Abstract
Heart failure with preserved ejection fraction has a higher prevalence in women versus men. There are several proposed mechanisms to explain this sex discrepancy including differences in cardiovascular adaptation to comorbidities and potential underlying etiologic mechanisms. In this review, we summarize sex differences in traditional risk factors, such as obesity, diabetes, hypertension, and coronary artery disease, which contribute to the development of heart failure with preserved ejection fraction in women. Furthermore, we explore female-specific risk factors, such as sex hormones, adverse pregnancy outcomes, and other reproductive factors, which may explain the predominance of heart failure with preserved ejection fraction in women. Beyond sex differences in risk factors, there are also significant sex differences in outcomes with women reporting lower quality of life but overall better survival versus men. Finally, while treatment options for patients with heart failure with preserved ejection fraction are still limited, sex differences have also been reported for the available therapies, with suggestion of preferential benefit of specific heart failure with preserved ejection fraction therapies in women. Further work is required to better understand sex differences in heart failure with preserved ejection fraction, including deeper understanding of pathophysiological mechanisms, derivation of more accurate risk stratification models, and increased representation of women in clinical trials.
Keywords: heart failure, HFpEF, sex differences
Introduction
Heart failure (HF) affects approximately six million Americans above the age of 20, with a projected prevalence of more than eight million by 2030.1,2 HF encompasses a broad range of phenotypes and is further subdivided into HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), both of which have unique epidemiology, risk factors, and treatment options. Secular trends from the Framingham Heart Study have demonstrated a declining incidence for HFrEF, but an increasing incidence for HFpEF over two decades (1990–1999 versus 2000–2009).1 Significant sex differences exist in the realm of HF, particularly when considering HFpEF. For example, the odds of HFpEF were 2.8-fold higher in women than in men within the Framingham Heart Study.3,4 The prevalence of HFpEF has also been demonstrated to be higher in women and increases with age (8%–10% in women and 4%–6% in men for individuals ⩾ 80 years of age).5 Epidemiologic analyses from large registries found that women comprise 55% of patients with HFpEF, but only 29% of patients with HFrEF.6
There is no clear consensus on the exact pathophysiological mechanisms to explain sex differences in prevalence, but hypotheses include greater inflammation and chronic microvascular dysfunction in women, two purported mechanistic drivers of HFpEF (Figure 1).7,8 Furthermore, traditional risk factors (obesity, diabetes, hypertension, and coronary artery disease (CAD)) preferentially contribute to development of HFpEF in women, and emerging data show that sex-specific risk factors including early menopause, adverse pregnancy outcomes (APOs), and other reproductive factors are also important risk factors for HFpEF in women. Finally, HFpEF is a clinical syndrome with considerable phenotypic heterogeneity, in part related to differences in patient profiles in men versus women. For example, previous studies have demonstrated notable phenotypic differences based on sex—women have more concentric left ventricular (LV) remodeling and more severe diastolic dysfunction with impaired LV relaxation versus men.9,10 A previous study analyzing data from three large HFpEF trials showed age-related differences in HFpEF: Younger patients were more often obese men and older patients were more often women with higher prevalence of comorbidities.11 Novel phenotyping tools are now able to identify distinct groups of HFpEF patients that differ in risk profile, outcomes, and clinical trajectories. One study leveraging these novel phenotyping tools found three separate phenogroups among a US-based cohort of 397 patients with HFpEF: (1) younger females with less adverse remodeling, (2) predominately females with obesity and diabetes, and (3) older men and women with chronic kidney disease and adverse LV remodeling.12 Beyond the sex differences in prevalence and clinical profile, there are also sex differences in outcomes within the HFpEF population, with women reporting lower quality of life (QOL) as compared to men13 but overall better survival.14
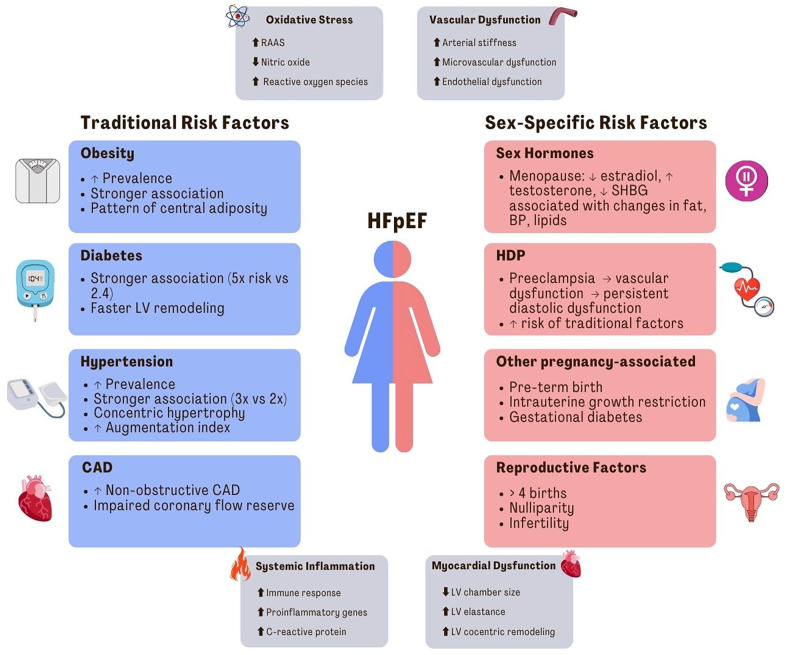
Figure 1.
