Abstract
Background
CogMed Working Memory Training (CWMT) is a computer-based program shown to improve working memory (WM) among those with cognitive impairments. No study to date has investigated its feasibility, acceptability, and satisfaction in adult patients with glioma, despite the well-documented incidence of WM impairment in this population.
Methods
Twenty patients with glioma and objective and/or perceived WM deficits enrolled in the study: 52% high-grade, 60% female, Mage = 47 (range = 21–72 years). Adverse events were monitored to determine safety. Feasibility and acceptability were assessed based on established metrics. Satisfaction was explored by exit-interviews. Neurocognitive tests and psychological symptoms were analyzed at baseline and post-CWMT to estimate effect sizes.
Results
Of 20 enrolled patients, 16 completed the intervention (80% retention rate). Reasons for withdrawal included time burden (n = 2); tumor-related fatigue (n = 1) or loss to follow-up (n = 1). No adverse events were determined to be study-related. Adherence was 69% with reasons for nonadherence similar to those for study withdrawal. The perceived degree of benefit was only moderate. Baseline to post-CWMT assessments showed medium to large effects on neurocognitive tasks. Psychological symptoms remained stable throughout the study period.
Conclusions
CWMT was found to be safe and acceptable in adult patients with glioma. Enrollment, retention rates, and treatment adherence were all adequate and comparable to studies recruiting similar populations. Only moderate perceived benefit was reported despite demonstrated improvements in objectively-assessed WM. This may indicate that the time commitment and intervention intensity (5 weeks of 50-min training sessions on 5 days/week) outweighed the perceived benefits of the program. (Trial Registration Number: NCT03323450 registered on 10/27/2017).
Keywords: Glioma, Working memory, Feasibility, Acceptability, CogMed, Cognitive rehabilitation
Introduction
Most individuals with gliomas show impairments in at least one domain of cognition [1–3] due to tumor mass effect [4] and associated treatments [5]. Insults to attention and executive functions are common [6], with working memory—the ability to take in, briefly hold onto, and manipulate information—a frequently affected cognitive system [7, 8]. Working memory is implicated in executing activities of daily living, successful learning and emotional regulation, with deficits capable of reducing functional independence [9–11]. As such, interventions that optimize cognitive performance are increasingly relevant, as even mild impairments can negatively affect quality of life [3, 12]. In addition to deficits on objective neurocognitive tests; individuals with glioma also report a high rate of subjective working memory complaints, which have significant implications for overall wellbeing [13]. As such, both objective and subjective deficits in working memory are likely to impact one’s confidence and ability to care for oneself [14].
CogMed Working Memory Training (CWMT) is a computerized program developed to improve working memory. Use of CWMT has been evaluated in a variety of medical populations for whom attention and executive dysfunction is common, such as ADHD [15], traumatic brain injury [16], and pediatric brain tumor [17]. Cognitive rehabilitation programs like CWMT rely on repeated simulation of a cognitive process (e.g., working memory) to strengthen the underlying neural networks responsible for it, promoting domain-specific capacity (i.e., near transfer) [18]. Any gains on objective measures related to those domain-specific capacities are referred to as near transfer outcomes (i.e., improvements in working memory scores after completing CWMT). Though it has been theorized that these improvements may generalized even further, and also result in improvements in other cognitive domains (i.e., far transfer), a recent meta-analysis evaluating CWMT emcacy revealed minimal to no effect on far transfer skills (e.g., academic achievement, language abilities) [19].
CWMT may be well-suited for adults with glioma given the high rates of objective and subjective executive dysfunction in this population and potential for improved quality of life. In a small case series of three patients with low grade glioma, preliminary evidence suggested improvements in working memory and attention (i.e., near transfer tasks) following CWMT [20]. A more recent trial in a mixed neurosurgery sample, including patients with glioma (46.7%), also found improvements on working memory tasks and self-reported quality of life, in addition to reduced depression and anxiety symptoms [21]. While these studies provide support for additional investigation of CWMT in adults with glioma, they did not directly address safety, feasibility, or acceptability of the intervention. Further, small and mixed-diagnosis samples make it diffcult to interpret the direct benefits or challenges of implementing CWMT within adult neuro-oncology.
While CWMT is a relatively low-risk intervention, there is a possibility that participation in this computer-based program could increase seizure incidence in patients with photosensitivity and associated seizure activity [22]. Approximately 40—60% of adults with glioma have a history of seizures [23]; thus, monitoring seizure frequency throughout CWMT protocol is critical in order to assess the safety of this intervention. While no adverse events as a result of CWMT have been previously reported in glioma patients [20, 21], given the increased fatigue and high disease burden, safety will be closely monitored. Assessing feasibility and acceptability for patients with both high and low grade tumors during ongoing treatment is relevant as working memory deficits are common across tumor grade [24, 25]. The NIH Science of Behavior Change ORBIT model provides a pathway for establishing behavioral interventions as evidenced-based treatments for chronic diseases based on the phases followed in medication trials [26]. Given that this standardized CWMT protocol has demonstrated positive effects on neurocognitive functioning in other medical populations [15–17, 21], the aims of the current study were to evaluate the safety, feasibility, acceptability, and preliminary emcacy of CWMT in a sample of low and high grade adult patients with glioma—a Phase Ila, proof-of-concept, study.
