SUMMARY
Lgr5+ intestinal stem cells (ISCs) depend on niche factors for their proper function. However, the source of these ISC niche factors and how they support ISCs in vivo remain controversial. Here, we report that ISCs depend on lymphatic endothelial cells (LECs) and RSPO3+GREM1+ fibroblasts (RGFs). In the intestine and colon, LECs are surrounded by RGFs and are located near ISCs at the crypt base. Both LECs and RGFs provide the critical ISC niche factor RSPO3 to support ISCs, where RSPO3 loss in both cell types drastically compromises ISC numbers, villi length, and repair after injury. In response to injury, LEC and RGF numbers expand and produce greater amounts of RSPO3 and other growth/angiocrine factors to foster intestinal repair. We propose that LECs represent a novel niche component for ISCs, which together with RGFs, serve as the major in vivo RSPO3 source for ISCs in homeostasis and injury-mediated regeneration.
eTOC Blurb
Goto and colleagues identify lymphatic endothelial cells (LECs) and RSPO3+GREM1+ fibroblasts (RGFs) as stromal niche components of intestinal stem cells (ISCs). LECs and RGFs sustain ISCs through the production RSPO3 and GREM1 in homeostasis. In injury, LECS and RGFs expand in number and produce growth/lymphangiocrine factors to orchestrate ISC-mediated repair.
Graphical Abstract
INTRODUCTION
Lgr5+ intestinal stem cells (ISCs) self-renew and give rise to the various specialized cell types of the intestinal epithelium (Gehart and Clevers, 2019). ISCs reside at crypt bases and depend on niche factors such as WNT, R-spondins (RSPO), BMP inhibitors (BMPi), and EGF for maintaining the stem cell state (Sato et al., 2009). The essentiality of these factors, for example, in supporting ISC function has been defined by their need to support intestinal organoid cultures. These ISC factors are produced by many of their neighboring cell types including epithelial and mucosal stromal cell types (Degirmenci et al., 2018; Greicius et al., 2018; McCarthy et al., 2020b; Sato et al., 2011; Shoshkes-Carmel et al., 2018). Epithelial Paneth and deep secretory cells adjacent to Lgr5+ ISCs in the small intestine and colon, respectively, produce WNT3 and EGF to support Lgr5+ ISCs (Sasaki et al., 2016; Sato et al., 2011; Yilmaz et al., 2012); however, there are redundant sources for WNT, including stromal cells - where co-culture of stromal cells with WNT3 deleted organoids permits organoid propagation (Farin et al., 2016; Farin et al., 2012). Finally, the absence of RSPO and BMPi production in epithelial cells further accentuates the role that stromal cells play as key constituents of the ISC niche (Greicius et al., 2018; Kosinski et al., 2007; Ogasawara et al., 2018).
In an attempt to define the stromal ISC niche, several mouse models and strategies to genetically manipulate or sort populations of stromal cells by cell surface markers have been developed and employed (Degirmenci et al., 2018; Greicius et al., 2018; Harnack et al., 2019; McCarthy et al., 2020a; McCarthy et al., 2020b; Shoshkes-Carmel et al., 2018; Stzepourginski et al., 2017). However, the source of these ISC niche factors and how they support ISCs in vivo remain controversial. First, direct visualization of these factors with precise cellular localization is technically challenging for low expressing genes or secreted factors. These approaches include in situ hybridization (ISH) or immunohistochemical approaches, which also fail to provide functional insights. Second, genetically engineered mouse models (GEMMs) used in these studies (e.g., Pdgfra-Cre, Foxl1-Cre, Gli1-CreERT2 mice) do not mark cell populations that produce specific ISC niche factors and many ISC niche factors are expressed by more than one cell type, making it difficult to ascribe in vivo loss of function phenotypes to specific cell types (Degirmenci et al., 2018; Greicius et al., 2018; McCarthy et al., 2020a; Shoshkes-Carmel et al., 2018). For example, telocytes, marked by Foxl1+ cells (Shoshkes-Carmel et al., 2018), Gli1+ cells (Degirmenci et al., 2018) or Pdgfrahigh cells (McCarthy et al., 2020b) in the stroma, have been proposed to serve as a major source of WNT in the intestinal stroma. Loss of active WNT in telocytes reduced crypt cell proliferation in the intestine, indicating that these cells are essential in providing WNT to intestinal stem and progenitor cells (Degirmenci et al., 2018; Shoshkes-Carmel et al., 2018). While Porcn deletion in Foxl1+ cells (i.e. telocytes) led to rapid crypt collapse in 3 days both in the small intestine and colon (Shoshkes-Carmel et al., 2018), Wls deletion in Gli1+ cells (i.e. telocytes) had minimal effect in the small intestine and necessitated 2–3 weeks to cause crypt attrition in the colon (Degirmenci et al., 2018). These distinct phenotypes likely reflect that Gli1 and Foxl1 positive stromal cells mark partially overlapping populations of cells as revealed by single cell analysis (Degirmenci et al., 2018; Kinchen et al., 2018; McCarthy et al., 2020b; Shoshkes-Carmel et al., 2018), highlighting the challenges of precisely manipulating growth factors using single promoter driven Cre recombinase models. Trophocytes, defined as CD81+Pdgfralow fibroblasts, are enriched for GREM1+ cells and support organoid growth through the production of BMPi (namely Grem1) and RSPO3 (McCarthy et al., 2020b). Although diphtheria toxin mediated cellular ablation of GREM1+ cells compromise ISC function, it is unclear whether ISC loss results from the lack of GREM1 production, loss of other growth factors such as RSPO in those cells, or secondary loss of other stromal ISC niche cell types. Thus, the development of additional tools to investigate and visualize specific stromal cell populations that correspond to specific ISC niche factors in vivo may help to clarify their in vivo role in fostering ISCs.
RSPOs boost WNT signaling by functioning as Lgr5 ligands (de Lau et al., 2014). RSPOs drive self-renewal of Lgr5+ ISCs in vivo (Yan et al., 2017) and are indispensable trophic factors for intestinal organoid growth in vitro (Sato et al., 2009). RSPOs are encoded by four paralogous genes: Rspo1-Rspo4 (Greicius et al., 2018). RSPO3 is the dominant R-spondin in the mammalian intestine and is significantly more potent than RSPO1 in enhancing WNT activation and supporting intestinal organoid growth (Greicius et al., 2018). Previous studies have proposed that RSPO3 is expressed by subepithelial myofibroblasts (Greicius et al., 2018; Stzepourginski et al., 2017), a subset that was later identified as telocytes (Shoshkes-Carmel et al., 2018), by Pdgfra+ fibroblasts (Greicius et al., 2018; McCarthy et al., 2020b), or by lymphatic endothelial cells (LECs) (Ogasawara et al., 2018); however, questions remain regarding which of these cell types is the major RSPO3 source for ISCs as previous studies have reported that RSPO3 deletion has minimal impact on intestinal homeostasis and integrity (Greicius et al., 2018; Harnack et al., 2019). In these studies, RSPO3 was either ablated during embryonic development or in a subset of RSPO3 producing cells, using Pdgfra-Cre; Rspo3 f/f (Greicius et al., 2018) or Myh11-CreERT2; Rspo3 f/f mice, respectively (Harnack et al., 2019). The first model disrupts Rspo3 with a constitutive Cre in a broad population of Pdgfra+ intestinal stromal cells and may be confounded by compensation from other RSPO family members given early embryonic deletion, and the second model disrupts Rspo3 in Myh11+ myofibroblasts, a population that does not show robust Rspo3 expression by scRNA-seq (Brugger et al., 2020; McCarthy et al., 2020b). While in both of these models RSPO3 loss either has mild effects of Lgr5+ ISC numbers and intestinal integrity in homeostasis (Greicius et al., 2018; Harnack et al., 2019), systemic overexpression of RSPO receptors that sequester in vivo RSPO proteins causes notable crypt degeneration (Yan et al., 2017), raising the possibility that Rspo3 has not been completely excised from the mucosal ISC niche in these in vivo loss of function models. Finally, it has been previously noted that LECs in the villi are RSPO3+ but whether this is the case near the crypts or how they support ISCs has not been explored (Ogasawara et al., 2018).
To address these issues, we employed multiple novel reporter mice to directly visualize and sort ISC niche factor-producing cells as well as to perform loss of function in vivo studies. We find that LECs represent a novel niche component for ISCs, which together with RSPO3+GREM1+ fibroblasts to serve as the major in vivo RSPO3 source for ISCs in homeostasis and injury.
RESULTS
RSPO3+ LECs reside close to Lgr5+ ISCs
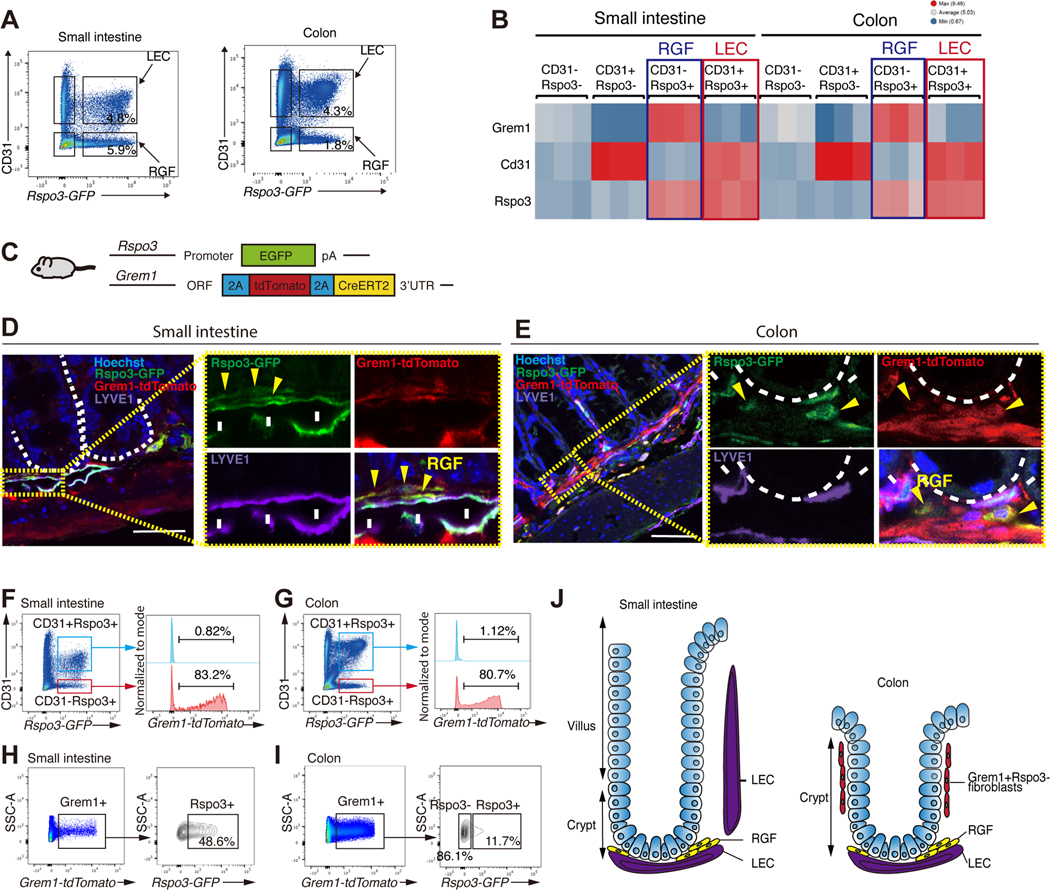
To visualize and characterize ISC niche factor-producing cells, we first focused on RSPO3 (a critical niche factor of Lgr5+ ISCs) and developed Rspo3−GFP BAC transgenic mice. Immunostaining for GFP in Rspo3-GFP intestines highlighted lymphatic channels in the small intestine and colon (Figure 1A) in a pattern that is consistent with Rspo3 mRNA expression (Figure S1A). To distinguish between LECs and vascular endothelial cells, we performed immunofluorescence (IF) for LEC-specific marker LYVE-1 and Rspo3−GFP and found that RSPO3 is expressed by LYVE-1+ LECs (Figure 1A) as previously demonstrated (Ogasawara et al., 2018).
Figure 1. RSPO3+ LECs reside close to Lgr5+ ISCs.