Risk factors and pathophysiology for HFpEF in women.
Summary of proposed pathophysiological mechanisms for HFpEF in women, including oxidative stress, vascular dysfunction, systemic inflammation, and myocardial dysfunction. Summary of traditional and sex-specific risk factors for HFpEF in women. HFpEF: heart failure with preserved ejection fraction; RAAS: renin angiotensin aldosterone system; LV: left ventricular; CAD: coronary artery disease; SHBG: sex hormone binding globulin; HDP: hypertensive disorders of pregnancy.
In this review, we summarize sex differences in traditional risk factors and explore female-specific risk factors, which may explain the predominance of HFpEF in women. We also review sex differences in outcomes and response to treatment options, while acknowledging existing gaps in knowledge and areas for future research.
Sex differences in traditional risk factors for HFpEF
Prediction models from longitudinal community-based cohorts have demonstrated important risk factors for the development of HFpEF including age, blood pressure, body mass index (BMI), and previous myocardial infarction (MI).15 Given that inflammation is thought to be central to the development of HFpEF, sex differences in prevalence of comorbidities that drive inflammation such as obesity, diabetes mellitus, and hypertension may explain the disproportionate risk in women.16,17 Cardiovascular risk factors are strongly associated with incident HF—both HFpEF and HFrEF—in both women and men, as demonstrated by a large analysis compiling data from four community-based cohorts; however, in this analysis, there were subtle sex-related differences with age (women: hazard ratio (HR) 2.07 (95% confidence interval (CI): 1.89–2.28) versus men: HR 1.80 (95% CI: 1.67–1.95)) and hypertension (women: HR 1.98 (95% CI: 1.68–2.34) versus men: HR 1.67 (95% CI: 1.45–1.93)) more strongly associated with HF risk in women.18 In this section, we review important differences between women and men in both the prevalence and the overall HFpEF risk conferred by traditional risk factors (Figure 1).
Obesity
Obesity has been an established risk factor for HF for many decades,19 and more recently has been associated with the risk of HFpEF in particular.15,16,20 Obesity is more common among women than men with existing HFpEF (46% versus 35%), as seen in baseline data from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial.21 It is also more strongly associated with risk of HFpEF. In a study of 22,681 patients, obesity was more strongly linked with the risk of HFpEF than HFrEF (HR 1.34 per 1 standard deviation increase in BMI (95% CI: 1.24–1.45) versus HR 1.18 (95% CI: 1.10–1.27)), and notably, this association was more pronounced among women versus men (p for difference HFpEF versus HFrEF = 0.01 in women and p = 0.34 in women).22 The proposed model for the relationship between obesity and HFpEF includes cardiometabolic factors, such as insulin resistance, leading to a state of systemic inflammation, endothelial dysfunction, and subsequent myocyte remodeling.23 Furthermore, the relative distribution of fat mass differs between women and men. Pre-menopausal women have more subcutaneous adiposity and less visceral adiposity than men.24 However, during the peri-menopausal transition, there is a rise in visceral adiposity due to the decline in estrogen levels.25 Estrogen may mediate signaling pathways that attenuate reactive oxygen species leading to downstream anti-inflammatory effects. Furthermore, estrogen may exert regulatory effects on adipocyte and cardiomyocyte gene expression at the post-transcriptional level by driving expression of non-coding ribonucleotide acids (RNA), such as microRNAs (miRNAs) and long-non-coding RNAs.26 Therefore, a decline in estrogen levels during menopause may result in loss of protective miRNAs and contribute to a state of systemic inflammation.26
Visceral adipose tissue, in particular, is associated with concentric LV remodeling27 and can also affect exercise intolerance which is a hallmark symptom of HFpEF. Haykowsky et al.28 found that intra-abdominal fat was significantly higher in patients with HFpEF when compared with healthy controls and was the strongest predictor of peak oxygen consumption (a marker of exercise performance). There were inverse associations between intra-abdominal fat with all measurements of physical function, including peak oxygen consumption and 6-min walk distance. This highlights the importance of specific management strategies aimed at not only promoting weight loss in general but also targeting specific fat stores, especially intra-abdominal.
