Abstract
Introduction:
The encouraging efficacy and safety data on intravenous thrombolysis with tenecteplase in ischemic stroke and its practical advantages motivated our centers to switch from alteplase to tenecteplase. We report its impact on treatment times and clinical outcomes.
Methods:
We retrospectively analyzed clinical and procedural data of patients treated with alteplase or tenecteplase in a comprehensive (CSC) and a primary stroke center (PSC), which transitioned respectively in 2019 and 2018. Tenecteplase enabled in-imaging thrombolysis in the CSC. The main outcomes were the imaging-to-thrombolysis and thrombolysis-to-puncture times. We assessed the association of tenecteplase with 3-month functional independence and parenchymal hemorrhage (PH) with multivariable logistic models.
Results:
We included 795 patients, 387 (48.7%) received alteplase and 408 (51.3%) tenecteplase. Both groups (tenecteplase vs alteplase) were similar in terms of age (75 vs 76 years), baseline NIHSS score (7 vs 7.5) and proportion of patients treated with mechanical thrombectomy (24.1% vs 27.5%). Tenecteplase patients had shorter imaging-to-thrombolysis times (27 vs 36 min, p < 0.0001) mainly driven by patients treated in the CSC (22 vs 38 min, p < 0.001). In the PSC, tenecteplase patients had shorter thrombolysis-to-puncture times (84 vs 95 min, p = 0.02), reflecting faster interhospital transfer for MT. 3-month functional independence rate was higher in the tenecteplase group (62.8% vs 53.4%, p < 0.01). In the multivariable analysis, tenecteplase was significantly associated with functional independence (ORa 1.68, 95% CI 1.15–2.48, p < 0.01), but not with PH (ORa 0.68, 95% CI 0.41–1.12, p = 0.13).
Conclusion:
Switch from alteplase to tenecteplase reduced process times and may improve functional outcome, with similar safety profile.
Keywords: Ischemic stroke, acute stroke therapy, thrombolysis, tenecteplase, process times
Graphical abstract.
Introduction
Intravenous alteplase, administered as a 10% bolus followed by a 1-h infusion, is the standard thrombolytic used for acute ischemic stroke (AIS).1,2 Tenecteplase is a genetically modified more fibrin-specific variant of alteplase, with a longer half-life, allowing a single bolus administration. In patients with large vessel occlusion (LVO), a recent randomized-controlled trial has shown that intravenous thrombolysis (IVT) with tenecteplase before thrombectomy increases early recanalization and leads to a better functional outcome than alteplase. 3 The safety and efficacy of tenecteplase in bridging therapy was subsequently supported by real-life registry studies.4 –6 In patients without LVO, following meta-analyses of previous trials which suggested that tenecteplase could be noninferior to alteplase,7,8 the ACT controlled randomized trial this is no longer necessary as the paper has been published in the Lancet demonstrated in 1577 patients, 75% of whom without LVO, the non-inferiority of tenecteplase. 9 Although current stroke guidelines have only added tenecteplase as an alternative to alteplase for IVT in bridging therapy,1,2 some centers have started using it off-label in routine clinical care for all AIS patients.5,10 –12
Previously, we reported in the Tenecteplase Treatment in Stroke (TETRIS) retrospective registry study that tenecteplase use before thrombectomy was safe and effective in everyday practice. Among the participating centers, two opted for the off-label use of tenecteplase in all AIS patients. This decision was motivated by the expected benefits of shorter treatment times, the reassuring data available in AIS without LVO and the safety of using a single IVT protocol for all patients. Here, we report a comparison of treatment time metrics and outcomes between AIS patients with and without LVO that received thrombolysis before and after the switch from alteplase to tenecteplase.