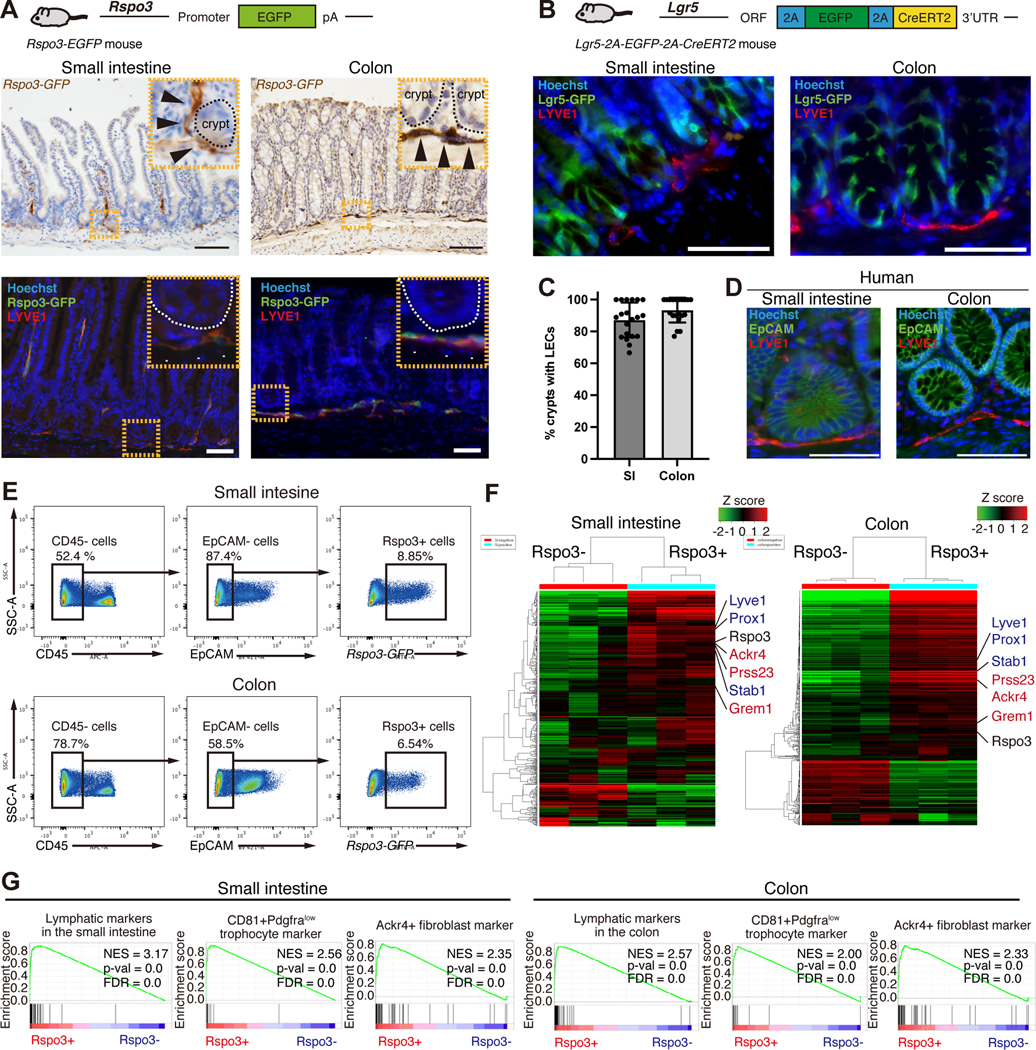
(A) Schematic of Rspo3−GFP mice (top). Rspo3−GFP expression by immunohistochemistry in the small and colon (middle). Black arrowheads indicate endothelial structures in close proximity to the crypt. Rspo3−GFP is expressed by LYVE1+ LECs (bottom). White arrowheads indicate the co-expression of Rspo3−GFP and LYVE1. (B) Schematic (top) of Lgr5–2A-GFP-2A-CreERT2 mice. IF shows the presence of LYVE1+ LECs residing near Lgr5+ ISCs in the small intestine and colon. (C) The percentage of the crypts with adjacent LYVE1+ LECs in the small intestine and colon. n = 20 from 6 mice per group. (D) IF for LYVE1 and EpCAM in the human small intestine and colon. (E) CD45-/EpCAM-/Rspo3−GFP+ cells from the small intestinal and colonic stromal cells of Rspo3−GFP mice by flow cytometry. (F) The heatmap of RNA-seq of sorted CD45−/EpCAM−/Rspo3−GFP− and CD45−/EpCAM−/Rspo3−GFP+ cells in the small intestine and colon. n = 3 mice per group. (G) GSEA of lymphatic markers, CD81+Pdgfralow trophocyte markers, and Ackr4+ fibroblast markers. FDR, false-discovery rate; NES, normalized enrichment score.
Scale bar, 50 μm (A, B, D). Data are mean ± SD.
See also Figure S1.
Given the role that RSPO3 plays in driving intestinal stemness, we next investigated the localization of LECs in relation to Lgr5+ ISCs. Because conventional Lgr5−EGFP-IRES-CreERT2 mice (Barker et al., 2007) have variegated reporter expression in the intestine (Schuijers et al., 2014), we developed Lgr5–2A-EGFP-2A-CreERT2 mice (Figure S1B), where reporter expression is observed in most crypts (Figure S1B). We also confirmed that Lgr5−GFP+ cells self-renew and differentiate for the long-term in the small intestine and colon by performing lineage tracing in Lgr5–2A-EGFP-2A-CreERT2; Rosa-LSL-tdTomato mice (Figure S1C). IF and confocal microscopy confirmed the presence of LYVE1+ LECs residing near small intestinal and colonic Lgr5+ ISCs (Figure 1B, C and movie S1). In the colon, LYVE1+ LECs are located near the crypt base, whereas in the small intestine, LYVE1+ LECs are located both at the crypt base and in the villus cores (also known as villus lacteals) (Cifarelli and Eichmann, 2019) (Figure 1A). These lacteals not only reside far from the epithelium but soluble RSPO3 likely exerts minimal effects on the Lgr5 non-expressing differentiated villus epithelium. Finally, analogous to the mouse intestine, we found that in the human small intestine and colon, LYVE1+ LECs are also located near crypt bottoms (Figures 1D and S1D).
Next, we performed RNA-seq on sorted Rspo3−GFP− and Rspo3−GFP+ cells from EpCAM-CD45-stromal cells of the small intestine and colon (Figures 1E, 1F, and S1E). Differential expression analysis and Gene Set Enrichment Analysis (GSEA) showed gene enrichment for LECs (e.g. Lyve1, Prox1, Stab1), Grem1+ fibroblasts (e.g., CD81+Pdgfralow trophocyte (McCarthy et al., 2020b) and Ackr4+ fibroblasts (Ackr4, Grem1, Prss23)(Thomson et al., 2018)) (Figure 1F, G), demonstrating heterogeneity of RSPO3+ cells. Reanalysis of single-cell RNA sequencing (scRNA-seq) data of the small intestinal (McCarthy et al., 2020b) and colonic (Kinchen et al., 2018) stroma also revealed that Rspo3 is not only expressed by LECs but also by a subset of Pdgfralow fibroblasts that co-express Grem1 (Figures S2A and S2B) that we denote as RSPO3+GREM1+ fibroblasts as “RG-fibroblasts (RGFs)”.
LECs and RGFs are the major cellular sources of mucosal RSPO3
To resolve the heterogeneity of RSPO3+ stromal cells, we performed scRNA-seq on sorted small intestinal Rspo3−GFP+ stromal cells (Figure 2A). Individual uniform manifold approximation and projection (UMAP) plots for each replicate demonstrate similar clustering patterns (Figure S1F). We detected two dominant clusters: a large cluster of Cd31+ cells (46%; 1,739/3,774) that express LEC markers (LEC cluster) and another cluster of Cd31- cells (52.5%; 1,983/3,774) that express Grem1 (RGF cluster) (Figure 2B). In addition, we also detect a very small cluster of telocytes (1.3%; 51/3774) that highly express Pdgfra as well as Foxl1, Bmp3 and Bmp5 (Figure 2B), as previously reported by (McCarthy et al., 2020b; Shoshkes-Carmel et al., 2018). Given that the telocyte cluster is quite small, telocytes likely account for a small proportion of RSPO3 production, in contrast to LECs and RGFs. There are 4 subclusters in LECs and 5 subclusters in RGFs (Figure 2A); all the subclusters are similar in terms of ISC niche factor production (Figure 2D). In RGFs, one cluster (RGF 2) is Cd81−Adamdec1+ while the other four clusters (RGF 1–1, 1–2, 1–3, 1–4) are CD81+Adamdec1− (Figure 2C). The former subset corresponds to CD81-Pdgfralow fibroblasts, while the latter subsets correspond to CD81+Pdgfralow trophocytes. Reanalysis of previously published scRNA-seq data (McCarthy et al., 2020b) of small intestinal Pdgfra+ cells confirmed the distribution of Rspo3+Grem1+ cells (RGFs) in both the Cd81-Pdgfralow fibroblast and Cd81+Pdgfralow trophocyte clusters (Figures S2C).
Figure 2. scRNA-seq of RSPO3+ cells in the small intestine and colon.
(A) UMAP of scRNA-seq of small intestinal Rspo3−GFP+ cells (3,774 cells from 2 mice). (B) Relative expression of Rspo3, LEC marker genes, and Grem1+ fibroblast marker genes, and telocyte marker genes projected onto the UMAP plot. (C) RGF 1 clusters are CD81+Adamdec1− whereas RGF 2 cluster is Cd81−Adamdec1+. (D) Dot plots of expression patterns of ISC niche factors in LEC and RGF subclusters. (E) UMAP of scRNA-seq of colonic Rspo3−GFP+ cells (7,901 cells from 2 mice). (F) Relative expression of Rspo3, LEC marker genes, and Grem1+ fibroblast marker genes onto the UMAP plot. (G) RGF 1 cluster is CD81+Adamdec1− whereas RGF 2 cluster is Cd81−Adamdec1+. (H) Dot plots of expression patterns of ISC niche factors in LEC and RGF subclusters.
See also Figures S1 and S2.
We also performed scRNA-seq on colonic Rspo3−GFP+ stromal cells. As in the small intestine, we detected two dominant clusters: LEC and RGF clusters (Figure 2E and 2F). Besides these clusters, there were small clusters of telocytes (0.89%; 71/7,901) and muscularis mucosa (1.67%; 132/7,901), the latter of which is a thin layer of muscle that separates the mucosal lamina propria from the submucosa. The muscularis mucosa cells highly express Acta2, Myh11, and Grem2 but not Pdgfra (Figure S1G). Consistent with a recent study (McCarthy et al., 2022), muscularis mucosa cells in addition to Grem1 and Rspo3 specifically express Grem2 (Figures S1G and 2F). Since these clusters (i.e., telocytes and muscularis mucosa cells) are small, the major sources of mucosal RSPO3 in the colon akin to the small intestine are LECs and RGFs. There are 4 subclusters in LECs and 2 subclusters in RGFs (Figure 2E); all of these subclusters are similar in terms of ISC niche factors (Figure 2H). As noted in the small intestine, RGFs can be subdivided into CD81+Adamdec1− (RGF 1) and Cd81- Adamdec1+ (RGF 2) subclusters (Figure 2G), corresponding to CD81+Pdgfralow trophocytes and CD81-Pdgfralow fibroblasts, respectively (Figure S2D) (Brugger et al., 2020). We also reanalyzed the scRNA-seq of human colon stromal cells (Kinchen et al., 2018): RGFs were a subset of PDGFRAlow cells (Figure S2E), as seen in the mouse small intestine and colon.
We next separately sorted LECs and RGFs (Figure 3A) by utilizing the Rspo3−GFP reporter mice and the cell surface marker CD31 that is expressed by LEC but not by RGFs (Figures 2B and 2F). RNA-seq on CD31−Rspo3−GFP−, CD31+Rspo3−GFP−, CD31−Rspo3−GFP+, and CD31+Rspo3−GFP+ cells confirmed that Grem1 is expressed by CD31−Rspo3−GFP+ cells (Figure 3B). GSEA and single sample GSEA (ssGSEA) (Barbie et al., 2009) revealed that CD31−Rspo3−GFP+ cells are enriched in RGF markers shared by CD81+Pdgfralow cells (McCarthy et al., 2020b) or Ackr4+ cells (Thomson et al., 2018); CD31+Rspo3−GFP+ cells in LEC markers (Kalucka et al., 2020); and CD31+Rspo3−GFP− cells in vascular endothelial cell markers (Kalucka et al., 2020) (Figures S3A and S3B), illustrating that LECs and RGFs can be separated and flow-sorted as CD31+Rspo3−GFP+ and CD31−Rspo3−GFP+ cells, respectively. Since bulk RNA-seq allows for the detection of lowly expressed genes, we next sought to assess the expression of ISC niche factors (besides Rspo3) in LECs and RGFs (Figure S3C- I): LECs and RGFs express Wnt family members some of which have been shown to support ISCs (Goss et al., 2009) (Figure S3I), indicating that these niche cells may support ISCs more broadly through the expression of Wnts. Lastly, RGFs also express Igf1 and Fgf7 (Figure S3D), growth factors that have been implicated in supporting organoid propagation (Fujii et al., 2018).
Figure 3. LECs and RGFs are the major cellular sources of mucosal RSPO3.
(A) Flow cytometry of EpCAM-CD45- stromal cells using Rspo3−GFP and CD31 in the small intestine and colon. (B) The heatmap of RNA-seq on CD31−Rspo3−GFP−, CD31+Rspo3−GFP−, CD31−Rspo3− GFP+, and CD31+Rspo3−GFP+ cells in the small intestine and colon. n = 3 mice per group. (C) Schematic of Rspo3−GFP; Grem1-tdTomato mouse. (D-E) Confocal microscopy images of IF for Rspo3−GFP, Grem1-tdTomato, and LYVE1 in the small intestine (D) and colon (E). Yellow arrowheads indicate RGFs. White arrows indicate LECs. (F-I) Flow cytometry of EpCAM0−CD45− stromal cells in the small intestine and colon of Rspo3−GFP; Grem1-tdTomato mice. (J) Schematic of RSPO3 and/or GREM1 positive cells in the small intestine and colon.
Scale bar, 10 μm (D) and 50 μm (E).
See also Figures S3 and S4.
RGFs reside close to crypt bottoms and surround lymphatic vasculature
To delineate the location of RGFs, we engineered Grem1-2A-tdTomato-2A-CreERT2 mice (Figure S4A). Grem1-tdTomato is expressed by subsets of lamina propria and submucosal stromal cells and is strongly expressed by Desmin+ muscularis mucosa and muscularis propria cells (Figure S4B). To visualize RGFs in the lamina propria, we generated Rspo3−GFP; Grem1-tdTomato mice (Figure 3C). Confocal microscopy of the small intestine revealed that RGFs are near LYVE+ LECs at the crypt bottoms (Figure 3D) and are less frequently detected in the villi cores next to lacteal LECs (Figure S4C). These villus RGFs co-express Adamdec1 (Figure S4C), indicating that they correspond to the RGF cluster 2 (Cd81−Adamdec1+ subcluster of RGFs) (Figure 2C). In the colon, RGFs again are detected adjacent to LYVE+ LECs both in the lamina propria above the muscularis mucosa and in the submucosal layer below the muscularis mucosa (Figure 3E). Given the proximity of LECs and RGFs to the stem cell zone of the crypt base, these stromal cells likely represent the main of source of RSPO3 and GREM1 for ISCs. Further away from the crypt bottom and consistent with our scRNA-seq analysis (Figure 2E), a small subset of RSPO3+GREM1+ cells also are detected in the muscularis mucosa (Figure 3E), where they may serve as a redundant source for these niche factors. Lastly, and of note, we observe many GREM1+RSPO3- cells at the middle-top zone of crypts in the colon but not of crypts in the small intestine (Figures S4D and 3J).