Diabetes mellitus
Diabetes mellitus is another traditional risk factor that has been established as having a more pronounced effect on HF in women as compared to men, with a fivefold associated increase in risk in women versus 2.4-fold increase in risk in men.29 The prevailing HFpEF hypothesis proposes that diabetes and other cardiometabolic risk factors contribute to HFpEF pathogenesis via systemic vascular inflammation and endothelial dysfunction.30 The production of reactive oxygen species31 and decreased bioavailability of nitric oxide leads to downstream lowering of soluble guanylate cyclase and protein kinase G (PKG) activity in cardiomyocytes. Deficient PKG activity subsequently impairs myocardial relaxation and induces cardiomyocyte hypertrophy.32 This process appears to occur earlier in the disease course in women versus men.33 Metabolic syndrome, thought to be a clinical precursor to diabetes, has been shown to be associated with echocardiographic measures of diastolic dysfunction.8,34
Hypertension
Hypertension is more prevalent among females with HFpEF versus males, especially in individuals age 75 years or older,14 and also confers greater risk of HF in women (threefold increase in risk in women versus twofold increase in men).35 Potential explanations include differential remodeling in these two populations. Hypertension more frequently leads to concentric hypertrophy in women, but eccentric remodeling in men.36,37 There are also intrinsic physiological characteristics in women that predispose them to HFpEF, including increased arterial stiffness and more pronounced age-related increase in arterial stiffness.9,38 In an analysis of 279 participants from the Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fraction (PARAMOUNT) study, women had higher arterial stiffness compared with men, though the difference was not significant after adjusting for height.38 In addition, Higashi et al.39 investigated the relationship between arterial stiffness and LV diastolic dysfunction in 446 participants and found higher carotid augmentation index (a parameter of arterial stiffness) was more strongly correlated with measures of LV diastolic function in women than men, even after controlling for confounding factors such as age and blood pressure. Overall, this hints toward a differential impact of aging and hypertension on cardiac structure and endothelial function between the sexes. Augmentation of blood pressure due to arterial stiffness increases LV systolic load and cardiac afterload, therefore, worsening myocardial oxygen demand, all of which can influence LV diastolic function. Additionally, elderly women with isolated systolic hypertension have sex-specific alterations of pulse wave reflection (related to increased LV systolic load) and LV remodeling, which may amplify the increased risk of HFpEF.40 Another study reported greater difference between central and peripheral blood pressure, or a higher augmentation index, in women compared to men (7.4 ± 5.2 versus 1.0 ± 6.9; p < 0.001), which may put women at a higher risk for end-organ damage such as LV hypertrophy.41
While hypertension contributes to HFpEF development via concentric hypertrophic in response to increased myocardial afterload, it has also been linked to increased microvascular inflammation which is known to promote downstream myocardial remodeling associated with HFpEF.30
Coronary artery disease
CAD and myocardial ischemia can lead to diastolic dysfunction and HFpEF. In a retrospective study of 376 patients with HFpEF, those with CAD were more likely to be men as compared to those without CAD (57% versus 25%).42 Several studies, including the I-PRESERVE study and the PARAMOUNT study, have demonstrated that women are less likely to have CAD versus men (38% versus 43% stable angina in I-PRESERVE and 22% versus 11% MI in PARAMOUNT).21,38 However, women are more likely to have non-obstructive CAD and coronary microvascular dysfunction, which has a high prevalence in HFpEF.7,43 In Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction, a prospective, multi-center study comprised of 263 patients, the prevalence of coronary microvascular dysfunction in HFpEF patients was 75% and was associated with systemic endothelial dysfunction as measured by peripheral arterial tonometry testing.7 Furthermore, in a study of 201 patients without obstructive CAD, impaired coronary flow reserve (a marker of chronic microvascular dysfunction) was associated with diastolic dysfunction (adjusted odds ratio (OR) 2.58, 95% CI: 1.22-5.48) and HFpEF hospitalizations (adjusted HR 2.47, 95% CI: 1.09-5.62).44 Finally, an analysis from the Multiethnic Study of Atherosclerosis (MESA), comprised of 6,809 healthy participants, reported that coronary artery calcium (CAC) scores greater than 300 were associated with increased risk of HFpEF, but only in women.45 Notably, after excluding participants who developed coronary heart disease events before HFpEF hospitalization, CAC greater than 300 was no longer a significant contributor to estimating risk of HFpEF in women, suggesting that ischemia and microvascular dysfunction may be mediators in women.45
Atrial fibrillation
Atrial fibrillation (AF) is prevalent in 25%–39% of HFpEF patients, and the two conditions share common risk factors including age and hypertension.46 Studies have also demonstrated an association between AF with death and HF hospitalization in HFpEF patients, an association that has not been observed in HFrEF patients.47 While AF is more common in men versus women with HFpEF, outcomes are worse in women with HFpEF and AF. For example, among participants enrolled in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial, the association between AF and hospitalization was stronger in women with HFpEF (HR 1.63, 95% CI: 1.40–1.91) versus men (HR 1.37, 95% CI: 1.18–1.58), p-interaction = 0.032.48
Iron deficiency and anemia
Anemia is a common comorbidity in HF and has been independently associated with greater risk of death and HF hospitalization across a range of ejection fractions,49,50 Potential mechanisms include adverse LV remodeling, neurohormonal activation, and higher levels of systemic inflammation.51 Previous studies have shown a prevalence of 21%–68% within the HFpEF population, with higher prevalence is HFpEF than HFrEF.49,52–54 Within the Get With the Guidelines-Heart Failure registry, prevalence of anemia was higher in women versus men for both HFrEF and HFpEF subgroups.55 Greater prevalence of iron deficiency in women in the general population has been hypothesized to predispose to HFpEF due to adverse effects on oxidative metabolism and immunological responses.56 Furthermore, in the Acute Decompensated Heart Failure Syndromes registry in Japan, there were sex-specific differences in the association between anemia and outcomes. Anemia was an independent predictor of all-cause death and cardiac death in women with HFpEF, but not in women with HFrEF, while the opposite was observed in men.57
Female-specific risk factors
Beyond sex differences in prevalence and conferred risk related to traditional risk factors, emerging data suggest that sex-specific risk factors may also contribute to the predominance of HFpEF in women (Figure 1).
