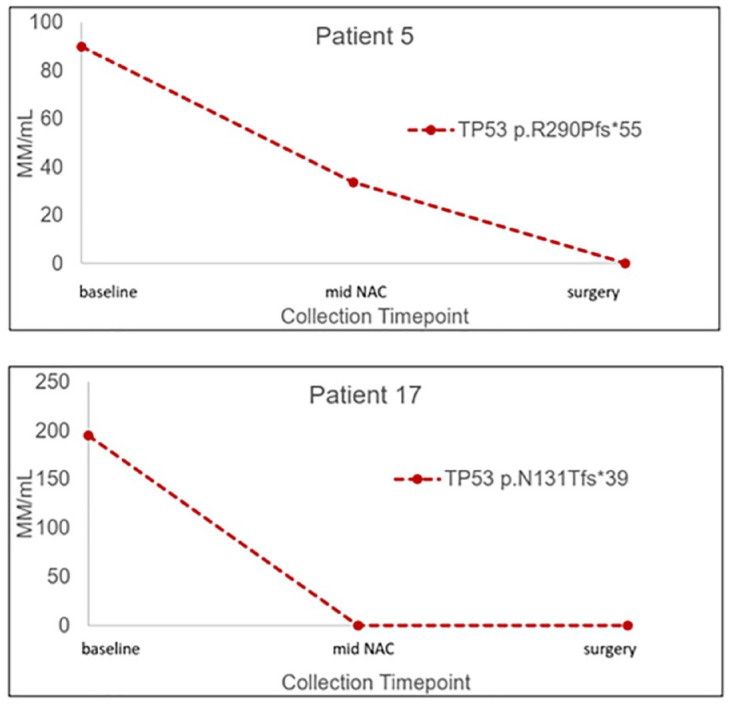
Representative plots of longitudinal ctDNA tracking. Two patients (patients 5 and 17) showed complete clearance of TP53 mutations in plasma before surgery, which associated with cCR to NAC.
cCR, clinical complete response; ctDNA, circulating tumor DNA; NAC, neoadjuvant chemotherapy.
Accuracy of pre-surgery ctDNA + cCR for pCR determination
Table 3 summarizes the performance of the combination of a ctDNA-negative result and achieving cCR (ctDNA + cCR) in predicting pCR after NAC. The status ctDNA and cCR correctly identified the pCR in 14 out of 15 patients (93.33%), whereas there was one false-positive case (patient 7), one false-negative case (patient 32), and three true-negative cases (patients 10, 26, and 31). This resulted in an accuracy of 89.5%, a false-positive rate of 3.8%, and a NPV of 75.0%.
Table 3.
Performance of the status of ctDNA and cCR in predicting pCR after NAC.
| Measure | |
|---|---|
| Accuracy | 89.5% |
| False-positive rate | 3.8% |
| PPV | 93.3% |
| NPV | 75.0% |
cCR, clinical complete response; ctDNA, circulating tumor DNA; NPV, negative predictive value; pCR, pathologic complete response; PPV, positive predictive value.
Post-surgery ctDNA status and recurrence
To assess whether any of the 15 patients achieving cCR showed any detectable ctDNA during a surveillance period following surgery, blood collections from these patients were performed at three time points after surgery (4 weeks, 6 months, and 12 months) and ctDNA testing was performed using the same patient-specific tissue-informed SafeSEQ assays used to monitor each patient during NAC. As shown in Table 4, in 40 samples collected from these 15 patients, none showed any detectable ctDNA at any point during the 12-month follow-up period. In addition, no ctDNA was detected after 24 months of follow-up in 13 out of 15 patients (two patients were not available for blood collection; data not shown) achieving cCR, and none of these patients were found to have recurrent disease. Only two patients experienced recurrent disease after longer clinical follow-up: patient 7 (time until recurrence, 22 months), who did not achieve cCR and had a ctDNA-positive result after NAC, and patient 10 (time until recurrence, 27 months), who did not achieve neither cCR nor pCR, but had NMD after NAC. Unfortunately, these two patients were not available for any follow-up blood collection to undergo ctDNA testing during the 12-month follow-up period after surgery. However, blood samples were obtained in patients 7 and 10 after recurrence, and ctDNA testing revealed the same ctDNA mutations detected during NAC. Both patients showed a significant increase in ctDNA, with patient 7 presenting the TP53 p.R110P mutation at 397.7 MM/ml, and patient 10 presenting both TP53 p.V173L (8.4 MM; 1.5 MM/ml) and PIK3CA p.H1047R (6.9 MM; 1.2 MM/ml) mutations. It was unfortunate that these patients were unavailable for ctDNA surveillance monitoring during the period shortly after surgery when positive detection of these mutations might have provided an early signal of recurrent disease.