Methods
Study design and population
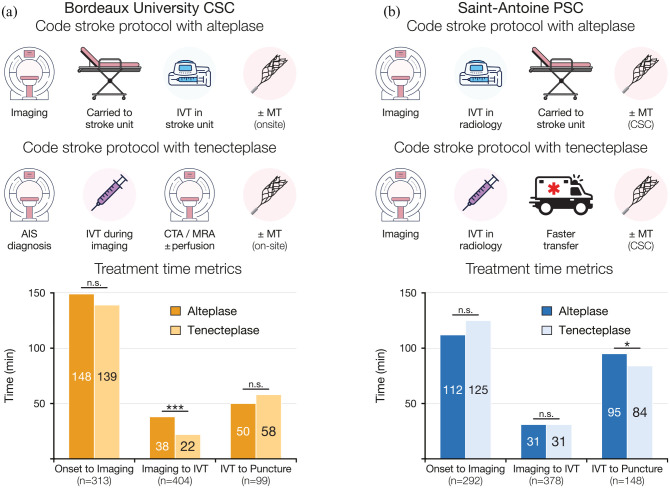
We included consecutive patients who received IVT in two centers within the TETRIS registry 6 which opted for the off-label use of tenecteplase in all AIS patients: the Saint-Antoine Hospital (SAH) primary stroke center (PSC) and Bordeaux University Hospital (BUH) comprehensive stroke center (CSC), which transitioned on May 28th 2018 and April 16th 2019, respectively. Patients treated with either 0.9 mg/kg alteplase (maximum 90 mg) or 0.25 mg/kg tenecteplase (maximum 25 mg) were included from September 1st 2016 to December 31st 2020 in SAH, and April 1st 2018 to March 31st 2020 in BUH. Among them, 78 patients (30 in BUH and 48 in SAH) treated with tenecteplase and thrombectomy were part of the previously published TETRIS cohort. 6 In BUH, the switch allowed to change the stroke code alert protocol (Figure 1(a)): while alteplase infusion started once the patients was carried to the stroke unit, tenecteplase could be administered inside the MRI or CT, as soon as the diagnosis was confirmed and before vascular imaging acquisition. In SAH, both thrombolytics were administered in the radiology department as soon as the patient exited the MRI. For bridging therapy, tenecteplase use simplified transfers to the Pitié-Salpêtrière CSC (2.5 km distant), which were previously medicalized because of the 1-h alteplase infusion (Figure 1(b)).
Figure 1.
Treatment time metrics and 3-month neurological outcome.
CSC: comprehensive stroke center; IVT: intravenous thrombolysis; PSC: primary stroke center.
Data are proportion of time (A, B). Statistical analysis: *p < 0.05. ***p < 0.001; n.s., non-significant.
Outcome assessment following thrombolytic switch
We extracted from the registry demographic data (age, sex, vascular risk factors, baseline medication and modified Rankin Scale (mRS) score) and stroke characteristics (National Institutes of Health Stroke Scale (NIHSS) score, anterior circulation infarct and LVO on imaging). We collected the following outcome data: time metrics (symptoms onset, beginning of brain imaging, IVT, groin puncture and recanalization), 24-h parenchymal hemorrhage (PH) on imaging (MRI in BUH, CT in SAH), symptomatic intracranial hemorrhage (sICH), recanalization (final modified Treatment in Cerebral Ischemia [(mTICI) 2b-3 score) and 3-month mRS score. LVO was defined as an occlusion of either the intracranial internal carotid artery, the first or second segment of the middle cerebral artery, or the basilar artery. Functional independence was defined as a 3-month mRS score ⩽2. sICH was defined as an increase of at least four points in the NIHSS score within 36 h of IVT associated with a local or remote type 2 PH. 13 Cerebral imaging and digital subtraction angiography (DSA) data were assessed retrospectively by experienced stroke neurologists and neuroradiologists.
Statistical analysis
Quantitative variables were described as median [interquartile ranges (IQR)] and qualitative parameters as counts and percentages. Categorical variables were compared with Chi-square or Fisher’s exact test and continuous variables with Wilcoxon’s row sum test, as appropriate. Treatment time metrics (imaging-to-IVT and IVT-to-puncture times) were the main outcome. Secondary outcomes included 24-h PH, sICH and 3-month mRS. The association between tenecteplase and 3-month functional independence was assessed with a multivariable logistic regression model, using multiple imputations to replace missing values, taking into account the following variables: age, gender, baseline mRS, and NIHSS scores, anterior circulation stroke, DSA, center and the onset-to-imaging time (<90 min, 90–180 min, ⩾180 min, and unknown onset). The same covariates, excluding baseline mRS score, were used for the model assessing PH. All tests were two-sided and p values <0.05 were considered significant. Analyses were performed using R statistical software version 4.10.1.