We also performed flow cytometry of EpCAM-CD45- stromal cells in Grem1-tdTomato; Rspo3– GFP mice. More than 80% of CD31−Rspo3−GFP+ cells express GREM1 both in the small intestine and colon whereas CD31+Rspo3−GFP+ cells do not express GREM1 (Figures 3F and 3G). While half of GREM1+ cells express RSPO3 in the small intestine, 86% of GREM1+ cells do not express RSPO3 in the colon (Figures 3H and 3I) and correspond to the GREM1+RSPO3- cells in the middle-top zone of the colonic crypts (Figures S4D and 3J). Thus, it is not possible to enrich for RGFs based solely on GREM1 expression. Although the distributions of RSPO3 and/or GREM1 positive cells differs in the differentiated regions of the villi or upper crypts of the small intestine and colon, respectively (Figure 3J), LECs and RGFs consistently reside adjacent to the ISC zone of the crypt base, highlighting the likely critical role that they play in fostering ISCs.
LECs and RGFs support ISCs in vitro
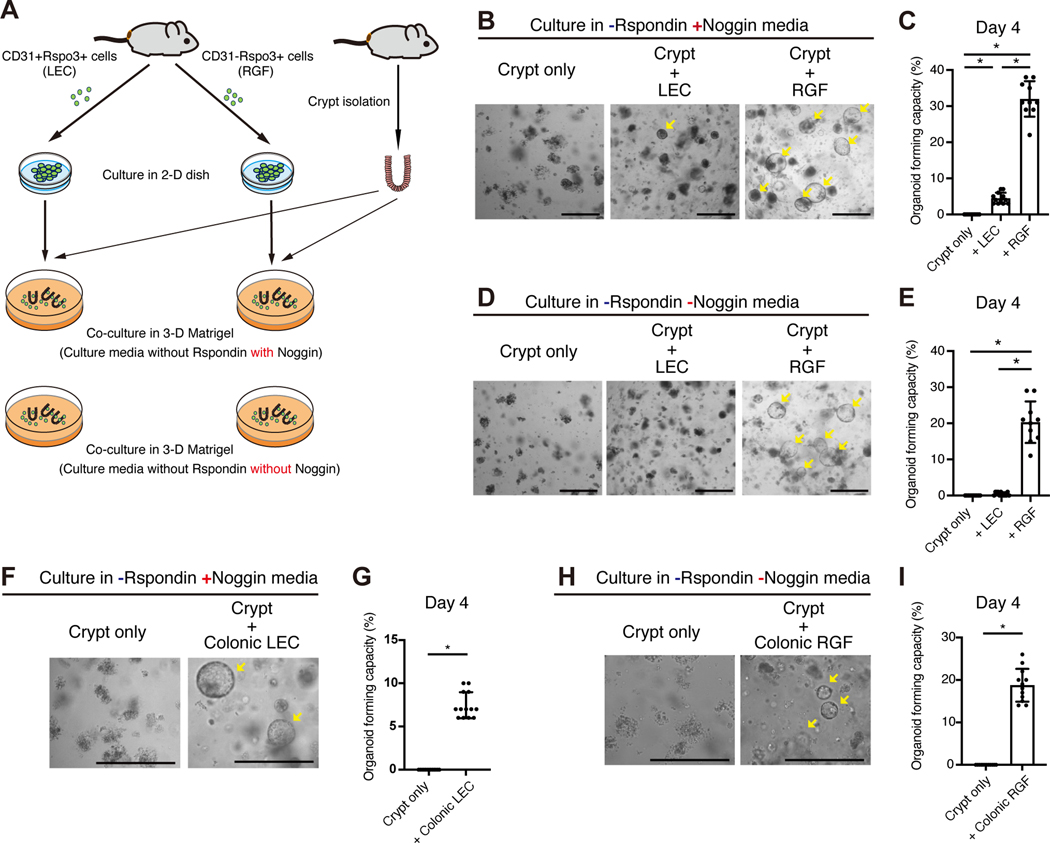
Next, to ascertain whether RSPO3+ stromal cells support ISCs, we set out to perform heterotypic co-culture experiments with RSPO3+ stromal cells and intestinal crypts. We adapted a protocol for the culture of dermal lymphatic endothelial cells (Lokmic, 2016), and successfully propagate sorted Rspo3−GFP+ cells both in 2-D and 3-D cultures (Figures S4E and S4F). We sorted CD31+Rspo3−GFP+ LECs and CD31−Rspo3−GFP+ RGFs, expanded them in 2-D cultures, and then performed heterotypic co- culture experiments with intestinal crypts in culture media supplemented with Noggin, but not with RSPO (Figure 4A). Both LECs and RGFs sustained organoid formation and growth (Figure 4B). The organoid forming capacity of crypts were significantly higher with RGFs than with LECs (Figure 4C), though the latter was still able to aid organoid propagation; the difference between these two niche populations ability in supporting organoid clonogenicity likely reflects that RGFs expand significantly more in culture than LECs (Figures S4G–I). In support of this, we confirmed that RSPO3+ stromal cells support organoid growth in a cell number-dependent manner (Figures S4J–L), highlighting that both of these niche cells can support ISC/early progenitors in organoid assays.
Figure 4. LECs and RGFs support ISCs in vitro.
(A) Schematic of heterotypic co-culture assay. (B-C) Representative images (B) and quantification (C) of co-culture of small intestinal LECs or RGFs with the crypts in the culture media supplemented with Noggin, but not with RSPO. n = 10 – 12 from 3 mice per group. (D-E) Representative images (D) and quantification (E) of co-culture of small intestinal LECs or RGFs with the crypts in the culture media without Noggin and RSPO. n = 10 – 12 from 3 mice per group. (F-G) Representative images (F) and quantification (G) of co-culture of colonic LECs with the crypts in the culture media supplemented with Noggin, but not with RSPO. n = 13 from 3 mice per group. (H-I) Representative images (H) and quantification (l) of co-culture of colonic RGFs with the crypts in the culture media without Noggin and RSPO. n = 12 from 3 mice per group.
One-way ANOVA (C, E). Unpaired two-tailed t-tests (G, I). Data are mean ± SD. *p < 0.05. Scale bar, 20 μm (B, D, F, H). Arrows indicate organoid formation.
See also Figure S4.
Because RGFs also produce BMPi GREM1, we deciphered whether RGFs can substitute for RSPO and BMPi Noggin. Whereas LECs did not support the organoid formation in culture media that lacks both RSPO and Noggin, RGFs robustly promoted organoid growth (Figures 4D and 4E), confirming that RGFs secrete two key niche factors (i.e., RSPO3 and GREM1) for ISCs. Finally, we also validated that colonic LECs akin to their small intestinal counterparts support organoids through RSPO production (Figures 4F and 4G) and that colonic RGFs support organoids through RSPO and Noggin production (Figures 4H and 4I).
LECs and RGFs support ISCs in vivo
We next sought out to ascertain the in vivo role and sources of RSPO3 in ISC maintenance. We took advantage of our newly engineered Grem1-CreERT2 driver (Figure S4A) and obtained Prox1-CreERT2 (a driver specific to lymphatic endothelium (Srinivasan et al., 2007)) and Rspo3 flox mice (Neufeld et al., 2012) to ablate Rspo3 in RGFs and LECs, respectively. To conditionally disrupt RSPO3 in LECs, we generated Prox1-CreERT2; Rspo3 f/f mice, and in RGFs, generated Grem1-CreERT2; Rspo3 f/f mice, and in both LECs and RGFs, generated Grem1-CreERT2; Prox1-CreERT2; Rspo3 f/f mice (Figures 5A and 5B). To assess Rspo3 mRNA expression in the intestines of these models, we performed Rspo3 ISH (Figure S5D). Rspo3 mRNA expression was essentially lost in Grem1-CreERT2; Prox1-CreERT2; Rspo3 f/f intestines, whereas residual Rspo3 mRNA expression was detected in the intestines of Grem1-CreERT2; Rspo3 f/f or Prox1-CreERT2; Rspo3 f/f mice, validating our scRNA-seq findings that the RSPO3 niche includes LECs and RGFs (Figures 2A and E). RSPO3 loss in either LECs or RGFs diminished numbers of Olfm4+ or Lgr5+ ISCs, but did not show any histological changes in the small intestine and the colon (Figures 5C–L), indicating compensation of RSPO3 from the unexcised niche population. In support of this notion, when Rspo3 was deleted in both LECs and RGFs using Grem1-CreERT2; Prox1-CreERT2; Rspo3 f/f mice, this caused a dramatic decrease in the numbers of Olfm4+ or Lgr5+ ISCs that was accompanied by shortened crypt-villus units and crypts in the small intestine and the colon, respectively (Figures 5C–L). These intestinal histological changes and loss of phenotypic ISCs that occur after RSPO loss in the LEC and RGF niche emulate what was reported in a new model of diphtheria toxin mediated ablation of Lgr5+ ISCs (Tan et al., 2021).
Figure 5. LECs and RGFs support ISCs in vivo.
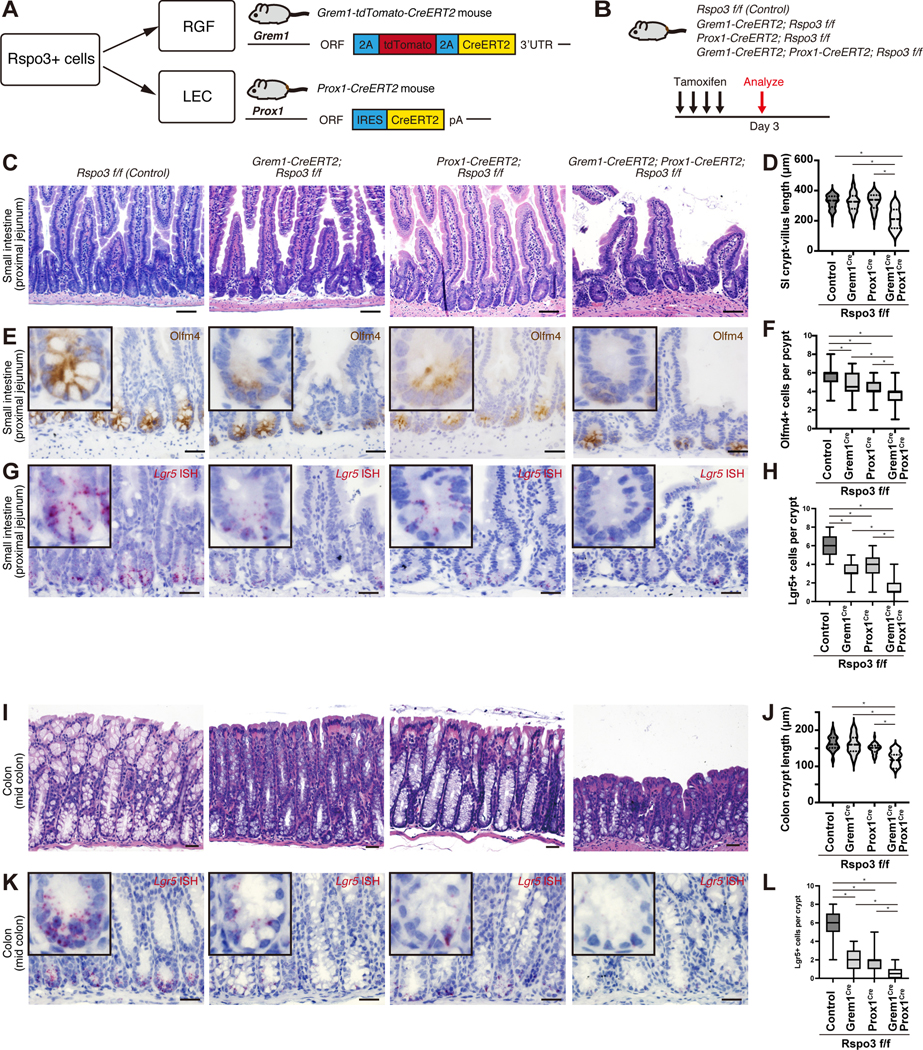
(A) Schematic of the Cre mouse models to target RSPO3+ cells. (B) Schematic of the mouse models of RSPO3 loss in LECs, RGFs, or both. (C-L) H&E staining of the small intestine (C) and colon (I); immunohistochemistry for Olfm4 in the small intestine (E); Lgr5 mRNA expression in the small intestine (G) and colon (K) by ISH after inducing RSPO3 loss in LECs, RGFs, or both. Quantification of the crypt- villus length in the proximal jejunum of the small intestine (D) (n = 100 crypt-villi from 6 mice per group); Olfm4+ cells in the proximal jejunum of the small intestine (F) (n = 60 crypts from 6 mice per group); Lgr5+ cells in the proximal jejunum of the small intestine (H) (n = 50 crypts from 6 mice per group); crypt length in the mid colon (J) (n = 40 crypts from 6 mice); Lgr5+ cells in the mid colon (L) (n = 50 crypts from 6 mice per group) after inducing RSPO3 loss in LECs, RGFs, or both.
One-way ANOVA (D, F, H, J, L). For box-and-whisker plots (F, H, L), data were expressed as box-and- whisker from the minimum to the maximum. *p < 0.05. Scale bar, 20 μm (C, E, G, I, K).See also Figure S5.