Table 4.
Surveillance ctDNA testing results for the 15 BC patients achieving cCR after NAC*.
| Patient ID | 10 weeks after surgery | 6 months after surgery | 12 months after surgery |
|---|---|---|---|
| 1 | NMD | NMD | NMD |
| 4 | NMD | NMD | NMD |
| 5 | NMD | NMD | NMD |
| 6 | NMD | NMD | NMD |
| 9 | NMD | NMD | NMD |
| 11 | NMD | NMD | NMD |
| 12 | NMD | NMD | NMD |
| 14 | NMD | NA | NMD |
| 17 | NMD | NA | NA |
| 28 | NMD | NMD | NMD |
| 29 | NMD | NMD | NMD |
| 30 | NMD | NMD | NMD |
| 32 | NMD | NMD | NMD |
| 33 | NMD | NMD | NA |
| 34 | NMD | NMD | NA |
All but one patient (patient 32) also achieved pCR.
BC, breast cancer; cCR, clinical complete response; ctDNA, circulating tumor DNA; MM, mutant molecules; NA, patient sample not available; NAC, neoadjuvant chemotherapy; NMD, no mutation detected; pCR, pathologic complete response.
Discussion
The effectiveness of NAC treatment regimens for BC has improved during recent years and has achieved high rates of pCR, particularly in HER2+ and TN tumors.24,25 These observations have prompted the notion that patients that achieve cCR following NAC might be spared breast surgery, as forgoing surgery would potentially reduce surgical complications, improve quality of life, and decrease healthcare costs. This idea of de-escalation of breast surgery in BC patients with an apparent complete response to NAC is gaining recognition as an alternative approach to patient management. However, there are current challenges with accurately determining the status of residual disease at the end of neoadjuvant treatment using the current clinico-radiological evaluations or image-guided biopsy techniques.8,9,26 Therefore, surgery after NAC is still considered the standard of care for primary BC, either to completely remove residual disease in non-pCR cases or to confirm a pCR.
In this pilot study, we identified certain HER2+ and TN BC patients showing clearance of ctDNA mutations as a potential correlate of complete response to NAC. In fact, 14 out of 15 patients meeting the criteria of absence of plasma mutations plus cCR based on clinical examination and imaging assessment had a proven pCR according to the histopathological evaluation of the surgical specimen (PPV = 93.3%). This observation leads to the tenable conclusion that these patients could have safely avoided breast surgery after NAC. Moreover, the patient that achieved cCR, but did not achieve a pCR (patient 32) did not showed detectable ctDNA after NAC and did not experience disease recurrence during the follow-up period, and furthermore did not present resurgence of tumor mutation in plasma. These observations are in accord with the conclusion that, in this patient, residual disease was minimal and did not spread to other organs before the surgery or disease was eliminated during NAC. With longer follow-up, an eventual reappearance of the patient’s specific mutation in plasma ctDNA may indicate microscopic occult disease before its detection/visualization by imaging techniques.27
In the future, if liquid biopsy negative results were considered as a correlate of pCR in the decision-making process, patients may not undergo surgery but instead would undergo an intensive follow-up (frequent imaging and/or liquid biopsy surveillance). In case of minimal residual disease, this might be addressed by radiotherapy and/or adjuvant treatment according to the standard practice. By contrast, patients that do not achieve cCR after NAC (five in our study; see patients ID in Table 3) should undergo breast surgery regardless of the presence or absence of plasma mutations detected. Moreover, three of five patients not achieving cCR had plasma mutations detected prior to surgery, which has been associated with poorer prognosis,28,29 and, in fact, two on these patients (ID 7 and ID 10) relapsed. Regrettably, both of these patients were not available for ctDNA testing during the 4-week, 6-month, and 12-month follow-up period post-surgery, and both patients showed markedly increase ctDNA levels at 24 months after recurrence. Although patient 10 showed clearance of both TP53 and PIK3CA mutations in ctDNA from baseline, the same mutations re-appeared after recurrence at 24 months. Similarly, patient 7 had NMD at mid-NAC but again the baseline mutation in the TP53 gene was present at the end of the treatment. These observations raise the possibility that these mutations may indicate underlying resistance mechanisms of the tumor.30