Sex hormones
Patients with HFpEF are more likely to be older females,21 with postmenopausal women having a higher incidence of LV diastolic dysfunction. This observation has led to the hypothesis that the decline in estradiol at menopause may contribute to the pathogenesis of HFpEF.58,59 Estradiol is an important regulator of inflammation, reactive oxygen species, nitric oxide signaling, and endothelial function.59 The decline in estradiol levels at menopause is associated with changes in body fat, blood pressure, and lipids, all of which are implicated in development of HFpEF.59 Furthermore, in a study from MESA, a more androgenic pattern of hormones with lower estradiol, higher free testosterone, and lower sex hormone binding globulin was associated with greater increase in LV mass in both women and men, but associated with concentric remodeling only in women.60
Pregnancy
Hypertensive disorders of pregnancy, such as preeclampsia, are risk factors for future cardiovascular disease including HFpEF.61,62 In a meta-analysis of twenty-two studies, preeclampsia was associated with a fourfold increase in future incident HF.63 Furthermore, in a Norwegian cohort study, preeclampsia was associated with HF with HR of 2 (95% CI: 1.50–2.68, p < 0.001).64 While most previous studies did not distinguish between HFrEF and HFpEF, a recent retrospective cohort study utilizing New York and Florida state data found that preeclampsia was independently associated with greater risk of HFpEF with an adjusted HR of 2.09 (95% CI: 1.80–2.44).62 Additionally, there is likely to be reciprocal interplay between traditional cardiovascular risk factors and sex-specific risk factors. For example, women with preexisting hypertension and diabetes are at increased risk of developing preeclampsia, and patients with preeclampsia are more likely to develop downstream hypertension and diabetes.62 Preeclampsia and resultant endothelial dysfunction can lead to diastolic dysfunction that persists up to 1 year postpartum in 51% of patients,65 highlighting the importance of identifying and monitoring these high-risk women for downstream cardiovascular complications. Endothelial dysfunction with low nitric oxide bioavailability and LV remodeling is the leading hypothesis explaining these associations.30 Breetveld et al.66 reported that women with former preeclampsia have lower endothelium-dependent flow-mediated dilation, a marker of endothelial dysfunction, compared with women with normal gestation along with threefold higher prevalence of HF. Another study demonstrated impaired coronary flow reserve in patients with preeclampsia.67 In addition, evidence of persisting vascular dysfunction has been described in women following pregnancy complicated by hypertensive disorder of pregnancy.68
Beyond hypertensive disorders of pregnancy, APOs that include pre-term birth, preeclampsia, and intrauterine growth restriction portent increased risk for future hypertension, LV hypertrophy, and vascular dysfunction. Proteomic analyses show that pathways of inflammation and coagulation are increased in both women with APOs and in HFpEF.69 Furthermore, normotensive pregnancies with fetal growth restriction also have impaired myocardial relaxation with one specific study showing a third of women with history of fetal growth restriction to have diastolic dysfunction.70 However, Hansen et al.71 revealed that in a population of 10,292 women, women with any APO had higher prevalence of hypertension, diabetes, coronary heart disease, but only hypertensive disorders of pregnancy were significantly associated with HFpEF (OR, 1.87; 95% CI: 1.32–2.65).
Gestational diabetes has been associated with future risk of HF among women, though the data are mixed. In a large population-based cohort study, women with gestational diabetes had a 62% higher risk of HF (HR 1.62, 95% CI: 1.28–2.05) even after adjustment for confounders.72 However, in an analysis from the Women’s Health Initiative, there was no association seen between gestational diabetes and HF. This may be due to the low prevalence of gestational diabetes in that study’s population (2.5% versus 7.6% prevalence in the United States).71,73
Reproductive factors
Other pregnancy-related features, including parity, are also associated with diastolic function. A study of 710 women demonstrated grand parity, which was defined as greater than four births, to be an independent risk factor for LV diastolic dysfunction. The exact mechanism remains to be elucidated.74 On the other end of the spectrum, nulliparity and a shorter total reproductive duration have also been shown to portend a higher risk of HFpEF.75,76 Potential explanations include shorter total reproductive duration and therefore lower cumulative exposure to endogenous sex hormones. Another sex-specific risk factor related to reproductive health is infertility. In a large prospective cohort from the Women’s Health Initiative, infertility was shown to be significantly associated with incident HFpEF (20% increased risk). This risk was not explained by established cardiovascular risk factors.77
Sex differences in outcomes
Studies have shown HFpEF outcomes differ by sex. In general, women with HFpEF have better outcomes than men,21,78 including lower in-hospital mortality (4.2% versus 4.6%, p < 0.01).14 However, a post hoc analysis of the TOPCAT trial showed no difference in cardiovascular and all-cause mortality between women and men, though women had worse patient-reported outcomes.79 Other studies have also demonstrated lower QOL in women with HFpEF, which was independent of age and HF severity.80
One potential explanation for worse QOL in women versus men with HFpEF is that women have lower exercise capacity, which is a quintessential feature of HFpEF. In a study of participants from the RELAX (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction) trial, women with HFpEF had worse exercise capacity than men, despite comparable resting cardiac function.81 Furthermore, a cross-sectional observational study using invasive hemodynamic exercise measures demonstrated that women have lower exercise peripheral oxygen extraction and ventricular reserve compared to men in spite of lower burden of cardiometabolic disease among women in that study’s population.82 Additionally, Beale et al.9 revealed that women had poorer diastolic reserve with higher measurements of LV filling pressures with exercise as well as systemic and pulmonary vascular dysfunction in a group of 161 patients with HFpEF. These sex differences in exercise hemodynamics highlight the need for further investigation into related pathophysiology to explain differences in muscle and vascular responses to exercise in women versus men with HFpEF.
