Abstract
Background
Inter-relationships between multimorbidity and geriatric syndromes are poorly understood. This study assesses heterogeneity in joint trajectories of somatic disease, functional status, cognitive performance, and depressive symptomatology.
Methods
We analyzed 16 years of longitudinal data from the Health and Retirement Study (HRS, 1998-2016) for n = 11,565 older adults (≥65 years) in the United States. Group-based mixture modeling identified latent clusters of older adults following similar joint trajectories across domains.
Results
We identified four distinct multidimensional trajectory groups: (1) Minimal Impairment with Low Multimorbidity (32.7% of the sample; mean = 0.60 conditions at age 65, 2.1 conditions at age 90) had limited deterioration; (2) Minimal Impairment with High Multimorbidity (32.9%; mean = 2.3 conditions at age 65, 4.0 at age 90) had minimal deterioration; (3) Multidomain Impairment with Intermediate Multimorbidity (19.9%; mean = 1.3 conditions at age 65, 2.7 at age 90) had moderate depressive symptomatology and functional impariments with worsening cognitive performance; (4) Multidomain Impairment with High Multimorbidity (14.1%; mean = 3.3 conditions at age 65; 4.7 at age 90) had substantial functional limitation and high depressive symptomatology with worsening cognitive performance. Black and Hispanic race/ethnicity, lower wealth, lower education, male sex, and smoking history were significantly associated with membership in the two Multidomain Impairment classes.
Conclusions
There is substantial heterogeneity in combined trajectories of interrelated health domains in late life. Membership in the two most impaired classes was more likely for minoritized older adults.
Keywords: Cognition, depression, multimorbidity, joint trajectories
Introduction
For older adults, having two or more chronic diseases (multimorbidity) is associated with poor health outcomes and high costs to the health care system which exceed the risks attributable to any individual constituent disease.1,2 Various studies document these relationships whether conditions are clinically-concordant (e.g., hypertension, heart disease), or discordant (e.g., arthritis, cancer).3,4 The development of multimorbidity is associated with additional clinical phenotypes marked by syndromes that involve impairment across multiple body systems.5 Still, inter-relationships between changes in multimorbidity and important geriatric syndromes—such as disability, frailty, and cognitive impairment—are not well-characterized. It is critical to better specify these relationships to understand aging processes, incorporate what matters to older adults,6,7 and engineer programs that preserve independence.7,8
The medical complexity and workload involved in managing various health maintenance tasks and coordinating care between multiple providers can be substantial and overwhelming for patients,9–11 especially during transitions to serious illness.12 This complexity has particularly significant effects among older adults with limited financial means, low levels of education, and inadequate access to timely health care services.9,13 Understanding the unique trajectories of aging that encompass multiple, person-centered health domains is therefore crucial to developing targeted interventions and guiding investments in programs that promote healthy aging.
Research has begun to focus on the synergistic relationships with other facets of wellbeing common in geriatric practice, such as mobility, mentation, and multicomplexity (elements of the “5 Ms Framework”).7,14,15 Older adults’ ability to manage chronic diseases, maintain physical and mental functioning, and address social-emotional needs is critical to attaining high quality of life. Nevertheless, comparatively little work has focused on longitudinal changes across multiple health domains simultaneously to examine aging processes from a comprehensive, whole-person perspective.16 Long-term assessment may clarify the relationship between domains important to sustaining independence. In addition, variability across multiple health domains may be strongly related to divergent experiences and differential access to socioeconomic resources over the life course. Such cumulative inequality is evident in findings that minoritized older adults are living extended periods of their lifespan in sicker, more disabled states.17–20 To develop and deploy interventions to remediate these observed disparities, it is important to assess multiple intersecting domains of health for vulnerable groups.
This study aims to identify distinct multidimensional trajectories of aging across four health domains that together, convey a holistic view of wellbeing in late life: multimorbidity burden, functional status, cognitive performance, and depressive symptomatology. Because we anticipate that these processes are heterogeneous for population groups due to cumulative inequality occurring across the lifespan, we hypothesize that minoritized older adults and those with low socioeconomic status are more likely to follow aging trajectories characterized by substantial impairment across these health domains.
Methods
Data Source
The Health and Retirement Study (HRS) is a nationally-representative prospective cohort study of community-dwelling adults age 51 years and older in the United States.21 The HRS is a biennial longitudinal study of over 43,000 people 51 years of age and older that began in 1992 and designed to follow middle-aged and older Americans in pre- and post-retirement age until death. Therefore the HRS provides rich population-based data that capture economic and health transitions of adults in mid and late life (https://hrs.isr.umich.edu/about). Because of cross-wave inconsistency in the assessment of key variables prior to 1998, we utilized all available waves from 1998 through 2016. This study was approved by our university’s Institutional Review Board (IRB ID# STUDY00017034, STUDY00019414).
Study Sample
All community-dwelling respondents aged 65-95 years who participated in the HRS survey during the study period (1998-2016) were eligible for inclusion. Similar to other studies, we limited our sample to respondents who provided a minimum of three observations to allow for stable estimation of non-linear trajectories across multiple outcomes.22,23 A total of 22,272 HRS respondents interviewed between 1998 and 2016 were age-eligible and living in the community. Of those, 15,030 respondents provided the required minimum of three waves of outcome data. We then excluded 382 respondents with proxy interviews at all interview waves and 2,712 respondents with clinically-inconsistent chronic disease self-reports that we were unable to adjudicate using a previously developed multistep adjudication method.24 Additionally, we excluded 230 non-Hispanic respondents who had missing race/ethnicity data or reported “other” as their racial/ethnic category because of heterogeneity within this racial/ethnic identity group, and 141 respondents with missing data on other baseline sociodemographic characteristics. Finally, we dropped proxy responses at the wave-level because measures of depressive symptoms were not assessed during proxy interviews. The final sample consisted of 11,565 respondents who contributed a total of 66,952 repeated observations between 1998 and 2016, with an average of 5.79 (SD=2.20) observations per respondent.
Outcome Measures
Multimorbidity was operationalized as a count of self-reported somatic diseases (range: 0–7) available and assessed at each wave: heart disease (including myocardial infarction, coronary heart disease, angina, congestive heart failure, other heart problems), hypertension, stroke (excluding transient ischemic attack [TIA]), diabetes, arthritis, lung disease (including chronic bronchitis, emphysema, excluding asthma), and cancer (including any malignant tumors excluding skin cancer). They were assessed at baseline with, “Has a doctor ever told you that you have…”, and at follow-up waves with, “Since we last talked with you, has a doctor told you that you have…”. Exclusion decisions (i.e, TIA, asthma, skin cancer) were made by the HRS.