The detection of ctDNA in plasma regardless of achieving cCR and pCR in five cases prompts the need to rigorously establish a clinically relevant level of ctDNA that triggers heightened suspicion of molecular residual disease (MRD). Moreover, clinical ctDNA cutoffs that comprise qualitative judgments of ‘MRD positive’ versus ‘MRD negative’ are likely to require more sophisticated algorithms to be developed that integrate both patient-specific mutations, as well as sample-level tumor mutational burden to gain greater certainties of an individual patient’s risk of recurrence/relapse or cure in response treatment. In this study, we have provided a framework of longitudinal testing in a small cohort of BC patients to begin establishing a clinically relevant cutoff using SafeSEQ technology. Further trials with greater numbers of patients will be required to ascertain both the frequency of testing, qualitative result, and quantities of ctDNA that will serve of a useful adjunct to determine both disease clearance and risk of disease recurrence. In managing the patient using ctDNA results, it will be critical to establish both durability of neoadjuvant response by determining consistency of negative ctDNA test results as well as a higher risk of recurrence triggered by consecutive positive and ascending values of ctDNA in plasma. Here, the serial sampling after the surgery was reassuring, as none of these five patients experienced elevations of ctDNA levels during the follow-up. Interpretation of the results at exceedingly low levels of ctDNA of detection should be done with caution, and bearing in mind that SafeSEQ is an ultra-sensitive technique able to detect as few as three mutants in 20,000 total DNA molecules22 Accordingly, the overall percent agreement between tissue and plasma mutations in this study was 95%, much higher than the observed with other techniques,31,32 even when low levels of ctDNA are usually not detectable in early disease.33 Another explanation for the presence of low levels of (clinically irrelevant) mutant molecules could be the occurrence of clonal hematopoiesis of indeterminate potential, which has been reported to be frequent for the TP53 gene.34,35
The results of this pilot study should be interpreted within the context of its strengths and limitations. An important limitation of this study is its small sample size, although this patient cohort is fairly representative of a typical neoadjuvant population. A second limitation of this study is that the post-surgery follow-up time was restricted to 12 months, so it is not possible to rule out any other potential recurrence in the future, as the recurrence window of HER2+ and TN BC is estimated to be 5 years, or even more in the case of luminal tumors.36 Given these limitations (only two patients had recurred at the writing of this manuscript), ctDNA levels could not be assessed as a prognostic factor for recurrence. In addition, we were unable to perform post-surgery surveillance blood testing of all patients and at all of the scheduled time points during the follow-up.
In summary, the SafeSEQ NGS technology identified 19/20 (95%) of BC patients with detectable mutations in plasma at baseline, enabling the vast majority of patients to be tracked via liquid biopsy during NAC. Clearance of ctDNA positivity and achieving cCR after NAC had favorable correlation with effective pathological response. Moreover, the presence of clearly detectable ctDNA levels is likely to be associated with the lack of clinical/pathological complete response in BC patients. These findings prompt further studies with larger patient cohorts to investigate the added value of ctDNA to predict pCR, aiding in determining which patients might be safely spared surgery after NAC. If this strategy is confirmed to be clinically useful, an approach of ‘watch and wait’ (including serial plasma testing) could be implemented in selected minimal-risk patients.