Materials and methods
Procedure
Eligibility for this single-arm Phase Ila proof-of-concept study was determined based on routine clinical neuropsychological evaluation, which also served as their baseline assessment, once enrolled. Baseline evaluations occurred 1 to 4 weeks before beginning the CWMT protocol. All who enrolled in the study underwent an in-person introduction to CWMT followed by a 5-week at-home intervention consisting of 50-min online training sessions 5 days per week. There was no comparison or control group. During the first 1–2 weeks, participants were offered weekly phone check-ins to troubleshoot difficulties (e.g., technological issues). Adverse events were monitored via weekly communication with patients as well as medical chart reviews. Program adherence was monitored by study staff. If participants missed more than 3 scheduled training sessions at any point during the intervention, a member of the study team attempted to contact them to identify the reason and offer support. Per CWMT protocol, 3 to 4 weeks following completion of at-home training, participants completed a post-CWMT neurocognitive evaluation including near-and far-transfer tasks as well as questionnaires assessing domains of quality of life including subjective cognitive concerns and psychological symptoms. The same outcome measures were completed at 3- and 6-months post-CWMT. Safety, feasibility, and acceptability, as well as satisfaction were determined throughout the entire study period, from baseline to 6-month follow-up. Statistical analyses to estimate effect sizes were conducted only from baseline to post-CWMT, as this was a proof-of-concept study.
Participants
Inclusion criteria:
Participants were patients at an NCI-designated cancer center who met the following criteria: (1) glioma diagnosis confirmed by histopathology report or medical neuro-oncology team documentation; (2) able to read and understand English; (3) age ≥ 18 years; (4) internet access; (5) completed radiation treatment; and (6) demonstrate either objective or subjective deficits in working memory. Objective deficits were defined as ≥ 1 SD below their estimated intelligence—as measured by Advanced Clinical Solutions Test of Premorbid Functioning (TOPF) [27]—on the Digit Span subtest of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [28]. This method was used to account for deterioration relative to pre-tumor functioning, rather than using a cutpoint based on established norms which can fail to capture declines in functioning for those with high or low average estimated intelligence. Subjective deficits were defined by an age-adjusted T-score ≥ 65 on the Working Memory subscale of the Behavioral Rating Inventory of Executive Function—Adult (BRIEF-A) [29]. There is precedence for these inclusion criteria in previous cognitive rehabilitation trials with glioma patients [30]—as they exhibit both objective [8] and subjective neurocognitive dysfunction in the domain of working memory [13]. If interested participants did not have a computer or tablet device, an iPad was loaned to them for the duration of CWMT. Chemotherapy treatment and tumor progression did not exclude participation as we were interested in the feasibility and acceptability of CWMT for our patients, many of whom remain on continuous chemotherapy and experience progression within a year of diagnosis.
Exclusion criteria:
Individuals were ineligible if: (1) their cognitive/functional status was deemed impaired during the eligibility neuropsychological evaluation (e.g., dementia, aphasia); (2) their estimated intelligence was < 75; or (3) they were unable to understand and provide informed consent.
Measures
Safety was measured as the number of adverse events (AE). AES were defined as any event that occurred over the course of the study protocol that led to physical or emotional deterioration (see Table 1). Once recorded, the AE was investigated to determine whether it could be attributed to CWMT or the study procedures [31].
Table 1.
Feasibility and acceptability evaluation metrics
| Domain | Metric |
|---|---|
| Safety | • Adverse event reporting |
| Recruitment feasibility | • Eligibility rate • Rates and reasons for ineligibility • Eligibility based on objective v. subjective working memory impairment • Enrollment rate |
| Acceptability of procedures | • Retention rates of the intention-to-treat sample, ≥70% was deemed acceptable • Differences in demographic, medical, and baseline measures between treatment completers and dropouts |
| Intervention acceptability | • Retention rates of treatment initiators, ≥70% was deemed acceptable • Reason for dropouts • Per protocol intervention adherence, completing 5 sessions per week for 5 weeks • Reported challenges/diffculties throughout the intervention • Intervention satisfaction survey |
Feasibility and acceptability were operationalized based on guidelines for Phase IIa proof-of-concept trials and previously published studies adhering to these recommendations, see Table 1 [26, 32–34].
Near transfer outcomes, were scores on neurocognitive tests measuring working memory. Far transfer outcomes were scores on tests of divided attention and delayed memory. Quality of life was measured using subjective working memory impairment and self-report surveys of psychiatric distress. See Table 2 for outcome measure details.
Table 2.
