Abstract
To determine whether an ongoing response to Leishmania major would affect the response to a non-cross-reacting, non-leishmanial antigen, susceptible BALB/c mice and resistant C3H mice were infected with L. major parasites expressing Escherichia coli β-galactosidase (β-GAL); this parasite was designated L. major-βGAL. BALB/c and C3H mice responded to infection with L. major-βGAL by mounting a CD4 T-cell response to both parasite antigens and to the reporter antigen, β-GAL. The phenotypes of these T cells were characterized after generating T-cell lines from infected mice. As expected, BALB/c mice responded to infection with L. major-βGAL by producing interleukin 4 in response to the parasite and C3H mice produced gamma interferon (IFN-γ) in response to the parasite and β-GAL. Interestingly, however, BALB/c mice produced IFN-γ in response to β-GAL. Taken together, these results demonstrate that priming of IFN-γ-producing cells can occur in BALB/c mice despite the fact the animals are simultaneously mounting a potent Th2 response to L. major.
The two major subsets of the CD4 T-cell compartment of mice, Th1 and Th2, produce distinct repertoires of cytokines. For example, Th1 cells secrete gamma interferon (IFN-γ) and interleukin 2 (IL-2) while Th2 cells secrete IL-4 and IL-5 (23). The functions of Th1 and Th2 cells are modulated by the reciprocal cross-regulatory properties of these cytokines. Moreover, other cells of the immune system, such as macrophages (Mφs), produce cytokines (e.g., IL-10, IL-12, and transforming growth factor β) that can influence the activities of T cells. Infection of mice with Leishmania major is perhaps the best-studied example of a disease in which selective activation of Th cells leads to opposite outcomes of infection. Most mouse strains (e.g., C57BL/6 and C3H) mount a Th1 response to the parasite and cure the infection, whereas susceptible mice (e.g., BALB/c) mount a Th2 response and succumb to infection (4, 20, 24, 25, 32).
These observations point to an interesting question regarding immunoregulation. How might a heavily skewed Th1 or Th2 parasite-specific response influence the concomitant response of T cells to a protein that was antigenically unrelated to Leishmania?
To address this question, we utilized techniques developed for introducing and expressing foreign genes in L. major (7). Using this approach, we expressed a bacterial antigen, namely Escherichia coli β-galactosidase (β-GAL), in L. major and then infected mice with this transfected parasite, which was designated L. major-β-GAL. Since the expressed β-GAL would be targeted to the same phagolysosome as L. major and be exposed to the same set of phagolysosomal degradative enzymes, it could serve as a “reporter antigen” to determine how a Th1 or Th2 response to L. major influences the immune response to β-GAL. Therefore, we followed the T-cell response to both L. major and β-GAL in mice infected with L. major-βGAL. We anticipated that the response to β-GAL would be type 1 in mice mounting a Th1 response to L. major, whereas it would be type 2 in mice mounting a Th2 response to the parasite.
MATERIALS AND METHODS
Parasites.
L. major promastigotes (LV39/Neal/P strain, clone 5 [22]) were maintained in M199 or NNN medium as previously described (8, 22). For experiments, promastigotes were harvested from stationary phase cultures which contained the infective (metacyclic) form of the parasite (26). To produce amastigotes, promastigotes were injected subcutaneously (s.c.) into the shaved rumps of BALB/c mice or BALB/c nu/nu mice, and amastigotes were isolated according to published techniques (11).
Molecular constructs and transfection.
To express β-GAL within L. major, we used the vector pXG (strain B1288 [12]). The E. coli lacZ coding region was introduced into the pXG expression site, yielding the plasmid pXG-βGAL (strain B1007; L. Borges and S. M. Beverley, unpublished data). Parasites freshly recovered from infected mice were transfected with pXG-NEO or pXG-βGAL by electroporation, and clonal lines were obtained by plating on semisolid media as previously described (15). Transfected promastigotes were maintained in medium containing 10 μg of Geneticin (Sigma, St. Louis, Mo.) per ml. Several transfectants were inoculated into mice to confirm that they remained infective, and one each bearing one or the other plasmid and showing wild-type infectivity was identified and used in this work. Infective L. major promastigotes containing pXG-NEO were designated L. major-NEO while those containing pXG-βGAL were designated L. major-βGAL.