Acknowledgments
The authors thank all the patients and their families who participated in this study. They thank Anabel Herrero, PhD, on behalf of Springer Healthcare Communications, for providing medical writing support for this manuscript. This medical writing assistance was funded by Sysmex Inostics, Inc.
Footnotes
ORCID iDs: Nikaoly Ciriaco  https://orcid.org/0000-0002-1151-554X
https://orcid.org/0000-0002-1151-554X
Esther Zamora  https://orcid.org/0000-0002-2233-367X
https://orcid.org/0000-0002-2233-367X
Santiago Escrivá-de-Romaní  https://orcid.org/0000-0001-7816-7589
https://orcid.org/0000-0001-7816-7589
José Jiménez Flores  https://orcid.org/0000-0002-2576-1776
https://orcid.org/0000-0002-2576-1776
Vicente Peg  https://orcid.org/0000-0002-5203-6166
https://orcid.org/0000-0002-5203-6166
Contributor Information
Nikaoly Ciriaco, Pathology Department, Hospital del Mar, Barcelona, Spain; Universidad Autónoma de Barcelona, Barcelona, Spain.
Esther Zamora, Universidad Autónoma de Barcelona, Barcelona, Spain; Breast Cancer Group, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain; Medical Oncology Department, Vall d’Hebron University Hospital, Barcelona, Spain.
Santiago Escrivá-de-Romaní, Breast Cancer Group, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain; Medical Oncology Department, Vall d’Hebron University Hospital, Barcelona, Spain.
Ignacio Miranda Gómez, Radiology Department, Vall d’Hebron University Hospital, Barcelona, Spain.
José Jiménez Flores, Molecular Oncology Lab. Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain.
Cristina Saura, Medical Oncology Department, Vall d’Hebron University Hospital, Barcelona, Spain.
Hillary Sloane, Sysmex Inostics, Inc., Baltimore, MD, USA; Sysmex Inostics GmbH, Hamburg, Germany.
Anna Starus, Sysmex Inostics, Inc., Baltimore, MD, USA; Sysmex Inostics GmbH, Hamburg, Germany.
Johannes Fredebohm, Sysmex Inostics, Inc., Baltimore, MD, USA; Sysmex Inostics GmbH, Hamburg, Germany.
Lucy Georgieva, Oxford Gene Technology, Oxford, UK.
Graham Speight, Oxford Gene Technology, Oxford, UK.
Frederick Jones, Sysmex Inostics, Inc., Baltimore, MD, USA; Sysmex Inostics GmbH, Hamburg, Germany.
Santiago Ramón y Cajal, Universidad Autónoma de Barcelona, Barcelona, Spain; Pathology Department, Vall d’Hebron University Hospital, Barcelona, Spain; Spanish Biomedical Research Network Centre in Oncology (CIBERONC), Madrid, Spain.
Martín Espinosa-Bravo, Breast Cancer Unit, Vall d’Hebron University Hospital, Barcelona, Spain.
Vicente Peg, Universidad Autónoma de Barcelona, Barcelona, Spain; Pathology Department, Vall d’Hebron University Hospital, Paseo Vall d’Hebron 119-129, Barcelona 08035, Spain; Spanish Biomedical Research Network Centre in Oncology (CIBERONC), Madrid, Spain.
Declarations
Ethics approval and consent to participate: The study protocol was approved by the medical ethical committee of the Hospital Universitario Vall d’Hebron on 30/09/2016 (ID PR(AG) 204/2016). All patients provided written informed consent.
Consent for publication: All authors have read the manuscript and approved its submission to Therapeutic Advances in Medical Oncology.
Author contribution(s): Nikaoly Ciriaco: Conceptualization; Data curation, Formal analysis; Writing – original draft; Writing – review & editing.
Esther Zamora: Conceptualization; Writing – original draft; Writing – review & editing.
Santiago Escrivá-de-Romaní: Conceptualization; Writing – original draft; Writing – review & editing.
Ignacio Miranda Gómez: Data curation; Writing – original draft; Writing – review & editing.
José Jiménez Flores: Data curation.
Cristina Saura: Conceptualization; Writing – original draft; Writing – review & editing.