Outcome measures
| Measure/activity | Construct assessed | Method | Scoring |
|---|---|---|---|
| RBANS - Digit Span | Working memory screener | In-person test | RBANS age-corrected norms [28] |
| WAIS-IV-Digit Span | Auditory working memory | In-person test | WAIS-IV age-corrected norms [43] |
| WMS-IV - Symbol Span | Visual working memory | In-person test | WMS-IV age-corrected norms [44] |
| Trail Making Test B | Task switching and Processing speed | In-person test | Halstead-Reitan age, gender, and race-corrected norms [45] |
| RBANS - DM | Verbal and visual delayed memory | In-person test | RBANS scoring manual [28] |
| BRIEF-A WM | Subjective working memory | Survey | BRIEF-A age-corrected norms [29] |
| PHQ-9 | Depressive symptoms | Survey | Raw scores [46–49] |
| BDI-II | Depressive symptoms | Survey | Raw scores [50] |
| GAD-7 | Generalized anxiety symptoms | Survey | Raw scores [47] |
| BAI | Anxiety symptoms | Survey | Raw scores [51] |
RBANS Repeatable Battery for the Assessment of Neuropsychological Status, WAIS-IV Wechsler Adult Intelligence Scale – 4th Edition, WMS-IV Wechsler Memory Scale – 4th Edition, DM Delayed Memory Index; BRIEF-A WM Behavior Rating Inventory of Executive Function – Adult Working Memory subscale, PHQ-9 Patients Health Questionnaire – 9-item, BDI-II Beck Depression Inventory – 2nd Edition, GAD-7 Generalized Anxiety Disorder – 7-item, BAI Beck Anxiety Inventory, BL Baseline, Post Post-CWMT, 3 month 3 month follow-up from post-CWMT, 6 month Follow-up from post CWMT
Data analytic plan
To determine whether patients were eligible based on objective dysfunction, TOPF and Digit Span subtest scores on the RBANS were converted to z-scores, and the procedure described above was followed. To explore safety, feasibility, and acceptability, descriptive statistics and independent samples t-tests were used. To investigate preliminary emcacy, repeated measures ANOVAs of within-group change from baseline to post-CWMT were conducted on near- and far-transfer measures as well as subjective working memory impairment and psychiatric distress. No within-group analyses were completed for the three- and six-month follow-up time points as this was a preliminary study. Partial eta squared statistics were used to estimate effect sizes such tha t ≥0.02 = small, ≥0.13 = medium, and ≥0.26 = large effect. If variables had a non-normal distribution, the Wilcoxon ranksum test was used to assess baseline to post-intervention changes. Effect size was estimated by calculating the r statistic, with ≥0.10 = small, ≥0.30 = medium, and ≥0.5 = large effect. Given the preliminary nature of these analyses, no corrections for multiple comparisons were made.
Results
A total of 20 participants were enrolled and completed baseline evaluations. Participants were primarily female (60%), White (95%), an average of 47 years old (SD= 12.11) and with an average of 16.21 years (SD = 2.23) of education. Most participants were diagnosed with either an oligoden-droglioma (45%) or a glioblastoma multiforme (30%). Half the sample was off treatment at baseline (50%) and most had no history of progression (85%) at the time of enrollment. Full demographic and medical information is provided in Supplemental Table.
Safety
Reported AES included 4 hospitalizations, 1 seizure, and 6 reports of increased fatigue. Upon consultation with the neuro-oncology team following each incident, it was determined that disease progression or worsening disease burden, instead of CWMT or study protocol, were responsible for all AEs.
Feasibility of recruitment
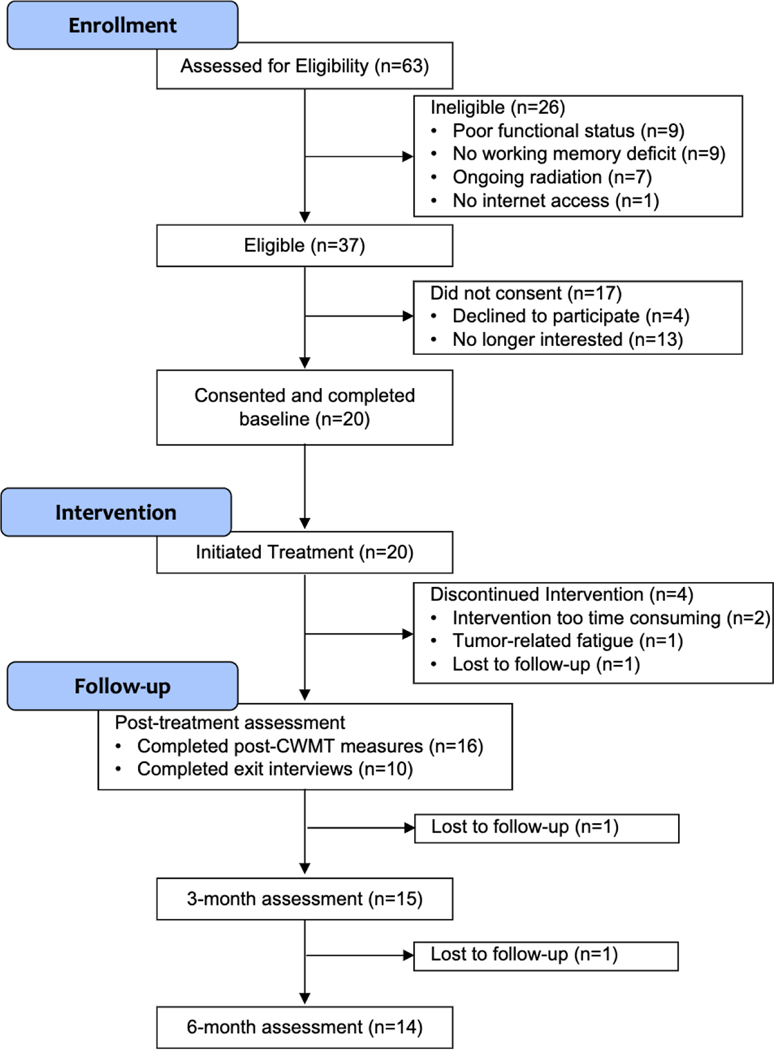
Recruitment occurred from October 2017 to May 2019. A total of 63 patients were screened, with 37 meeting study eligibility criteria (59% eligibility rate). Of these, 9 (23%) met criteria based on objective working memory impairment, 18 (49%) met criteria based on subjective impairment and 10 (27%) met criteria based on both. Of the 37 eligible individuals referred to the study, 20 consented to participate, resulting in a 54% enrollment rate (see Fig. 1); four met criteria for objective working memory deficits (20%), 11 for subjective working memory deficits (55%), and 5 for both (25%). A chi-square test compared the differences between enrollment status (enrolled or not) by objective versus subjective working memory impairment. Those who met criteria for both were excluded for this analysis. There was no significant difference χ2(1) = 1.3, p = 0.26.