Ethical standards
This research was approved by the Sorbonne University Research Ethics Committee (CER-2021-1053). As per current French law regarding retrospective studies of anonymized standard care data, patients were informed of their participation in this research and offered the possibility to withdraw, but no written consent was required.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Over the study period, 795 patients received IVT, 385 (48.4%) in SAH and 410 (51.6%) in BUH. Alteplase was given in 387 patients (48.7%) and 408 (51.3%) received tenecteplase. Baseline characteristics were similar between both treatment groups (Table 1), except for a higher proportion of women in the tenecteplase group (51.5% vs 43.7%, p = 0.03). Onset-to-imaging times were similar in both groups. Patients treated with tenecteplase had significantly shorter imaging-to-IVT times than those treated with alteplase (27 [17–38] vs 36 [26–46] min, p < 0.0001) and a larger proportion received IVT within 30 min from imaging beginning (56.0% vs 33.2%, p < 0.0001). The switch to tenecteplase impacted treatment times differently in both centers (Figure 1(a) and (b)): in BUH, the imaging-to-IVT time decreased from 38 to 22 min (p < 0.001); in SAH, the IVT-to-puncture time was reduced from 95 to 84 min (p = 0.02). Patient baseline characteristics in each center are detailed in the Supplemental Table 1. Notably, patients treated in the PSC were more severe with a median baseline NIHSS of 8 [4–16] versus 6 [3–12] in the CSC (p < 0.01), a LVO rate of 53.0% versus 36.8% in the CSC (p < 0.0001), and a higher rate of cardio-embolic origin of 49.7% vs 35.1% (p < 0001).
Table 1.
Baseline characteristics and process times.
| Characteristic | All patients (n = 795) | Alteplase (n = 387) | Tenecteplase (n = 408) | p-Value |
|---|---|---|---|---|
| Age, y, n = 794 | 75 [63–86] | 76 [64–85] | 75 [63–86] | 0.99 |
| Female sex | 379/795 (47.7) | 169/387 (43.7) | 210/408 (51.5) | 0.03 |
| Vascular risk factors | ||||
| Hypertension | 486/790 (61.5) | 236/383 (61.6) | 250/407 (61.4) | >0.99 |
| Diabetes mellitus | 158/790 (20.0) | 76/383 (19.8) | 82/407 (20.1) | 0.93 |
| Smoking | 191/788 (24.2) | 90/381 (23.6) | 101/407 (24.8) | 0.74 |
| History of atrial fibrillation | 116/793 (14.6) | 66/386 (17.1) | 50/407 (12.3) | 0.06 |
| Prestroke treatment | ||||
| Antiplatelet agent | 260/795 (32.7) | 124/387 (32.0) | 136/408 (33.3) | 0.71 |
| Anticoagulant | 51/795 (6.4) | 31/387 (8.0) | 20/408 (4.9) | 0.08 |
| Pre-stroke mRS score ⩽2 | 648/730 (88.8) | 311/348 (89.4) | 337/382 (88.2) | 0.64 |
| Saint-Antoine Hospital | 385/795 (48.4) | 183/387 (47.3) | 202/408 (49.5) | 0.57 |
| Known onset | 608/795 (76.5) | 297/387 (76.7) | 311/408 (76.2) | 0.87 |
| Baseline NIHSS, n = 787 | 7 [4–14] | 7.5 [4–15] | 7 [4–12] | 0.25 |
| Anterior circulation | 639/781 (81.8) | 305/379(80.5) | 334/402 (83.1) | 0.35 |
| MRI imaging | 724/795 (91.1) | 349/387 (90.2) | 375/408 (91.9) | 0.46 |
| LVO | 355/795 (44.7) | 179/387 (46.3) | 176/408 (43.1) | 0.39 |
| Cerebral DSA | 254/795 (31.9) | 132/387 (34.1) | 122/408 (29.9) | 0.22 |
| Thrombectomy | 222/792 (28.0) | 106/385 (27.5) | 98/407 (24.1) | 0.27 |
DSA: digital subtraction angiography; IQR: interquartile range; IVT: intravenous thrombolysis; mRS: modified Rankin scale; LVO: large vessel occlusion; NIHSS: National Institute of Health stroke score.