Functional status was measured at each survey wave assessing self-reported limitations in performing six activities of daily living (ADLs) (walking across a room, dressing, bathing, eating, getting in or out of bed, and using the toilet), and five instrumental activities of daily living (IADLs) (using a telephone, managing money, taking medications, shopping for groceries, and preparing hot meals). Each item was coded as 0 if the respondent reported no difficulty and 1 if the reported having any difficulty in performing the activity. Items were summed to yield a combined score (range 0-11), with higher scores indicating greater functional limitation.25
Cognitive performance was measured at each survey wave using the 27-point HRS cognitive scale26–28 consisting of: 1) immediate and delayed 10-noun free recall to assess memory; 2) serial sevens subtraction to assess working memory; and 3) counting backwards to assess speed of mental processing. Lower scores represent worse cognitive performance, with a score of ≤11 indicative of cognitive impairment.28
Depressive symptoms were measured at each wave using the 8-item Centers for Epidemiologic Research Depression (CES-D) scale,29–31 which consists of eight dichotomous items assessing the presence of specific symptoms for much of the prior week. The total number of endorsed symptoms are summed to scores ranging from 0-8. Higher scores indicate greater depressive symptoms. Previous research has shown that a score of four or more is a valid indicator of clinically-significant depressive symptomatology.32
Predictors
We compared distributions of sociodemographic and health-related factors between identified multidimensional trajectory groups for: mutually-exclusive race/ethnicity categories for non-Hispanic White (White), non-Hispanic Black (Black), and Hispanic; female sex; educational attainment (less than high school education, high school graduate, some college or greater than college education); nativity (foreign-born); household wealth quartile (derived from baseline net worth in US dollars); baseline coupled status (married/living with a partner); baseline smoking status (current, previous, or never smoker), and baseline body mass index (BMI) category (underweight, healthy weight, overweight, obese). BMI was calculated according to the established formula (BMI=weight [pounds] x 703 / height^2 [inches]). The BMI categories were defined as underweight (BMI < 18.5), healthy weight (BMI=18.5 to < 25.0), overweight (BMI=25 to < 30.0), and obese (BMI ≥ 30).33 Years of age were used as the time metric in the trajectory models, therefore, age was not included as a potential predictor of trajectory group membership.
Statistical Analysis
We used group-based trajectory modeling (GBTM) to identify subgroups that followed distinct patterns of joint change in trajectories of multimorbidity, functional status, cognitive performance, and depressive symptoms. Briefly, GBTM is a semi-parametric, finite mixture modeling approach designed to identify clusters of individuals who follow similar trajectories over time regarding some outcome of interest.34 In the current study, we employ a recent generalization of GBTM, group-based multidimensional trajectory modeling, which identifies clusters of individuals who share common trajectories across multiple longitudinal indicators.35 Model parameters are estimated via maximum likelihood and are robust to missing data under the missing at random (MAR) assumption.
Based on the empirical distributions of the outcome measures, we selected the most appropriate link function for each outcome from those currently implemented in GBTM software.34 Specifically, we used a censored normal model specification for multimorbidity and cognitive performance, and a zero-inflated Poisson model for functional status and depressive symptoms. We modeled the trajectory of each outcome as a quadratic function of age. Because the model search process is computationally challenging when examining trajectories across multiple outcomes, we used a two-step approach to identify the best-fitting multidimensional trajectory model.35 First, we fit a series of separate GBTM models for each outcome, successively increasing the number of trajectory groups from one to six, then we identified the optimal number of groups for each outcome using the following criteria: 1) reduction in Bayesian information criterion (BIC); 2) average posterior probability of group membership > 0.7 for all groups; 3) odds of correct classification > 5.0 for all groups; 4) group size >5% of total sample.35 Next, we fit multidimensional trajectory models across the range of optimal group numbers identified in the first step and selected the final model by comparing the fit indices and qualitatively evaluating whether each increase in group number resulted in the identification of a trajectory group that was substantively distinct from those previously identified.
After selecting the optimal multidimensional trajectory model, we assigned each respondent to the trajectory group for which they had the highest posterior probability of membership (modal assignment). We calculated descriptive statistics stratified by trajectory group and conducted multinomial logistic regression to examine the association of baseline factors with assigned trajectory group membership. As our primary factor of interest, we examined the association of race/ethnicity with group membership in an unadjusted model. We then adjusted this model for the predictors described above and examined interactions between race/ethnicity and sex, education, and net worth. To assess for potential bias in either coefficient estimates or standard errors introduced by the probabilistic nature of trajectory group assignment, we conducted sensitivity analyses comparing the results of the multinomial regression model with identically specified models where: 1) group membership was determined by modal assignment and respondents were weighted using the posterior probability of assigned group membership, and 2) group membership was determined by proportional assignment, where respondents were assigned to each trajectory group in proportion to their posterior probability of group assignment. Analyses were conducted in Stata 17, with multidimensional trajectory models fit using the ‘traj’ add-on package.36
Results
Sample characteristics
Characteristics of the study population are shown in Table 1. The mean age at baseline was 69.3 (SD=5.5) years. Fifty-eight percent of respondents were female, 79% were White, 13% were Black, and 8% were Hispanic. High school was the highest level of educational attainment among 54% of respondents, while 24% reported less than a high school education and 22% reported completing at least some college or higher level of education. Many respondents were either overweight (41%) or obese (27%) at baseline. The average age of death for decedents was 83.9 (SD=7.3).
Table 1.
Baseline descriptive characteristics for participants overall and by multidimensional trajectory groups, Health & Retirement Study 1998-2016.
| Overall Total (N = 11,565) | Minimal Impairment with Low Multimorbidity (N = 3771) | Minimal Impairment with High Multimorbidity (N = 3878) | Multidomain Impairment with Intermediate Multimorbidity (N = 2291) | Multidomain Impairment with High Multimorbidity (N = 1625) | |
|---|---|---|---|---|---|
| Non-Hispanic White, n (%) | 9125 (78.90) | 3287 (87.17) | 3269 (84.30) | 1535 (67.00) | 1034 (63.63) |
| Non-Hispanic Black, n (%) | 1550 (13.40) | 283 (7.50) | 410 (10.57) | 474 (20.69) | 383 (23.57) |
| Hispanic, n (%) | 890 (7.70) | 201 (5.33) | 199 (5.13) | 282 (12.31) | 208 (12.80) |
| Age, mean (SD) | 69.32 (5.52) | 70.79 (6.34) | 68.28 (4.70) | 69.74 (5.59) | 67.81 (4.12) |
| Male, n (%) | 4805 (41.55) | 1537 (40.76) | 1792 (46.21) | 899 (39.24) | 577 (35.51) |
| Female, n (%) | 6760 (58.45) | 2234 (59.24) | 2086 (53.79) | 1392 (60.76) | 1048 (64.49) |
| < High school, n (%) | 2798 (24.19) | 581 (15.41) | 576 (14.85) | 932 (40.68) | 709 (43.63) |
| High school graduate, n (%) | 6192 (53.54) | 2109 (55.93) | 2256 (58.17) | 1083 (47.27) | 744 (45.78) |
| ≥ Some college, n (%) | 2575 (22.27) | 1081 (28.67) | 1046 (26.97) | 276 (12.05) | 172 (10.58) |
| Wealth (1000s USD), median (IQR) | 179.60 (396.75) | 271.00 (487.50) | 223.00 (455.38) | 111.00 (244.60) | 59.80 (169.20) |
| US Born, n (%) | 961 (8.31) | 3471 (92.04) | 3639 (93.84) | 2034 (88.78) | 1460 (89.85) |
| Foreign born, n (%) | 10604 (91.69) | 300 (7.96) | 239 (6.16) | 257 (11.22) | 165 (10.15) |
| Coupled, n (%) | 7884 (68.17) | 2620 (69.48) | 2857 (73.67) | 1443 (62.99) | 964 (59.32) |
| Not coupled, n (%) | 3681 (31.83) | 1151 (30.52) | 1021 (26.33) | 848 (37.01) | 661 (40.68) |
| Never smoker, n (%) | 4919 (42.53) | 1842 (48.85) | 1515 (39.07) | 966 (42.16) | 596 (36.68) |
| Past smoker, n (%) | 5268 (45.55) | 1593 (42.24) | 1962 (50.59) | 970 (42.34) | 743 (45.72) |
| Current smoker, n (%) | 1378 (11.92) | 336 (8.91) | 401 (10.34) | 355 (15.50) | 286 (17.60) |
| Under weight, n (%) | 141 (1.22) | 66 (1.75) | 30 (0.77) | 22 (0.96) | 23 (1.42) |
| Normal weight, n (%) | 3612 (31.23) | 1644 (43.60) | 966 (24.91) | 723 (31.56) | 279 (17.17) |
| Overweight, n (%) | 4705 (40.68) | 1540 (40.84) | 1681 (43.35) | 949 (41.42) | 535 (32.92) |
| Obese, n (%) | 3107 (26.87) | 521 (13.82) | 1201 (30.97) | 597 (26.06) | 788 (48.49) |
| Heart disease, n (%) | 2785 (24.08) | 312 (8.27) | 1312 (33.83) | 307 (13.40) | 854 (52.55) |
| Hypertension, n (%) | 6456 (55.82) | 1056 (28.00) | 2894 (74.63) | 1139 (49.72) | 1367 (84.12) |
| Stroke, n (%) | 635 (5.49) | 24 (0.64) | 226 (5.83) | 64 (2.79) | 321 (19.75) |
| Diabetes, n (%) | 1967 (17.01) | 104 (2.76) | 908 (23.41) | 237 (10.34) | 718 (44.18) |
| Lung Disease, n (%) | 1018 (8.80) | 65 (1.72) | 448 (11.55) | 90 (3.93) | 415 (25.54) |
| Arthritis, n (%) | 7076 (61.19) | 1300 (34.48) | 2871 (74.03) | 1463 (63.86) | 1442 (88.74) |
| Cancer, n (%) | 1428 (12.35) | 205 (5.44) | 757 (19.52) | 149 (6.50) | 317 (19.51) |
| Age at death, mean (SD) | 83.90 (7.25) | 87.52 (7.08) | 83.12 (6.77) | 83.77 (6.58) | 79.33 (5.98) |
Abbreviations: SD= standard deviation; IQR= interquartile range.