Fig. 1.
CONSORT flow diagram; CWMT CogMed Working Memory Training
Acceptability of study procedures
Retention was 80% at post-CWMT assessment (16/20), 75% at 3-month follow-up (15/20), and 70% at 6-month follow-up (14/20). The two patients who dropped out in the follow-up period cited disease burden (e.g., fatigue) that would preclude cognitive testing. Compared to treatment completers, those who dropped out had higher self-reported depression [BDI-II: t(19)= –2.65, p = 0.02] and anxiety [GAD-7: [t(18)= – 2.46, p = 0.03]; no other differences were found (Supplemental Table).
Acceptability of the intervention
All patients who enrolled in the study completed at least one CWMT training session, for a total of 20 treatment initiators; 16 completed the intervention for an 80% treatment retention rate. Reasons for dropout included time commitment too great given other responsibilities of work and family (n = 2), brain tumor treatment-related fatigue (n = 1) and loss to follow-up (n = 1). Of the 16 treatment completers, 11 (68.8%) completed CWMT per protocol. Those who did not complete per protocol all eventually completed, but did so over a longer period of time than the requisite 5 weeks (ranging from 6 to 11 weeks). Reasons for nonadherence included self-reported brain tumor disease burden (n = 2), too time intensive (n = 2), and technological diffculties specific to the CWMT platform (n = 1).
Two patients dropped out during the follow-up period. Of those 14 patients who completed all study procedures, 10 responded to the investigator’s inquiry about their satisfaction with the intervention. Nine out of 10 reported benefit from the training (YIN), with an average rating of 6.7 out of 10 (1 = no benefit and the most benefit; mode = 5). Eight out of 10 patients reported benefit to their working memory skills specifically (YIN), with an average rating of 5.8/10 (mode = 5). Eight out of 10 patients reported they would do the process again, and 9 out of 10 would recommend CWMT to someone else.
Preliminary effects of CWMT
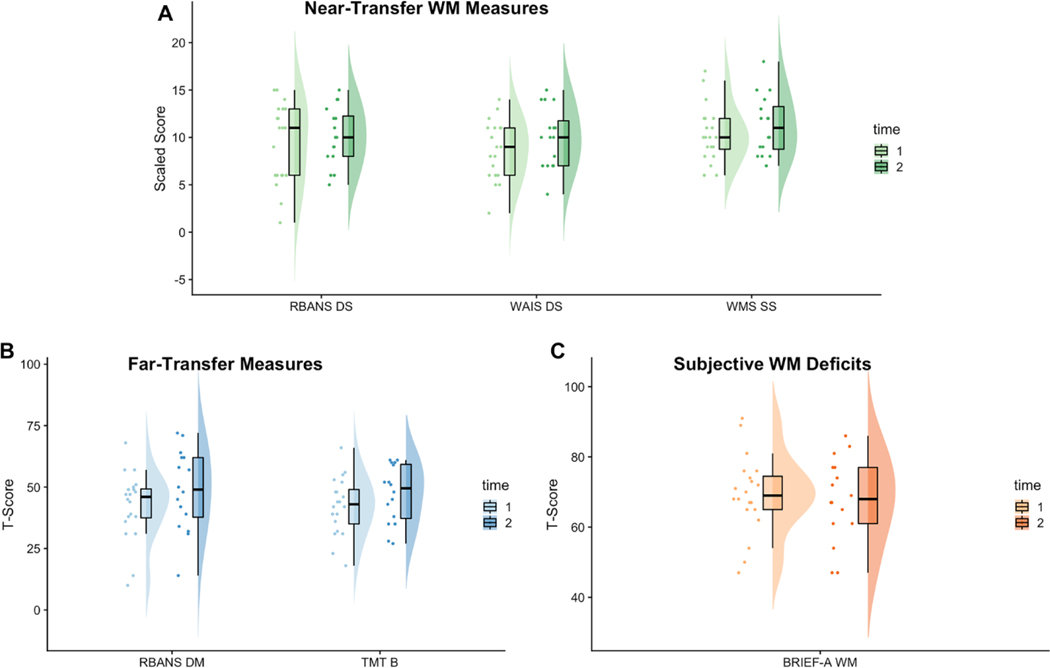
Distributions of scores on near-transfer, far-transfer, and subjective working memory measures at baseline and post-CWMT are depicted in Fig. 2 and results of statistical comparisons are presented in Table 3. For near-transfer measures, there were medium to large effects and significant increases on WAIS Digit Span (ηp2 = 0.35, p = 0.01) and WMS Symbol Span (ηp2 = 0.25, p = 0.04) from baseline to post-CWMT. There was a small effect and no significant change on RBANS Digit Span (ηp2 = 0.05, p = 0.40). With respect to far-transfer measures, there were medium effects but no statistically significant increases on TMT-B (ηp2 = 0.20, p = 0.07) and RBANS-DM (ηp2 = 0.16, p = 0.12). There were no changes on subjective working memory (i.e., BRIEF-A Working Memory; ηp2 = 0.01, p = 0.79). For measures of psychological distress, there was a small but nonsignificant effect of improvement on PHQ-9 (ηp2 = 0.06, p = 0.35) and BDI-II (ηp2 = 0.03, p = 0.55). There was no effect on GAD-7 (r = 0.08, p = 0.75) or BAI (r=0.08, p = 0.75).
Fig. 2.