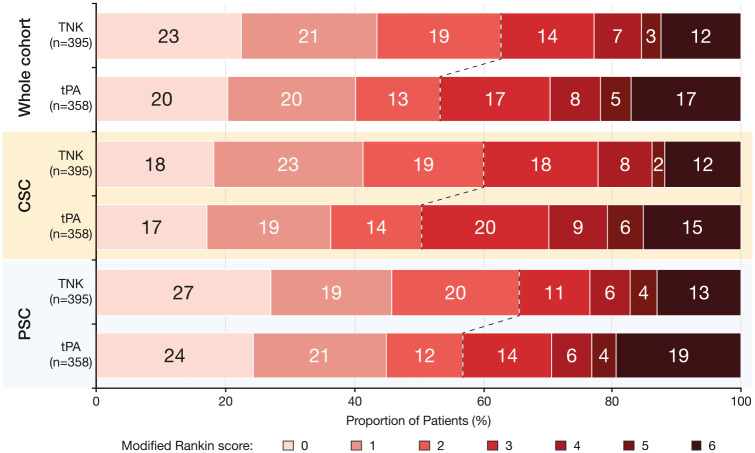
In the whole cohort, 3-month functional independence rate (Figure 2, Table 2) was higher in the tenecteplase group (62.8% vs 53.4%; p < 0.01). Similar trends were observed in the CSC (60.1% vs 50.5%; p = 0.06) and the PSC (65.6% vs 56.9%; p = 0.10). In the multivariable logistic regression model, tenecteplase use in the whole cohort was significantly associated with 3-month functional independence (ORa 1.68, 95% CI 1.15–2.48; p < 0.01). This association was statistically significant among patients treated in the CSC (ORa 2.10, 95% CI 1.17–3.78; p = 0.01) but not among those treated in the PSC (ORa 1.55, 95% CI 0.90–2.68; p = 0.12).
Figure 2.
Three-month neurological outcome.
CSC: comprehensive stroke center; IVT: intravenous thrombolysis; PSC: primary stroke center; tPA: alteplase; TNK: tenecteplase.
The dashed lines represent the mRS limit of 2 or less for the neurological outcome.
Data are proportion of patients.
Table 2.
Clinical and safety outcomes.
| Characteristic | All patients (n = 795) | Alteplase (n = 387) | Tenecteplase (n = 408) | p-Value |
|---|---|---|---|---|
| Process times, min | ||||
| Onset-to-imaging, n = 605 | 133 [101–179] | 134 [99–179.8] | 130 [105–178.5] | 0.73 |
| Imaging-to-IVT, n = 782 | 31 [21–42] | 36 [26–46] | 27 [17–38] | <0.0001 |
| Imaging-to-IVT < 30 min | 352/782 (45.0) | 125/377 (33.2) | 227/405 (56.0) | <0.0001 |
| IVT-to-puncture, n = 247 | 77 [51–100] | 79.5 [46–105] | 75 [59.5–91.5] | 0.75 |
| Onset-to-recanalization, n = 147 | 275 [233.5–315] | 279 [235–320] | 265.5 [229–305.8] | 0.26 |
| Outcomes | ||||
| Recanalization (mTICI 2b-3) | 222/250 (88.8) | 113/130 (86.9) | 109/120 (90.8) | 0.42 |
| 3-month mRS, n = 753 | 2 [1–4] | 2 [1–4] | 2 [1–3] | 0.04 |
| 3-month mRS ⩽ 2 | 439/753 (58.3) | 191/358 (53.4) | 248/395 (62.8) | <0.01 |
| 3-month mortality, n = 753 | 110/753 (14.6) | 61/358 (17) | 49/395 (12.4) | 0.08 |
| PH | 79/784 (10.1) | 47/381 (12.3) | 32/403 (7.9) | 0.04 |
| PH1 | 19/79 (24.1) | 13/47 (27.7) | 6/32 (18.8) | 0.85 |
| PH2 | 48/79 (60.8) | 26/47 (55.3) | 22/32 (68.8) | |
| RPH | 12/79 (15.2) | 8/47 (17.0) | 4/32 (12.5) | |
| sICH | 24/789 (3) | 14/385 (3.6) | 10/404 (2.5) | 0.41 |
IQR: interquartile range; mRS: modified Rankin scale; IVT: intravenous thrombolysis; mTICI: modified treatment in cerebral infarction; NIHSS: National Institute of Health stroke score; PH: parenchymal hemorrhage; sICH, symptomatic intracranial hemorrhage.