While exercise capacity may be one explanation to explain variances in QOL, another study demonstrated no association between exercise capacity with QOL in women with HFpEF. In this study which examined echocardiographic and QOL data from the RELAX and NEAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trials in men versus women, diastolic dysfunction, ischemic heart disease, and exercise capacity predicted QOL in men with HFpEF, while only age and BMI predicted QOL for women. More investigation is required to ascertain these differences, including potential contributions from psychosocial factors and other social determinants of health.83
Sex differences in treatment
While treatment options for HFpEF are not as widespread as for HFrEF, sex differences have been reported for the available HFpEF therapies (Table 1). In a post hoc analysis of the TOPCAT trial, there was a reduction in all-cause mortality for spironolactone in women (HR 0.66; 95% CI: 0.48–0.9; p = 0.01), but not in men (sex–treatment interaction p = 0.02). These results were only hypothesis generating, but warrant further studies on the use of spironolactone in women with HFpEF. The authors of the analysis hypothesized that differential effects of mineralocorticoid antagonists on cardiac remodeling in women versus men may have explained the sex-specific finding.79 In a prespecified subgroup analysis of the Prospective Comparison of ARNI With ARB Global Outcomes in Heart Failure With Preserved Ejection Fraction (PARAGON-HF) trial, sacubitril-valsartan reduced the risk of HF hospitalization by 26% in women, but no effect was seen in men.84,85 Similar to TOPCAT, there is no clear mechanistic evidence to explain these findings, but sex differences in natriuretic peptide (NP) biology and crosstalk with sex hormones have been proposed.85 Specifically, the authors hypothesize that sacubitril-valsartan-induced NP production may preferentially benefit women versus men with HFpEF given lower baseline levels of NP in women with HFpEF.85 Lower NP levels are associated with reduced cyclic guanosine monophosphate-protein kinase (cGMP) signaling. This reduction in signaling may be further accentuated by loss of estrogen-dependent endothelial nitric oxide synthase activation of cGMP pathway following menopause. NP activation by sacubitril-valsartan may explain preferential benefit in women with HFpEF who are cGMP deficient. Sodium glucose transporter-2 inhibitors (SGLT2i) are another class of medications that have emerged as treatment options for HFpEF. SGLT2i do not lead to NP activation, but rather work through several distinct mechanisms. Most recently, in the EMPEROR-PRESERVED (Empagliflozin in Heart Failure with a Preserved Ejection Fraction) trial and DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial, which investigated the SGLT2 inhibitors empagliflozin and dapagliflozin, respectively, there was no difference in treatment effect among women versus men.86,87
Table 1.
Sex differences in recent HFpEF treatment trials.
| Trial (year) | Number of patients | % female | Intervention | EF cut-off | Type of analysis | Baseline differences (women versus men) | Sex-specific results |
|---|---|---|---|---|---|---|---|
| TOPCAT (2019) | 1767 | 49.9% | Spironolactone | 45% | Post hoc, non-pre-specified | Older Fewer comorbidities Higher LVEF, BP, BMI Lower GFR, Hgb Higher NYHA class Lower KCCQ score |
• Rates of primary outcome and HF hospitalization not different • Significant reduction in all-cause mortality w/spironolactone in women (HR 0.66; 95% CI: 0.48–0.9; p = 0.01), but not in men; sex-treatment interaction p = 0.024. |
| PARAGON-HF (2020) | 4796 | 51.7% | Sacubitril-valsartan | 45% | Prespecified subgroup | Older More obesity Fewer comorbidities Lower median NT-proBNP level Lower GFR Higher LVEF Higher NYHA class Lower KCCQ score Less likely on MRA |
• In control group, women had higher rates of HF hospitalization and lower rates of CV death • Rate ratio for primary outcome was 0.73 (95% CI: 0.59–0.90) in women and 1.03 (95% CI: 0.84–1.25) in men with p interaction = 0.017, predominately related to HF hospitalization (33% RRR, p interaction = 0.005) |
| EMPEROR-PRESERVED (2021) | 5988 | 44.7% | Empagliflozin | 40% | Prespecified subgroup | – | • No difference between women and men • HR for male: 0.81 (95% CI: 0.69–0.96) and for female: 0.75 (95% CI: 0.64–0.92) |
| DELIVER (2022) | 6263 | 43.9% | Dapagliflozin | 40% | Prespecified subgroup | – | • No difference between women and men • HR for male: 0.82 (95% CI: 0.71–0.96) and for female: 0.81 (95% CI: 0.67–0.97) |
Summary of sex-specific results in recent HFpEF treatment trials, including TOPCAT, PARAGON-HF, and EMPEROR-PRESERVED.