We further investigated whether niche RSPO3 loss influences differentiation and proliferation in the small intestine and colon. RSPO3 loss in LECs, RGFs, or both did not alter secretary differentiation as assessed by PAS staining (goblet cells) and immunostaining for Lysozyme (Paneth cells) (Figures S5B, S5C, and S5G). The numbers of Ki67+ proliferative crypt cells significantly decreased only when RSPO3 was deleted in both LECs and RGFs (Figures S5A and S5G), consistent with the shortened crypt-villus units observed in the intestines of Grem1-CreERT2; Prox1-CreERT2; Rspo3 f/f mice (Figures 5C, D, I, and J). To assess whether stromal RSPO3 loss affects the expression of other WNT target genes, we performed Axin2 and Ascl2 ISH. Niche RSPO3 loss diminished Axin2 and Ascl2 expression in crypt cells (Fig S5E and F), illustrating that niche RSPO3 loss dampens WNT signaling in the crypt. To test whether niche RSPO3 loss sensitizes ISCs and early progenitors to further WNT inhibition, we administered LGK974 (a Porcupine inhibitor that prevents the post-translational activation of WNTs) (Liu et al., 2013) to our RSPO3 mouse models. Whereas control intestines or partial niche RSPO3 loss intestines showed no histological changes, complete niche RSPO3 loss intestines (i.e., Grem1-CreERT2; Prox1-CreERT2; Rspo3 f/f mice) illustrated significant crypt degeneration/drop-out as determined on day 8 post-treatment (Figure S5H). These findings conceptually align with a prior study (Huels et al., 2018) that proposed that the combination of a Porcupine inhibitor in the setting of reduced WNT signaling results in crypt loss. Taken together, these results demonstrate that LECs and RGFs play an overlapping role in providing niche RSPO3 to Lgr5+ ISCs and that niche RSPO3 loss reduces not only ISC numbers but also functional WNT signaling and proliferation in the crypt.
LECs and RGFs support post-injury regeneration in vivo
To assess how injury affects compromised Lgr5+ ISC function upon RSPO3 loss in LECs, RGFs or both, we exposed these mice to 12 Gy irradiation after tamoxifen administration (Figure 6A). At day 3 post-injury, whereas enlarged/hyperplastic regenerative crypts were seen in the control mice, areas of crypt attrition/loss lesions and reduced regenerative crypts were noted in the Grem1-CreERT2; Rspo3 f/f and Prox1-CreERT2; Rspo3 f/f models (Figures 6B and C). When RSPO3 is disrupted in both LECs and RGFs (that is, in Grem1-CreERT2; Prox1-CreERT2; Rspo3 f/f mice), there is a pronounced increase in ulcerated mucosa that lacks intact crypts (Figures 6B and C). Since the colonic epithelium is more resistant to irradiation induced damage as compared to the small intestine (Hua et al., 2017), colonic crypt attrition/loss day 3 post-injury was not detected in control, Grem1-CreERT2; Rspo3 f/f or Prox1-CreERT2; Rspo3 f/f mice but was only seen when RSPO3 was ablated in both LECs and RGFs (Figure 6D). To distinguish whether these phenotypes are due to dampened regeneration or hypersensitivity to irradiation, we evaluated apoptotic cells at day 1 post-injury. Notably, the numbers of cleaved Caspase-3 (CCS3)+ cells were not significantly different between the groups (Figures 6E and F), indicating that irradiation in the setting of RSPO3 niche loss does not lead to en masse or synchronous death of ISCs/progenitors. These findings together with our day 3 post-irradiation findings, which showed severely compromised crypt numbers, indicate that Rspo3 loss in the niche most likely stymies crypt-mediated regeneration rather than increasing sensitivity to irradiation.
Figure 6. LECs and RGFs support post-injury regeneration in vivo.
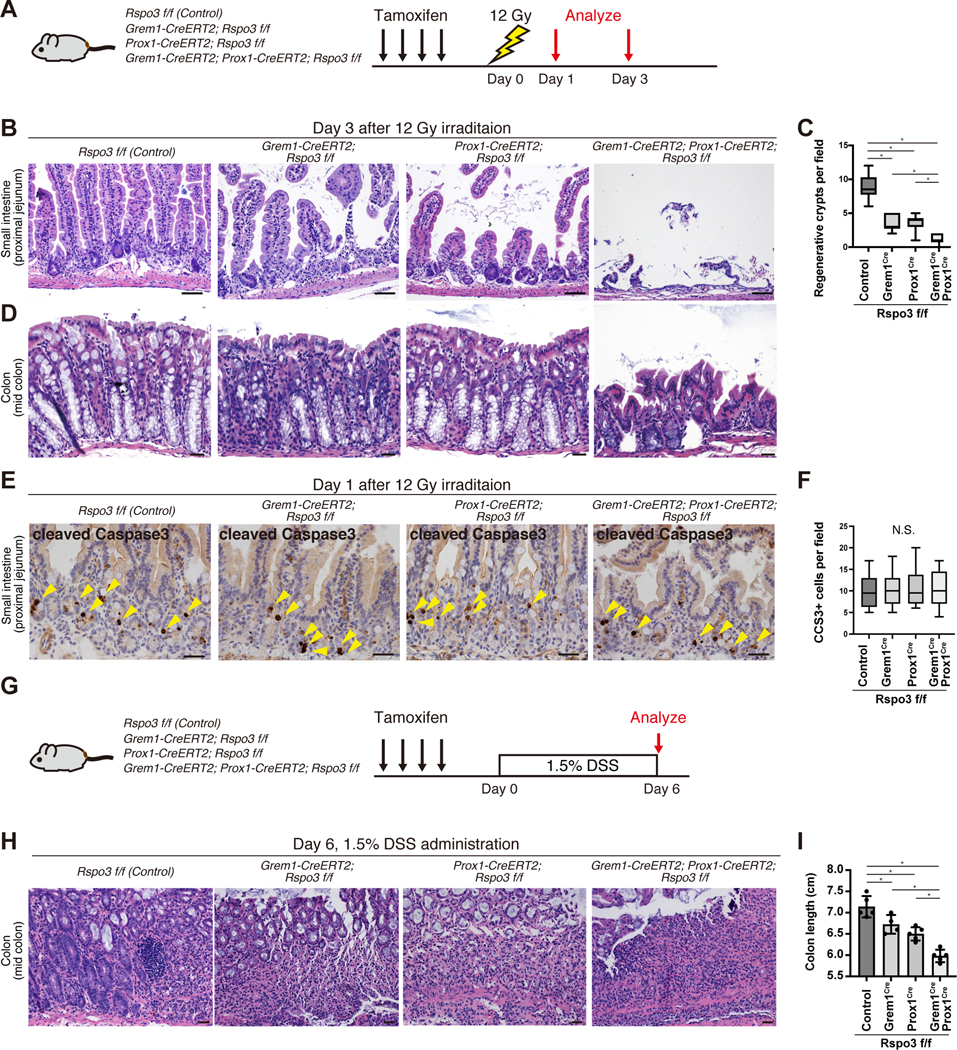
(A) Schematic of irradiation experiments. (B-D) H&E staining of the small intestine (B) and colon (D) and quantification of regenerative crypts in the proximal jejunum (C) (n = 10 fields from 6 mice per group) day 3 post-irradiation following RSPO3 loss in LECs, RGFs, or both. (E-F) immunohistochemistry for CCS3 in the small intestine (E) and quantification of CCS3+ cells (F) day 1 post-irradiation following RSPO3 loss in LECs, RGFs, or both (n = 20 fields from 3 mice per group). (G-I) Schematic of DSS-induced colitis experiments (G). H&E staining of the colon (H) and the colon length (I) at day 6 (n= 4 – 5 mice per group).
One-way ANOVA (C, F, I). For box-and-whisker plots (C, F), data were expressed as box-and-whisker from the minimum to the maximum. *p < 0.05. N.S. not significant. Scale bar, 20 μm (B, D, E, H).
To test whether RSPO3 loss dampens regeneration capacity in other injury models, we assessed the impact of dextran sulfate sodium (DSS), where we administered 1.5% DSS to our GEMMs and harvested the colons on day 6 (Figure 6G). The extent of RSPO3 loss in the stromal niche correlated with colonic shortening due to colitis (Figures 6H and I). Collectively, these results illustrate that, regardless of the mode of intestinal injury, niche RSPO3 is critical for intestinal regeneration and that LECs and RGFs play redundant roles in providing niche RSPO3 for post-injury repair.
Cross-regulation between RGFs and LECs
The proximity of RGFs to LECs prompted us to explore possible cross-regulation between these two populations. LECs expressed Vegfr3, whereas its ligand, Vegfd, is highly expressed by RGFs (Figure S6A), suggesting that RGFs may support LECs through VEGF-D production (an essential factor for lymphangiogenesis (Karaman et al., 2018)). Furthermore, as mentioned earlier, RGFs also highly express Igf1 and Fgf7 (Figure S3D), growth factors that not only enhance organoid propagation (Fujii et al., 2018) but also aid LEC function in vitro and in vivo (Bjorndahl et al., 2005; Chang et al., 2004; Lokmic, 2016), indicating that they may play a regulatory role in LECs.
To investigate whether RGFs support the maintenance of LECs in vivo, we generated Grem1- tdTomato-CreERT2; Rosa DTA mice, which allow for the inducible ablation of RGFs with TM (Figure S6C). Five days after TM injections, depletion of Grem1-tdTomato+ cells (RGFs and Grem1+ muscle layer) led to decreased numbers of LYVE1+ or PROX1+ LECs close to the crypt base (Figures S6D–G) (Kalucka et al., 2020). To directly assess the role that LECs have on ISCs, we administered soluble VEGFR3 receptor, which is an intervention that dampens VEGFR3 signaling and leads to lymphatic regression (Makinen et al., 2001), to Lgr5–2A-EGFP-2A-CreERT2 mice (Figure S6H). Soluble VEGFR3 receptor administration significantly decreased Lgr5+ cell numbers (Figures S6I and J), demonstrating that LECs support Lgr5+ ISCs and that the reduction of ISCs seen after the ablation of RGFs (and Grem1+ muscle layer) may in part occur via diminished LEC numbers (Figure S6W). It is important to point out that Grem1+ ablation not only depletes RGFs but also the Grem1+ muscle layer (Fig S6D) as was previously reported by (McCarthy et al., 2020b), thus future work will be needed to distill the relative contribution and cross-talk of these two Grem1+ compartments on the regulation of LECs.
Conversely, we identified REELIN (RELN), which is known to play a role in heart regeneration (Liu et al., 2020), as a possible LEC-produced ligand that may influence RGFs. REELIN is expressed by LECs and whose receptor, Vldlr, is expressed by RGFs (Figure S6B). REELIN administration to cultured RGFs upregulates Rspo3 expression, but not Grem1 expression (Figure S6K–M). In crypt and RGF co-cultures, REELIN exposure modestly elevated organoid formation in media without RSPO, but not in media with RSPO (Figure S6N–P), indicating that REELIN likely acts by boosting RSPO3 in RGFs. REELIN pretreatment of RGFs also modestly boosted their ability to support sorted (Lgr5−GFPhigh) ISC- derived organoid formation in media without RSPO (Figures S6Q–S). However, when REELIN was administered to epithelial crypt cultures with supplementation of RSPO and Noggin, but without RGFs (Figure S6T), REELIN itself did not significantly affect the organoid formation (Figure S6U and V), indicating that LEC-derived REELIN likely indirectly supports ISCs via RSPO3 production in RGFs (Figure S6W).
LECs and RGFs expand to facilitate epithelial regeneration after irradiation-induced damage
Given the significant role of LEC and RGF derived RSPO3 in ISC function after injury (Figures 6A–I), we next investigated how these niche cells respond to irradiation mediated injury. Interestingly, irradiation expanded lymphatic vascular channels near the crypt base as noted by histologic examination (Figure 6B) and IF for LYVE1 (Figure 7A). We independently validated that CD31+Rspo3− GFP+ LECs and CD31−Rspo3−GFP+ RGFs were expanded by flow cytometric quantification day 3 post-irradiation (Figures 7B and 7C). To better visualize the expanded RGFs, we similarly irradiated Rspo3−GFP; Grem1-tdTomato mice and found by confocal microscopy that LECs and RGFs are present in greater quantities near regenerating crypts (Figure 7D). We also confirmed the expansion of LECs and RGFs in a DSS–induced colitis injury model (Figure S7A), indicating that post-injury regeneration is often accompanied by stromal niche expansion.
Figure 7. LECs and RGFs expand to facilitate epithelial regeneration after irradiation induced damage.
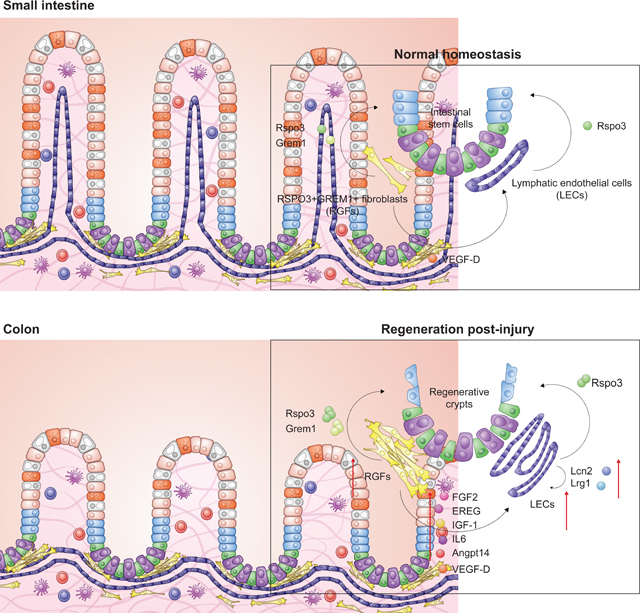
(A) IF for LYVE1 in the small intestine post-irradiation. (B-C) Flow cytometry (B) and quantification (C) of LECs and RGFs from the small intestinal EpCAM-CD45- stromal cells. n = 5 mice per group. (D) Confocal microscopy of IF for Rspo3−GFP, Grem1-tdTomato, and LYVE1 in the small intestine of Rspo3−GFP; Grem1-tdTomato mouse day 3 post-irradiation. Yellow arrowheads indicate expanded RGFs. White arrows indicate LECs. (E) UMAP of scRNA-seq of small intestinal Rspo3−GFP+ cells 3 days post-irradiation (Control: 6,171 cells from 2 mice, irradiation: 11,706 cells from 2 mice). (F) Violin plots for Rspo3 expression. (G) Violin plots for Il1r1 expression. (H) GSEA of IL-1-mediated signaling pathway genes. (I-J) Schematic for IL-1a treatment of cultured RGFs (I). qRT-PCR of Rspo3 mRNA expression in RGFs (J). n = 3 mice per group. (K-M) Schematic of co-culture experiments of IL-1a- pretreated RGFs and crypts (K). Representative images (L) and quantification of organoid forming capacity (M) in the co-culture. n = 12 from 3 mice per group. (N) Model of how LECs and RGFs support ISCs in homeostasis and injury.