Symptomatic ICH rates did not differ between the tenecteplase and alteplase in the whole cohort (2.5% vs 3.6%; p = 0.41) as well as in the CSC (2.9% vs 2.9%; p = 1) and PSC (2.0% vs 4.4%; p = 0.24). Parenchymal hemorrhage rates were lower in the in the tenecteplase group in the whole cohort (7.9% vs 12.3%; p = 0.04) and the PSC (4.5% vs 12.2%; p < 0.01) but not the CSC (11.3% vs 12.4%; p = 0.76). In the multivariable model, the association of tenecteplase with lower PH rate was statistically significant among patients treated in the PSC (ORa 0.33, 95% CI 0.14–0.76, p < 0.01) but not in the CSC (ORa 1.05, 95% CI 0.55–2.01, p = 0.89) nor in the whole cohort (ORa 0.68, 95% CI 0.41–1.12, p = 0.13).
Discussion
In our study, we have shown that the switch from alteplase to tenecteplase can reduce both imaging-to-IVT and, in a drip-and-ship paradigm, IVT-to-puncture times. Moreover, compared to alteplase, patients treated with tenecteplase achieved a better 3-month functional outcome with similar safety.
Reduced treatment times are a key expected benefit of tenecteplase, thanks to its simple and fast administration, but discrepant data are available in the literature. While Zhong et al. found similar door-to-IVT times with both thrombolytics, 5 two recent studies have shown that tenecteplase was associated with shorter door-to-IVT times compared with alteplase.11,12 The present study confirms that tenecteplase use speeds up the thrombolysis procedure and shows more specifically in two centers with different stroke code protocols how this can be achieved. BUH opted for in-imaging administration of tenecteplase instead of in-stroke-unit infusion of alteplase. Although reduced treatment times have been previously reported with the in-imaging administration of alteplase, 14 its application in France has been limited by the widespread use of MRI. Indeed, while some centers start IVT within the MRI, either with only the bolus which can significantly impact serum alteplase levels, 15 or for fewer centers also the 1-h infusion using long tubing or MRI-compatible pumps, most centers initiate IVT outside the MRI. 15 Here, the major modification of the thrombolytic administration process was facilitated by the use of tenecteplase and was associated with a significant reduction of the imaging-to-IVT time (16 min, 95% CI 12–19). Conversely, stroke code alert protocol was unchanged in SAH and the imaging-to-IVT time did not differ between alteplase- and tenecteplase-treated patients. However, in the drip-and-ship paradigm treatment of SAH patients, the switch from alteplase to tenecteplase was associated with a significant IVT-to-puncture time reduction (11 min, 95% CI 3–23) likely reflecting the easier interhospital transfer when the 1-h infusion of alteplase is not needed. Tenecteplase use may be particularly beneficial in areas, as in France, where medicalized transport is required for patients receiving continuous infusion.