Multidimensional trajectories
Evaluation of fit indices (Table 2) for individual trajectory models of functional status, cognitive performance, and depressive symptoms each indicated that a four-group model was preferred. There were only marginal improvements in BIC scores between four- and five-group models and reduced posterior probabilities and group sizes in the five-group model. The exception was multimorbidity, which had the most favorable fit indices for the five-group model. Given these results, we compared multidimensional trajectory models with three to five groups. The BIC scores showed the greatest improvement between the three- and four-group models and plateau in the five-group model. While the five-group model had a nominally lower BIC compared with the four-group model, the five-group model split a group into two relatively indistinct subgroups. Therefore, we chose the four-group model as the most informative and parsimonious of the models tested. The average posterior probabilities of group membership in the four-group model ranged from 0.92 to 0.96 across trajectory groups and the odds of correct classification ranged from 26.5 to 156.99, both metrics indicating excellent fit.
Table 2.
Fit statistics for the three-, four-, and five-group multidimensional trajectory model, Health and Retirement Study 1998-2016
| Average Posterior Probability | Odds of Correct Classification | Group Size (%) | BIC | |
|---|---|---|---|---|
| Three-group model | −460070.3 | |||
| Group 1 | 0.956 | 33.3 | 39.5 | |
| Group 2 | 0.936 | 20.1 | 42.0 | |
| Group 3 | 0.960 | 107.6 | 18.5 | |
| Four-group model | −452322.5 | |||
| Group 1 | 0.915 | 43.0 | 20.2 | |
| Group 2 | 0.958 | 47.9 | 32.7 | |
| Group 3 | 0.930 | 26.5 | 32.9 | |
| Group 4 | 0.963 | 157.0 | 14.2 | |
| Five-group model | −444597.9 | |||
| Group 1 | 0.949 | 60.3 | 23.8 | |
| Group 2 | 0.965 | 193.8 | 12.3 | |
| Group 3 | 0.940 | 29.4 | 34.7 | |
| Group 4 | 0.922 | 52.6 | 18.3 | |
| Group 5 | 0.957 | 181.8 | 10.9 |
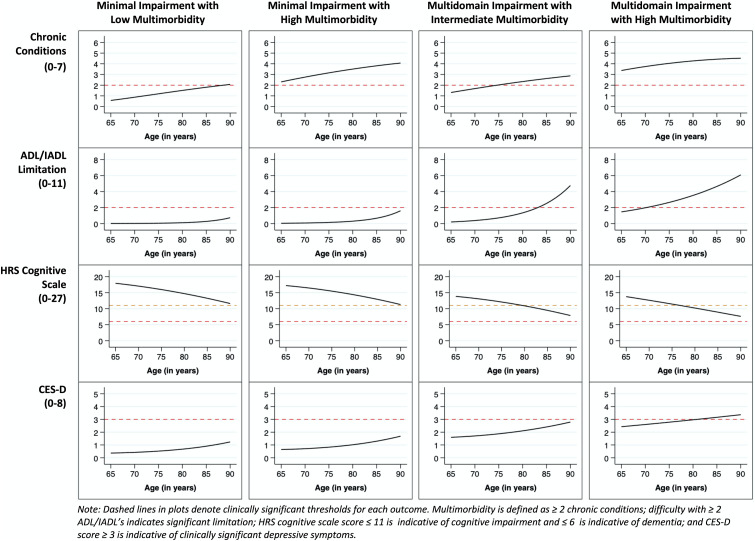
The four groups of distinct joint trajectories of multimorbidity accumulation, functional status, cognitive performance, and depressive symptoms are displayed in Figure 1 and the estimated trajectory parameters (intercepts, linear terms, and quadratic terms) are provided in Table 3. Minimal Impairment with Low Multimorbidity (Group 1), representing 32.7% (n=3771) of the sample, had, on average, low rates of chronic disease at age 65 and little accumulation over time; no ADL/IADL limitation at age 65 and low levels of limitation in late life; high cognitive performance at age 65 that, while declining, does not cross the threshold for cognitive impairment by age 90; and almost no depressive symptomatology throughout the observed age span.
Figure 1.
Multidimensional trajectories of functional limitations, number of chronic conditions, cognitive performance, and depressive symptoms, Health and Retirement Study 1998-2016.
Table 3.
Group-based multitrajectory model parameter estimates, Health and Retirement Study 1998-2016
| Minimal Impairment with Low Multimorbidity | Minimal Impairment with High Multimorbidity | Multidomain Impairment with Intermediate Multimorbidity | Multidomain Impairment with High Multimorbidity | |
|---|---|---|---|---|
| Multimorbidity (Count of chronic conditions), censored normal model | ||||
| Intercept | 0.6904*** | 2.428*** | 1.2304*** | 3.2812*** |
| Linear term | 0.077*** | 0.0971*** | 0.0783*** | 0.0755*** |
| Quadratic term | −0.0005** | −0.0011*** | −0.0007*** | −0.0014*** |
| Functional status (ADL/IADL limitations), zero-inflated Poisson model | ||||
| Intercept | −4.2541*** | −2.9063*** | −1.7751*** | 0.3905*** |
| Linear term | 0.1029*** | 0.1059*** | 0.1176*** | 0.0578*** |
| Quadratic term | 0.002*** | 0.0013*** | 0.0004** | −0.0000 |
| Cognitive function (HRS cognitive scale), censored normal model | ||||
| Intercept | 17.8362*** | 16.8455*** | 14.7908*** | 13.7962*** |
| Linear term | −0.1506*** | −0.1303*** | −0.1393*** | −0.2267*** |
| Quadratic term | −0.0036*** | −0.0037*** | −0.0045*** | −0.0014 |
| Depressive symptoms (CES-D), zero-inflated Poisson model | ||||
| Intercept | −1.1882*** | −0.5955*** | 0.5726*** | 0.9476*** |
| Linear term | 0.0223*** | 0.0222*** | 0.0086* | 0.0132*** |
| Quadratic term | 0.0011*** | 0.0009*** | 0.0005*** | −0.0001 |
Abbreviations: ADL = activities of daily living; IADL = instrumental activities of daily living; CES-D = Center for Epidemiological Studies Depression scale. *p < 0.05, **p < 0.01, ***p < 0.001.
