Abstract
Antigens characteristic for Mycobacterium avium subspecies paratuberculosis were identified by crossed immunoelectrophoresis (CIE) and by absorbing out cross-reactive antigens by using a polyclonal and polyvalent Mycobacterium avium subspecies avium antiserum. Two antigens were present in M. avium subsp. paratuberculosis and not detected in Mycobacterium avium subsp. avium. They were identified as antigens 17 and 20 in a CIE reference system for M. avium subsp. paratuberculosis antigens. Purified antigen 20 was identified as alkyl hydroperoxide reductase C (AhpC) while the N-terminal part of purified antigen 17 showed 80% homology with alkyl hydroperoxide reductase D (AhpD) of Mycobacterium tuberculosis. AhpC had a nonreduced mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis corresponding to a molecular mass of 45 kDa and is probably a homodimer linked with disulfide bridges in its native form. AhpD had a mobility corresponding to 19 kDa. Monospecific rabbit antiserum against AhpC and AhpD reacted with 9 strains of M. avium subsp. paratuberculosis but not with 20 other mycobacterial strains except for a Mycobacterium gordonae strain, against which a weak cross-reactive band was produced. Goats experimentally infected with M. avium subsp. paratuberculosis had strong gamma interferon (IFN-γ) responses toward both AhpC and AhpD, and they also had antibodies against AhpC. The ability of AhpC and AhpD to induce IFN-γ production shows that these proteins potentially could be used in future vaccines or in diagnostic assays. These results further show that AhpC and AhpD are immunologically important proteins which are constitutively and highly expressed in M. avium subsp. paratuberculosis without the bacteria being submitted to oxidative stress and that the specificities of antigens can be a matter of different levels of protein expression in various species as well as distinct structural differences.
Mycobacterium avium subsp. paratuberculosis causes a chronic granulomatous infection of the intestines characterized by persistent diarrhea and emaciation in ruminants. The bacterium has also been proposed as an etiologic agent of Crohn's disease in humans (8, 34). Paratuberculosis in ruminants has a long incubation time and most animals remain subclinically infected. The immune responses in paratuberculosis resemble the immune responses towards other mycobacteria such as Mycobacterium leprae, Mycobacterium bovis and Mycobacterium tuberculosis (5, 14, 28). Protective immunity is characterized by strong Th1-cell responses, while animals with fulminating disease have strong antibody responses and weak cellular responses.
The diagnosis of paratuberculosis in living ruminants is based on several tests, and the detection of antibodies by a complement fixation test or enzyme-linked immunosorbent assay and the cultivation of feces are routine laboratory methods. The culture is confirmed to be M. avium subsp. paratuberculosis by the identification of the IS900 element by PCR analysis. The PCR method has also been used directly on feces, but so far this method has not shown sufficient sensitivity for diagnostic use (44). Both cultivation of feces and antibody assays have a low sensitivity, particularly in the early stage of the infection (11, 38). This is strongly related to the finding that animals with minimal disease have low antibody responses but elicit strong Th1-cell responses as determined by the antigen-specific stimulation of cells. These responses can be measured by the gamma interferon (IFN-γ) enzyme immunoassay which originally was designed for the diagnosis of tuberculosis in cattle (Bovigam; CSL, Parkville, Australia) (6, 33, 39, 47).
The specificity of tests for cellular immunity relies on the qualities of the antigen used in the assays. Tests may be improved by selecting antigens or epitopes that are characteristic of M. avium subsp. paratuberculosis. Although several antigens in M. avium subsp. paratuberculosis have been identified (1, 3, 24, 29, 43), only a few of these antigens have been further characterized, including antigen A (a member of the Ag 85 complex), antigen D (7, 41, 45), lipoarabinomannan (42), and the A36 complex with a 34-kDa antigen which was reported to be species specific (13). The antibody responses in infected cattle against some of these antigens have been investigated, but few reports concerning cellular immune responses against purified antigens are available (13, 21, 23).
The close genetic relationship between M. avium subsp. avium and M. avium subsp. paratuberculosis is well established (35, 49), and it is a major challenge to differentiate between infections caused by the two organisms. The two bacteria produce different disease complexes; M. avium subsp. paratuberculosis causes a chronic inflammation in the intestines of ruminants while M. avium subsp. avium is pathogenic for birds and can cause disease in immunocompromised individuals. Even though infections with M. avium subsp. avium or other related mycobacteria usually do not cause disease in ruminants, such infections can give a high number of false-positive results in immunologic diagnostic testing for paratuberculosis in animals. The close genetic relationship between M. avium subsp. paratuberculosis and M. avium subsp. avium resembles that between M. bovis and M. tuberculosis. In the latter pair, there are striking examples of proteins expressed in large quantities by M. bovis that are expressed only in small quantities by M. tuberculosis (26, 30, 46). Similar differences in the patterns of protein expression between M. avium subsp. avium and M. avium subsp. paratuberculosis could be expected to exist. Proteins expressed in large amounts by M. avium subsp. paratuberculosis and in small amounts by M. avium subsp. avium would be valuable for the diagnosis of the disease and may also be important in the pathogenesis of paratuberculosis.
A comparison of M. bovis and M. tuberculosis recently revealed 11 regions (encompassing 91 open reading frames) of M. tuberculosis H37Rv that were absent from one or more virulent strains of M. bovis. These potential open reading frames encode proteins that are thus species specific for M. tuberculosis (4). It is a distinct possibility that species-specific antigens also exist in M. avium subsp. paratuberculosis that are not represented by homologous genes in M. avium subsp. avium. The aim of this work was to identify species-specific antigens of M. avium subsp. paratuberculosis or antigens that show major quantitative differences.
MATERIALS AND METHODS
Strains and antisera.
M. bovis AN5, M. tuberculosis H37rv, and Mycobacterium bovis BCG Moreau were obtained from the National Hospital, Oslo, Norway. Reference strains of 13 mycobacterial species, 16 clinical isolates belonging to the M. avium-Mycobacterium intracellulare complex, and 14 other related bacterial species (Table 1) were obtained from the National Veterinary Institute, Oslo, Norway. The strains used for the purification of antigens were M. avium subsp. paratuberculosis strain 2E and M. avium subsp. avium strain D4. Polyclonal, polyvalent rabbit antisera against M. avium subsp. paratuberculosis strain 2E (batch B312) and M. avium subsp. avium strain D4 were obtained from Dako, Glostrup, Denmark. Monospecific rabbit antiserum was made by immunizing rabbits with precipitation lines formed between purified proteins and polyclonal M. avium subsp. paratuberculosis antiserum. The lines were cut out from crossed immunoelectrophoresis (CIE) gels and mixed with Freund's incomplete adjuvant, and two rabbits were immunized with each antigen (25).