Determining β-GAL activity.
Pellets containing 2.5 × 107 parasites (promastigotes or amastigotes) were snap frozen in liquid nitrogen and then resuspended in 100 μl of TPI buffer (19) containing 0.01% sodium dodecyl sulfate (SDS). β-GAL activity was determined as previously described (19), taking care to ensure that the amount of extract and the time of the assay were in the linear range of the assay. Extract aliquots were diluted to 80 μl with TPI and added to 320 μl of reaction buffer containing 0.3 mM 4-methyl-umbelliferyl β-d-galactoside (Sigma). Aliquots of 40 μl were taken at 10-min intervals and added to 200 μl of stop buffer. The fluorescence of the 4-methylumbelliferone (4-MU) product was measured in a Bio-Rad Fluoromark microplate fluorometer and compared to that of known concentrations of 4-MU (Sigma). In these assays, 2,700 fluorescence units corresponds to 1 nmol of 4-MU product. Assays of purified E. coli β-GAL (Sigma) showed a specific activity of 1.5 × 108 fluorescence units/min/mg with the 4-MU-β-GAL substrate. All assay time points and samples were done in duplicate.
Reagents.
β-GAL was purchased from Sigma (G 6008) and dialyzed extensively against sterile double-distilled water before use. The protein content in the dialyzed preparation was determined by the micro bicinchoninic acid assay (Pierce, Rockford, Ill.), and then the preparation was aliquoted and stored at −70°C until use. Methyl[3H]thymidine (5 Ci/mmol) was purchased from Amersham (Arlington Heights, Ill.).
Infection and lymphocyte stimulation assay.
Mice were infected s.c. in one hind footpad with 2.5 × 106 L. major-NEO or L. major-βGAL amastigotes, in a final volume of 50 μl. At intervals thereafter, the draining popliteal and inguinal lymph node cells (LNC) were restimulated (6) in vitro (4 × 105/well) with L. major promastigotes (106/ml) or β-GAL (100 μg/ml) in Dulbecco's modified Eagle medium (DMEM) containing 0.5% normal mouse serum in 96-well flat-bottom plates (Costar, Cambridge, Mass.). After 5 days of culture (optimal time), LNC proliferation was measured by pulsing the plates with 1 μCi of [3H]thymidine per well, harvesting 24 h later on an automated sample harvester, and assaying the incorporated radioactivity by scintillation counting. Triplicate cultures were used in all experiments. In addition, supernatants were harvested at 48 h and assayed by capture enzyme-linked immunosorbent assays for their content of IFN-γ and IL-4 by published techniques (5, 6, 30).
Generating antigen-specific T-cell blasts and flow cytometry.
The LNC (5 × 106/ml) draining lesions on infected mice were cultured in 24-well flat-bottom plates (Costar) and stimulated with L. major (5 × 105/ml) promastigotes or β-GAL (100 μg/ml). The blast cells were isolated on Percoll gradients (29).
Methods used to analyze the cell surface phenotype of murine lymphocytes are described elsewhere (6, 30).
Activating β-GAL-specific T-cell hybridoma 1E3.03.H4 to produce IL-2.
The T-cell hybridoma 1E3.03.H4 (kindly provided by J. Langhorne, Imperial College, London, England, and I. Muller, University of Notre Dame, Notre Dame, Ind.) is I-Ad restricted and β-GAL specific. As a result, it produces IL-2 when activated by BALB/c Mφs presenting β-GAL (10). 1E3.03.H4 was maintained in DMEM containing 5% fetal calf serum.