Minimal Impairment with High Multimorbidity (Group 2; n=3878; 32.9%), is distinguished from the minimal impairment group by a higher multimorbidity level from the outset and throughout the observed age span. This group reaches the multimorbidity threshold by age 65, on average, and has greater accumulation of chronic conditions with increasing age. However, despite higher multimorbidity burden, members of this group developed few ADL/IADL limitations, maintained cognitive performance, and had few depressive symptoms across the observed age span.
Older adults in Multidomain Impairment with Intermediate Multimorbidity (Group 3; n=2291; 20.2%) had higher average multimorbidity burden than Group 1, Minimal Impairment with Low Multimorbidity—but lower average multimorbidity burden than Group 2, Minimal Impairment with High Multimorbidity. This group also displayed poor cognitive performance at age 65, rapid cognitive decline with advancing age, and high depressive symptomatology across the observed age span. Notably, while this group had, on average, good ADL/IADL function in early aging (65-75 years), the number of functional limitations sharply increases after age 75.
Finally, 14.2% (n=1625) of the sample comprised Multidomain Impairment with High Multimorbidity (Group 4). Respondents in this group had, on average, high levels of multimorbidity—more than three of seven chronic conditions at age 65 and the highest number of chronic conditions by age 90. Similar to the Multidomain Impairment with Intermediate Multimorbidity, members of this group demonstrated cognitive performance that was, on average, lower at age 65 than in the two low impairment groups, and exhibited rapid declination with advancing age. Compared with all other groups, this group experienced the greatest ADL/IADL functional limitation across the entire observed age span. Members of this group had, on average, two existing ADL/IADL limitations at age 65 and six by age 90. Additionally, this group had, on average, the highest number of depressive symptoms at age 65 and across the observed age span.
Multidimensional trajectory group membership characteristics
Sociodemographic and health-related characteristics for each multidimensional trajectory group are also presented in Table 1. There was a disproportionate number of Black and Hispanic respondents in both the Multidomain Impairment with Intermediate Multimorbidity and the Multidomain Impairment with High Multimorbidity groups. Overall, respondents from minoritized racial/ethnic backgrounds, low educational attainment, low wealth, current or prior smokers, and obese respondents were overrepresented in the two Multidomain Impairment trajectory groups (Groups 3 and 4). Tables A1-2 present outcome and chronic disease means and medians at selected ages—65, 75, and 90 years of age—for each of the trajectory groups. Mean outcome levels were comparatively worse at each reported age across outcomes for the Multidomain Impairment with High Multimorbidity group, especially when compared with the Minimal Impairment with Low Multimorbidity group (Table A1). Equivalently aged participants in the Multidomain Impairment with High Multimorbidity group had much higher prevalence of each of the chronic diseases compared with the other three groups (Table A2). The Minimal Impairment with High Multimorbidity group had higher prevalence across all diseases and particularly for heart disease, hypertension, diabetes, lung disease, and cancer compared with the Minimal Impairment with Low Multimorbidity and the Multidomain Impairment with Intermediate Multimorbidity groups.
Multidimensional trajectory group membership predictors
In unadjusted and adjusted multinomial logistic regression models, the healthiest and most functional group, Minimal Impairment with Low Multimorbidity (Group 1), served as the reference group. Consistent with the high posterior probabilities observed for assigned class membership, sensitivity analyses examining the impact of uncertainty in class assignment found negligible differences in the magnitude and statistical significance of coefficients between weighted and unweighted models, and, therefore, we report the results from unweighted models using modal class assignment. Compared with White respondents, Black respondents had greater odds of being in Multidomain Impairment with Intermediate Multimorbidity (Group 3) (OR=3.59, 95% CI=3.06,4.21) or Multidomain Impairment with High Multimorbidity (Group 4) (OR=4.30, 95% CI=3.63,5.09) than being in the reference group in the unadjusted model. Similarly, compared with White respondents, Hispanic respondents had greater odds of being in Multidomain Impairment with Intermediate Multimorbidity (Group 3) (OR=3.0, 95% CI=2.48,3.64) or Multidomain Impairment with High Multimorbidity (Group 4) (OR=3.29, 95% CI=2.68,4.04) in the unadjusted model.
In the covariate-adjusted multinomial logistic regression model (Table 4), relative to White respondents, Black respondents had greater odds of being in Multidomain Impairment with Intermediate Multimorbidity (Group 3) (OR=2.05, 95% CI=1.73,2.44) or in Multidomain Impairment with High Multimorbidity (Group 4) (OR=1.70, 95% CI=1.40,2.05) than being in the Minimal Impairment with Low Multimorbidity group. Similarly, Hispanic respondents were more likely than White respondents of being in either of the Multidomain Impairment (Groups 3 and 4) rather than the reference group, with odds ratios of 1.65 (95% CI=1.30,2.08) and 1.50 (95% CI=1.15,1.96), respectively. We found no significant interactions between race/ethnicity and sex, education, or household wealth.
Table 4.
Multinomial logistic regression of multidimensional trajectory group membership, Health and Retirement Study 1998-2016
| Minimal Impairment with High Multimorbidity OR (95% CI) | Multidomain Impairment with Intermediate Multimorbidity OR (95% CI) | Multidomain Impairment with High Multimorbidity OR (95% CI) | |
|---|---|---|---|
| Non-Hispanic White | Ref | Ref | Ref |
| Non-Hispanic Black | 1.152 (0.973,1.365) | 2.053*** (1.727,2.440) | 1.693*** (1.401,2.045) |
| Hispanic | 0.983 (0.779,1.241) | 1.648*** (1.304,2.082) | 1.499** (1.147,1.958) |
| Male | Ref | Ref | Ref |
| Female | 1.000 (0.904,1.107) | 1.100 (0.974,1.241) | 1.327*** (1.151,1.531) |
| < High School | 0.831** (0.724,0.955) | 2.251*** (1.962,2.582) | 2.122*** (1.816,2.480) |
| High School | Ref | Ref | Ref |
| ≥ College | 0.996 (0.891,1.113) | 0.623*** (0.532,0.730) | 0.714*** (0.587,0.867) |
| US born | Ref | Ref | Ref |
| Foreign born | 0.797* (0.653,0.973) | 0.901 (0.727,1.118) | 0.781 (0.602,1.012) |
| Wealth quartile 4 (high) | Ref | Ref | Ref |
| Wealth quartile 3 | 1.044 (0.923,1.180) | 1.217* (1.033,1.433) | 1.445** (1.152,1.811) |
| Wealth quartile 2 | 1.248** (1.092,1.425) | 1.630*** (1.379,1.926) | 2.584*** (2.076,3.216) |
| Wealth quartile 1 (low) | 1.618*** (1.378,1.899) | 2.336*** (1.936,2.817) | 5.935*** (4.717,7.468) |
| Not coupled | Ref | Ref | Ref |
| Coupled | 1.319*** (1.180,1.475) | 1.103 (0.972,1.251) | 1.190* (1.029,1.376) |
| Never smoker | Ref | Ref | Ref |
| Previous smoker | 1.463*** (1.323,1.618) | 1.226*** (1.086,1.384) | 1.607*** (1.393,1.855) |
| Current smoker | 1.697*** (1.436,2.006) | 1.959*** (1.637,2.345) | 3.024*** (2.467,3.707) |
| Under weight | 0.783 (0.503,1.220) | 0.664 (0.398,1.109) | 1.654 (0.974,2.808) |
| Normal weight | Ref | Ref | Ref |
| Overweight | 1.825*** (1.638,2.034) | 1.350*** (1.189,1.533) | 2.093*** (1.765,2.483) |
| Obese | 3.857*** (3.378,4.405) | 2.352*** (2.015,2.745) | 8.360*** (6.980,10.01) |
Notes. Reference trajectory group is Minimal Impairment with Low Multimorbidity group. Referent wealth quartile 4 represents highest wealth quartile.