Interestingly, we found that tenecteplase is associated with a higher rate of 3-month functional independence (62.8% vs 53.4%). Although this association was only statistically significant in the CSC in the multivariable analysis, similar trends (60.1% vs 50.5% in the CSC and 65.6% vs 56.9% in the PSC) were observed in both centers in the univariable analysis. Association of tenecteplase with better functional outcome has already been reported, in clinical trials of patients with LVO,3,16 and in recent real-life studies. 11 Several mechanisms could explain our result. First, the shorter treatment times with tenecteplase could play a key part, as it has been demonstrated that shorter door-to-needle times in AIS patients is associated with lower mortality rates.17,18 Second, the faster and more efficient reperfusion associated with tenecteplase, well documented in LVO AIS,3,4,6,16 is likely to be beneficial to patients with LVO and also those with more distal occlusions. The lack of significant association in the PSC could result from key differences between patients treated in both centers. Indeed, patients treated in the PSC had more severe strokes, with a higher rate of LVO and thrombectomy, which could level the treatment effect of tenecteplase. Additionally, it cannot be excluded that other confounding factors unaccounted for in our model, such as the higher rate of cardio-embolic strokes, could play a role in this result.
Additionally, as reported in previous studies and meta-analyses,3,4,6,7 we observed reassuring safety data and found equivalent sICH rates (2.5% vs 3.6%) in both tenecteplase and alteplase groups. In the multivariable analysis, we observed a significant association with a lower rate of PH in the PSC but not in the CSC. However, each center mostly used a different imaging modality for the 24-h control, MRI in the CSC and CT in the PSC. Although it has been reported that MRI and CT were equivalent to detect PH, 19 it cannot be excluded that it still played a part in this difference.
Our study has limitations linked to its retrospective nature. It is not randomized and we cannot exclude that non-analyzed confounding factors may have participated to the time metrics or functional outcome improvement. For instance, temporal trends which generally tend toward improvement, could also explain the better treatment metrics and functional outcome rates. Indeed, it stands in contrast with the recent results reported from the ACT controlled randomized trial, which did not demonstrate the superiority of tenecteplase over alteplase in a secondary analysis of 1577 patients. On the other hand, a real-life study may be the best design to assess time metrics as they would be impacted by the patient information and consent process required in clinical trials.
Conclusion
In our study, we found that the switch from alteplase to tenecteplase was associated with a reduction of treatment times for AIS patients managed in both a CSC and PSC. Moreover, in the post-switch cohort, better 3-month functional independence rates were observed with similar safety. We have shown in this study that tenecteplase, thanks to its ease of use, can enable in-imaging administration and is associated to time metrics reduction. In a drip-and-ship paradigm, we have found that tenecteplase is associated with faster patient transfer to a CSC for thrombectomy. Finally, functional outcome improvement associated with tenecteplase will have to be confirmed by further clinical trials.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873221113729 for Treatment times, functional outcome, and hemorrhage rates after switching to tenecteplase for stroke thrombolysis: Insights from the TETRIS registry by Gaspard Gerschenfeld, Jean-Sébastien Liegey, François-Xavier Laborne, Marion Yger, Victoire Lyon, Thomas Checkouri, Bertille Tricard-Dessagne, Gaultier Marnat, Frédéric Clarençon, Nicolas Chausson, Guillaume Turc, Igor Sibon, Sonia Alamowitch and Stéphane Olindo in European Stroke Journal
Acknowledgments
None.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All reported disclosures were outside the submitted work. Dr. Laborne reports personal fees from Boehringer Ingelheim. Dr. Marnat reports personal fees from Stryker, Medtronic and Microvention. Prof. Clarençon reports personal fees from Medtronic, Guerbet, Balt Extrusion and Penumbra. Dr. Chausson reports a grant and personal fees from Boehringer Ingelheim and Bristol Myers Squibb. Prof. Sibon reports personal fees from Astra-Zeneca, Bayer, BMS-Pfizer, Boehringer Ingelheim, Elsevier, Novonordisk, Servier and Medtronic. Prof. Alamowitch reports personal fees from the Astra-Zeneca, Bayer, BMS-Pfizer and Elsevier. No other disclosures were reported.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: As per current French law regarding retrospective studies of anonymized standard care data, patients were informed of their participation in this research and offered the possibility to withdraw, but no written consent was required.
Ethical approval: This research was approved by the Sorbonne University Research Ethics Committee (CER-2021-1053).