TOPCAT: Treatment of Preserved Cardiac Function Heart Failure with Aldosterone Antagonist; PARAGON-HF: Prospective Comparison of ARNI With ARB Global Outcomes in Heart Failure With Preserved Ejection Fraction; EMPEROR-PRESERVED: Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; EF: ejection fraction; CAD: coronary artery disease; AF: atrial fibrillation; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; BP: blood pressure; BMI: body mass index; GFR: glomerular filtration rate; Hgb: hemoglobin; NYHA: New York Heart Association; KCCQ: Kansas City Cardiomyopathy Questionnaire; NT-proBNP: N-terminal pro brain natriuretic peptide; MRA: mineralocorticoid; HR: hazard ratio; CI: confidence interval; RRR: relative risk reduction.
Conclusion and gaps in knowledge
The disproportionate prevalence of HFpEF in women stems from a myriad of factors, including higher rates of traditional risk factors, unique pathophysiological consequences that put women at higher risk from these traditional risk factors, and distinct sex-specific effects. While women represent the majority of patients with HFpEF, there is still a lack of adequate representation in clinical trials. Given the heterogeneity of HFpEF, further work is needed to create more accurate risk stratification models that encompass both traditional and sex-specific risk markers. Additionally, we are yet to thoroughly understand the differences in pathophysiological mechanisms as well as responses to comorbidities between women and men.
Given these challenges, we propose a series of future directions to address the higher prevalence of HFpEF in women and improve outcomes:
Existing evidence suggests that sex-specific miRNA networks may mediate the relationship between comorbidity-induced inflammatory activation and HFpEF pathogenesis in women. Future research on miRNA-induced mechanisms may shed light on unique pathways that contribute to HFpEF development in women.
Improved mechanistic understanding of sex-specific inflammatory cascades and effects of sex hormones may enable greater precision of targeted therapies for women with HFpEF. Specifically, identification of novel circulating biomarkers related to tissue remodeling, inflammation, and neurohormonal regulation may refine risk prediction in women with HFpEF.
Baseline sex differences in LV ejection fraction and steeper trajectories with age in women are well established, but diagnostic cutoffs remain sex-agnostic.88 We propose sex-specific diagnostic cutoffs in ejection fraction.8
A preventive strategy should be adopted to decrease the rising incidence and prevalence of HFpEF in women.
Acknowledgments
None.
Footnotes
ORCID iD: Emily Lau  https://orcid.org/0000-0001-9361-6397
https://orcid.org/0000-0001-9361-6397
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Gurleen Kaur: Conceptualization; Writing – original draft; Writing – review & editing.
Emily Lau: Conceptualization; Writing – original draft; Writing – review & editing.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: E.L. is supported by the NIH K23-HL159243 and the American Heart Association (853922).
Competing interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation 2022; 145: e153–e639. [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3): 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ho JE, Gona P, Pencina MJ, et al. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J 2012; 33(14): 1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2022; 145: e895–e1032. [DOI] [PubMed] [Google Scholar]
- 5. Ceia F, Fonseca C, Mota T, et al. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail 2002; 4(4): 531–539. [DOI] [PubMed] [Google Scholar]
- 6. Stolfo D, Uijl A, Vedin O, et al. Sex-based differences in heart failure across the ejection fraction spectrum: phenotyping, and prognostic and therapeutic implications. JACC Heart Fail 2019; 7(6): 505–515. [DOI] [PubMed] [Google Scholar]
- 7. Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018; 39: 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beale AL, Meyer P, Marwick TH, et al. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018; 138: 198–205. [DOI] [PubMed] [Google Scholar]
- 9. Beale AL, Nanayakkara S, Segan L, et al. Sex differences in heart failure with preserved ejection fraction pathophysiology: a detailed invasive hemodynamic and echocardiographic analysis. JACC Heart Fail 2019; 7(3): 239–249. [DOI] [PubMed] [Google Scholar]
- 10. Sotomi Y, Hikoso S, Nakatani D, et al. Sex differences in heart failure with preserved ejection fraction. J Am Heart Assoc 2021; 10: e018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tromp J, Shen L, Jhund PS, et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 2019; 74: 601–612. [DOI] [PubMed] [Google Scholar]
- 12. Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis EF, Lamas GA, O’Meara E, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail 2007; 9(1): 83–91. [DOI] [PubMed] [Google Scholar]
- 14. Goyal P, Paul T, Almarzooq ZI, et al. Sex- and race-related differences in characteristics and outcomes of hospitalizations for heart failure with preserved ejection fraction. J Am Heart Assoc 2017; 6: e003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho JE, Enserro D, Brouwers FP, et al. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail 2016; 9(6): e003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eaton CB, Pettinger M, Rossouw J, et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail 2016; 9(10): e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daubert MA, Douglas PS. Primary prevention of heart failure in women. JACC Heart Fail 2019; 7: 181–191. [DOI] [PubMed] [Google Scholar]
- 18. Suthahar N, Lau ES, Blaha MJ, et al. Sex-specific associations of cardiovascular risk factors and biomarkers with incident heart failure. J Am Coll Cardiol 2020; 76: 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. New Engl J Med 2002; 347: 305–313. [DOI] [PubMed] [Google Scholar]