AbbreviationsOR = odds ratio; CI = confidence interval; NH = non-Hispanic.
* p < 0.05, ** p < 0.01, *** p < 0.001.
After adjusting for covariates, female respondents were more likely than male respondents to be in Multidomain Impairment with High Multimorbidity (Group 4) (OR=1.33, 95% CI=1.15,1.53) rather than in Minimal Impairment with Low Multimorbidity (Group 1), although there were no significant associations between sex and membership in other trajectory groups. Being coupled was associated with greater odds of being in Minimal Impairment with High Multimorbidity (Group 2) (OR=1.32, 95% CI=1.18,1.48) or Multidomain Impairment with High Multimorbidity (Group 4) (OR=1.19, 95% CI=1.029,1.38) relative to the reference.
Educational attainment and household wealth were both significant factors of group membership after controlling for other variables in the model. Relative to high school graduates, respondents with greater than a high school education were significantly less likely to be in Multidomain Impairment with Intermediate Multimorbidity (Group 3) (OR=0.62, 95% CI=0.53,0.73) or Multidomain Impairment with High Multimorbidity (Group 4) (OR=0.71, 95% CI=0.59,0.87). Conversely, respondents who did not finish high school were less likely to be in Minimal Impairment with High Multimorbidity (Group 2) (OR=0.83, 95% CI=0.72,0.96) but more likely to be in Multidomain Impairment with Intermediate Multimorbidity (Group 3) (OR=2.25, 95% CI=1.96,2.58) or Multidomain Impairment with High Multimorbidity (Group 4) (OR=2.12, 95% CI=1.82,2.48). There was a significant wealth gradient in group membership, with lower wealth associated with significantly greater odds of membership in either of the two Multidomain Impairment groups (Groups 3 and 4). Compared with the wealthiest respondents, persons in the lowest wealth quartile had 134% greater odds of being in Multidomain Impairment with Intermediate Multimorbidity (Group 3) (OR=2.34, 95% CI=1.94,2.82) and 494% greater odds of being in Multidomain Impairment with High Multimorbidity (Group 4) (OR=5.94, 95% CI=4.72,7.47).
Smoking history and increased BMI were both significantly associated with greater likelihood of being in the Multidomain Impairment groups (Groups 3 and 4). Compared with never smokers, both previous and current smokers had greater odds of being in Multidomain Impairment with Intermediate Multimorbidity (Group 3) and Multidomain Impairment with High Multimorbidity (Group 4), with current smokers having 96% greater odds and 202% greater odds, respectively. Of all the characteristics included in the fully adjusted model, obesity had the strongest association with membership in Multidomain Impairment with High Multimorbidity (Group 4), with obese respondents having markedly greater odds (OR=8.37, 95% CI=6.98,10.01) than healthy weight respondents of being in this group.
Discussion
This study broadens the understanding of the relationships between several important and interrelated health domains in late life. Our study identified four distinct latent classes of multidimensional trajectories of chronic disease, functional status, cognitive performance, and emotional wellbeing which, when examined in combination, reflected varying courses of multimorbidity burden and impairment. Older adults in the Minimal Impairment with Low Multimorbidity group were consistent with a healthy aging trajectory along the multiple domains of health examined in this study. Respondents in this group had high levels of function across multiple domains into late life. Similarly, older adults in the Minimal Impairment with High Multimorbidity fared well on many domains of health as they aged despite experiencing high multimorbidity burden.
Notably, while the Minimal Impairment with High Multimorbidity group had high and rising levels of multimorbidity, members appeared resilient to these high disease burdens because they exhibited preserved functional and cognitive status and experienced low depressive symptomatology well into old age. Relative to the referent minimally impaired group, membership in this group was associated with sociodemographic characteristics such as having lower wealth, and living in a coupled partnership, as well as lifestyle factors such as smoking and being overweight or obese. It is interesting that these correlates hint at mechanisms for high levels of multimorbidity—having fewer material resources, smoking, and obesity have been linked to the development of several chronic diseases37—but also suggest potential compensatory mechanisms—such as being in a coupled partnership—that may be associated with observed resilience on other health domains. While members may experience high disease burdens, the social support gained from these partnerships may provide pathways to functional, cognitive, and emotional health maintenance in the face of managing multiple chronic conditions. Future work should examine coupled partnerships more fully, including assessing the role of relationship quality and disruptions to partnerships on multidimensional aspects of health.
In contrast to the two Minimal Impairment groups, the remaining two latent classes had demonstrably worse trajectories of aging across multiple health domains. The Multidomain Impairment with Intermediate Multimorbidity group was characterized by poor cognitive performance throughout the ages observed, and a rapid uptick in the difficulty to perform daily life functions. Collectively, these multiple trajectories reinforce that impaired ADL/IADL function in later life may be associated with cognitive decline for this group. Finally, the Multidomain Impairment with High Multimorbidity group fared comparatively worse on every domain examined from age 65 onward. Members of this group demonstrated high levels of chronic somatic and mental health morbidity and poor function—cognitive as well as daily life function—throughout older age. The high levels of impairment across all four outcomes throughout the observed age span suggest that exposures earlier in the lifecourse (early life, early adulthood, and midlife) should be examined to further understand critical periods in these multidimensional courses of health and wellbeing into old age. This group was also more likely to have obese BMI at baseline and had the youngest average age at death. Members of this group may benefit from earlier life interventions to prevent obesity and chronic conditions associated with obesity and earlier mortality.
In multinomial logistic regression models comparing the four identified groups, we found that socially disadvantaged and minoritized populations were more likely to be members of the Multidomain Impairment groups. Black and Hispanic older adults were significantly more likely than White older adults to be members of either of the two Multidomain Impairment multidimensional trajectory groups that experienced high levels and rates of impairment and disease accumulation. Similarly, older adults with low socioeconomic status were significantly more likely to follow trajectories of substantial impairment across multiple health domains into old age. These vulnerable population groups are at higher risk of multidomain decline and as such, should be the focus of clinical interventions and public health programs aimed at modulating these declines.