TABLE 1.
Detection of AhpC and AhpD by SDS-PAGE with Western blotting of 43 bacteria
| Bacterial species (no. of isolates) | Strain | Source(s) of isolate | Detection ofa:
|
|
|---|---|---|---|---|
| AhpC | AhpD | |||
| M. avium subsp. paratuberculosis (1) | 2E | 3+ | 3+ | |
| M. avium subsp. paratuberculosis (1) | ATCC 19698b | 3+ | 3+ | |
| M. avium subsp. paratuberculosis (7) | Clinical isolates | Goat; cattle | 3+ | 3+ |
| M. avium subsp. avium (1) | D4 | − | − | |
| M. avium subsp. avium (4) | Clinical isolates | Goat | − | − |
| M. intracellulare (1) | MNC 72c | − | − | |
| M. intracellulare (5) | Clinical isolates | Pig | − | − |
| M. scrofulaceum (1) | MNC 95 | − | − | |
| M. kansasii (1) | MNC 861 | − | − | |
| M. gordonae (1) | MNC 64 | +/− | +/− | |
| M. fortuitum (1) | MNC 103 | − | − | |
| M. phlei (1) | MNC 115 | − | − | |
| M. smegmatis (1) | MNC 13 | − | − | |
| M. bovis (1) | AN5 | − | − | |
| M. bovis BCG (1) | Moreau | − | − | |
| M. tuberculosis (1) | H37Rv | − | − | |
| A. pyogenes (3) | Clinical isolates | Cattle; sheep | − | − |
| R. equi (1) | Clinical isolate | Foal | 1+ | − |
| R. equi (2) | Clinical isolates | Foal | − | − |
| N. asteroides (1) | Clinical isolate | Cattle | − | − |
| C. pseudotuberculosis (2) | Clinical isolates | Goat; unknown | − | − |
| C. renale (3) | Clinical isolates | Cattle | − | − |
| C. flavescens (1) | ATCC 10340 | − | 1+ | |
| C. vitarumen (1) | ATCC 10234 | − | − | |
The band strength was evaluated visually (see examples of Western blots in Fig. 1). −, no visible band; +/−, barely visible band; 1+, weak band; 2+, moderate band; 3+, strong band.
American Type Culture Collection, Rockville, Md.
Mycobacteria Nocardia Collection, Statens Serum Institut, Copenhagen, Denmark.
Bacterial culture and antigen preparation.
M. avium subsp. paratuberculosis strains were cultivated as surface pellicles on liquid synthetic Reid's medium with mycobactin J (2 μg/ml) (Allied Monitor, Fayette, Missouri) until there was sufficient growth at 37°C, and the other mycobacterial species were cultivated as surface pellicles on liquid synthetic Sauton medium for 4 weeks at 37°C. M. avium subsp. avium strain D4 and M. intracellulare strain MNC 72 were cultivated on both Reid's and Sauton medium. Corynebacterium pseudotuberculosis, Corynebacterium renale, Corynebacterium flavescens, Corynebacterium vitarumen, Nocardia asteroides, Arcanobacterium pyogenes, and Rhodococcus equi were cultivated aerobically on brain heart infusion broth on a shaker for 2 to 4 days at 37°C. Harvested bacteria were washed three times in phosphate-buffered saline (PBS) and suspended in PBS at a concentration of 200 mg/ml. The bacteria were kept on ice and sonicated for 20 × 1 min. Sonicated samples were centrifuged at 20,000 × g for 15 min, and the supernatant was filtered (pore size, 0.22 μm) to remove residual particulate material.
Absorption of M. avium subsp. paratuberculosis or M. avium subsp. avium antigens.
M. avium subsp. avium antiserum (800 μl; 45 mg of immunoglobulins [Ig]/ml) was adsorbed to a HiTrap protein G column (Pharmacia, Uppsala, Sweden). Approximately 2 mg of sonicated proteins of M. avium subsp. paratuberculosis 2E and M. avium subsp. avium strain D4 was subsequently applied to the column. Bound IgG-antigen complexes were eluted with glycine-HCl buffer (pH 2.7). The primary effluent and eluate fractions were collected. Between each step, the column was washed with 0.2 mM Na phosphate buffer (pH 7.0). The protein content in the output was monitored by measuring optical density (OD) at 280 nm.
CIE.
CIE with intermediate gels was performed with 1% agarose gels on 5- by 7-cm glass plates according to standard procedures (10). The first dimension was run at 8 V/cm for approximately 1 h until Evan's blue albumin indicator had moved 3 cm. The second dimension was run at 3 V/cm overnight. In the reference plate, 10 μg of M. avium subsp. paratuberculosis sonicate proteins was used as an antigen, and 100 μl of M. avium subsp. paratuberculosis antiserum was incorporated into the top gel. The intermediate gel contained 0.9% NaCl or 100 μl of M. avium subsp. avium antiserum. After second-dimension electrophoresis, the plates were washed and pressed four times, followed by Coomassie brilliant blue (CBB) staining.
SDS-PAGE with immunoblotting.
The antigens were separated under reducing or nonreducing conditions by horizontal sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in precast gradient Excel gel in an 8 to 18% concentration (Pharmacia), using a Multiphor II unit 2117 (Pharmacia). After separation, the gel was stained with CBB or the proteins were transferred to a nitrocellulose membrane (pore size, 0.2 μm) by electroblotting at 0.8 mA/cm2 for 1 h or by diffusion blotting (32). The membranes were blocked with PBS containing 2% bovine serum albumin and 1% gelatin and incubated with serum overnight. Bound antibodies were recognized by horseradish peroxidase-labelled anti-rabbit or anti-goat Ig. As a substrate, 3,3′ diaminobenzidine was added to visualize the bound antibodies.
Molecular masses of AhpC and AhpD by SDS-PAGE.
To determine the molecular masses of these antigens, the precipitation lines were cut out from CIE gels and dissolved in SDS buffer (31). The samples were then sonicated for 15 s and incubated at 37°C overnight before they were subjected to SDS-PAGE and electroblotted onto a nitrocellulose membrane as described above.