Unpaired two-tailed t-tests (C, J, M). Wilcox test (F, G). Data are mean ± SD. *p < 0.05. Scale bar, 50 μm (A) and 20 μm (D, L). Arrows indicate organoid formation.
See also Figure S7.
To gain molecular insights into how RSPO3+ stromal cells expand during regeneration and influence ISCs, we performed scRNA-seq in sorted Rspo3−GFP+ cells three days post-irradiation. We then leveraged multi-dataset integration (Stuart et al., 2019) to detect corresponding clusters from the normal and irradiated samples (Figure S7B). Like what we observed in the homeostatic intestine (Figure 2A), major sources of RSPO3 after irradiation include LECs and RGFs, the latter of which consists of RGF 1 (CD81+Adamdec1−) and RGF 2 (Cd81−Adamdec1+) (Figures 7E and S7C). Irradiation not only increased the RGF numbers (Figures 7B–D), but also significantly upregulated Rspo3 expression in both RGF subsets (Figure 7F).
We, then, performed differential gene expression analysis and GSEA between irradiated and control mice in each RGF subset. Among the top differentially expressed genes in them was Il1r, whose expression while low in homeostasis was notably elevated after irradiation (Figures 7G and 7H). Interesting, recent work has implicated macrophage-derived IL-1 as a signal that activates IL-1R1 mediated signaling in mesenchymal cells to boost to RSPO3 production as a mechanism for improving intestinal recovery after DSS–induced colitis (Cox et al., 2021). To test whether IL-1 signaling pathway triggers RSPO3 production in RGFs, we administered IL-1a to cultured RGFs (Figure 7I) and observed that Rspo3 expression was significantly activated upon IL-1a exposure (Figure 7J). Furthermore, in co- culture experiments, IL-1a-pretreatment of RGFs (Figure 7K) boosted their ability to support the organoid-forming capacity of crypts (Figure 7L and M). However, the irradiation of cultured RGFs did not enhance the ability of RGFs to support organoid growth (Figures S7D–F), implicating neighboring cells in the in vivo environment as the source of IL-1a that triggers RSPO3 production in RGFs. Overall, these results reveal that IL-1a augments RSPO3 production in in vitro RGFs and that IL-1r signaling in RGFs might be involved in mediating aspects of ISC regeneration in vivo.
In addition to Il1r, Igf1, Fgf2, and Ereg were among the most highly differentially expressed genes after irradiation in RGFs (Figure S7G). The induction of these growth factors may support the maintenance or expansion of LECs and ISCs as IGF-1 and FGFs are requisite growth factors for propagating LECs in vitro (Bjorndahl et al., 2005; Chang et al., 2004; Lokmic, 2016) (Figure S4E) and present in intestinal organoid media (Fujii et al., 2018). GSEA analysis also showed upregulation of gene sets in “HALLMARK_ANGIOGENESIS” (Figure S7H) and the expression of Il6 and Angptl4-– factors that facilitate angiogenesis (Cohen et al., 1996; Gopinathan et al., 2015)—as being upregulated in irradiated RGFs (Figure S7I), raising the possibility that RGFs may help coordinate the response of LECs and ISCs by providing growth/angiogenic factors during regeneration. Similar to Rspo3, Il6 and Angptl4 upregulation occurs in an IL-1a dependent manner in cultured RGFs (Figures S7J and K), indicating the IL-1a may elicit many of the adaptive changes noted in RGFs. Lastly, in LECs, Lcn2 and Lrg1, other essential regulators of angiogenesis (Nguyen et al., 2020; Wang et al., 2013; Yang et al., 2013), are among the most differentially upregulated genes after irradiation (Figure S7L), implying autocrine mechanisms may also underly LEC expansion. Collectively, our data demonstrate that LECs and RGFs expand to facilitate epithelial regeneration after injury (Figure 7N) and suggest that RGFs, partially through IL-1a mediated signaling, may serve as a hub to coordinate ISC and LEC responses to injury that promote regeneration through the heightened production of RSPO3 and growth/angiogenic factors such as IGF-1, FGF2 and Angptl4 (Figure 7N).
DISCUSSION
Understanding the cell types and trophic/growth factors that support stem cells in diverse tissues is of paramount importance for delineating how stem cells coordinate tissue remodeling or regeneration with organismal needs. This process is of particular importance in the intestine where rapidly renewing Lgr5+ ISCs dynamically sustain the turnover of the intestine in homeostasis, aging, and diverse dietary inputs (Barker et al., 2007; Beyaz et al., 2016; Cheng et al., 2019; Mana et al., 2021; Pentinmikko et al., 2019; Sato et al., 2009). Here, we propose that two mesenchymal populations, namely LECs and RGFs, that reside in close proximity to ISCs are the dominant sources of intestinal RSPO3, which is the ligand of Lgr4/5 receptors on ISCs and early progenitors. Furthermore, by leveraging newly engineered fluorescent RSPO3 and GREM1 reporter mice, we find that RGFs, which include previously characterized CD81+Pdgfralow trophocytes (McCarthy et al., 2020b), produce both RSPO3 and GREM1 and are in close physical proximity to LECs, the other primary source of RSPO3. Finally, these niche populations by altering their production of RSPO3 and other growth/angiocrine factors orchestrate ISC adaptation to diverse injuries.
Addressing the necessity of RSPO3 in ISC maintenance with GEMMs has been challenging as previous studies have demonstrated that it has at best a moderate impact on intestinal homeostasis and integrity (Greicius et al., 2018; Harnack et al., 2019). To date, none of the earlier experimental models disrupted RSPO3 in both RGFs and LECs. We found that conditional RSPO3 loss in either LECs and RGFs decreased ISC numbers and when co-deleted in both cellular populations led to shortened villi-crypt units, near complete absence of Lgr5+ ISCs, and ulcerated mucosa after irradiation induced damage. These results illustrate that LECs and RGFs represent redundant RSPO3 sources.
The proximity of RGFs to LECs implies cross-regulation between these two populations. We show that RGFs and LECs not only produce ISC niche factors but also produce factors that may regulate each other. RGFs produce VEGF-D, which may support LEC maintenance, while LECs produce REELIN, which may control RSPO3 production in RGFs to support ISCs. Furthermore, in addition to providing homeostatic support to ISCs, we find that RGFs and LECs help aid ISC-mediated repair after irradiation. In response to injury, both populations doubled in size relative to all mucosal cells to increase the RSPO3 niche pool to help bolster ISC function. Additionally, we found that IL-1a can augment RSPO3 production in in vitro RGFs and might be involved in mediating aspects of ISC regeneration in vivo (Figures 7G–M). Notably, IL-1a is released after damage in the colon, skin, and lung and its signaling pathway (i.e., the IL-1-mediated singling pathway) has been implicated in regeneration (Cox et al., 2021; Katsura et al., 2019; Lee et al., 2017). Interestingly, injury adapted RGFs and LECs also augment their expression of growth/angiogenic factors (Figure 7N), that may represent additional juxtacrine and autocrine mechanisms by which RGFs and LECs expand their numbers in injury-induced repair.
It is surprising to find that LECs are located close to crypt bottoms in the human small intestine and colon (Figures 1D and S1D), because intramucosal colorectal carcinoma (i.e., early-stage colon cancer confined to the mucosa above the muscularis mucosae) seldom metastasizes to the regional lymph nodes or distant organs in the clinic. From clinical studies for colorectal cancer, it is known that the depth of invasion of the tumor beyond the mucosa increases the risk of lymph node and distant organ metastasis. It is likely that lymphovascular invasion depends more on the aggressive genotype and invasiveness of the carcinoma than on the presence of lymphatics in the mucosa or at the crypt bases.
Finally, a recent study showed that hair follicle stem cells remodel lymphatics (Gur-Cohen et al., 2019; Pena-Jimenez et al., 2019); yet, whether LECs can reciprocally influence stemness has not been explored. Our data demonstrate that LECs serve as a critical in vivo reservoir for RSPO3 production for ISCs in homeostasis and injury-mediated repair and may plausibly do so in other tissues, especially those maintained by Lgr5-expressing stem cells such as the stomach and hair follicle. Accompanying studies by (Palikuqi et al., 2022) and (Niec et al., 2022) provide additional support regarding the role that LECs play as ISC niche components in homeostasis and injury. While LECs and RGFs sustain ISCs in homeostasis and in promoting repair after injury, future work with the tools developed in this study will be required to decipher the role that these niche cells play in coordinating stem cell function in response to diverse diet-induced organismal physiologies, aging, and disease states such as cancer initiation and progression.
Limitations of the Study
Here we propose that LECs and RGFs constitute the RSPO3 niche for ISCs. Although we demonstrate that RSPO3 loss in LECs and RGFs compromises ISCs in homeostasis and injury- induced regeneration, our data and model raise many important questions and limitations that need to be explored in the future. One such limitation is the extent to which LECs and RGFs cross-regulate one another as well as fine-tune ISC function. We propose that RGFs influence LECs numbers in vivo, but these studies in part depended on GREM1+ cellular ablation studies, which not only ablate RGFs but also GREM1+ muscularis mucosae and propria cells. Loss of GREM1+ RGFs and muscularis cells decreased LEC numbers. Future studies will need to distill the relative contribution of RGFs and GREM1+ muscularis cells in the control of LECs and ISCs. In addition, we identified that VEGF-D is a lymphangiocrine factor produced by RGFs. When we administered soluble VEGFR3 receptor to sequester VEGF-D as well as other VEGF family members, this reduced ISC numbers. Future studies will need to specifically disrupt VEGF-D and its other family members in RGFs to ascertain their specific role in the ISC niche, including the regulation of LECs by RGFs. Similarly, we proposed that REELIN, a LEC-derived factor, cell non-autonomously controls ISCs by triggering RSPO3 production in RGFs. These studies relied on organoid culture assays and may reflect partially what happens in vivo. Future studies will need to specifically ablate REELIN in LECs versus other niche cells in vivo to decipher its precise role in the ISC niche. Finally, we found that IL-1 signaling pathway is upregulated in RGFs after irradiation-injury and that IL-1a augments the ability of RGFs to support organoid growth in vitro. Although these findings suggest that IL-1a might be involved in mediating aspects of ISC regeneration in vivo, this needs to be tested using GEMMs to delete IL-1R specifically in RGFs.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Ömer H. Yilmaz (ohyilmaz@mit.edu, (Ö.H.Y.)).
Materials availability
All in-house generated mouse strains generated for this study are available from the Lead Contacts with a completed Materials Transfer Agreement.