- 20. Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013; 34(19): 1424–1431. [DOI] [PubMed] [Google Scholar]
- 21. Lam CS, Carson PE, Anand IS, et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 2012; 5: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savji N, Meijers WC, Bartz TM, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018; 6(8): 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tromp J, Westenbrink BD, Ouwerkerk W, et al. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2018; 72: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 24. Karastergiou K, Smith SR, Greenberg AS, et al. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ 2012; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ambikairajah A, Walsh E, Tabatabaei-Jafari H, et al. Fat mass changes during menopause: a metaanalysis. Am J Obstet Gynecol 2019; 221(5): 393.e50–409.e50. [DOI] [PubMed] [Google Scholar]
- 26. Florijn BW, Bijkerk R, van der Veer EP, et al. Gender and cardiovascular disease: are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc Re 2018; 114: 210–225. [DOI] [PubMed] [Google Scholar]
- 27. Neeland IJ, Gupta S, Ayers CR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging 2013; 6(5): 800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haykowsky MJ, Nicklas BJ, Brubaker PH, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail 2018; 6(8): 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974; 34(1): 29–34. [DOI] [PubMed] [Google Scholar]
- 30. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 31. Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011; 124: 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Heerebeek L, Hamdani N, Falcão-Pires I, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012; 126: 830–839. [DOI] [PubMed] [Google Scholar]
- 33. Galderisi M, Anderson KM, Wilson PW, et al. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol 1991; 68: 85–89. [DOI] [PubMed] [Google Scholar]
- 34. Kim HL, Kim MA, Oh S, et al. Sex difference in the association between metabolic syndrome and left ventricular diastolic dysfunction. Metab Syndr Relat Disord 2016; 14(10): 507–512. [DOI] [PubMed] [Google Scholar]
- 35. Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275: 1557–1562. [PubMed] [Google Scholar]
- 36. Garavaglia GE, Messerli FH, Schmieder RE, et al. Sex differences in cardiac adaptation to essential hypertension. Eur Heart J 1989; 10(12): 1110–1114. [DOI] [PubMed] [Google Scholar]
- 37. Kuch B, Muscholl M, Luchner A, et al. Gender specific differences in left ventricular adaptation to obesity and hypertension. J Hum Hypertens 1998; 12(10): 685–691. [DOI] [PubMed] [Google Scholar]
- 38. Gori M, Lam CS, Gupta DK, et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16(5): 535–542. [DOI] [PubMed] [Google Scholar]
- 39. Higashi H, Okayama H, Saito M, et al. Relationship between augmentation index and left ventricular diastolic function in healthy women and men. Am J Hypertens 2013; 26(11): 1280–1286. [DOI] [PubMed] [Google Scholar]
- 40. Tadic M, Cuspidi C, Plein S, et al. Sex and heart failure with preserved ejection fraction: from pathophysiology to clinical studies. J Clin Med 2019; 8: 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chester R, Sander G, Fernandez C, et al. Women have significantly greater difference between central and peripheral arterial pressure compared with men: the Bogalusa Heart Study. J Am Soc Hypertens 2013; 7(5): 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014; 63: 2817–2827. [DOI] [PubMed] [Google Scholar]
- 43. Elgendy IY, Pepine CJ. Heart failure with preserved ejection fraction: is ischemia due to coronary microvascular dysfunction a mechanistic factor? Am J Med 2019; 132(6): 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018; 39: 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sharma K, Al Rifai M, Ahmed HM, et al. Usefulness of coronary artery calcium to predict heart failure with preserved ejection fraction in men versus women (from the multi-ethnic study of atherosclerosis). Am J Cardiol 2017; 120: 1847–1853. [DOI] [PubMed] [Google Scholar]
- 46. Zakeri R, Chamberlain AM, Roger VL, et al. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation 2013; 128: 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Linssen GC, Rienstra M, Jaarsma T, et al. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 2011; 13(10): 1111–1120. [DOI] [PubMed] [Google Scholar]
- 48. O’Neal WT, Sandesara P, Hammadah M, et al. Gender differences in the risk of adverse outcomes in patients with atrial fibrillation and heart failure with preserved ejection fraction. Am J Cardiol 2017; 119: 1785–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Savarese G, Jonsson Å, Hallberg AC, et al. Prevalence of, associations with, and prognostic role of anemia in heart failure across the ejection fraction spectrum. Int J Cardiol 2020; 298: 59–65. [DOI] [PubMed] [Google Scholar]
- 50. Berry C, Poppe KK, Gamble GD, et al. Prognostic significance of anaemia in patients with heart failure with preserved and reduced ejection fraction: results from the MAGGIC individual patient data meta-analysis. QJM 2016; 109(6): 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation 2018; 138: 80–98. [DOI] [PubMed] [Google Scholar]
- 52. Lund LH, Donal E, Oger E, et al. Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16: 992–1001. [DOI] [PubMed] [Google Scholar]
- 53. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014; 64: 2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 55. Hsich EM, Grau-Sepulveda MV, Hernandez AF, et al. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J 2012; 163(3): 430–437, 437.e1–437.e3. [DOI] [PubMed] [Google Scholar]