Purification of AhpC and AhpD.
The purification procedures for AhpC and AhpD were developed as a combination of ion-exchange chromatography, hydrophobic interaction chromatography, and gel filtration using a Gradi Frac system equipped with a P-50 pump, a UV monitor, and a conductivity monitor (Pharmacia). Fractions were analyzed by SDS-PAGE with diffusion blotting (32). One imprint was incubated with polyvalent M. avium subsp. paratuberculosis antiserum and another was incubated with polyvalent M. avium subsp. avium antiserum. Proteins remaining in the gel after blotting were stained with CBB. The fractions containing proteins with the estimated molecular masses that did react with the M. avium subsp. paratuberculosis antiserum and not the M. avium subsp. avium antiserum were collected.
Purification of AhpD.
In a typical run, the initial protein concentrate (140 mg of M. avium subsp. paratuberculosis culture filtrate proteins) was applied to a Q Sepharose Fast Flow column (Pharmacia) with a bed volume of 15 ml and eluted at 7.5 ml/min with a gradient of 0 to 500 mM NaCl with 30 mM Tris-HCl [pH 8.7] and 3% Methyl Cellosolve (Sigma). The fractions containing AhpD were diluted 1:5 with the same buffer and separated on a 6-ml IEC Resource Q column (Pharmacia) with a linear gradient from 200 to 700 mM NaCl. The fractions with AhpD were diluted 1:2 with 30 mM Tris-HCl (pH 7.5) with 2 M ammonium sulfate, giving a salt concentration of 1 M ammonium sulfate. The pH was adjusted to 7.5, and the sample was run through a Butyl HiTrap (Pharmacia) hydrophobic interaction column. The flowthrough fraction containing AhpD was dialyzed against Tris-HCl buffer (pH 7.5) overnight and then separated on the Resource Q column with a linear gradient from 0 to 0.5 M ammonium sulfate with 30 mM Tris-HCl (pH 7.5) with a flow rate of 3 ml/min. The fractions containing the 19-kDa protein were separated on a Superdex 75 26/60 gel filtration column in PBS with a flow rate of 2 ml/min.
Purification of AhpC.
The first step corresponds to that described for AhpD, but different fractions were recovered for further processing. The fractions containing AhpC were diluted 1:2 with 30 mM Tris-HCl (pH 7.5) with 2 M ammonium sulfate, giving a salt concentration of 1 M ammonium sulfate. The pH was adjusted to 7.5 and the sample was separated on 6 ml of Source phenyl medium (Pharmacia) that was packed in an XK16 column. The proteins were eluted at 4 ml/min with a gradient of 1 to 0 M ammonium sulfate with 30 mM Tris-HCl (pH 7.5). The recovered fractions containing AhpC were separated on a Resource Q column with at a flow rate of 3 ml/min with a gradient of 0 to 0.5 M ammonium sulfate in the same buffer, followed by a hydrophobic interaction on a Resource phenyl column at a flow rate of 2 ml/min with a gradient of 1 to 0 M ammonium sulfate.
N-terminal amino acid sequencing.
Automatic Edman degradation was performed on a 477A protein sequencer with an online 120-A phenylthiohydantoin amino acid analyzer (Applied Biosystems, Foster City, Calif.).
IFN-γ production.
Goat kids between 2 and 3 months of age were infected with M. avium subsp. paratuberculosis three times a week for 9 weeks. The infection model has been described briefly elsewhere (40) and will be described in detail later. One year after infection, when the goats still showed no sign of clinical disease, peripheral blood leukocytes were assayed for IFN-γ production. One milliliter of heparinized blood was dispensed into 24-well tissue culture trays and IFN-γ production was measured after stimulation with antigens. The samples were stimulated with purified AhpC (2.5 μg/ml), purified AhpD (2.5 μg/ml), and Johnin purified protein derivative (PPD) (10 μg/ml) and incubated at 37°C in 5% CO2 in air for 24 h. The plasma was removed and assayed for IFN-γ with a bovine IFN-γ enzyme immunoassay (Bovigam). The OD values in response to the various antigens minus the OD values in a nonstimulated control well containing cells from the same animal sample gave the corrected OD values. According to the manufacturer's instructions, the test was considered negative when corrected OD values were below 0.05.
RESULTS
Identification of antigens characteristic for M. avium subsp. paratuberculosis with CIE.
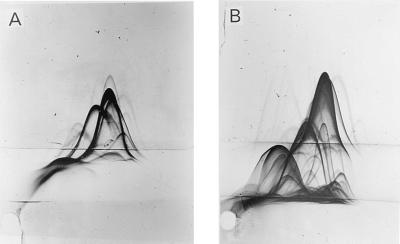
A reference plate was made as described previously and several precipitation lines were seen (Fig. 1A). To identify antigens highly expressed by M. avium subsp. paratuberculosis but not by M. avium subsp. avium, a polyclonal and polyvalent M. avium subsp. avium antiserum was incorporated into the intermediate gel on a reference plate (Fig. 1B). Cross-reactive precipitation lines were pulled down into the intermediate gel. However, several precipitation lines remained in the same position in the top gel, demonstrating virtually no antibody reactivity against these antigens in anti-M. avium subsp. avium. The most distinct of these precipitation lines showed a characteristic “inward-feet reaction” indicating a weak cross-reactivity with M. avium subsp. avium.
FIG. 1.
CIE of M. avium subsp. paratuberculosis sonicate. (A) Sonicated proteins were separated in the first dimension. Polyclonal M. avium subsp. paratuberculosis antiserum was incorporated into the top gel and NaCl (0.9%) was incorporated into the intermediate gel. (B) Sonicated proteins were separated in the first dimension. Polyclonal M. avium subsp. paratuberculosis antiserum was incorporated into the top gel while polyclonal anti-M. avium subsp. avium antiserum was incorporated into the intermediate gel.
Preparative isolation of M. avium subsp. paratuberculosis-specific antigens.