Our study reinforces the importance of identifying changes across functional, physiological, and emotional health in older age.13,38–43 Wickrama and colleagues (2013)42 assessed multidimensional health trajectories across chronic disease and functional domains, and found three latent classes characterized by maintained, persistently high, and deteriorating levels across these domains. In accordance with our study findings, socioeconomically disadvantaged and racial/ethnic minoritized groups were more likely to belong to adverse trajectory classes, reflecting significant health inequalities in late life. Also largely in alignment with our findings, Xu et al. (2015)43 assessed cumulative changes across multiple physiological systems with the evaluation of multidimensional trajectories across physiological and functional health domains. They identified four distinct patterns of joint changes in functional, emotional, and cognitive performance and found stark educational differences in later old age, suggestive of social stratification in aging. Our findings extend beyond these studies by evaluating trajectories across a long follow-up period, and evaluating the intertwined courses of multimorbidity and common geriatric syndromes (disability and cognition) for older adults.
The study has several strengths. First, it contributes to the rapidly evolving multimorbidity literature by evaluating concurrent changes in key health domains. By identifying distinct classes of older adults who experience changes in their somatic multimorbidity burden alongside critical aspects to “what matters most” to older adults, our findings highlight heterogeneity across multiple markers of health in late life that need to be addressed. Second, the HRS is a large, ongoing, prospective health interview survey representative of the U.S. population of middle-aged and older adults. By virtue of the long follow-up time, multiple repeated observations for a large sample of Americans permits the study of longitudinal changes of these important health domains. Third, the prospective design and oversampling of minoritized older adults in HRS enables the examination of group membership characteristics for these groups.
Several limitations should also be noted. First, the data are self-reported. While this is a feature for many of the measures employed in these analyses (i.e., assessed cognitive performance, and reported depressive symptoms), chronic disease self-reports may be subject to misreporting. However, multiple studies have shown adequate concordance between patient self-reports and objectively-ascertained diagnoses.44–46 Second, we were limited by the number of chronic diseases assessed in the HRS. These diagnoses cover many common aging-related conditions, but there may be important distinctions with examination of more specific diagnoses. Future studies should also evaluate multidimensional trajectories that include both more specific diagnoses (e.g., ischemic heart disease) and a wider range of pertinent chronic diseases consistent with multimorbidity measurement frameworks.47,48 On a related matter, the biennial interview schedule may not sufficiently capture important transitions and end-of-life terminal declines. Additional work should evaluate functional changes in data sources that conduct more frequent assessments. Third, there is substantial heterogeneity in the Hispanic ethnicity category in country of origin or ancestry and which is associated with substantial divergent health status between Hispanic subgroups.49 Fourth, there were insufficient numbers of other racial and ethnic groups to provide adequate power for modeling. Assessment of multidimensional trajectories among understudied racial/ethnic groups and within large racial/ethnic classifications should be examined in data sources that permit these evaluations. Furthermore, the exclusion of respondents who contributed fewer than three waves of data to facilitate the modeling of non-linear trajectories may have resulted in a sample biased toward more healthy respondents, and the presence of differential attrition rates across identified trajectory groups may have resulted in biased estimates of trajectory group size.50 Finally, these analyses are not intended to assess causal effects. Rather, the multinomial regression analyses identify between-group differences in key sociodemographic and health-related factors. There may be important mediational relationships between included model covariates such as BMI and education or income that should be the focus of future work.
Our study has important implications. It suggests that high multimorbidity burden can be present with heterogeneous impacts on other aspects of aging in American older adults. While some distinct classes of older adults may experience high multimorbidity, there are two divergent pathways characterized by either resilience or substantial impairment throughout old age in relation to these high burdens. Our findings highlight the importance of targeting and remediating poor progression of multimorbidity, depressive symptoms, cognitive, and functional health domains for minoritized and low socioeconomic older adults in the US. This involves addressing upstream social factors and structural inequities that precede downstream morbidity, functional, cognitive, and affective health outcomes. These results would logically support early interventions to prevent the development and worsening of chronic disease burden, depressive symptoms, as well as cognitive and functional impairment, and involve not only healthcare interventions, but also those targeting key social determinants of health, for example, improving the quality of early-life education particularly in lower-income communities, and facilitating engagement with community-based services and supports into old age. Finally, our study points toward the need for future research to identify factors that enable resilience to high multimorbidity, particularly modifiable factors amenable to therapeutic intervention. There is a need to prioritize healthy aging initiatives that improve early detection, prevention, management, and support for physiological, emotional, cognitive, and functional health needs, particularly among minoritized and low socioeconomic groups.
Appendix
Table A1.
Mean and median values for outcomes by multidimensional trajectory group at selected ages, Health and Retirement Study 1998-2016
| Minimal Impairment with Low Multimorbidity | Minimal Impairment with High Multimorbidity | Multidomain Impairment with Intermediate Multimorbidity | Multidomain Impairment with High Multimorbidity | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Chronic Conditions | ||||||||
| Age 65 | 0.6(0.55) | 1(1) | 2.3(0.86) | 2(1) | 1.33(0.70) | 1(1) | 3.34(1.19) | 3(1) |
| Age 75 | 1.26(0.78) | 1(1) | 3.14(0.90) | 3(1) | 2.02(0.80) | 2(1) | 3.95(1.08) | 4(2) |
| Age 90 | 2.1(0.98) | 2(2) | 4.01(0.77) | 4(0) | 2.72(0.87) | 3(1) | 4.65(0.98) | 5(1) |
| ADL/IADL Limitations | ||||||||
| Age 65 | 0.01(0.11) | 0(0) | 0.05(0.24) | 0(0) | 0.21(0.57) | 0(0) | 1.52(2.12) | 1(2) |
| Age 75 | 0.05(0.27) | 0(0) | 0.14(0.47) | 0(0) | 0.7(1.24) | 0(1) | 2.68(2.55) | 2(3) |
| Age 90 | 0.75(1.30) | 0(1) | 1.51(1.92) | 1(2) | 4.34(2.35) | 4(3.5) | 5.96(2.69) | 5(3) |
| HRS Cognitive Scale | ||||||||
| Age 65 | 18.08(3.33) | 18(4) | 17.14(3.44) | 17(5) | 13.78(4.15) | 14(6) | 13.63(4.46) | 14(7) |
| Age 75 | 16.06(3.65) | 16(5) | 15.44(3.53) | 16(5) | 11.78(4.37) | 12(6) | 11.45(4.66) | 12(7) |
| Age 90 | 11.64(3.92) | 12(6) | 11.73(3.86) | 12(5) | 7.67(4.40) | 8(6) | 7.35(3.23) | 8(4) |
| CES-D | ||||||||
| Age 65 | 0.41(0.85) | 0(1) | 0.71(1.10) | 0(1) | 1.58(1.59) | 1(2) | 2.49(1.90) | 2(3) |
| Age 75 | 0.55(0.92) | 0(1) | 0.88(1.32) | 0(1) | 1.89(1.77) | 1(3) | 2.85(1.96) | 3(3) |
| Age 90 | 1.18(1.42) | 1(2) | 1.4(1.46) | 1(2) | 2.57(1.74) | 2(3) | 2.98(1.92) | 3(3) |
Table A2.