Data and code availability
Bulk RNA-seq data and single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Olfm4 | Cell Signaling Technologies | Cat#: 39141S RRID:AB_2650511 |
| Goat polyclonal anti-GFP | Abcam | Cat#: ab6673 RRID:AB_305643 |
| Rat monoclonal anti-LYVE1 | Thermo Fisher Scientific | Cat#: 14-0443-82 RRID:AB_1633414 |
| Rabbit polyclonal anti-LYVE1 | Sigma Aldrich | Cat#: HPA042953 RRID:AB_2678238 |
| Rabbit polyclonal anti-Lysozyme | Thermo Fisher Scientific | Cat#: RB-372-A RRID:AB_138388 |
| Goat polyclonal anti-Prox1 | R&D | Cat#:AF2727 RRID:AB_2170716 |
| Rabbit monoclonal anti-Cleaved Caspase-3 | Cell Signaling | Cat#:9664 RRID:AB_2070042 |
| Mouse monoclonal anti-EpCAM | Biolegend | Cat#: 324201 RRID:AB_756075 |
| Mouse monoclonal anti-Ki67 | BD | Cat#: 550609 RRID:AB_393778 |
| Mouse monoclonal anti-Desmin | Abcam | Cat#: ab6322 RRID:AB_305423 |
| Mouse monoclonal anti-ADAMDEC1 | Thermo Fisher Scientific | Cat#: 6C4 RRID:AB_2543628 |
| Rabbit polyclonal anti-RFP | Rockland | Cat#: 600-401-379 RRID:AB_2209751 |
| Biotin-conjugated secondary donkey anti-rabbit IgG (H+L) | Jackson ImmunoResearch | Cat#: 711-066-152 RRID:AB_2340594 |
| Biotin-conjugated secondary donkey anti-goat IgG (H+L) | Jackson ImmunoResearch | Cat#: 705-066-147 RRID:AB_2340398 |
| Donkey Anti-Goat IgG Alexa Fluor 488 conjugated | Thermo Fisher Scientific | Cat#: A11055 RRID:AB_2534102 |
| Donkey Anti-Mouse IgG Alexa Fluor 488 conjugated | Thermo Fisher Scientific | Cat#: A21202 RRID:AB_141607 |
| Donkey Anti-Rabbit IgG Alexa Fluor 568 conjugated | Thermo Fisher Scientific | Cat#: A10042 RRID:AB_2534017 |
| Donkey Anti-Mouse IgG Alexa Fluor 647 conjugated | Thermo Fisher Scientific | Cat#: A31571 RRID:AB_162542 |
| Donkey Anti-Rat IgG Alexa Fluor 647 conjugated | Thermo Fisher Scientific | Cat#: A48272 RRID:AB_2893138 |
| Rat monoclonal anti-CD45-PE | Thermo Fisher Scientific | Cat#: 12-0451-83 RRID:AB_465669 |
| Rat monoclonal anti-CD31-eFluor450 | Thermo Fisher Scientific | Cat#: 48-0311-82 RRID:AB_10598807 |
| Rat monoclonal anti-EpCAM-APC | Thermo Fisher Scientific | Cat#: 17-5791-82 RRID:AB_2716944 |
| Chemicals, peptides, and recombinant proteins | ||
| Tamoxifen | Sigma Aldrich | T5648-1G |
| Sunflower Seed Oil | Spectrum | S1929 |
| Matrigel | Corning | 356231 |
| Recombinant Murine Noggin | Peprotech | 250-38 |
| Recombinant Human R-spondin1 | Peprotech | 120-38 |
| Recombinant Human VEGF-C Protein | R&D | 9199-VC-025 |
| Recombinant Murine REELIN | R&D | 3820-MR |
| Recombinant Murine IL-1a | Peprotech | 211-11A |
| B27 | Life Technologies | 17504044 |
| Y-27632 dihydrochloride | Sigma Aldrich | Y0503 |
| EGM-2 MV BulletKit | Lonza | CC-3202 |
| RPMI 1640 | Thermo Fisher Scientific | 11875093 |
| Liberase TM | Roche | 5401127001 |
| DNAse I | Roche | 10104159001 |
| ACK lysis buffer | Thermo Fisher Scientific | A1049201 |
| Accutase | STEMCELL technologies | 07920 |
| 7AAD | Thermo Fisher Scientific | A1310 |
| qScript cDNA SuperMix | Quantabio | 95048-100 |
| PerfeCTa SYBR green fast mix | Quantabio | 95072-012 |
| Elite ABC HRP Kit | Vector Laboratories | PK6100 |
| Signalstain DAB Substrate Kit | Cell Signaling Technologies | 8049S |
| Signalstain Antibody Diluent | Cell Signaling Technologies | 8112L |
| Dextran sulfate sodium salt | MP Biomedicals | 0216011050 |
| LGK974 | MedChemExpress | HY-17545 |
| Recombinant Mouse VEGFR3 Fc Chimera Protein | R&D | 743-R3-100 |
| Deposited data | ||
| RNA sequencing Data | This manuscript | GSE185348 |
| RNA sequencing Data | This manuscript | GSE185350 |
| scRNA sequencing Data | This manuscript | GSE185354 |
| Experimental models: Organisms/strains | ||
| Lgr5−2A-EGFP-2A-CreERT2 | This manuscript | N/A |
| Rosa26-LSL-tdTomato | The Jackson Laboratory | JAX strain 007914 |
| Lgr5-2A-EGFP-2A-CreERT2; Rosa26-LSL-tdTomato | This manuscript | N/A |
| Rspo3−GFP | Gene Expression Nervous System Atlas Project | https://www.mmrrc.org/catalog/sds.php?mmrrc_id=34606 |
| Grem1-2A-tdTomato-2A-CreERT2 | This manuscript | N/A |
| Rspo3−GFP; Grem1-2A-tdTomato-2A-CreERT2 | This manuscript | N/A |
| Rspo3 flox mice | The Jackson Laboratory | JAX strain 027313 |
| Prox1-CreERT2 | The Jackson Laboratory | JAX strain 022075 |
| Prox1-CreERT2; Rspo3 flox/flox | This manuscript | N/A |
| Grem1-2A-tdTomato-2A-CreERT2; Rspo3 flox/flox | This manuscript | N/A |
| Grem1-2A-tdTomato-2A-CreERT2; Prox1-CreERT2; Rspo3 flox/flox | This manuscript | N/A |
| Rosa26-LSL-DTA | The Jackson Laboratory | JAX strain 009669 |
| Oligonucleotides | ||
| Primers for RT-PCR, see METHOD DETAILS | Genewiz | N/A |
| Software and algorithms | ||
| FlowJo v10 | FlowJo LLC. | https://www.flowjo.com/ |
| FACSDiva software version 8.0 | BD Biosciences | https://www.bdbiosciences.com/en-us/products/software/instrument-software/bd-facsdiva-software#Overview |
| GraphPad Prism 9 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| ImageJ-Fiji | National Institutes of Health, USA | https://fiji.sc/ |
| R version 3.6.1, version 4.0.2 | (R Core Team 2021) | https://www.r-project.org |
| Salmon version 1.1.0, version 1.3.0 | (Patro et al., 2017) | https://github.com/COMBINE-lab/salmon |
| Tximport version 1.21.1, version 1.16.1 | (Soneson et al., 2015) | https://bioconductor.org/packages/release/bioc/html/tximport.html |
| DESeq2 | (Love et al., 2014) | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| Tibco Spotfire Analyst 7.6.1 | TIBCO | https://www.tibco.com/products/tibco-spotfire |
| javaGSEA version 4.1.0 | (Mootha et al., 2003) | https://www.gsea-msigdb.org/gsea/index.jsp |
| Cell ranger version 6.0.1 | 10X Genomics | https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger |
| Seurat version 4.0.3 | (Stuart et al., 2019) | https://github.com/satijalab/seurat/releases |
| Other | ||
| In situ hybridization probe for mouse Rspoo3: Mm-Rspo3 | Advanced Cell Diagnostics | Ref#402011 |
| In situ hybridization probe for mouse Lgr5: Mm-Lgr5 | Advanced Cell Diagnostics | Ref#312171 |
| In situ hybridization probe for mouse Axin2: Mm-Axin2 | Advanced Cell Diagnostics | Ref#400331 |
| In situ hybridization probe for mouse Ascl2: Mm-Ascl2 | Advanced Cell Diagnostics | Ref#412211 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal models
Mice were under the husbandry care of the Department of Comparative Medicine in the Koch Institute for Integrative Cancer Research. All procedures were conducted in accordance with the American Association for Accreditation of Laboratory Animal Care and approved by MIT’s Committee on Animal Care. Lgr5–2A-EGFP-2A-CreERT2 mice was generated by inserting P2A-EGFP-T2A-CreERT2 cassette in the endogenous Lgr5 gene locus immediately at the 5′ end of the endogenous stop codon using CRISPR-Cas9 technology and zygote microinjection. Grem1-2A-tdTomato-2A-CreERT2 mouse was generated by inserting P2A-tdTomato-T2A-CreERT2 cassette in the endogenous Grem1 gene locus immediately at the 5′ end of the endogenous stop codon using CRISPR-Cas9 technology and zygote microinjection. Successful targeting was validated by Southern blotting and PCR analysis. Rspo3−GFP BAC transgenic mouse was obtained from the Gene Expression Nervous System Atlas Project (Heintz, 2004) and backcrossed to C57BL/6J mice for more than 10 generations. Rspo3 flox mice (Neufeld et al., 2012) (JAX strain 027313), Prox1-CreERT2 mice (Srinivasan et al., 2007) (JAX strain 022075), Rosa26-LSL-DTA mice (JAX strain 009669) and Rosa26-LSL-tdTomato mice (JAX strain 007914) were obtained from the Jackson Laboratory. These mice were maintained in a C57BL/6 background. In this study, both male and female mice were used at the ages of 8–12 wks. For induction of Cre-mediated recombination, 200 μl of 20 mg/ml tamoxifen in corn oil (Figure S1C), or 100 μl of 20 mg/ml tamoxifen over 4 consecutive days (Figures 5, 6, S5, and S6D–G) were intraperitoneally injected.
Human intestinal samples
Resected human colonic and small intestinal sections that were diagnosed as normal were obtained from 8 patients. Inclusion criteria were male or female adults aged 20–90 years. The Massachusetts General Hospital (MGH) Institutional Review Board committee approved the study protocol.
METHOD DETAILS
Immunohistochemistry (IHC) and immunofluorescence (IF)
Tissues were fixed in 10% formalin, paraffin embedded and sectioned in 4–5 micron sections as previously described (Beyaz et al., 2016; Cheng et al., 2019; Mana et al., 2021). Antigen retrieval was performed using Borg Decloaker RTU solution (Biocare Medical, BD1000G1) and a pressurized Decloaking Chamber (Biocare Medical, NxGen). Antibodies and respective dilutions used for immunohistochemistry are as follows: rabbit monoclonal anti-OLFM4 (1:10,000, CST, 39141), rabbit polyclonal anti-Lysozyme (1:2,000, Thermo Fisher Scientific, RB-372-A), mouse monoclonal anti-Ki67 (1:400, BD, 550609), rabbit monoclonal anti-cleaved Caspase-3 (1:200, Cell Signaling, 9664), and goat polyclonal anti-GFP (1:500, abcam, ab6673). Biotin-conjugated secondary donkey anti-rabbit or anti- goat antibodies were used (1:500, Jackson ImmunoResearch). Vectastain Elite ABC immunoperoxidase detection kit (Vector Laboratories, PK6100) was followed by Signalstain DAB substrate kit for visualization (CST, 8049S). All antibody dilutions were performed in Signalstain Antibody Diluent (CST, 8112L). The following primary antibodies were used for immunofluorescence: goat polyclonal anti-GFP (1:500, abcam, ab6673), rat monoclonal anti-LYVE1 (1:1000, Thermo Fisher Scientific, 14–0443-82), rabbit polyclonal anti-LYVE1 (1:1000, Sigma Aldrich, HPA042953), mouse monoclonal anti-Desmin (1:200, abcam, ab6322), mouse monoclonal anti-ADAMDEC1 (1:100, Thermo Fisher Scientific, 6C4), mouse monoclonal anti-EpCAM (1:4,000, Biolegend, 324201), goat polyclonal anti-Prox1 (1:400, R&D, AF2727) and rabbit polyclonal anti-RFP (1:400, Rockland 600–401-379). Alexa Fluor secondary antibodies, anti-goat 488, anti-mouse 488, anti-rabbit 568, anti-mouse 647, and anti- rat 647 (1:500, Thermo Fisher Scientific), were used for visualization. Slides were stained with Hoechst for 10 min and covered with Prolong Gold (Life Technologies, P36930) mounting media. Images were acquired using a Nikon Eclipse 90i upright microscope equipped with a Hamamatsu Orca-ER CCD camera, and APC line 1200 light source. For the acquisition of high-resolution confocal images, Nikon A1R Ultra-Fast Spectral Scanning Confocal Microscope was used.
Intestinal stromal cell isolation and flow cytometry
Intestinal stromal cells were isolated as previously reported with a slight modification (McCarthy et al., 2020b; Ogasawara et al., 2018). The small intestines and the colons were removed, washed with cold PBS, opened longitudinally, cut into approximately 5 mm pieces, and then incubated with PBS plus EDTA (10 mM) for 40 min at 37°C. Crypts were mechanically removed from the tissue by shaking and washing with PBS. The remaining tissue was digested on a shaker at 400 rpm for 40 min at 37°C in 100 μg/ml Liberase TM (Roche, 5401127001) and 100 μg/ml DNAse I (Roche, 10104159001) diluted in RPMI 1640 media (Thermo Fisher Scientific, 11875093), containing 2% fetal bovine serum (FBS). Every 20 min, the tissue suspension was passed through an 14-gauge needle using a 10-ml syringe for mechanical dissociation. Extracted cells were passed through a 40 μm filter, centrifuged at 500 g for 5 min, and washed with RPMI 1640 medium containing 2% FBS. Cells were resuspended in ACK lysis buffer (Thermo Fisher Scientific, A1049201), incubated on ice for 4 minutes to remove red blood cells, washed with RPMI-1640 medium containing 2% FBS, and resuspended in the same buffer. Dissociated single cells were treated with the following antibody cocktail for flow cytometry analysis: CD45-PE (1:200, ThermoFisher,12-0451-83), CD31−eFluor450 (1:500, ThermoFisher, 48–0311-82), EpCAM-APC (1:300, ThermoFisher, 17–5791-82). 7AAD (ThermoFisher, A1310) was used a viability dye to exclude dead cells from the analysis. Fluorescence-activated cell sorting was performed using a FACS Aria II (BD Biosciences). The data were analyzed using FlowJo software (version 10, TreeStar) and FACSDiva software (version 8.0, BD Biosciences).
Culture of Rspo3+ stromal cells
Sorted Rspo3+, CD31−Rspo3−, CD31+Rspo3−, CD31−Rspo3+, and CD31+Rspo3+ cells were centrifuged at 500 g for 5 min and resuspended in 500 μl endothelial cell media: EGM-2 MV Bullet Kit (Lonza, cc-3202) supplemented with 50 ng/mL VEGF-C (R&D, 2179-VC-025). The kit contains EGF, hydrocortisone, gentamicin (GA-1000), FBS, VEGF, FGF-b, IGF-1, and ascorbic acid. Cells were plated in a fibronectin-coated 24-well plate in total volume of 500 μl with 2×104 cells per well. Endothelial cell media were changed every other day. Once the cells reached 80% confluence, the cells are detached from the wells with Accutase (STEMCELL technologies, 07920) for passage and co-culture with the intestinal crypts. Because LEC growth in culture is slower than that of RGFs (Figure S4G and H), LECs were expanded for more than 14 days after sorting until their expansion becomes stable (Figure S4I). For IL-1a administration experiments, sorted CD31−Rspo3+ cells were cultured and recombinant murine IL-1a (10 ng/ml, Peprotech, 211–11A) was administered to each well 24 hours prior to RNA extraction and co-culture experiments. For REELIN administration experiments, sorted CD31−Rspo3+ cells were cultured and recombinant murine REELIN (100 ng/ml, R&D, 3820-MR) was administered to each well 48 hours prior to RNA extraction and co-culture experiments.
Intestinal crypt isolation and co-culture of intestinal crypts with stromal cells.