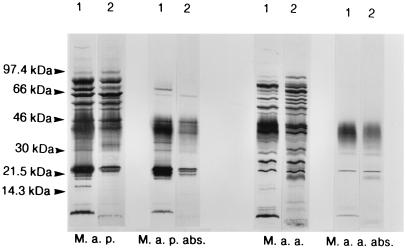
The CIE experiment indicated that a crude preparation of M. avium subsp. paratuberculosis-specific antigens could be made by a one-step affinity chromatographic procedure. Cross-reactive antigens in the M. avium subsp. paratuberculosis sonicate were removed as described. The collected fractions were investigated by SDS-PAGE with Western blotting. Peptides with molecular masses of 18, 31, 34, and 70 to 80 kDa in the primary effluent fraction of M. avium subsp. paratuberculosis reacted with the polyclonal M. avium subsp. paratuberculosis antiserum and not with the polyclonal M. avium subsp. avium antiserum in Western blotting (Fig. 2). There were no bands of corresponding molecular masses in the primary effluent of the M. avium subsp. avium sonicate. Several bands in the 22- to 25-kDa region could be detected in the primary effluent of M. avium subsp. paratuberculosis with both of the polyclonal antisera. The reactions were stronger and there were more bands detected with M. avium subsp. paratuberculosis antibodies. In the molecular mass range of 41 to 43 kDa, there was strong reactivity with M. avium subsp. paratuberculosis antiserum and weak, if any, reaction with M. avium subsp. avium antiserum. The bands that still could be detected with M. avium subsp. avium antiserum in the primary effluent of M. avium subsp. paratuberculosis and M. avium subsp. avium can be explained by inadequate absorption or reaction with Ig fractions that did not bind to the protein G column.
FIG. 2.
SDS-PAGE with Western blotting of sonicated M. avium subsp. paratuberculosis and M. avium subsp. avium before and after absorption on polyclonal M. avium subsp. avium antiserum. M. a. p., M. avium subsp. paratuberculosis; M. a. a., M. avium subsp. avium; M. a. p. abs., absorbed M. avium subsp. paratuberculosis (primary effluent fraction of M. avium subsp. paratuberculosis); M. a. a. abs., absorbed M. avium subsp. avium (primary effluent fraction of M. avium subsp. avium). Lanes 1, incubated with M. avium subsp. paratuberculosis antiserum; lanes 2, incubated with M. avium subsp. avium antiserum.
Identification of AhpC and AhpD.
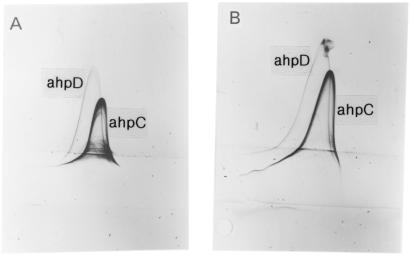
The primary effluent fraction was also tested in CIE (Fig. 3A). Two distinct precipitation lines were seen in addition to several weak lines. M. avium subsp. avium antiserum was then put in the intermediate gel to identify cross-reactivity with this antiserum (Fig. 3B). The M. avium subsp. avium antiserum did not affect the position of the two distinct precipitates, but one of these lines gave a characteristic inward-feet reaction as also seen in Fig. 1B, indicating that weakly cross-reactive antibodies were present in the M. avium subsp. avium antiserum. The migration distance and the shape in the first dimension of this line, as well as the weak cross-reactivity, resemble antigen 20 as described by Gunnarsson and Fodstad (24). They also described another antigen (antigen 17) in the proximity of antigen 20 that seemed to be specific for M. avium subsp. paratuberculosis. They found that three M. avium subsp. paratuberculosis antisera reacted with antigen 17 and antigen 20, and that M. avium subsp. avium and BCG antisera did not. These observations correspond very closely with our results. The N-terminal amino acid sequences described later show that antigen 20 is homologous to AhpC and antigen 17 is homologous to AhpD, so we have used these names.
FIG. 3.
CIE of crude preparation of specific M. avium subsp. paratuberculosis antigens. (A) The primary effluent fraction of M. avium subsp. paratuberculosis was separated in the first dimension. Polyclonal M. avium subsp. paratuberculosis antiserum was incorporated into the top gel while NaCl (0.9%) was incorporated into the intermediate gel. (B) The primary effluent fraction of M. avium subsp. paratuberculosis was separated in the first dimension. Polyclonal M. avium subsp. paratuberculosis antiserum was incorporated into the top gel while polyclonal M. avium subsp. avium antiserum was incorporated into the intermediate gel.
Molecular masses of AhpC and AhpD.
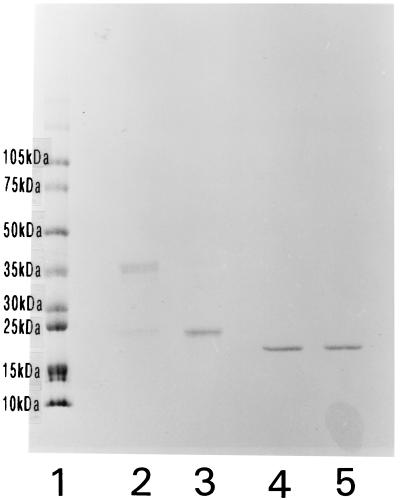
The precipitation lines representing AhpC and AhpD were prepared for SDS-PAGE as described above to determine the molecular masses of the peptides. In Western blotting with M. avium subsp. paratuberculosis antiserum, AhpC was seen as broad band at about 45 kDa and a broad band at about 24 kDa. AhpD migrated with an apparent molecular mass of 19 kDa. However, by gel filtration on Superdex 75, AhpC could be detected in the void volume fraction, indicating a molecular mass larger than 70 kDa and that AhpC was part of a larger complex. To investigate the connection between the different bands in AhpC, a sample was separated under reducing and nonreducing conditions by SDS-PAGE with subsequent staining with CBB. Distinct differences could be observed. When purified AhpC was separated under nonreducing conditions, the most distinct band was seen at 45 kDa and a weaker band was seen at 24 kDa. Under reducing conditions, the peptide at 24 kDa became dominant and only a faint band was detected at 45 kDa (Fig. 4). These results indicate that AhpC is linked with disulfide bridges and that it exists as a homodimer in its native form. AhpD migrated as a single band under reducing and nonreducing conditions.
FIG. 4.
SDS-PAGE with CBB staining of purified AhpC and AhpD separated under reducing or nonreducing conditions. Lanes: 1, molecular mass standard; 2, AhpC-nonreducing conditions; 3, AhpC-reducing conditions; 4, AhpD-nonreducing conditions; 5, AhpD-reducing conditions.
Purification and purity of AhpC and AhpD samples.