Scores on Outcome Measures from Baseline to Post-CWMT. Raincloud plots depict the distribution of scores on near-transfer (A, green), far-transfer (B, blue), and subjective working memory (C, orange) measures from baseline assessment (time 1, lighter shade) to post-CWMT (time 2, darker shade). Points represent individual patient scores and boxplots show the median and quartiles. RBANS DS RBANS Digit Span subtest, WAIS DS WAIS-IV Digit Span subtest, WMS SS WMS-IV Symbol span, RBANS DM RBANS Delayed Memory subtest, TMT B Trail Making Test B, BRIEF-A WM BRIEF-A Working Memory. †p<0.01, *p <0.05, **p<0.01
Table 3.
Analyses of change from baseline to post-CWMT (n = 16)
| Outcome variable | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline M (SD) | Post-CWMT M (SD) | F | P | ηp2 | 3 mos M (SD) | 6 mos M (SD) | |
| Near-transfer | |||||||
| RBANS Digit Span | 9.40 (4.19) | 10.13 (3.07) | 0.77 | 0.40 | 0.05 | 10.53 (3.02) | 10.21 (4.08) |
| WAIS Digit Span | 8.70 (3.08) | 10.00 (3.18) | 8.18 | 0.01* | 0.35 | 10.80 (2.86) | 10.07 (2.99) |
| WMS Symbol Span | 10.35 (2.94) | 11.25 (3.15) | 5.00 | 0.04* | 0.25 | 10.80(2.18) | 10.00 (2.83) |
| Far-transfer | |||||||
| TMT-B | 42.25 (11.68) | 47.19 (11.81) | 3.81 | 0.07 | 0.20 | 46.73 (8.01) | 46.29 (10.68) |
| RBANS-DM | 92.35 (13.64) | 98.81 (16.23) | 2.79 | 0.12 | 0.16 | 93.40 (13.89) | 95.93 (18.63) |
| Subjective executive dysfunction | |||||||
| BRIEF- working memory | 69.30 (11.09) | 68.00 (11.89) | 0.07 | 0.79 | 0.01 | 61.47 (14.28) | 64.38 (14.23) |
| Psychiatric distress | |||||||
| PHQ-9 | 6.63 (4.79) | 3.81 (5.00) | 0.93 | 0.35 | 0.06 | 5.53 (3.76) | 5.50 (3.66) |
| BDI-II | 11.60 (6.44) | 9.27 (4.73) | 0.38 | 0.55 | 0.03 | 7.53 (4.12) | 8.25 (4.39) |
| Z | p | r | |||||
| GAD-7 | 5.68 (4.91) | 4.63 (3.44) | −0.46 | 0.65 | 0.12 | 3.93 (3.26) | 3.67 (3.47) |
| BAI | 8.95 (7.04) | 8.07 (6.77) | −0.32 | 0.75 | 0.08 | 4.80 (5.12) | 7.46 (3.99) |
Repeated measure ANOVAs only conducted on baseline to post-CWMT. BRIEF Behavior Rating Inventory of Executive Functioning, RBANS Repeatable Battery for the Assessment of Neuropsychological Status, TMT-B Trail Making Test B, WAIS Weschler Adult Intelligence Scale, WMS Weschler Memory Scale, PHQ-9 Patient Health Questionnaire – 9, GAD-7 Generalized Anxiety Disorder – 7, BDI-II Beck Depression Inventory, BAI Beck Anxiety Inventory
Discussion
This was the first evaluation of safety, feasibility, and acceptability of CWMT in adult patients with gliomas. We also investigated emcacy on near- and far-transfer neurocognitive tasks and measures of quality of life to estimate preliminary effect sizes for future randomized controlled trials.
With respect to safety, no adverse events were deemed to be study-related. Therefore, CWMT was found to be safe for these patients, even for those with a notable history of seizure. This finding is consistent with prior research showing CWMT to be safe for cancer patients with seizure history [17], though individuals with photosensitive seizures should continue to be excluded from future studies.
Nearly half of the adult patients with glioma seen for standard-of-care neuropsychological evaluation were eligible for participation in this study. The most common reason for ineligibility was no working memory impairment. Among those who enrolled, a higher percentage demonstrated subjective (55%) rather than objective working memory impairment (20%). Considered together, this suggests that limiting eligibility criteria to objective working memory deficits would yield a much smaller sample of potential participants. Furthermore, subjective working memory impairments may increase motivation to enroll in CWMT or, alternatively, objective working memory impairments may reduce ability to participate in CWMT. More research on reasons for declining participation as well as differences in eligibility on enrollment, retention, and adherence could help to clarify these possibilities. Ultimately, over half (54%) of those who met criteria consented to participate, demonstrating a relatively high enrollment rate for a neuro-oncology sample [30, 35], which suggests that the eligibility requirements and demand for CWMT may be adequate for larger scale trials.
Procedures were found to be acceptable based on dropout rates at each endpoint. There were no differences on sample characteristics or neurocognitive functioning between dropouts and treatment completers. However, dropouts reported higher levels of depression and anxiety symptoms. This is not surprising given the burden inherent in CWMT adherence, which may prevent those with psychological distress from engaging in the intervention. Therefore, the current procedures can be considered acceptable in those without significant psychological distress. In the presence of such distress, it may be appropriate to recommend psychotherapy prior to recommending CWMT.