The small intestines were removed, washed with cold PBS, opened longitudinally and then incubated on ice with PBS plus EDTA (10 mM) for 45 min. Tissues were then moved to PBS. Crypts were then mechanically separated from the connective tissue by shaking, and then filtered through a 100 μm mesh into a 50 mL conical tube to remove villus material and tissue fragments. For co-cultures, 100 crypts were cultured in 48-well tissue culture plates loaded with 10 μl drops of Matrigel (Corning) together with 1×105 stromal cells detached from the short-term culture after sorting as described above. Endothelial cell media supplemented with B27 1X (Life Technologies) and Y-27632 dihydrochloride monohydrate 10 μM (Sigma-Aldrich) were added to the wells with or without 100 ng/mL Noggin (Peprotech, 250–38) and 500 ng/mL RSPO1 (Peprotech, 120–38). Culture media were changed every other day, and cell cultures were maintained at 37°C in fully humidified chambers containing 5% CO2.
qRT-PCR and in situ hybridization
Total RNA was isolated using RNeasy Mini Kit (QIAGEN, 74104) or RNeasy Micro Kit (QIAGEN, 74004) according to the manufacturer’s instructions. RNA was converted to cDNA using qScript cDNA SuperMix (Quantabio, 95048–100). Quantitative RT-PCR (qRT-PCR) reaction was performed using cDNA with SYBR green fast mix (Quantabio, PerfeCTa, 95072–012) on a Roche lightcycler (Roche, LightCycler 480 II). The following primers used for qRT-PCR: Gapdh forward, 5’- AGGTCGGTGTGAACGGATTTG-3’; Gapdh reverse, 5’-TGTAGACCATGTAGTTGAGGTCA-3’; Rspo3 forward,5’-ATGCACTTGCGACTGATTTCT-3’; Rspo3 reverse, 5’- GCAGCCTTGACTGACATTAGGAT-3’; Grem1 forward, 5’- CTGGGGACCCTACTGCCAA-3’; Grem1 reverse, 5’- TTTGCACCAATCTCGCTTCAG-3’; Il6 forward, 5’-TAGTCCTTCCTACCCCAATTTCC-3’;Il6 reverse, 5’-TTGGTCCTTAGCCACTCCTTC-3’; Agptl4 forward, 5′- CATCCTGGGACGAGATGAACT-3′; Agptl4 reverse, 5′- TGACAAGCGTTACCACAGGC-3′.
Single molecule in situ hybridization was performed using Advanced Cell Diagnostics RNAscope 2.5 HD Detection Kit. The in situ hybridization probes used in this study are as follows: Mm-Rspo3 (Ref 402011), Mm-Lgr5 (Ref 312171), Mm-Axin2 (Ref 400331), Mm-Ascl2 (Ref 412211).
RNA-seq data processing and differential expression analysis
Single-end RNA-seq data from GSE185348 was used to quantify transcripts from the mm10 mouse assembly with the Ensembl version 98 annotation using Salmon version 1.1.0 (Patro et al., 2017). Paired-end RNA-seq data from GSE185350 was used to quantify transcripts from the mm10 mouse assembly with the Ensembl version 101 annotation using Salmon version 1.3.0 (Patro et al., 2017). Gene level summaries for GSE185350 were prepared using tximport version 1.16.1 (Soneson et al., 2015) running under R version 4.0.2 (R Core Team 2021). The tximport and R versions for GSE185348 were 1.21.1 and 3.6.1 respectively. Differential expression analysis was done with DESeq2 version 1.24.0 (Love et al., 2014) and differentially expressed genes were defined as those having an absolute apeglm (Zhu et al., 2019) log2 fold change greater than 1 and an adjusted p-value less than 0.05. Data parsing and some visualizations was done using Tibco Spotfire Analyst 7.6.1. Mouse genes were mapped to human orthologs using Mouse Genome Informatics (http://www.informatics.jax.org/) orthology report and preranked Gene Set Enrichment Analysis (Mootha et al., 2003) was done using javaGSEA version 4.1.0 with a custom gene sets or sets from MSigDB version 7.2 (Subramanian et al., 2005). The custom gene sets are provided in Supplementary Table 1.
scRNA-seq data processing and analysis
scRNA-seq data was produced using the 10x genomics platform. Data were processed using cell ranger version 6.0.1 with alignment to a modified version of the GENCODE mouse genome (GRCm38), version M23 (Ensembl 98) target provided by 10x genomics. The modification was the addition of the GFP selectable marker. Cellranger filtered data was imported into R version 4.1.0 (R Core Team 2021) and analyzed with Seurat version 4.0.3 (Stuart et al., 2019). Cells with > 500 and < 5,500 detected genes, >= 200 molecules, and <= 10% mitochondrial transcripts were retained. Datasets were merged utilizing the “merge” function and data were normalized using the “NormalizeData” function in Seurat. For clustering of the cells, the “FindNeighbors” and “FindClusters” functions in Seurat are used with the resolutions of 0.7 (Figure 2A), 0.4 (Figure 2E), and 0.2 (Figure 7E). To define the cell type in each cluster, the data were queried for known stromal cell-specific genes (Kinchen et al., 2018; McCarthy et al., 2020b), and the specific expression of the marker genes in each cluster is confirm by UMAP plots with gene expression using the “FeaturePlot” function in Seurat (Figures 2B, 2F, and S1G). For the integrated analyses between normal and irradiation conditions (Figure 7E), datasets are integrated after normalization using the “IntegrateData” function in Seurat.
For Figure 2A (small intestine, normal condition), sorted Rspo3−GFP+ cells from two separate mice (2 replicates) were used. We confirmed that the same clustering patterns in the small intestine are detected in both of the individual UMAP plots (Figure S1F). For Figure 2E (colon), two animals were pooled into a single 10X run. For Figure 7E (small intestine, normal condition vs irradiation), two independent replicates from two independent mice are used for each condition (control: 2 replicates from 2 mice, injury: 2 replicates from 2 mice, Figure S7B). To avoid the batch effect, all the samples in Figure 7E were prepared on the same day, and sequencing was performed in the same lane of a NexSeq 500 System (Illumina).
Mouse treatments
Mice were given 1.5% DSS (MP Biomedicals, 0216011050) in drinking water for 6 consecutive days. The Porcupine inhibitor LGK974 (MedChemExpress, HY-17545) was administered by daily oral gavage in a concentration of 5 mg/kg in a vehicle of 0.5% Tween-80/0.5% methylcellulose for 8 consecutive days. Recombinant VEGFR3-Fc chimera (R&D, 743-R3–100; 10 μg in 100 μl PBS) was intradermally injected for 4 consecutive days.
Irradiation experiments
Mice were challenged by 12 Gy of irradiation. Tissue was collected 24 or 72 hours post-irradiation. Numbers of surviving crypts were enumerated in the proximal jejunum from Hematoxylin and Eosin stained tissue and identified as robust crypt structures with dense nuclei and presence of Paneth cells. RGFs were irradiated at 2 or 6 Gy and then co-cultured with intestinal crypts.
QUANTIFICATION AND STATISTICAL ANALYSIS
Unless otherwise specified in the figure legends or Method Details, all experiments reported in this study were repeated at least three independent times. For organoid co-culture assays, 2–5 wells per group with at least 3 different mice were analyzed. All sample number (n) of biological replicates and technical replicates, definition of center, and dispersion and precision measures can be found in the figure legends. The images for H&E, immunofluorescence, and immunohistochemistry represent one of > 6 biological replicates unless otherwise stated. Flow cytometry plots represent one of > 3 biological replicates. All values are presented as mean ± SD unless otherwise stated. Intergroup comparisons were performed using unpaired two-tailed t-tests or one-way analysis of variance (ANOVA) with post- hoc Tukey’s multiple comparison. P values of < 0.05 were considered to be significant. Statistical analysis was performed by GraphPad Prism. No sample or animals were excluded from analysis. Age- and sex-matched mice were randomly assigned to groups. Studies were not conducted blind with the exception of all histological analyses. Please note that statistical details are found in the figure legends.
Supplementary Material
Supplementary Table 1: Custom gene sets for Gene Set Enrichment Analysis (GSEA), Related to Figure 1 and Figure 3
Supplementary Video 1: 3-D reconstitution of confocal microscopy images of immunofluorescence for Lgr5-GFP and LYVE1 in the mouse small intestine, Related to Figure 1
Highlights.
LECs and RGFs are the major sources of mucosal RSPO3 in the small intestine and colon RGFs reside close to ISCs and surround LECs LECs and RGFs support ISCs and post-injury regeneration in vivo LECs and RGFs expand to facilitate epithelial regeneration after injury
ACKNOWLEDGEMENTS
We thank the Swanson Biotechnology Center at the Koch Institute, which encompasses the Flow Cytometry, Histology, Microscopy and Genomics & Bioinformatics Core facilities (NCI P30-CA14051). We thank Charlie Whittaker and Dikshant Pradhan for analysis and helpful discussions regarding RNA sequencing data. We thank the Department of Comparative Medicine for mouse husbandry support. We thank Sven Holder and members of the Hope Babette Tang (1983) Histology Facility for substantial histology support. We thank members of the Yilmaz laboratory for discussions, Kerry Kelley for laboratory management, and Liz Galoyan for administrative assistance. N.G. is supported by Postdoctoral Fellowship for Research Abroad from Japan Society for the Promotion of Science. Ö.H.Y. is supported by R01CA211184, R01CA034992, and U54CA224068; a Pew-Stewart Trust scholar award; the Kathy and Curt Marble cancer research award; a Koch Institute-Dana-Farber/Harvard Cancer Center Bridge Project grant; and AFAR. Ö.H.Y. receives support from the MIT Stem Cell Initiative via Fondation MIT.
Footnotes
DECLARATION OF INTERESTS
Ö.H.Y. holds equity and is a SAB member in Ava Lifesciences.
Ö.H.Y. receives research support from Microbial Machines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, et al. (2009). Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, et al. (2016). High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, and Cao Y. (2005). Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A 102, 15593–15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger MD, Valenta T, Fazilaty H, Hausmann G, and Basler K. (2020). Distinct populations of crypt- associated fibroblasts act as signaling hubs to control colon homeostasis. PLoS Biol 18, e3001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, and Kaipainen A. (2004). Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A 101, 11658–11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, Tripathi S, Calibasi-Kocal G, Rickelt S, Butty VL, et al. (2019). Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell 178, 1115–1131 e1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifarelli V, and Eichmann A. (2019). The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell Mol Gastroenterol Hepatol 7, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem LW, Neufeld G, and Levi BZ (1996). Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 271, 736–741. [DOI] [PubMed] [Google Scholar]
- Cox CB, Storm EE, Kapoor VN, Chavarria-Smith J, Lin DL, Wang L, Li Y, Kljavin N, Ota N, Bainbridge TW, et al. (2021). IL-1R1-dependent signaling coordinates epithelial regeneration in response to intestinal damage. Sci Immunol 6. [DOI] [PubMed] [Google Scholar]
- de Lau W, Peng WC, Gros P, and Clevers H. (2014). The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev 28, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, and Basler K. (2018). GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558, 449–453. [DOI] [PubMed] [Google Scholar]
- Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, de Punder K, Angers S, Peters PJ, Maurice MM, et al. (2016). Visualization of a short-range Wnt gradient in the intestinal stem-cell niche.Nature 530, 340–343. [DOI] [PubMed] [Google Scholar]
- Farin HF, Van Es JH, and Clevers H. (2012). Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143, 1518–1529 e1517. [DOI] [PubMed] [Google Scholar]
- Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, Nishikori S, Sugimoto S, and Sato T. (2018). Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 23, 787–793 e786. [DOI] [PubMed] [Google Scholar]
- Gehart H, and Clevers H. (2019). Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 16, 19–34. [DOI] [PubMed] [Google Scholar]
- Gopinathan G, Milagre C, Pearce OM, Reynolds LE, Hodivala-Dilke K, Leinster DA, Zhong H, Hollingsworth RE, Thompson R, Whiteford JR, et al. (2015). Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res 75, 3098–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, and Morrisey EE (2009). Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut.Dev Cell 17, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius G, Kabiri Z, Sigmundsson K, Liang C, Bunte R, Singh MK, and Virshup DM (2018). PDGFRalpha(+) pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci U S A 115, E3173–E3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ, and Fuchs E. (2019). Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnack C, Berger H, Antanaviciute A, Vidal R, Sauer S, Simmons A, Meyer TF, and Sigal M. (2019). R- spondin 3 promotes stem cell recovery and epithelial regeneration in the colon. Nat Commun 10, 4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. (2004). Gene expression nervous system atlas (GENSAT). Nat Neurosci 7, 483. [DOI] [PubMed] [Google Scholar]
- Hua G, Wang C, Pan Y, Zeng Z, Lee SG, Martin ML, Haimovitz-Friedman A, Fuks Z, Paty PB, and Kolesnick R. (2017). Distinct Levels of Radioresistance in Lgr5(+) Colonic Epithelial Stem Cells versus Lgr5(+) Small Intestinal Stem Cells. Cancer Res 77, 2124–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huels DJ, Bruens L, Hodder MC, Cammareri P, Campbell AD, Ridgway RA, Gay DM, Solar-Abboud M, Faller WJ, Nixon C, et al. (2018). Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nat Commun 9, 1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, et al. (2020). Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 180, 764–779 e720. [DOI] [PubMed] [Google Scholar]