The yield from one run of 140 mg of crude protein was approximately 1 mg of AhpC and 300 μg of AhpD. The purified antigens were tested by SDS-PAGE with CBB staining and Western blotting with polyclonal antisera against M. avium subsp. paratuberculosis and M. avium subsp. avium. Only the bands representing AhpC and AhpD could be detected in the representative samples by Western blotting with the M. avium subsp. paratuberculosis antiserum and by CBB staining. The purified AhpC seemed to contain some degradation products, since two peptides could be seen at 23 to 24 kDa. These peptides were inseparable throughout the purification procedure, and the clear signal obtained by N-terminal sequencing of AhpC suggests that these are degradation products of the same protein.
To identify the purified proteins in CIE, AhpC and AhpD were put in the intermediate gels of two different CIE plates with the primary effluent fraction of M. avium subsp. paratuberculosis separated in the first dimension and polyclonal M. avium subsp. paratuberculosis antiserum in the top gel. The purified proteins formed horizontal precipitation lines that fused with and elevated the AhpC and AhpD precipitation lines, respectively. These results confirm the identity between the purified proteins and the lines representing AhpC and AhpD (results not shown).
N-terminal amino acid sequence.
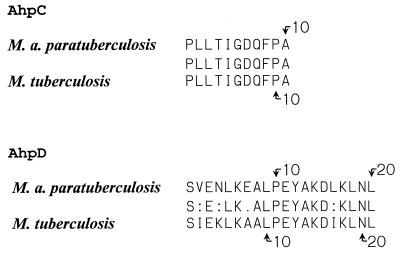
Purified AhpC and AhpD were subjected to N-terminal amino acid sequencing. The first 11 amino acids were identified in AhpC and the first 20 amino acids were identified in AhpD. The amino acid sequences are given in Fig. 5.
FIG. 5.
Primary N-terminal amino acid sequences of AhpC and AhpD from M. avium subsp. paratuberculosis aligned with M. tuberculosis AhpC and AhpD, respectively. Arrows indicate the positions of amino acids.
Database searches to identify homologous genes.
GenBank and EMBL release no. 109 and 56 were searched for homologous peptides with the N-terminal amino acid sequences from AhpD and AhpC. An N-terminal amino acid sequence identical to AhpC was found in M. avium subsp. avium and in M. tuberculosis. The molecular masses of the homologous peptides were 21.7 kDa. The overall homology between AhpC from these two species was 90% by a Lipman-Pearson pairwise protein alignment (27). A peptide with 80% homology to AhpD which had an estimated molecular mass of 19 kDa was found in M. tuberculosis. The genes encoding AhpC and AhpD are located adjacent to each other on the M. tuberculosis genome (12) (Rv2428 and Rv2429, respectively).
Preparation of monospecific rabbit antibodies to AhpC and AhpD.
Monospecific polyclonal antiserum against purified AhpC and AhpD was made in rabbits and the resulting antibodies were characterized by CIE and Western blotting. In CIE, the crude antigen-specific preparation of M. avium subsp. paratuberculosis was applied to the antigen well, and M. avium subsp. paratuberculosis antiserum was incorporated into the top gel. The same precipitation lines that were elevated when AhpC and AhpD were incorporated into the intermediate gel were pulled down by the monospecific antisera when the sera were put into the intermediate gel, confirming that the antisera reacted with the corresponding precipitation lines. Western blotting of M. avium subsp. paratuberculosis sonicate showed that the monospecific antisera reacted only with the bands representing AhpC or AhpD.
Interspecies cross-reactivity of polyclonal AhpC and AhpD antibodies.
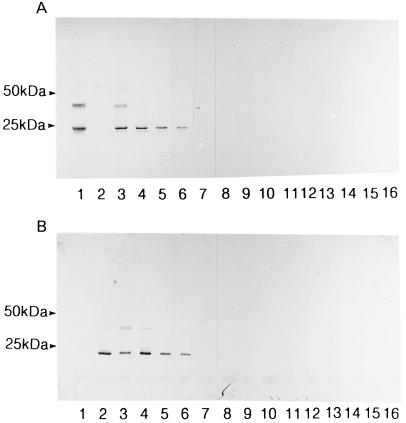
Several mycobacterial and other related bacterial species were tested by SDS-PAGE with Western blotting with the specific antisera against AhpC and AhpD (Fig. 6 and Table 1). The antiserum against AhpC reacted with all the M. avium subsp. paratuberculosis strains at 23 and 24 kDa. A broad band at 43 to 45 kDa could be seen in some of the paratuberculosis strains but was most prominent in strain 2E. The antiserum against AhpD also reacted with all the M. avium subsp. paratuberculosis strains, showing a distinct band at 19 kDa. In addition, one or two faint bands at 41 kDa could be seen in these strains. M. avium subsp. avium (D4) and M. intracellulare (MNC 72) did not react with AhpC and AhpD antisera when cultivated on Reid's or Sauton medium, showing that there is no difference in expression of these proteins due to differences between Reid's and Sauton media. The two antisera did not react with any of the other mycobacterium strains, except for producing faint bands in Mycobacterium gordonae (invisible in copy) that migrated with corresponding molecular masses, showing that AhpC and AhpD were expressed in very low amounts by this species. The AhpC antiserum reacted with one R. equi strain and the AhpD antiserum reacted with C. flavescens, also giving bands at the expected positions of AhpC and AhpD.
FIG. 6.
SDS-PAGE under reducing conditions with Western blotting of different mycobacterial species. (A) The membrane containing the separated proteins was incubated with monospecific antiserum against AhpC. (B) The membrane containing the separated proteins was incubated with monospecific antiserum against AhpD. Lanes: 1, purified AhpC; 2, purified AhpD; 3, M. avium subsp. paratuberculosis 2E; 4, M. avium subsp. paratuberculosis ATCC 19698; 5, M. avium subsp. paratuberculosis 173p (goat clinical isolate); 6, M. avium subsp. paratuberculosis (goat clinical isolate); 7, M. avium subsp. avium D4; 8, M. intracellulare MNC 72; 9, Mycobacterium scrofulaceum MNC 95; 10, Mycobacterium kansasii MNC 861; 11, M. gordonae MNC 64; 12, Mycobacterium fortuitum MNC 103; 13, Mycobacterium phlei MNC 115; 14, M. bovis AN5; 15, M. tuberculosis H37Rv; 16, M. bovis BCG Moreau.
IFN-γ production.