To stimulate 1E3.03.H4, BALB/c starch-elicited peritoneal exudate cells (31) were cultured (DMEM plus 5% fetal calf serum) in 24-well plates (106 cells/well, 1 ml of total volume) overnight at 37°C. The wells were rinsed with warm DMEM to remove nonadherent cells. The remaining adherent cells (consisting of 95% Mφs [5]) were cultured for an additional 4 h with either medium alone or 100 U of IFN-γ (13) plus 10 ng of lipopolysaccharide (LPS)/ml (E. coli 055:B55W; Difco, Detroit, Mich.) (10 ng of LPS/ml is a subactivating dose [28]). Cultures were rinsed, and Mφs were cultured for another 4 h at 34°C with either β-GAL (50 μg/ml) or various forms of L. major (see text for details). Finally, the cultures were rinsed, 1E3.03.H4 cells were added (106 cells/well), and the cultures were incubated for 18 h. The supernatants of the cultures were collected and analyzed for their content of IL-2 by capture enzyme-linked immunosorbent assay using published techniques (5, 6, 30) as an indicator of the degree of activation of 1E3.03.H4.
RESULTS
Generating L. major expressing high levels of β-GAL throughout the infectious cycle.
We chose β-GAL as our reporter antigen since, when emulsified in complete Freund's adjuvant and injected into mice, β-GAL elicits a potent type 1 response (data not shown).
In previous studies, we utilized the pX expression vector to express high levels of β-GAL in L. major and Leishmania mexicana (18, 21). However, subsequent data suggested that β-GAL expression was down-regulated in lesion amastigotes (21; L. Borges and S. M. Beverley, unpublished data). In this work, we used a related expression vector, pXG-NEO, which yields consistently higher expression in the amastigote stage (L. Borges and S. M. Beverley, unpublished data).
We introduced pXG-NEO and pXG-βGAL into L. major promastigotes and identified infective clonal transfectants bearing a plasmid. Those bearing pXG-NEO were designated L. major-NEO while those containing pXG-βGAL were designated L. major-βGAL. L. major-βGAL promastigotes expressed high levels of β-GAL, 46 ng/106 cells (about 2% of the total cell protein). The β-GAL in these L. major-βGAL parasites was exclusively intracellular, as revealed by the absence of staining of colonies by the sensitive chromogenic substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; data not shown).
To confirm that the production of β-GAL remained high in the amastigote form of L. major, L. major-βGAL amastigotes were purified from cutaneous lesions of BALB/c mice 3 weeks after infection. These amastigotes showed high levels of β-GAL, 10 ng/106 cells, or about 22% of the level present in promastigotes when they were injected into mice. Preliminary studies suggest that the majority of the decrease relative to the levels in promastigotes arose from a decline in the episomal pXG-βGAL plasmid copy number during the period of growth in vivo, rather than specific developmental regulation of pXG-βGAL expression (data not shown). Thus, L. major-βGAL showed a sustained ability to express β-GAL throughout the parasite infectious cycle in mice infected with the parasite. As a result, L. major-βGAL was an ideal candidate for the experiments presented here.
L. major-NEO induces only a leishmanial-specific response, but L. major-βGAL induces both a leishmanial and β-GAL-specific response in BALB/c and C3H mice.