Hillary Sloane: Formal analysis; Methodology; Writing – original draft.
Anna Starus: Formal analysis; Methodology; Writing – original draft.
Johannes Fredebohm: Formal analysis; Methodology; Writing – review & editing.
Lucy Georgieva: Formal analysis; Methodology; Writing – original draft.
Graham Speight: Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Frederick Jones: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Santiago Ramón y Cajal: Writing – original draft; Writing – review & editing.
Martín Espinosa-Bravo: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Vicente Peg: Conceptualization; Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from Sysmex Inostics, Inc. The sponsor coordinated data collection from study centers, and funded the statistical analysis and medical writing assistance.
E.Z. has received fees as consultant, participated in advisory boards or received travel grants from Roche/Genentech, Eisai Europe, and Daiichi Sankyo/AstraZeneca; S.E.R has received fees as consultant, participated in advisory boards or had an investigator role from Daiichi Sankyo/AstraZeneca, Pfizer, Roche, Seagen, Byondis, Lilly, MedSIR, and Synthon; C.S has served as consultant, participated in advisory boards or received travel grants from Byondis, AstraZeneca, Daiichi Sankyo, Eisai, Exact Sciences, Exeter Pharma, F. Hoffmann – La Roche Ltd, MediTech, Merck Sharp & Dohme, Novartis, Pfizer, Philips, Pierre Fabre, Puma biotechnology, Sanofi-Aventis, SeaGen and Zymeworks; V.P. has received fees as consultant, participated in advisory boards or received travel grants from Sysmex, Roche, Merck Sharp & Dohme, AstraZeneca, Bayer and Exact Sciences.
The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Data supporting the results presented in this study are available from the corresponding author upon reasonable request.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Ataseven B, von Minckwitz G. The impact of neoadjuvant treatment on surgical options and outcomes. Ann Surg Oncol 2016; 23: 3093–3099. [DOI] [PubMed] [Google Scholar]
- 3. Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 2020; 26: 2838–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colleoni M, Viale G, Zahrieh D, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors. Clin Cancer Res 2004; 10: 6622–6628. [DOI] [PubMed] [Google Scholar]
- 5. Kuerer HM, Rauch GM, Krishnamurthy S, et al. A clinical feasibility trial for identification of exceptional responders in whom breast cancer surgery can be eliminated following neoadjuvant systemic therapy. Ann Surg 2018; 267: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mamounas EP. Omitting surgery in complete responders after neoadjuvant chemotherapy: the quest continues. Ann Surg Oncol 2018; 25: 3119–3122. [DOI] [PubMed] [Google Scholar]
- 7. Heil J, Kuerer HM, Pfob A, et al. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: current evidence and future challenges. Ann Oncol 2020; 31: 61–71. [DOI] [PubMed] [Google Scholar]
- 8. Schaefgen B, Mati M, Sinn HP, et al. Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol 2016; 23: 789–795. [DOI] [PubMed] [Google Scholar]
- 9. Zhang K, Li J, Zhu Q, et al. Prediction of pathologic complete response by ultrasonography and magnetic resonance imaging after neoadjuvant chemotherapy in patients with breast cancer. Cancer Manag Res 2020; 12: 2603–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Zhou Y, Mao F, et al. The diagnostic performance of minimally invasive biopsy in predicting breast pathological complete response after neoadjuvant systemic therapy in breast cancer: a meta-analysis. Front Oncol 2020; 10: 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alimirzaie S, Bagherzadeh M, Akbari MR. Liquid biopsy in breast cancer: a comprehensive review. Clin Genet 2019; 95: 643–660. [DOI] [PubMed] [Google Scholar]
- 12. Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med 2018; 379: 1754–1765. [DOI] [PubMed] [Google Scholar]
- 13. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015; 7: 302ra133. [DOI] [PubMed] [Google Scholar]
- 15. McDonald BR, Contente-Cuomo T, Sammut S-J, et al. Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Sci Transl Med 2019; 11: eaax7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moss J, Zick A, Grinshpun A, et al. Circulating breast-derived DNA allows universal detection and monitoring of localized breast cancer. Ann Oncol 2020; 31: 395–403. [DOI] [PubMed] [Google Scholar]