To test the immunogenicity of the two purified proteins, heparinized blood from experimentally infected goats was stimulated with AhpC, AhpD, and PPD. Cells from all the infected animals produced IFN-γ when stimulated with AhpC, AhpD, or PPD while cells from the uninfected control did not (Table 2). The results are given in corrected OD values (ODantigen-stimulated − ODnonstimulated) representing the specific elevation of IFN-γ production by the various antigens. The levels of IFN-γ in the nonstimulated samples were low in all the animals. The amount of IFN-γ production in response to the different antigens correlated positively; the animal with the highest response to PPD also had the highest response to the two purified antigens. The response was always highest when the cells were stimulated with PPD, followed by AhpC and then AhpD. The response to AhpD was relatively low compared to the response to AhpC. The response to AhpC was comparatively high, considering that 10 μg of PPD per ml was used, compared to a concentration of 2.5 μg/ml for the purified proteins. None of the antigens induced IFN-γ production in cells from the uninfected control. These results show that T-cell epitopes are present on AhpC and AhpD and that the proteins induce a specific, probably T-cell-mediated, response in peripheral blood leukocytes from animals with subclinical paratuberculosis.
TABLE 2.
IFN-γ responses toward different antigens in experimentally infected goats (corrected OD values)
| Animal | Antigen (concn)
|
||
|---|---|---|---|
| AhpC (2.5 μg/ml) | AhpD (2.5 μg/ml) | PPD (10 μg/ml) | |
| 705 | 0.07 | 0.06 | 0.50 |
| 711 | 0.97 | 0.15 | 2.25 |
| 718 | 0.43 | 0.11 | 1.50 |
| 708a | 0.0 | 0.01 | 0.0 |
Animal 708 is a healthy noninfected control.
Antibody responses.
Antibody production against purified AhpC and AhpD in four experimentally infected goats and one control goat was tested 20 months postinfection by Western blotting. Sera from all the infected animals reacted with AhpC but no reaction against AhpD could be detected, showing that B-cell epitopes are present on AhpC. Antibodies against the proteins were not detected in the control animal.
DISCUSSION
The aim of this study was to identify species-specific proteins in M. avium subsp. paratuberculosis or proteins that are expressed in much larger amounts in this bacterium than in other closely related mycobacteria. We have identified two antigens which are homologous to AhpC and AhpD from M. tuberculosis. AhpC and AhpD were expressed in large amounts in M. avium subsp. paratuberculosis and could not be detected by Western blotting of other mycobacterial species except for minor quantities in M. gordonae when the bacilli were grown without exposure to oxidative stress. The high expression of AhpC and AhpD in M. avium subsp. paratuberculosis without the need for peroxide induction is a unique feature of this bacterium. This indicates that these enzymes may be important for the particular adaptation of this organism as an extremely slow-growing intestinal pathogen and for the virulence of M. avium subsp. paratuberculosis.
AhpC and AhpD are detoxifying enzymes that are important for protection against reactive nitric and oxidative metabolites. Expression of these proteins together with other detoxifying enzymes are controlled by the central regulator OxyR (9, 22). In gram-negative bacteria, the oxyR gene can be activated by low doses of hydrogen peroxide, which induces the production of several proteins that can protect the cell from a subsequent lethal dose of peroxide (9, 15). oxyR and ahpC homologues are also present in several mycobacterial species, but only saprophytic Mycobacterium smegmatis shows a similar protective response, with the induction of a novel protein synthesis when exposed to low doses of hydrogen peroxide (37). In contrast, pathogenic mycobacteria (M. tuberculosis and M. avium subsp. avium) seem to lack the capacity for a protective oxyR response of the type originally defined for Escherichia coli and Salmonella.
In M. tuberculosis, the oxyR gene is nonfunctional due to deletions and point mutations (16, 17, 37) and as a result, AhpC is usually not expressed in M. tuberculosis. However, some isoniazid-resistant strains that lack KatG activity have been shown to overproduce AhpC as a compensatory mechanism (36).
The oxyR gene appears intact in M. avium subsp. avium and the expression of AhpC increases markedly when exposed to hydrogen peroxide (37). The first report on AhpC, designated Avi3, from M. avium subsp. avium described the detection of this protein in M. avium subsp. avium but not in other mycobacteria, including M. avium subsp. paratuberculosis (2, 48). Our findings do not support these observations. We observed that AhpC and AhpD were highly expressed in M. avium subsp. paratuberculosis and not detected in M. avium subsp. avium when the bacilli were grown without exposure to oxidative stress. In the earlier studies, a monoclonal antibody made against AhpC from M. avium subsp. avium was used when screening the various mycobacterial species, and minor differences in amino acid sequence could explain the failure of this monoclonal antibody to react with M. avium subsp. paratuberculosis. However, in the present study monospecific polyclonal antiserum against purified proteins was used that would have reacted with corresponding proteins in the M. avium subsp. avium strains if these proteins were expressed in this species. Another indication of the low expression of AhpC and AhpD in M. avium subsp. avium and their high expression in M. avium subsp. paratuberculosis was the strong reaction of AhpC and AhpD with the polyclonal antiserum made in rabbits against M. avium subsp. paratuberculosis. An equivalent antiserum against M. avium subsp. avium had very low levels of cross-reactive antibodies against AhpC and no cross-reaction was seen with AhpD. Elsaghier et al. have also shown specifically elevated antibody responses in M. avium subsp. paratuberculosis-infected mice but not in M. avium subsp. avium-infected mice towards a protein that later was identified as AhpC. This shows that AhpC is also expressed by M. avium subsp. paratuberculosis in vivo (19, 20). The N-terminal amino acid sequence from the purified AhpC of M. avium subsp. paratuberculosis is identical to the N-terminal sequence of AhpC from M. avium subsp. avium, and the homology between AhpC from M. avium subsp. avium and M. tuberculosis is about 90%. It is thus unlikely that major differences are present further along the peptide, considering the close genetic relationship between M. avium subsp. paratuberculosis and M. avium subsp. avium. We interpret our results as indicating that AhpC and AhpD are expressed in much larger amounts in M. avium subsp. paratuberculosis but we cannot exclude the possibility that differences in amino acid sequence exist between the proteins from the two species. Sequencing of the genes encoding AhpC and AhpD and the OxyR transcriptional regulator in M. avium subsp. paratuberculosis and comparison with the equivalent sequences in M. avium subsp. avium are necessary in order to clarify these questions.