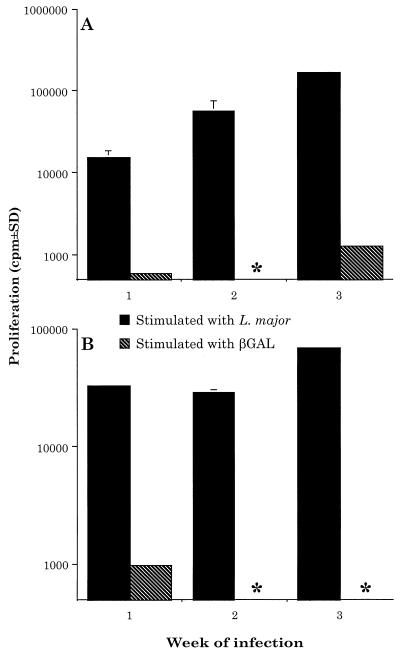
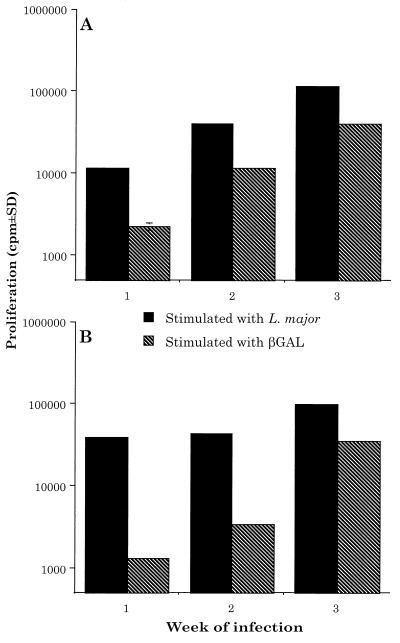
We chose to use BALB/c or C3H mice as our experimental model since they represent mice that are highly susceptible or highly resistant to infection with L. major, respectively (2, 27). These mice were infected with either 2.5 × 106 amastigotes of control parasites (L. major-NEO) or L. major-βGAL s.c. in one hind footpad. At various times thereafter, the draining LNC were removed and restimulated in vitro with either L. major or β-GAL. As determined in vitro, BALB/c and C3H mice responded to infection with L. major-NEO by generating a vigorous parasite-specific response, but little or no response to β-GAL (Fig. 1). In contrast, BALB/c and C3H mice responded to both L. major and β-GAL in vitro following infection with L. major-βGAL. At 3 weeks of infection, the β-GAL-specific response was approximately 40% of the response to the parasite (Fig. 2).
FIG. 1.
L. major-NEO induces only a leishmanial-specific response in BALB/c and C3H mice. BALB/c mice (A) and C3H mice (B) were injected with 2.5 × 106 L. major-NEO amastigotes s.c. in one hind footpad. At the indicated times thereafter, the draining LNC were plated at the rate of 4 × 105 per well. The LNC were stimulated with L. major (106/ml) or β-GAL (100 μg/ml). The degree of proliferation was assessed by scintillation counting; see Materials and Methods for detailed techniques. Results are from triplicate wells (means ± standard deviations) and are representative of three independent experiments. ∗, no proliferation detected.
FIG. 2.
L. major-βGAL induces both a leishmanial- and β-GAL-specific response in BALB/c and C3H mice. BALB/c mice (A) and C3H mice (B) were infected with 2.5 × 106 L. major-βGAL amastigotes, and the draining LNC were treated as described in the legend of Fig. 1. Results are representative of three independent experiments.
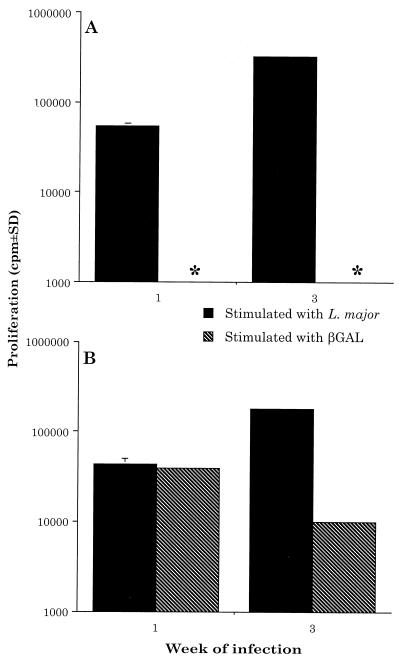
It should be noted that amastigotes were utilized for the experiments presented here since very few amastigotes, relative to promastigotes, are lysed when they are injected into mice (14). This approach minimized the possibility that β-GAL released from lysing L. major-βGAL promastigotes at the inception of the infection might have influenced our results. However, to directly test this possibility, 2.5 × 106 L. major-NEO control parasites were purposely contaminated with soluble β-GAL (100 μg), and these were coinjected s.c. into the footpads of BALB/c and C3H mice. At varying times thereafter, the draining LNC were removed and restimulated in vitro with either L. major or β-GAL. Importantly, this approach did not prime BALB/c mice to β-GAL and produced only a short-lived response to β-GAL in C3H mice (Fig. 3).