Overall, results support the acceptability of CWMT for patients with glioma. There was an adequate retention rate of 80% at the end of CWMT, comparable to other clinical trials of cognitive rehabilitation programs in adults with gliomas [36, 37]. Ten percent of the treatment-initiator dropouts did so due to time burden, and 31 % of treatment completers were unable to adhere to the CWMT procedures largely due to a combination of high intervention burden and high disease burden. Taken together, it may be worth considering a less burdensome CWMT protocol, perhaps investigating a less time intensive intervention with respect to both frequency and length of training sessions. Individuals with high disease burden (e.g., progression, initiation of new treatments) may want to delay initiation of the intervention protocol. Technological issues were reported by almost one-third of the treatment completers and should be explored further in future trials, including the cause (e.g., user error or platform related) and possible solutions.
Satisfaction surveys revealed that, on forced yes–no questions, patients were willing to complete the training program again (80%) and would recommend it to others (90%). However, when asked to rate the degree of benefit, it was only moderate. In combination with the reasons cited for drop out (e.g., high burden, fatigue), diffculty keeping pace with treatment protocol, and feedback given by participants throughout the intervention, adaptations are likely necessary to further improve perceived benefit. It is possible that social desirability may have inflated satisfaction ratings as these were administered over the phone and not in a written self-report survey. It could also be that while individuals may appreciate engaging in strategies to improve their cognitive functioning, the intervention as implemented is too burden-some, thus contributing to lower perceived benefit.
Preliminary emcacy was assessed for near- and far-transfer neurocognitive outcomes. Two of the three near-transfer tasks demonstrated improvements following CWMT with medium to large effect sizes. However, there was a small effect and no significant change on the RBANS Digit Span, one of the near-transfer tasks. This may represent a test-specific issue, given that the RBANS is a screener and may not be comprehensive enough to capture statistical change due to a ceiling effect. Taken together, our results provide evidence in line with previous literature on CWMT’s potential to improve near-transfer tasks [17, 19, 21]. The medium effect size found for far-transfer outcomes is particularly promising given previous mixed results in non-glioma samples. Our results demonstrated improvement on divided attention and delayed memory, important abilities for daily functioning and maintained independence (e.g., management of finances). The near- and far-transfer benefits of CWMT for neurocognitive abilities are observable in the shifting distribution of scores (Fig. 2), despite the small sample in this proof-of-concept study. These findings support future evaluation of near- and far-transfer outcomes following CWMT in patients with glioma and encourage investigation of ecologically valid measures of cognition. Future research should also consider the potential effects of disease burden on intervention efficacy (e.g., tumor grade, disease progression, treatment regimen). While not assessed within this study, given that patients with high grade gliomas often undergo continuous treatment, assessing the role of these variables is necessary for future trials.
There was no evidence of improvement in subjective working memory following CWMT, which is surprising considering the high rates of subjective working memory impairment at baseline coupled with observed working memory improvements on neurocognitive tests. It may be tempting to interpret this as a function of low insight; however, research on subjective executive dysfunction in neuro-oncology patients has consistently demonstrated preserved insight based on patient-informant agreement [38–40]. The lack of improvement on subjective working memory impairment is in alignment with the neutral rating on perceived benefit from CWMT. Our findings also demonstrated little-to-no effect of CWMT on self-reported measures of psychological distress. Taken together, it may be that CWMT successfully trains aspects of neurocognitive function but these improvements did not transfer to improved patient-reported outcomes. This could be due to intervention burden masking the positive effects on cognition. That is, it may be that despite successfully training on neurocognitive outcomes, the intensity of the intervention protocol coupled with glioma treatment and fatigue outweighed the potential benefit on quality of life. These findings contribute to the conclusion that despite some documented feasibility and acceptability along with promising effects on cognition, future work should investigate less demanding intervention protocols for improving quality of life.
This study has several limitations. First, the RBANS Digit Span subtest was used to determine objective working memory impairment; the RBANS subtests have demonstrated inferior psychometric properties when compared to more comprehensive neuropsychological measures, thus this may have affected recruitment feasibility as well as sample characteristics in unknown ways. Nevertheless, the method of comparing performance on the RBANS Digit Span to estimated intelligence to determine eligibility may have addressed the pathognomic tendency of the RBANS [41]. Second, in accordance with the ORBIT model, this phase IIa proof-of-concept did not include a comparison group (e.g., active or wait-list control) and findings are preliminary. Another limitation pertains to non-specific intervention elements which may contribute to outcomes: in the current study, CWMT coaching was used at study initiation and participant non-adherence. It is possible that the frequent communication and attention from study-team members may represent an unaccounted-for active element, with the potential to influence feasibility and acceptability outcomes. However, CWMT coaching may be difficult for many neuro-oncology programs to provide and could prevent successful dissemination and implementation. Additionally, the satisfaction surveys were not completed by those who dropped out, which likely skewed the results in a positive direction. Finally, the majority of the sample were women despite the majority of glioma patients being men [42], reducing the generalizability of the sample.
In conclusion, CWMT was safe for adults with glioma. Recruitment was feasible, largely due to the addition of subjective working memory complaints as eligibility criteria. Study procedures were acceptable for patients with minimal-to-mild psychological distress. Importantly, intervention burden and technological challenges were recorded in a small, but substantial, subset. Satisfaction may have been dampened by intervention burden with only moderate perceived benefit. Finally, moderate-to-large effects were found on near- and far-transfer neurocognitive tasks. However, this did not extend to patient-reported outcome measures, which showed little-to-no effect. Future studies should consider investigating less time-intensive CWMT protocols to accommodate the needs of adults with gliomas.
Supplementary Material
Acknowledgements
Thank you to all of our patients. We would also ike to thank our research assistants Kelcie Willis and Julia Brechbiel for their contributions to this project
Funding VCU School of Medicine Deans Academic Enhancement Award. The project described was also supported by CTSA award No KL2TR002648 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the omcial views of the National Institutes of Health.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11060-021-03839-y.