- 56. Macdougall IC, Canaud B, de Francisco AL, et al. Beyond the cardiorenal anaemia syndrome: recognizing the role of iron deficiency. Eur J Heart Fail 2012; 14(8): 882–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kajimoto K, Minami Y, Sato N, et al. Gender differences in anemia and survival in patients hospitalized for acute decompensated heart failure with preserved or reduced ejection fraction. Am J Cardiol 2017; 120: 435–442. [DOI] [PubMed] [Google Scholar]
- 58. Gökçe M, Karahan B, Erdöl C, et al. Left ventricular diastolic function assessment by tissue Doppler echocardiography in relation to hormonal replacement therapy in postmenopausal women with diastolic dysfunction. Am J Ther 2003; 10(2): 104–111. [DOI] [PubMed] [Google Scholar]
- 59. Sabbatini AR, Kararigas G. Menopause-related estrogen decrease and the pathogenesis of HFpEF: JACC review topic of the week. J Am Coll Cardiol 2020; 75: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 60. Subramanya V, Zhao D, Ouyang P, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: the multi-ethnic study of atherosclerosis (MESA). Maturitas 2018; 108: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med 2018; 169: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williams D, Stout MJ, Rosenbloom JI, et al. Preeclampsia predicts risk of hospitalization for heart failure with preserved ejection fraction. J Am Coll Cardiol 2021; 78: 2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017; 10(2): e003497. [DOI] [PubMed] [Google Scholar]
- 64. Honigberg MC, Riise HKR, Daltveit AK, et al. Heart failure in women with hypertensive disorders of pregnancy: insights from the cardiovascular disease in Norway project. Hypertension 2020; 76: 1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Melchiorre K, Sutherland GR, Liberati M, et al. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 2011; 58: 709–715. [DOI] [PubMed] [Google Scholar]
- 66. Breetveld NM, Ghossein-Doha C, van Neer J, et al. Decreased endothelial function and increased subclinical heart failure in women several years after pre-eclampsia. Ultrasound Obstet Gynecol 2018; 52(2): 196–204. [DOI] [PubMed] [Google Scholar]
- 67. Ciftci FC, Caliskan M, Ciftci O, et al. Impaired coronary microvascular function and increased intima-media thickness in preeclampsia. J Am Soc Hypertens 2014; 8(11): 820–826. [DOI] [PubMed] [Google Scholar]
- 68. Grand’Maison S, Pilote L, Okano M, et al. Markers of vascular dysfunction after hypertensive disorders of pregnancy: a systematic review and meta-analysis. Hypertension 2016; 68: 1447–1458. [DOI] [PubMed] [Google Scholar]
- 69. Lane-Cordova AD, Khan SS, Grobman WA, et al. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol 2019; 73: 2106–2116. [DOI] [PubMed] [Google Scholar]
- 70. Melchiorre K, Sutherland GR, Liberati M, et al. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension 2012; 60: 437–443. [DOI] [PubMed] [Google Scholar]
- 71. Hansen AL, Søndergaard MM, Hlatky MA, et al. Adverse pregnancy outcomes and incident heart failure in the women’s health initiative. JAMA Netw Open 2021; 4: e2138071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Echouffo-Tcheugui JB, Guan J, Retnakaran R, et al. Gestational diabetes and incident heart failure: a cohort study. Diabet Care 2021; 44: 2346–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res Clin Pract 2018; 141: 200–208. [DOI] [PubMed] [Google Scholar]
- 74. Keskin M, Avşar Ş, Hayıroğlu Mİ, et al. Relation of the number of parity to left ventricular diastolic function in pregnancy. Am J Cardiol 2017; 120: 154–159. [DOI] [PubMed] [Google Scholar]
- 75. Hall PS, Nah G, Howard BV, et al. Reproductive factors and incidence of heart failure hospitalization in the women’s health initiative. J Am Coll Cardiol 2017; 69: 2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Parikh NI, Cnattingius S, Dickman PW, et al. Parity and risk of later-life maternal cardiovascular disease. Am Heart J 2010; 159(2): 215.e6–221.e6. [DOI] [PubMed] [Google Scholar]
- 77. Lau ES, Wang D, Roberts M, et al. Infertility and risk of heart failure in the women’s health initiative. J Am Coll Cardiol 2022; 79: 1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. O’Meara E, Clayton T, McEntegart MB, et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Circulation 2007; 115: 3111–3120. [DOI] [PubMed] [Google Scholar]
- 79. Merrill M, Sweitzer NK, Lindenfeld J, et al. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail 2019; 7(3): 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Faxén UL, Hage C, Donal E, et al. Patient reported outcome in HFpEF: sex-specific differences in quality of life and association with outcome. Int J Cardiol 2018; 267: 128–132. [DOI] [PubMed] [Google Scholar]
- 81. Mauricio R, Patel KV, Agusala V, et al. Sex differences in cardiac function, biomarkers and exercise performance in heart failure with preserved ejection fraction: findings from the RELAX trial. Eur J Heart Fail 2019; 21(11): 1476–1479. [DOI] [PubMed] [Google Scholar]
- 82. Lau ES, Cunningham T, Hardin KM, et al. Sex differences in cardiometabolic traits and determinants of exercise capacity in heart failure with preserved ejection fraction. JAMA Cardiol 2020; 5: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Honigberg MC, Lau ES, Jones AD, et al. Sex differences in exercise capacity and quality of life in heart failure with preserved ejection fraction: a secondary analysis of the RELAX and NEAT-HFpEF trials. J Card Fail 2020; 26(3): 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. New Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 85. McMurray JJV, Jackson AM, Lam CSP, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 2020; 141: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. New Engl J Med 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 87. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. New Engl J Med 2022; 387: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 88. Chung AK, Das SR, Leonard D, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation 2006; 113: 1597–1604. [DOI] [PubMed] [Google Scholar]