Chronic disease prevalence by multitrajectory group and age, Health and Retirement Study 1998-2016
| Minimal Impairment with Low Multimorbidity | Minimal Impairment with High Multimorbidity | Multidomain Impairment with Intermediate Multimorbidity | Multidomain Impairment with High Multimorbidity | Total | |
|---|---|---|---|---|---|
| Heart disease | n=yes/total n (%) | n=yes/total n (%) | n=yes/total n (%) | n=yes/total n (%) | n=yes/total n (%) |
| Age 65-66 | 63/1696 (3.71) | 704/2495 (28.22) | 70/1117 (6.27) | 546/1083 (50.42) | 1383/6391 (21.64) |
| Age 75-76 | 277/2255 (12.28) | 1161/2299 (50.50) | 312/1424 (21.91) | 570/862 (66.13) | 2320/6840 (33.92) |
| Age 85-86 | 371/1393 (26.63) | 565/752 (75.13) | 266/600 (44.33) | 175/202 (86.63) | 1377/2947 (46.73) |
| Hypertension | |||||
| Age 65-66 | 361/1696 (21.29) | 1864/2495 (74.71) | 558/1117 (49.96) | 923/1083 (85.23) | 3706/6391 (57.99) |
| Age 75-76 | 990/2255 (43.90) | 2000/2299 (86.99) | 919/1424 (64.54) | 798/862 (92.58) | 4707/6840 (68.82) |
| Age 85-86 | 810/1393 (58.15) | 694/752 (92.29) | 454/600 (75.67) | 196/202 (97.03) | 2154/2947 (73.09) |
| Stroke | |||||
| Age 65-66 | 3/1696 (0.18) | 97/2495 (3.89) | 17/1117 (1.52) | 198/1083 (18.28) | 315/6391 (4.93) |
| Age 75-76 | 41/2255 (1.82) | 257/2299 (11.18) | 94/1424 (6.60) | 266/862 (30.86) | 658/6840 (9.62) |
| Age 85-86 | 77/1393 (5.53) | 163/752 (21.68) | 114/600 (19.00) | 92/202 (45.54) | 446/2947 (15.13) |
| Diabetes | |||||
| Age 65-66 | 30/1696 (1.77) | 582/2495 (23.33) | 103/1117 (9.22) | 503/1083 (46.45) | 1218/6391 (19.06) |
| Age 75-76 | 145/2255 (6.43) | 810/2299 (35.23) | 254/1424 (17.84) | 496/862 (57.54) | 1705/6840 (24.93) |
| Age 85-86 | 106/1393 (7.61) | 297/752 (39.49) | 98/600 (16.33) | 101/202 (50.00) | 602/2947 (20.43) |
| Lung Disease | |||||
| Age 65-66 | 14/1696 (0.83) | 259/2495 (10.38) | 41/1117 (3.67) | 307/1083 (28.35) | 621/6391 (9.72) |
| Age 75-76 | 83/2255 (3.68) | 387/2299 (16.83) | 105/1424 (7.37) | 294/862 (34.11) | 869/6840 (12.70) |
| Age 85-86 | 73/1393 (5.24) | 183/752 (24.34) | 58/600 (9.67) | 58/202 (28.71) | 372/2947 (12.62) |
| Arthritis | |||||
| Age 65-66 | 517/1696 (30.48) | 1863/2495 (74.67) | 696/1117 (62.31) | 983/1083 (90.77) | 4059/6391 (63.51) |
| Age 75-76 | 1079/2255 (47.85) | 1964/2299 (85.43) | 1091/1424 (76.62) | 820/862 (95.13) | 4954/6840 (72.43) |
| Age 85-86 | 888/1393 (63.75) | 707/752 (94.02) | 516/600 (86.00) | 201/202 (99.50) | 2312/2947 (78.45) |
| Cancer | |||||
| Age 65-66 | 57/1696 (3.36) | 443/2495 (17.76) | 55/1117 (4.92) | 223/1083 (20.59) | 778/6391 (12.17) |
| Age 75-76 | 256/2255 (11.35) | 689/2299 (29.97) | 169/1424 (11.87) | 250/862 (29.00) | 1364/6840 (19.94) |
| Age 85-86 | 241/1393 (17.30) | 332/752 (44.15) | 123/600 (20.50) | 79/202 (39.11) | 775/2947 (26.30) |
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging at the National Institutes of Health (Grant Number: R01AG055681 and RF1AG05845 to ARQ). Additional funding: National Institute on Aging/National Institutes of Health (R01AG047891 to HGA who also contributed from the Yale Claude D. Pepper Older Americans Independence Center P30AG021342 and P30AG066508; the University of Michigan Claude D. Pepper Older Americans Independence Center AG024824 and the Michigan Institute for Clinical and Health Research UL1TR000433 support to AB; and the Oregon Alzheimer’s Disease Research Center P30AG008017, P30AG066518 and P30AG024978 to JK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Lochner KA, Cox CS. Prevalence of Multiple Chronic Conditions Among Medicare Beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10. doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barile JP, Thompson WW, Zack MM, Krahn GL, Horner-Johnson W, Bowen SE. Multiple chronic medical conditions and health-related quality of life in older adults, 2004-2006. Preventing chronic disease. 2013;10:E162. doi: 10.5888/pcd10.120282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinetti ME, Fried T. The end of the disease era. The American Journal of Medicine. 2004;116(3):179-185. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Quiñones AR, Markwardt S, Botoseneanu A. Multimorbidity Combinations and Disability in Older Adults. The Journals of Gerontology: Series A. 2016;71(6):823-830. doi: 10.1093/gerona/glw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vetrano DL, Foebel AD, Marengoni A, et al. Chronic diseases and geriatric syndromes: The different weight of comorbidity. European Journal of Internal Medicine. 2016;27:62-67. doi: 10.1016/j.ejim.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ, Edgman-Levitan S. Shared Decision Making — The Pinnacle of Patient-Centered Care. New England Journal of Medicine. 2012;366(9):780-781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 7.Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: Interactions and Outcomes across the Care Continuum. J Aging Health. 2021;33(7-8):469-481. doi: 10.1177/0898264321991658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetrano DL, Palmer K, Marengoni A, et al. Frailty and Multimorbidity: A Systematic Review and Meta-analysis. The Journals of Gerontology: Series A. 2019;74(5):659-666. doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 9.Spencer-Bonilla G, Quiñones AR, Montori VM, International Minimally Disruptive Medicine Workgroup . Assessing the Burden of Treatment. J Gen Intern Med. 2017;32(10):1141-1145. doi: 10.1007/s11606-017-4117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May CR, Eton DT, Boehmer K, et al. Rethinking the patient: using Burden of Treatment Theory to understand the changing dynamics of illness. BMC Health Services Research. 2014;14:281. doi: 10.1186/1472-6963-14-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran V-T, Montori VM, Ravaud P. Is My Patient Overwhelmed?: Determining Thresholds for Acceptable Burden of Treatment Using Data From the ComPaRe e-Cohort. Mayo Clinic Proceedings. 2020;95(3):504-512. doi: 10.1016/j.mayocp.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182-192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zang E, Shi Y, Wang X, Wu B, Fried TR. Trajectories of physical functioning among US adults with cognitive impairment. Age and Ageing. 2022;51(6):afac139. doi: 10.1093/ageing/afac139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd C, Smith CD, Masoudi FA, et al. Decision Making for Older Adults With Multiple Chronic Conditions: Executive Summary for the American Geriatrics Society Guiding Principles on the Care of Older Adults With Multimorbidity. J Am Geriatr Soc. 2019;67(4):665-673. doi: 10.1111/jgs.15809. [DOI] [PubMed] [Google Scholar]
- 15.Tinetti M, Huang A, Molnar F. The Geriatrics 5M’s: A New Way of Communicating What We Do. Journal of the American Geriatrics Society. 2017;65(9):2115. doi: 10.1111/jgs.14979. [DOI] [PubMed] [Google Scholar]
- 16.Marengoni A, Calderon-Larrañaga A. Health inequalities in ageing: towards a multidimensional lifecourse approach. The Lancet Public Health. 2020;5(7):e364-e365. doi: 10.1016/S2468-2667(20)30093-1. [DOI] [PubMed] [Google Scholar]