Declarations
Conflict of interest The authors have no conflict of interests to disclose.
Data availability The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical approval Approval was obtained from the ethics committee of Virginia Commonwealth University and Massey Cancer Center. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate Informed consent was obtained from all individual participants included in the study.
References
- 1.Tucha O, Smely C, Preier M, Lange KW (2000) Cognitive deficit before treatment among patients with brain tumors. Neurosurgery 47:324–333. 10.1097/00006123-200008000-00011 (discussion 333—334) [DOI] [PubMed] [Google Scholar]
- 2.Zucchella C, Bartolo M, Di Lorenzo C et al. (2013) Cognitive impairment in primary brain tumors outpatients: a prospective cross-sectional survey. J Neurooncol 112:455–460 [DOI] [PubMed] [Google Scholar]
- 3.Meyers CA, Hess KR, Yung WK, Levin VA (2000) Cognitive function as a predictor of survival in patients with recurrent malig-nant glioma. J Clin Oncol 18:646–650. 10.1200/jco.2000.18.3.646 [DOI] [PubMed] [Google Scholar]
- 4.Noll KR, Ziu M, Weinberg JS, Wefel JS (2016) Neurocognitive functioning in patients with glioma of the left and right temporal lobes. J Neurooncol 128:323–331. 10.1007/s11060-016-2114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAleer MF, Brown PD (2015) Neurocognitive function following therapy for low-grade gliomas. Semin Radiat Oncol 25:210–218. 10.1016/j.semradonc.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 6.Pendergrass C, Targum S, Harrison J (2018) Cognitive impairment associated with Cancer: A brief review. Innov Clin Neurosci 15:36–44 [PMC free article] [PubMed] [Google Scholar]
- 7.Teixidor P, Gatignol P, Leroy M et al. (2007) Assessment of verbal working memory before and after surgery for low-grade glioma. J Neurooncol 81:305–313. 10.1007/s11060-006-9233-y [DOI] [PubMed] [Google Scholar]
- 8.Gehrke AK, Baisley MC, Sonck ALB et al. (2013) Neurocognitive deficits following primary brain tumor treatment: systematic review of a decade of comparative studies. J Neurooncol 115:135–142 [DOI] [PubMed] [Google Scholar]
- 9.Cowan N (2014) Working memory underpins cognitive development, learning, and education. Educ Psychol Rev 26:197–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groves NB, Kofler MJ, Wells EL et al. (2020) An examination of relations among working memory, ADHD symptoms, and emotion regulation. J Abnorm Child Psychol 48:525–537. 10.1007/s10802-019-00612-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mlinac ME, Feng MC (2016) Assessment of activities of daily living, self-care, and independence. Arch Clin Neuropsychol 31 :506–516. 10.1093/arclin/acw049 [DOI] [PubMed] [Google Scholar]
- 12.Schagen SB, Klein M, Reijneveld JC et al. (2014) Monitoring and optimising cognitive function in cancer patients: present knowledge and future directions. Eur J Cancer Suppl 12:29–40. 10.1016/j.ejcsup.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loughan AR, Braun SE, Lanoye A (2019) Executive dysfunction in neuro-oncology: behavior rating inventory of executive function in adult primary brain tumor patients. Appl Neuropsychol Adult. 10.1080/23279095.2018.1553175 [DOI] [PubMed]
- 14.Loughan AR, Allen DH, Baumstarck K et al. (2018) Quality of life in neuro-oncology. Handb Brain Tumor Chemother Mol Ther Immunother. 10.1016/B978-O-12-812100-9.00061-9 [DOI]
- 15.Klingberg T, Fernell E, Olesen PJ et al. (2005) Computerized training of working memory in children with ADHD - a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 44:177–186. 10.1097/00004583-200502000-00010 [DOI] [PubMed] [Google Scholar]
- 16.Björkdahl A, Åkerlund E, Svensson S, Esbjörnsson E (2013) A randomized study of computerized working memory training and effects on functioning in everyday life for patients with brain injury. Brain Inj 27:1658–1665. 10.3109/02699052.2013.830196 [DOI] [PubMed] [Google Scholar]
- 17.Hardy KK, Willard VW, Allen TM, Bonner MJ (2013) Working memory training in survivors of pediatric cancer: a randomized pilot study. Psychooncology 22:1856–1865. 10.1002/pon.3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raskin S (2011) Neuroplasticity and rehabilitation. Guilford Publications [Google Scholar]
- 19.Aksayli ND, Sala G, Gobet F (2019) The cognitive and academic benefits of Cogmed: a meta-analysis. Educ Res Rev 27:229–243 [Google Scholar]
- 20.Sacks-Zimmerman A, Duggal D, Liberta T (2015) Cognitive remediation therapy for braintumor survivors with cognitive deficits. Cureus. 10.7759/cureus.350 [DOI] [PMC free article] [PubMed]
- 21.Liberta TA, Kagiwada M, Ho K et al. (2020) An investigation of COGMED working memory training for neurological surgery patients. Interdiscip Neurosurg Adv Tech Case Manag. 10.1016/j.inat.2020.100786 [DOI]
- 22.Poleon S, Szaflarski JP (2017) Targeted review photosensitivity in generalized epilepsies f. 10.1016/j.yebeh.2016.10.040 [DOI] [PubMed]