FIG. 3.
Injecting L. major-NEO plus β-GAL does not prime BALB/c mice to β-GAL and induces a short-lived response to β-GAL in C3H mice. BALB/c mice (A) and C3H mice (B) were injected with 2.5 × 106 L. major-NEO amastigotes plus 100 μg of β-GAL. At the indicated times thereafter, draining LNC were restimulated as described in the legend of Fig. 1. Results are representative of two independent experiments. ∗, no proliferation detected.
β-GAL induces IFN-γ production in BALB/c mice.
Next, we determined the cytokines secreted by LNC harvested from BALB/c mice infected with L. major-βGAL. Figure 4 shows that LNC from BALB/c mice responded to parasite antigens in the anticipated fashion; namely, they produced a substantial amount of IL-4 and little IFN-γ. In contrast, these LNC produced predominantly IFN-γ and little IL-4 when restimulated with β-GAL in vitro. Therefore, (i) β-GAL-specific cells were primed in BALB/c mice infected with L. major-βGAL, (ii) these cells proliferated to β-GAL stimulation in vitro, and (iii) these cells made IFN-γ when stimulated with β-GAL.
FIG. 4.
L. major-βGAL induces a β-GAL-specific type 1 response in BALB/c mice. BALB/c or C3H mice were infected with L. major-βGAL amastigotes, and LNC were harvested at the indicated week postinfection. Techniques for supernatant collection and analysis of cytokines secreted are given in Materials and Methods. Similar results were obtained with LNC stimulated with a soluble preparation (frozen and thawed) of L. major organisms rather than living parasites. Results are representative of two independent experiments. SD, standard deviation.
In C3H mice infected with L. major-βGAL, IFN-γ was produced in response to both leishmanial antigens and to β-GAL, whereas little or no IL-4 was produced in response to either antigen (Fig. 4).
L. major-βGAL primes CD4 L. major- and β-GAL-specific T cells.
Since the production of IFN-γ in response to β-GAL was not the result expected from the BALB/c mice, we wished to characterize this response further. The principal cell that responds to infection with L. major in mice is a CD4 T cell (4, 20, 24, 25, 32). Therefore, we determined whether the cells responding in our system were CD4 T cells. LNC draining the site of infection with L. major-βGAL were stimulated for 5 days in vitro with either L. major or β-GAL, and the responding T-cell blasts were isolated. Fluorescence-activated cell sorting analysis (Table 1) revealed that the surface phenotype of the cells that responded to either L. major or β-GAL was largely that of CD4 T cells. However, some cells were CD8 (12 to 15%). Thus, it is likely that CD4 cells, and perhaps CD8 cells, were the source of the IFN-γ shown in Fig. 4.
TABLE 1.
L. major-βGAL induces CD4 L. major- and β-GAL-specific T cells
| Antigenic stimulus | % Positive cellsa
|
||||
|---|---|---|---|---|---|
| CD4 | CD8 | T-cell receptor
|
B220 | ||
| αβ | γδ | ||||
| L. major | 82 | 12 | 76 | 3 | 4 |
| β-GAL | 80 | 15 | 78 | 2 | 3 |
Techniques used for obtaining T-cell lines and fluorescence-activated cell sorting analysis of the surface phenotype of the cells are given in Materials and Methods. The experiments were performed with LNC originally obtained from the lymph nodes draining lesions of L. major-βGAL in BALB/c mice infected with the parasite for 3 weeks. The data are representative of three independent experiments.