The immune response against detoxifying enzymes has received little attention, but it has been shown that AhpC from M. avium subsp. avium can elicit both delayed-type hypersensitivity responses and lymphocyte proliferation in immunized guinea pigs (48). Deretic et al. (18) have suggested that the silencing of oxyR in M. tuberculosis may have played a role in the evolution of this species and its adaptation to macrophage parasitism. These investigators suggest that several genes are shut down when M. tuberculosis enters the macrophage in order to avoid interactions with the immune system and thus prevent stimulation of a vigorous Th1 response, which is important for protective immunity. Interestingly, purified AhpC and AhpD from M. avium subsp. paratuberculosis elicited a strong IFN-γ response in goats with experimental subclinical paratuberculosis. Strong IFN-γ responses were produced by both antigens, especially by AhpC, indicating that these proteins might play a role in protection against paratuberculosis. AhpC also elicited B-cell responses in experimentally infected goats at a later stage of infection. The strong IFN-γ response against AhpC and AhpD shows that these proteins can potentially be used in the diagnosis of paratuberculosis with an IFN-γ-based test. However, the amount of individual variation of IFN-γ response to some proteins is large, so further testing is needed to see whether these proteins can be used for diagnostic purposes. Even though AhpC and AhpD are expressed in large amounts in M. avium subsp. paratuberculosis in vitro, the situation in vivo may differ. The presence of homologous genes makes it necessary to test animals infected with different mycobacterial species to evaluate the potential of these proteins in diagnostic assays. The ability of AhpC and AhpD to induce IFN-γ production also makes these proteins good candidates for inclusion in new vaccines against paratuberculosis.
ACKNOWLEDGMENT
This work was supported by grants from the Norwegian Research Council (project no. 116086).
REFERENCES
- 1.Abbas B, Riemann H P, Lonnerdal B. Isolation of specific peptides from Mycobacterium paratuberculosis protoplasm and their use in an enzyme-linked immunosorbent assay for the detection of paratuberculosis (Johne's disease) in cattle. Am J Vet Res. 1983;44:2229–2236. [PubMed] [Google Scholar]
- 2.Abe C, Saito H, Tomioka H, Fukasawa Y. Production of a monoclonal antibody specific for Mycobacterium avium and immunological activity of the affinity-purified antigen. Infect Immun. 1989;57:1095–1099. doi: 10.1128/iai.57.4.1095-1099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bech-Nielsen S, Burianek L L, Spangler E, Heider L E, Hoffsis G F, Dorn C R. Characterization of Mycobacterium paratuberculosis antigenic proteins. Am J Vet Res. 1985;46:2418–2420. [PubMed] [Google Scholar]
- 4.Behr M A, Wilson M A, Gill W P, Salamon H, Schoolnik G K, Rane S, Small P M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 5.Bendixen P H. Immunological reactions caused by infection with Mycobacterium paratuberculosis. A review. Nord Vetmed. 1978;30:163–168. [PubMed] [Google Scholar]
- 6.Billman-Jacobe H, Carrigan M, Cockram F, Corner L A, Gill I J, Hill J F, Jessep T, Milner A R, Wood P R. A comparison of the interferon gamma assay with the absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust Vet J. 1992;69:25–28. doi: 10.1111/j.1751-0813.1992.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 7.Brooks B W, Young N M, Watson D C, Robertson R H, Sugden E A, Nielsen K H, Becker S A. Mycobacterium paratuberculosis antigen D: characterization and evidence that it is a bacterioferritin. J Clin Microbiol. 1991;29:1652–1658. doi: 10.1128/jcm.29.8.1652-1658.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiodini R J, Van Kruiningen H J, Thayer W R, Merkal R S, Coutu J A. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig Dis Sci. 1984;29:1073–1079. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- 9.Christman M F, Morgan R W, Jacobson F S, Ames B N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 10.Closs O, Harboe M, Axelsen N H, Bunch-Christensen K, Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12:249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 11.Cocito C, Gilot P, Coene M, de Kesel M, Poupart P, Vannuffel P. Paratuberculosis. Clin Microbiol Rev. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 13.de Kesel M, Gilot P, Misonne M C, Coene M, Cocito C. Cloning and expression of portions of the 34-kilodalton-protein gene of Mycobacterium paratuberculosis: its application to serological analysis of Johne's disease. J Clin Microbiol. 1993;31:947–954. doi: 10.1128/jcm.31.4.947-954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lisle G W, Duncan J R. Bovine paratuberculosis III. An evaluation of a whole blood lymphocyte transformation test. Can J Comp Med. 1981;45:304–309. [PMC free article] [PubMed] [Google Scholar]
- 15.Demple B, Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983;304:466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- 16.Deretic V, Pagan-Ramos E, Zhang Y, Dhandayuthapani S, Via L E. The extreme sensitivity of Mycobacterium tuberculosis to the front-line antituberculosis drug isoniazid. Nat Biotechnol. 1996;14:1557–1561. doi: 10.1038/nbt1196-1557. [DOI] [PubMed] [Google Scholar]
- 17.Deretic V, Philipp W, Dhandayuthapani S, Mudd M H, Curcic R, Garbe T, Heym B, Via L E, Cole S T. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol. 1995;17:889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- 18.Deretic V, Song J, Pagan-Ramos E. Loss of oxyR in Mycobacterium tuberculosis. Trends Microbiol. 1997;5:367–372. doi: 10.1016/S0966-842X(97)01112-8. [DOI] [PubMed] [Google Scholar]
- 19.Elsaghier A, Nolan A, Allen B, Ivanyi J. Distinctive Western blot antibody patterns induced by infection of mice with individual strains of the Mycobacterium avium complex. Immunology. 1992;76:355–361. [PMC free article] [PubMed] [Google Scholar]
- 20.Elsaghier A, Prantera C, Moreno C, Ivanyi J. Antibodies to Mycobacterium paratuberculosis-specific protein antigens in Crohn's disease. Clin Exp Immunol. 1992;90:503–508. doi: 10.1111/j.1365-2249.1992.tb05874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Zaatari F A, Naser S A, Graham D Y. Characterization of a specific Mycobacterium paratuberculosis recombinant clone expressing 35,000-molecular-weight antigen and reactivity with sera from animals with clinical and subclinical Johne's disease. J Clin Microbiol. 1997;35:1794–1799. doi: 10.1128/jcm.35.7.1794-1799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farr S B, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilot P, de Kesel M, Coene M, Cocito C. Induction of cellular immune reactions by A36, an antigen complex of Mycobacterium paratuberculosis: comparison of A36 and johnin components. Scand J Immunol. 1992;36:811–821. doi: 10.1111/j.1365-3083.1992.tb03143.x. [DOI] [PubMed] [Google Scholar]