- 17. Beaver JA, Jelovac D, Balukrishna S, et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res 2014; 20: 2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013; 368: 1199–1209. [DOI] [PubMed] [Google Scholar]
- 19. Li S, Lai H, Liu J, et al. Circulating tumor DNA predicts the response and prognosis in patients with early breast cancer receiving neoadjuvant chemotherapy. JCO Precis Oncol 2020; 4: 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radovich M, Jiang G, Hancock BA, et al. Association of circulating tumor DNA and circulating tumor cells after neoadjuvant chemotherapy with disease recurrence in patients with triple-negative breast cancer: preplanned secondary analysis of the BRE12-158 randomized clinical trial. JAMA Oncol 2020; 6: 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodriguez BJ, Córdoba GD, Aranda AG, et al. Detection of TP53 and PIK3CA mutations in circulating tumor DNA using next-generation sequencing in the screening process for early breast cancer diagnosis. J Clin Med 2019; 8: 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kinde I, Wu J, Papadopoulos N, et al. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A 2011; 108: 9530–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rago C, Huso DL, Diehl F, et al. Serial assessment of human tumor burdens in mice by the analysis of circulating DNA. Cancer Res 2007; 67: 9364–9370. [DOI] [PubMed] [Google Scholar]
- 24. Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2018; 19: 115–126. [DOI] [PubMed] [Google Scholar]
- 25. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603. J Clin Oncol 2015; 33: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Loevezijn AA, van der Noordaa MEM, van Werkhoven ED, et al. Minimally invasive complete response assessment of the breast after neoadjuvant systemic therapy for early breast cancer (MICRA trial): interim analysis of a multicenter observational cohort study. Ann Surg Oncol 2021; 28: 3243–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mesquita A, Costa JL, Schmitt F. Utility of circulating tumor DNA in different clinical scenarios of breast cancer. Cancers (Basel) 2020; 12: 3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Y-H, Hancock BA, Solzak JP, et al. Next-generation sequencing of circulating tumor DNA to predict recurrence in triple-negative breast cancer patients with residual disease after neoadjuvant chemotherapy. NPJ Breast Cancer 2017; 3: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ortolan E, Appierto V, Silvestri M, et al. Blood-based genomics of triple-negative breast cancer progression in patients treated with neoadjuvant chemotherapy. ESMO Open 2021; 6: 100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Z, Sun T, Yang Z, et al. Monitoring treatment efficacy and resistance in breast cancer patients via circulating tumor DNA genomic profiling. Mol Genet Genomic Med 2020; 8: e1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madic J, Kiialainen A, Bidard F-C, et al. Circulating tumor DNA and circulating tumor cells in metastatic triple negative breast cancer patients. Int J Cancer 2015; 136: 2158–2165. [DOI] [PubMed] [Google Scholar]
- 32. Oshiro C, Kagara N, Naoi Y, et al. PIK3CA mutations in serum DNA are predictive of recurrence in primary breast cancer patients. Breast Cancer Res Treat 2015; 150: 299–307. [DOI] [PubMed] [Google Scholar]
- 33. Board RE, Wardley AM, Dixon JM, et al. Detection of PIK3CA mutations in circulating free DNA in patients with breast cancer. Breast Cancer Res Treat 2010; 120: 461–467. [DOI] [PubMed] [Google Scholar]
- 34. Razavi P, Li BT, Brown DN, et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat Med 2019; 25: 1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ptashkin RN, Mandelker DL, Coombs CC, et al. Prevalence of clonal hematopoiesis mutations in tumor-only clinical genomic profiling of solid tumors. JAMA Oncol 2018; 4: 1589–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suppan C, Posch F, Mueller HD, et al. Patterns of recurrence after neoadjuvant therapy in early breast cancer, according to the residual cancer burden index and reductions in neoadjuvant treatment intensity. Cancers (Basel) 2021; 13: 2492. [DOI] [PMC free article] [PubMed] [Google Scholar]