- 17.Angel RJ, Angel JL, Hill TD. Longer Lives, Sicker Lives? Increased Longevity and Extended Disability Among Mexican-Origin Elders. The Journals of gerontologySeries B, Psychological Sciences and Social Sciences. November 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quiñones AR, Botoseneanu A, Markwardt S, et al. Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLoS One. 2019;14(6):e0218462. doi: 10.1371/journal.pone.0218462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calderón-Larrañaga A, Santoni G, Wang HX, et al. Rapidly developing multimorbidity and disability in older adults: does social background matter? Journal of Internal Medicine. 2018;283(5):489-499. doi: 10.1111/joim.12739. [DOI] [PubMed] [Google Scholar]
- 20.Collins DM, Downer B, Kumar A, et al. Impact of Multiple Chronic Conditions on Activity Limitations Among Older Mexican-American Care Recipients. Prev Chronic Dis. 2018;15. doi: 10.5888/pcd15.170358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heeringa SG, Connor JH. Technical Description of the Health and Retirement Survey Sample Design.; 1995. [Google Scholar]
- 22.Hsu H-C, Jones BL. Multiple Trajectories of Successful Aging of Older and Younger Cohorts. The Gerontologist. 2012;52(6):843-856. doi: 10.1093/geront/gns005. [DOI] [PubMed] [Google Scholar]
- 23.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA : the journal of the American Medical Association. 2014;311(5):490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cigolle CT, Nagel CL, Blaum CS, Liang J, Quiñones AR. Inconsistency in the Self-report of Chronic Diseases in Panel Surveys: Developing an Adjudication Method for the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci. 2018;73(5):901-912. doi: 10.1093/geronb/gbw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spector WD, Fleishman JA. Combining activities of daily living with instrumental activities of daily living to measure functional disability. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1998;53(1):S46-S57. [DOI] [PubMed] [Google Scholar]
- 26.Langa KM, Ryan LH, McCammon RJ, et al. The Health and Retirement Study Harmonized Cognitive Assessment Protocol Project: Study Design and Methods. NED. September 2019:1-11. doi: 10.1159/000503004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weir DR, Langa KM, Ryan LH. 2016. Harmonized Cognitive Assessment Protocol (HCAP) Study Protocol Summary. Ann Arbor, MI: Health and Retirement Study, Survey Research Center, Institute for Social Research, University of Michigan; 2016:1-15. [Google Scholar]
- 28.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of Cognition Using Surveys and Neuropsychological Assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66B(suppl_1):i162-i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385-401. [Google Scholar]
- 30.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D. American Journal of Preventive Medicine. 1994. [PubMed] [Google Scholar]
- 31.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and aging. 1997;12(2):277. [DOI] [PubMed] [Google Scholar]
- 32.Steffick DE. Documentation of Affective Functioning Measures in the Health and Retirement Study. Ann Arbor, Michigan: Institute for Social Research, University of Michigan; 2000. [Google Scholar]
- 33.CDC . Defining Adult Overweight and Obesity. Centers for Disease Control and Prevention. https://www.cdc.gov/obesity/adult/defining.html. Published June 7, 2021. Accessed November 29, 2021. [Google Scholar]
- 34.Nagin D. Group-Based Modeling of Development. Harvard University Press; 2005. doi: 10.4159/9780674041318. [DOI] [Google Scholar]
- 35.Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27(7):2015-2023. doi: 10.1177/0962280216673085. [DOI] [PubMed] [Google Scholar]
- 36.Jones BL, Nagin DS. A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociological Methods & Research. 2013;42(4):608-613. doi: 10.1177/0049124113503141. [DOI] [Google Scholar]
- 37.Nyberg ST, Singh-Manoux A, Pentti J, et al. Association of Healthy Lifestyle With Years Lived Without Major Chronic Diseases. JAMA Intern Med. April 2020. doi: 10.1001/jamainternmed.2020.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noël PH, Williams JW, Unützer J, et al. Depression and Comorbid Illness in Elderly Primary Care Patients: Impact on Multiple Domains of Health Status and Well-being. The Annals of Family Medicine. 2004;2(6):555-562. doi: 10.1370/afm.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen K-M, Hung H-M, Lin H-S, Haung H-T, Yang Y-M. Development of the model of health for older adults. Journal of Advanced Nursing. 2011;67(9):2015-2025. doi: 10.1111/j.1365-2648.2011.05643.x. [DOI] [PubMed] [Google Scholar]
- 40.Chang W-C, Lu F-P, Lan T-Y, Wu S-C. Multidimensional health-transition patterns among a middle-aged and older population. Geriatrics & Gerontology International. 2013;13(3):571-579. doi: 10.1111/j.1447-0594.2012.00937.x. [DOI] [PubMed] [Google Scholar]
- 41.Luo MS, Li LW. Are Self-perceptions of Aging Associated With Health Trajectories Among Middle-Aged and Older Adults? The Gerontologist. 2020;60(5):841-850. doi: 10.1093/geront/gnz092. [DOI] [PubMed] [Google Scholar]
- 42.Wickrama KKAS, Mancini JA, Kwag K, Kwon J. Heterogeneity in multidimensional health trajectories of late old years and socioeconomic stratification: a latent trajectory class analysis. J Gerontol B Psychol Sci Soc Sci. 2013;68(2):290-297. doi: 10.1093/geronb/gbs111. [DOI] [PubMed] [Google Scholar]
- 43.Xu X, Liang J, Bennett JM, Botoseneanu A, Allore HG. Socioeconomic stratification and multidimensional health trajectories: evidence of convergence in later old age. The journals of gerontologySeries B, Psychological sciences and social sciences. 2015;70(4):661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weir D. Elastic Powers. National Academies Press (US); 2008. https://www.ncbi.nlm.nih.gov/books/NBK62437/. Accessed September 11, 2020. [Google Scholar]
- 45.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance Between Respondent Self-reports and Medical Records for Chronic Conditions: Experience From the Veterans Health Study. The Journal of Ambulatory Care Management. 2005;28(2):102-110. [DOI] [PubMed] [Google Scholar]
- 46.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of clinical epidemiology. 2004;57(10):1096-1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Calderón-Larrañaga A, Vetrano DL, Onder G, et al. Assessing and Measuring Chronic Multimorbidity in the Older Population: A Proposal for Its Operationalization. J Gerontol A Biol Sci Med Sci. 2017;72(10):1417-1423. doi: 10.1093/gerona/glw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodman RA, Posner S, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Preventing chronic disease. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daviglus ML, Talavera GA, Avilés-Santa ML, et al. Prevalence of Major Cardiovascular Risk Factors and Cardiovascular Diseases Among Hispanic/Latino Individuals of Diverse Backgrounds in the United States. JAMA. 2012;308(17):1775-1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haviland AM, Jones BL, Nagin DS. Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociological Methods & Research. 2011;40(2):367-390. [Google Scholar]