Guarantor: GG
Author Contributions: GG, SA and SO researched literature and conceived the study. GG, MY and SA were involved in gaining ethical approval. GG, JSL, MY, VL, TC, BTD, GM and FC were involved in patient recruitment and data collection. FXL performed statistical analysis. GG wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Gaspard Gerschenfeld  https://orcid.org/0000-0002-2456-704X
https://orcid.org/0000-0002-2456-704X
Guillaume Turc  https://orcid.org/0000-0001-5059-4095
https://orcid.org/0000-0001-5059-4095
Supplemental material: Supplemental material for this article is available online.
References
- 1. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021; 6: I–LXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 3. Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. New Engl J Medicine 2018; 378: 1573–1582. [DOI] [PubMed] [Google Scholar]
- 4. Seners P, Caroff J, Chausson N, et al. Recanalization before thrombectomy in tenecteplase vs. alteplase-treated Drip-and-Ship patients. J Stroke 2019; 21: 105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhong CS, Beharry J, Salazar D, et al. Routine use of tenecteplase for thrombolysis in acute ischemic stroke. Stroke 2021; 52: 1087–1090. [DOI] [PubMed] [Google Scholar]
- 6. Gerschenfeld G, Smadja D, Turc G, et al. Functional outcome, recanalization, and hemorrhage rates after large vessel occlusion stroke treated with tenecteplase before thrombectomy. Neurology 2021; 97: e2173–e2184. [DOI] [PubMed] [Google Scholar]
- 7. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke 2019; 50: 2156–2162. [DOI] [PubMed] [Google Scholar]
- 8. Kheiri B, Osman M, Abdalla A, et al. Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. J Thromb Thrombolysis 2018; 46: 440–450. [DOI] [PubMed] [Google Scholar]
- 9. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. Epub ahead of print 2022. DOI: 10.1016/s0140-6736(22)01054-6. [DOI] [PubMed]
- 10. Warach SJ, Saver JL. Stroke thrombolysis with tenecteplase to reduce emergency department spread of Coronavirus disease 2019 and shortages of alteplase. JAMA Neurol 2020; 77: 1203–1204. [DOI] [PubMed] [Google Scholar]
- 11. Mahawish K, Gommans J, Kleinig T, et al. Switching to tenecteplase for stroke thrombolysis: real-world experience and outcomes in a regional stroke network. Stroke 2021; 52: e590–e593. [DOI] [PubMed] [Google Scholar]
- 12. Hall J, Thon JM, Heslin M, et al. Tenecteplase improves door-to-needle time in real-world acute stroke treatment. Stroke Vasc Intervent Neurol 2021; 1: 1–9. [Google Scholar]
- 13. Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 14. Meretoja A, Weir L, Ugalde M, et al. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology 2013; 81: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 15. Smith C, Al-Nuaimi Y, Wainwright J, et al. The influence of bolus to infusion delays on plasma tissue plasminogen activator levels. Int J Stroke 2014; 9: 939–942. [DOI] [PubMed] [Google Scholar]
- 16. Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. New Engl J Med 2012; 366: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 17. Man S, Xian Y, Holmes DN, et al. Association between thrombolytic Door-to-Needle time and 1-Year mortality and readmission in patients with acute ischemic stroke. JAMA 2020; 323: 2170–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fonarow GC, Zhao X, Smith EE, et al. Door-to-Needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a Quality Improvement Initiative. JAMA 2014; 311: 1632–1640. [DOI] [PubMed] [Google Scholar]
- 19. Arnould M-C, Grandin CB, Peeters A, et al. Comparison of CT and three MR sequences for detecting and categorizing early (48 hours) hemorrhagic transformation in hyperacute ischemic stroke. AJNR Am J Neuroradiol 2004; 25: 939–944. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873221113729 for Treatment times, functional outcome, and hemorrhage rates after switching to tenecteplase for stroke thrombolysis: Insights from the TETRIS registry by Gaspard Gerschenfeld, Jean-Sébastien Liegey, François-Xavier Laborne, Marion Yger, Victoire Lyon, Thomas Checkouri, Bertille Tricard-Dessagne, Gaultier Marnat, Frédéric Clarençon, Nicolas Chausson, Guillaume Turc, Igor Sibon, Sonia Alamowitch and Stéphane Olindo in European Stroke Journal
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.