- 23.Vecht CJ, Kerkhof M, Duran-Pena A (2014) Seizure prognosis in brain tumors: new insights and evidence-based management.Oncologist 19:751–759. 10.1634/theoncologist.2014-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boele FW, Zant M, Heine ECE et al. (2014) The association between cognitive functioning and health-related quality of life in low-grade glioma patients. Neuro-oncology. 10.1093/nop/npu007 [DOI] [PMC free article] [PubMed]
- 25.Habets EJJ, Kloet A, Walchenbach R et al. (2014) Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir (Wien) 156:1451–1459. 10.1007/s00701-014-2115-8 [DOI] [PubMed] [Google Scholar]
- 26.Czajkowski SM, Powell LH, Adler N et al. (2015) From ideas to emcacy: The ORBIT model for developing behavioral treatments for chronic diseases. Heal Psychol 34:971–982. 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson (2009) Advanced Clinical Solutions for WAIS-IV and WMS-IV
- 28.Randolph C (2009) RBANS Update Repeatable Battery for the Assessment of Neuropsychological Status: Manual. Pearson [Google Scholar]
- 29.Roth RM, Isquith PM, Giola GA (2005) BRIEF—A: behavior rating inventory of executive function— adult version. Psychological Assessment Resources, Lutz, FL [Google Scholar]
- 30.Richard NM, Bernstein LJ, Mason WP et al. (2019) Cognitive rehabilitation for executive dysfunction in brain tumor patients: a pilot randomized controlled trial. J Neurooncol 142:565–575. 10.1007/s11060-019-03130-1 [DOI] [PubMed] [Google Scholar]
- 31.Peterson AL, Roache JD, Raj J, Young-McCaughan S (2013) The need for expanded monitoring of adverse events in behavioral health clinical trials. Contemp Clin Trials. 10.1016/j.cct.2012.10.009 [DOI] [PubMed]
- 32.Lancaster GA, Dodd S, Williamson PR (2004) Design and analysis of pilot studies: recommendations for good practice. J Eval [DOI] [PubMed]
- 33.Leon AC, Davis LL, Kraemer HC (2011) The role and interpretation of pilot studies in clinical research. J Psychiatr Res 45:626–629. 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vranceanu A-M, Jacobs C, Lin A et al. (2019) Results of a feasibility randomized controlled trial (RCT) of the Toolkit for Optimal Recovery (TOR): a live video program to prevent chronic pain in at-risk adults with orthopedic injuries. Pilot feasibility Stud 5:30. 10.1186/s40814-019-0416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassler MR, Elandt K, Preusser M et al. (2010) Neurocognitive training in patients with high-grade glioma: a pilot study. J Neurooncol 97: 109–115. 10.1007/s11060-009-0006-2 [DOI] [PubMed] [Google Scholar]
- 36..Gehring K, Sitskoorn MM, Gundy CM et al. (2009) Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol 27:3712–3722. 10.1200/JCO.2008.20.5765 [DOI] [PubMed] [Google Scholar]
- 37.Lonkhuizen PJC, Klaver KM, Wefel JS et al. (2019) Interventions for cognitive problems in adults with brain cancer: a narrative 13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agar M, Koh ES, Gibbs E et al. (2016) Validating self-report and proxy reports of the Dexamethasone Symptom Questionnaire -Chronic for the evaluation of longer-term corticosteroid toxicity. Support Care Cancer. 10.1007/s00520-015-2897-O [DOI] [PubMed]
- 39.Cantisano N, Menei P, Roualdes V et al. (2020) Patient-reported functional executive challenges and caregiver confirmation in adult brain tumor survivors. J Cancer Surviv. 10.1007/s1764-020-00961-0 [DOI] [PubMed]
- 40.van der Linden SD, Gehring K, De Baene W et al. (2020) Assessment of executive functioning in patients with meningioma and low-grade glioma: a comparison of self-report, proxy-report, and test performance. J Int Neuropsychol Soc. 10.1017/S1355617719001164 [DOI] [PubMed]
- 41.Duff K, Schoenberg MR, Mold JW et al. (2007) Normative and retest data on the RBANS cortical/subcortical index in older adults. J Clin Exp Neuropsychol. 10.1080/13803390601147629 [DOI] [PubMed]
- 42.Krogh Rasmussen B, Hansen S, Laursen RJ et al. (2017) Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I-IV in the the Danish Neuro-Oncology Registry. J Neurooncol 135:571–579. 10.1007/s11060-017-2607-5 [DOI] [PubMed] [Google Scholar]
- 43.Sattler J, Ryan J (2009) Assessment with the WAIS-IV
- 44.Drozdick L, Holdnack J, Hilsabeck R (2011) Essentials of WMS-IV assessment
- 45.Heaton RK, Miller S, Taylor M, Grant I (2004) Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults scoring programs
- 46.Kroenke K, Spitzer RL, Williams JBW (2001) The PHQ-9. J Gen Intern Med 16:605–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spitzer RL, Kroenke K, Williams JBW, Löwe B (2006) A brief measure for assessing generalized anxiety disorder. Arch Intern Med 166:1092. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 48.Beck AT, Steer RA, Brown GK (1996) BDI-II, Beck depression inventory : manual, Second edition
- 49.Wilson KA, de Beurs E, Palmer CA, Chambless DL (1999) Beck Anxiety Inventory. In: Maruish ME (ed) The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers, Mahwah, NJ, US [Google Scholar]
- 50.Beck AT, Steer RA, Brown GK (1996) Manual for the beck depression inventory-second edition. The Psychological Corporation, San Antonio, TX [Google Scholar]
- 51.Beck AT, Steer RA (1993) BAI, Beck anxiety inventory: manual, 1993rd edn. Psychological Corporation, San Antonio [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.