- 24.Gunnarsson E, Fodstad F H. Analysis of antigens in Mycobacterium paratuberculosis. Acta Vet Scand. 1979;20:200–215. doi: 10.1186/BF03546612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harboe M, Closs O, Deverill J. Production of monospecific antisera against antigenic components of Mycobacterium bovis (BCG) Scand J Immunol. 1976;5:861–866. doi: 10.1111/j.1365-3083.1976.tb03035.x. [DOI] [PubMed] [Google Scholar]
- 26.Harboe M, Nagai S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am Rev Respir Dis. 1984;129:444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- 27.Lipman D J, Pearson W R. Rapid and sensitive protein similarity searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- 28.Merkal R S, Kopecky K E, Larsen A B. Immunologic mechanisms in bovine paratuberculosis. Am J Vet Res. 1970;31:475–485. [PubMed] [Google Scholar]
- 29.Mutharia L M, Moreno W, Raymond M. Analysis of culture filtrate and cell wall-associated antigens of Mycobacterium paratuberculosis with monoclonal antibodies. Infect Immun. 1997;65:387–394. doi: 10.1128/iai.65.2.387-394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai S, Matsumoto J, Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981;31:1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norrild B. Radioimmunoelectrophoresis for determination of molecular size of polypeptides. Scand J Immunol. 1983;17:279–282. [Google Scholar]
- 32.Olsen I, Wiker H G. Diffusion blotting for rapid production of multiple identical imprints from sodium dodecyl sulfate polyacrylamide gel electrophoresis on a solid support. J Immunol Methods. 1998;220:77–84. doi: 10.1016/s0022-1759(98)00147-1. [DOI] [PubMed] [Google Scholar]
- 33.Rothel J S, Jones S L, Corner L A, Cox J C, Wood P R. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust Vet J. 1990;67:134–137. doi: 10.1111/j.1751-0813.1990.tb07730.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanderson J D, Moss M T, Tizard M L, Hermon-Taylor J. Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Gut. 1992;33:890–896. doi: 10.1136/gut.33.7.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxegaard F, Baess I, Jantzen E. Characterization of clinical isolates of Mycobacterium paratuberculosis by DNA-DNA hybridization and cellular fatty acid analysis. APMIS. 1988;96:497–502. [PubMed] [Google Scholar]
- 36.Sherman D R, Mdluli K, Hickey M J, Arain T M, Morris S L, Barry III C E, Stover C K. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science. 1996;272:1641–1643. doi: 10.1126/science.272.5268.1641. [DOI] [PubMed] [Google Scholar]
- 37.Sherman D R, Sabo P J, Hickey M J, Arain T M, Mahairas G G, Yuan Y, Barry III C E, Stover C K. Disparate responses to oxidative stress in saprophytic and pathogenic mycobacteria. Proc Natl Acad Sci USA. 1995;92:6625–6629. doi: 10.1073/pnas.92.14.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sockett D C, Conrad T A, Thomas C B, Collins M T. Evaluation of four serological tests for bovine paratuberculosis. J Clin Microbiol. 1992;30:1134–1139. doi: 10.1128/jcm.30.5.1134-1139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stabel J R. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J Vet Diagn Investig. 1996;8:345–350. doi: 10.1177/104063879600800311. [DOI] [PubMed] [Google Scholar]
- 40.Storset A K, Hasvold H J, Bernsten G, Knagenhjelm S K H, Larsen H J S. Early immune responses of goats during experimental Mycobacterium avium ss paratuberculosis infection. Scand J Immunol. 1998;47:615–660. [Google Scholar]
- 41.Sugden E A, Brooks B W, Young N M, Watson D C, Nielsen K H, Corner A H, Turcotte C, Michaelides A, Stewart R B. Chromatographic purification and characterization of antigens A and D from Mycobacterium paratuberculosis and their use in enzyme-linked immunosorbent assays for diagnosis of paratuberculosis in sheep. J Clin Microbiol. 1991;29:1659–1664. doi: 10.1128/jcm.29.8.1659-1664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugden E A, Samagh B S, Bundle D R, Duncan J R. Lipoarabinomannan and lipid-free arabinomannan antigens of Mycobacterium paratuberculosis. Infect Immun. 1987;55:762–770. doi: 10.1128/iai.55.3.762-770.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tizard M L, Moss M T, Sanderson J D, Austen B M, Hermon-Taylor J. p43, the protein product of the atypical insertion sequence IS900, is expressed in Mycobacterium paratuberculosis. J Gen Microbiol. 1992;138:1729–1736. doi: 10.1099/00221287-138-8-1729. [DOI] [PubMed] [Google Scholar]
- 44.van der Giessen J W, Haring R M, Vauclare E, Eger A, Haagsma J, van der Zeijst B A. Evaluation of the abilities of three diagnostic tests based on the polymerase chain reaction to detect Mycobacterium paratuberculosis in cattle: application in a control program. J Clin Microbiol. 1992;30:1216–1219. doi: 10.1128/jcm.30.5.1216-1219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiker H G, Harboe M, Nagai S, Patarroyo M E, Ramirez C, Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81:307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- 46.Wiker H G, Nagai S, Hewinson R G, Russell W P, Harboe M. Heterogenous expression of the related MPB70 and MPB83 proteins distinguishes various substrains of Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv. Scand J Immunol. 1996;43:374–380. doi: 10.1046/j.1365-3083.1996.d01-61.x. [DOI] [PubMed] [Google Scholar]
- 47.Wood P R, Corner L A, Plackett P. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res Vet Sci. 1990;49:46–49. [PubMed] [Google Scholar]
- 48.Yamaguchi R, Matsuo K, Yamazaki A, Takahashi M, Fukasawa Y, Wada M, Abe C. Cloning and expression of the gene for the Avi-3 antigen of Mycobacterium avium and mapping of its epitopes. Infect Immun. 1992;60:1210–1216. doi: 10.1128/iai.60.3.1210-1216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimura H H, Graham D Y. Nucleic acid hybridization studies of mycobactin-dependent mycobacteria. J Clin Microbiol. 1988;26:1309–1312. doi: 10.1128/jcm.26.7.1309-1312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]