- Karaman S, Leppanen VM, and Alitalo K. (2018). Vascular endothelial growth factor signaling in development and disease. Development 145. [DOI] [PubMed] [Google Scholar]
- Katsura H, Kobayashi Y, Tata PR, and Hogan BLM (2019). IL-1 and TNFalpha Contribute to the Inflammatory Niche to Enhance Alveolar Regeneration. Stem Cell Reports 12, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, Ashley N, Cubitt L, Mellado-Gomez E, Attar M, et al. (2018). Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 175, 372–386 e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, et al. (2007). Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A 104, 15418–15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Gund R, Dutta A, Pincha N, Rana I, Ghosh S, Witherden D, Kandyba E, MacLeod A, Kobielak K, et al. (2017). Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of gammadeltaT-cells. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al. (2013). Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A 110, 20224–20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, De la Cruz E, Gu X, Balint L, Oxendine-Burns M, Terrones T, Ma W, Kuo HH, Lantz C, Bansal T, et al. (2020). Lymphoangiocrine signals promote cardiac growth and repair. Nature 588, 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokmic Z. (2016). Isolation, Identification, and Culture of Human Lymphatic Endothelial Cells. Methods Mol Biol 1430, 77–90. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, et al. (2001). Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med 7, 199–205. [DOI] [PubMed] [Google Scholar]
- Mana MD, Hussey AM, Tzouanas CN, Imada S, Barrera Millan Y, Bahceci D, Saiz DR, Webb AT, Lewis CA, Carmeliet P, et al. (2021). High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep 35, 109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy N, Kraiczy J, and Shivdasani RA (2020a). Cellular and molecular architecture of the intestinal stem cell niche. Nat Cell Biol 22, 1033–1041. [DOI] [PubMed] [Google Scholar]
- McCarthy N, Manieri E, Storm EE, Saadatpour A, Luoma AM, Kapoor VN, Madha S, Gaynor LT, Cox C, Keerthivasan S, et al. (2020b). Distinct Mesenchymal Cell Populations Generate the Essential Intestinal BMP Signaling Gradient. Cell Stem Cell 26, 391–402 e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy N, Tie G, Madha S, Kraiczy J, Maglieri A, and Shivdasani RA (2022). Delineation and birth of a multi-layer intestinal stem cell niche. bioRxiv, 2021.2009.2028.462142. [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, et al. (2003). PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34, 267–273. [DOI] [PubMed] [Google Scholar]
- Neufeld S, Rosin JM, Ambasta A, Hui K, Shaneman V, Crowder R, Vickerman L, and Cobb J. (2012). A conditional allele of Rspo3 reveals redundant function of R-spondins during mouse limb development. Genesis 50, 741–749. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Farman N, Palacios-Ramirez R, Sbeih M, Behar-Cohen F, Aractingi S, and Jaisser F. (2020). Cutaneous Wound Healing in Diabetic Mice Is Improved by Topical Mineralocorticoid Receptor Blockade. J Invest Dermatol 140, 223–234 e227. [DOI] [PubMed] [Google Scholar]
- Niec RE, Chu T, Schernthanner M, Gur-Cohen S, Hidalgo L, Pasolli HA, Luckett KA, Wang Z, Bhalla SR, Cambuli F, et al. (2022). Lymphatics act as a signaling hub to regulate intestinal stem cell activity. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara R, Hashimoto D, Kimura S, Hayase E, Ara T, Takahashi S, Ohigashi H, Yoshioka K, Tateno T, Yokoyama E, et al. (2018). Intestinal Lymphatic Endothelial Cells Produce R-Spondin3. Sci Rep 8, 10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikuqi B, Rispal J, Vaka D, Boffelli D, and Klein O. (2022). Lymphangiocrine signals are required for proper intestinal repair after cytotoxic injury. bioRxiv, 2022.2003.2008.479995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, and Kingsford C. (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14, 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Jimenez D, Fontenete S, Megias D, Fustero-Torre C, Grana-Castro O, Castellana D, Loewe R, and Perez-Moreno M. (2019). Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J 38, e101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentinmikko N, Iqbal S, Mana M, Andersson S, Cognetta AB 3rd, Suciu RM, Roper J, Luopajarvi K, Markelin E, Gopalakrishnan S, et al. (2019). Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 571, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Sachs N, Wiebrands K, Ellenbroek SI, Fumagalli A, Lyubimova A, Begthel H, van den Born M, van Es JH, Karthaus WR, et al. (2016). Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proceedings of the National Academy of Sciences of the United States of America 113, E5399–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, and Clevers H. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Schuijers J, van der Flier LG, van Es J, and Clevers H. (2014). Robust cre-mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Reports 3, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Toth B, Kondo A, Massasa EE, Itzkovitz S, and Kaestner KH (2018). Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 557, 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneson C, Love MI, and Robinson MD (2015). Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, and Oliver G. (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21, 2422–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, and Satija R. (2019). Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902 e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stzepourginski I, Nigro G, Jacob JM, Dulauroy S, Sansonetti PJ, Eberl G, and Peduto L. (2017). CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A 114, E506–E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SH, Phuah P, Tan LT, Yada S, Goh J, Tomaz LB, Chua M, Wong E, Lee B, and Barker N. (2021). A constant pool of Lgr5(+) intestinal stem cells is required for intestinal homeostasis. Cell Rep 34, 108633. [DOI] [PubMed] [Google Scholar]
- Thomson CA, van de Pavert SA, Stakenborg M, Labeeuw E, Matteoli G, Mowat AM, and Nibbs RJB (2018). Expression of the Atypical Chemokine Receptor ACKR4 Identifies a Novel Population of Intestinal Submucosal Fibroblasts That Preferentially Expresses Endothelial Cell Regulators. J Immunol 201, 215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Abraham S, McKenzie JAG, Jeffs N, Swire M, Tripathi VB, Luhmann UFO, Lange CAK, Zhai Z, Arthur HM, et al. (2013). LRG1 promotes angiogenesis by modulating endothelial TGF-beta signalling. Nature 499, 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, et al. (2017). Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature 545, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, McNeish B, Butterfield C, and Moses MA (2013). Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J 27, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, et al. (2012). mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Ibrahim JG, and Love MI (2019). Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Custom gene sets for Gene Set Enrichment Analysis (GSEA), Related to Figure 1 and Figure 3
Supplementary Video 1: 3-D reconstitution of confocal microscopy images of immunofluorescence for Lgr5-GFP and LYVE1 in the mouse small intestine, Related to Figure 1
Data Availability Statement
Bulk RNA-seq data and single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Olfm4 | Cell Signaling Technologies | Cat#: 39141S RRID:AB_2650511 |
| Goat polyclonal anti-GFP | Abcam | Cat#: ab6673 RRID:AB_305643 |
| Rat monoclonal anti-LYVE1 | Thermo Fisher Scientific | Cat#: 14-0443-82 RRID:AB_1633414 |
| Rabbit polyclonal anti-LYVE1 | Sigma Aldrich | Cat#: HPA042953 RRID:AB_2678238 |
| Rabbit polyclonal anti-Lysozyme | Thermo Fisher Scientific | Cat#: RB-372-A RRID:AB_138388 |
| Goat polyclonal anti-Prox1 | R&D | Cat#:AF2727 RRID:AB_2170716 |
| Rabbit monoclonal anti-Cleaved Caspase-3 | Cell Signaling | Cat#:9664 RRID:AB_2070042 |
| Mouse monoclonal anti-EpCAM | Biolegend | Cat#: 324201 RRID:AB_756075 |
| Mouse monoclonal anti-Ki67 | BD | Cat#: 550609 RRID:AB_393778 |
| Mouse monoclonal anti-Desmin | Abcam | Cat#: ab6322 RRID:AB_305423 |
| Mouse monoclonal anti-ADAMDEC1 | Thermo Fisher Scientific | Cat#: 6C4 RRID:AB_2543628 |
| Rabbit polyclonal anti-RFP | Rockland | Cat#: 600-401-379 RRID:AB_2209751 |
| Biotin-conjugated secondary donkey anti-rabbit IgG (H+L) | Jackson ImmunoResearch | Cat#: 711-066-152 RRID:AB_2340594 |
| Biotin-conjugated secondary donkey anti-goat IgG (H+L) | Jackson ImmunoResearch | Cat#: 705-066-147 RRID:AB_2340398 |
| Donkey Anti-Goat IgG Alexa Fluor 488 conjugated | Thermo Fisher Scientific | Cat#: A11055 RRID:AB_2534102 |
| Donkey Anti-Mouse IgG Alexa Fluor 488 conjugated | Thermo Fisher Scientific | Cat#: A21202 RRID:AB_141607 |
| Donkey Anti-Rabbit IgG Alexa Fluor 568 conjugated | Thermo Fisher Scientific | Cat#: A10042 RRID:AB_2534017 |
| Donkey Anti-Mouse IgG Alexa Fluor 647 conjugated | Thermo Fisher Scientific | Cat#: A31571 RRID:AB_162542 |
| Donkey Anti-Rat IgG Alexa Fluor 647 conjugated | Thermo Fisher Scientific | Cat#: A48272 RRID:AB_2893138 |
| Rat monoclonal anti-CD45-PE | Thermo Fisher Scientific | Cat#: 12-0451-83 RRID:AB_465669 |
| Rat monoclonal anti-CD31-eFluor450 | Thermo Fisher Scientific | Cat#: 48-0311-82 RRID:AB_10598807 |
| Rat monoclonal anti-EpCAM-APC | Thermo Fisher Scientific | Cat#: 17-5791-82 RRID:AB_2716944 |
| Chemicals, peptides, and recombinant proteins | ||
| Tamoxifen | Sigma Aldrich | T5648-1G |
| Sunflower Seed Oil | Spectrum | S1929 |
| Matrigel | Corning | 356231 |
| Recombinant Murine Noggin | Peprotech | 250-38 |
| Recombinant Human R-spondin1 | Peprotech | 120-38 |
| Recombinant Human VEGF-C Protein | R&D | 9199-VC-025 |
| Recombinant Murine REELIN | R&D | 3820-MR |
| Recombinant Murine IL-1a | Peprotech | 211-11A |
| B27 | Life Technologies | 17504044 |
| Y-27632 dihydrochloride | Sigma Aldrich | Y0503 |
| EGM-2 MV BulletKit | Lonza | CC-3202 |
| RPMI 1640 | Thermo Fisher Scientific | 11875093 |
| Liberase TM | Roche | 5401127001 |
| DNAse I | Roche | 10104159001 |
| ACK lysis buffer | Thermo Fisher Scientific | A1049201 |
| Accutase | STEMCELL technologies | 07920 |
| 7AAD | Thermo Fisher Scientific | A1310 |
| qScript cDNA SuperMix | Quantabio | 95048-100 |
| PerfeCTa SYBR green fast mix | Quantabio | 95072-012 |
| Elite ABC HRP Kit | Vector Laboratories | PK6100 |
| Signalstain DAB Substrate Kit | Cell Signaling Technologies | 8049S |
| Signalstain Antibody Diluent | Cell Signaling Technologies | 8112L |
| Dextran sulfate sodium salt | MP Biomedicals | 0216011050 |
| LGK974 | MedChemExpress | HY-17545 |
| Recombinant Mouse VEGFR3 Fc Chimera Protein | R&D | 743-R3-100 |
| Deposited data | ||
| RNA sequencing Data | This manuscript | GSE185348 |
| RNA sequencing Data | This manuscript | GSE185350 |
| scRNA sequencing Data | This manuscript | GSE185354 |
| Experimental models: Organisms/strains | ||
| Lgr5−2A-EGFP-2A-CreERT2 | This manuscript | N/A |
| Rosa26-LSL-tdTomato | The Jackson Laboratory | JAX strain 007914 |
| Lgr5-2A-EGFP-2A-CreERT2; Rosa26-LSL-tdTomato | This manuscript | N/A |
| Rspo3−GFP | Gene Expression Nervous System Atlas Project | https://www.mmrrc.org/catalog/sds.php?mmrrc_id=34606 |
| Grem1-2A-tdTomato-2A-CreERT2 | This manuscript | N/A |
| Rspo3−GFP; Grem1-2A-tdTomato-2A-CreERT2 | This manuscript | N/A |
| Rspo3 flox mice | The Jackson Laboratory | JAX strain 027313 |
| Prox1-CreERT2 | The Jackson Laboratory | JAX strain 022075 |
| Prox1-CreERT2; Rspo3 flox/flox | This manuscript | N/A |
| Grem1-2A-tdTomato-2A-CreERT2; Rspo3 flox/flox | This manuscript | N/A |
| Grem1-2A-tdTomato-2A-CreERT2; Prox1-CreERT2; Rspo3 flox/flox | This manuscript | N/A |
| Rosa26-LSL-DTA | The Jackson Laboratory | JAX strain 009669 |
| Oligonucleotides | ||
| Primers for RT-PCR, see METHOD DETAILS | Genewiz | N/A |
| Software and algorithms | ||
| FlowJo v10 | FlowJo LLC. | https://www.flowjo.com/ |
| FACSDiva software version 8.0 | BD Biosciences | https://www.bdbiosciences.com/en-us/products/software/instrument-software/bd-facsdiva-software#Overview |
| GraphPad Prism 9 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| ImageJ-Fiji | National Institutes of Health, USA | https://fiji.sc/ |
| R version 3.6.1, version 4.0.2 | (R Core Team 2021) | https://www.r-project.org |
| Salmon version 1.1.0, version 1.3.0 | (Patro et al., 2017) | https://github.com/COMBINE-lab/salmon |
| Tximport version 1.21.1, version 1.16.1 | (Soneson et al., 2015) | https://bioconductor.org/packages/release/bioc/html/tximport.html |
| DESeq2 | (Love et al., 2014) | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| Tibco Spotfire Analyst 7.6.1 | TIBCO | https://www.tibco.com/products/tibco-spotfire |
| javaGSEA version 4.1.0 | (Mootha et al., 2003) | https://www.gsea-msigdb.org/gsea/index.jsp |
| Cell ranger version 6.0.1 | 10X Genomics | https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger |
| Seurat version 4.0.3 | (Stuart et al., 2019) | https://github.com/satijalab/seurat/releases |
| Other | ||
| In situ hybridization probe for mouse Rspoo3: Mm-Rspo3 | Advanced Cell Diagnostics | Ref#402011 |
| In situ hybridization probe for mouse Lgr5: Mm-Lgr5 | Advanced Cell Diagnostics | Ref#312171 |
| In situ hybridization probe for mouse Axin2: Mm-Axin2 | Advanced Cell Diagnostics | Ref#400331 |
| In situ hybridization probe for mouse Ascl2: Mm-Ascl2 | Advanced Cell Diagnostics | Ref#412211 |