L. major-βGAL-infected Mφs do not activate β-GAL-specific T cells unless the Mφs are activated with IFN-γ plus LPS.
Taken as a whole, the data presented show that when L. major is transfected with the Th1 antigen β-GAL, the transfected parasite (L. major-βGAL) induces a T-cell response to itself and to β-GAL in BALB/c mice (Fig. 2). Moreover, this β-GAL priming appears to occur only after L. major-βGAL is internalized by phagocytic cells (e.g., Mφs) which then present β-GAL to responding T cells. The latter conclusion is supported by the data of Fig. 2 and 3, wherein it is shown (i) that L. major purposely contaminated with soluble β-GAL does not induce a β-GAL response in BALB/c mice (therefore, possible early extracellular lysis of injected L. major-βGAL parasites with the release of β-GAL was not responsible for the β-GAL response observed [Fig. 3]) and (ii) that the response to β-GAL in L. major-βGAL-infected BALB/c mice was greatest at 3 weeks of infection (Fig. 2)—a time by which L. major-βGAL has presumably long since been internalized by phagocytes in the mice.
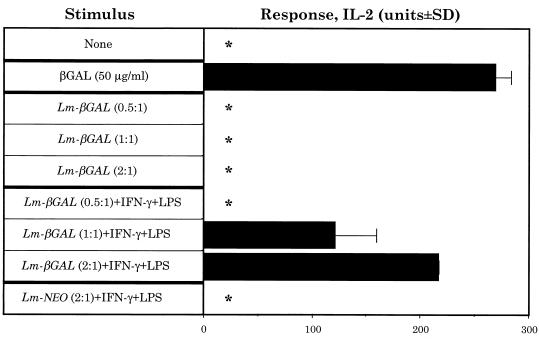
To directly test whether L. major-βGAL-infected BALB/c Mφs could present β-GAL to β-GAL-specific T cells, we infected BALB/c Mφs in vitro with the parasite and tested whether these Mφs could activate the I-Ad-restricted, IL-2-producing β-GAL-specific T cell hybridoma, 1E3.03.H4. Following incubation with soluble β-GAL, starch-elicited peritoneal BALB/c Mφs induced a marked IL-2 response from 1E3.03.H4 (Fig. 5). In contrast, following infection with L. major-βGAL, BALB/c Mφs were unable to present β-GAL unless the cells were activated with IFN-γ plus LPS (Fig. 5). This was a dose-dependent response, in that the greater the degree of infection with L. major-βGAL, the greater was the IL-2 signal secreted by 1E3.03.H4 (Fig. 5). Finally, this IL-2 response was specific for β-GAL since no IL-2 was secreted when BALB/c Mφs were infected with L. major-NEO and activated with IFN-γ plus LPS (Fig. 5).
FIG. 5.
L. major-βGAL infected Mφs do not activate β-GAL-specific T cells unless the Mφs are activated with IFN-γ plus LPS. Mφs were cultured with (i) soluble β-GAL or (ii) either L. major-βGAL (Lm-βGAL) or L. major-NEO (Lm-NEO) (indicated infection rates, 0.5 parasite per Mφ, 1 parasite per Mφ, etc.). After rinsing away β-GAL or nonphagocytized parasites, the β-GAL-specific, IL-2-secreting T-cell hybridoma, 1E3.03.H4, was added. The degree of activation of 1E3.03.H4 was determined by measuring the levels of IL-2 in the culture supernatants. For details of these techniques, see Materials and Methods. Results are representative of four independent experiments. ∗, no IL-2 detected. To ensure that the results obtained were not due to differences in the uptake of L. major-βGAL parasites by unstimulated Mφs versus Mφs treated with IFN-γ plus LPS (and thus variation in the ability of the Mφs to stimulate 1E3.03.H4), we counted the number of intracellular parasites in the Mφs (techniques in reference 31) at the end of the experiments when supernatants were harvested for IL-2 testing. Unstimulated Mφs (infected with a ratio of 2 parasites/Mφ) contained 170 ± 41 (mean ± standard deviation) intracellular L. major-βGAL per 100 Mφs, while IFN-γ plus LPS-treated Mφs contained 121 ± 21 parasites.
DISCUSSION
We used an L. major parasite that expresses a non-leishmanial reporter Th1 antigen (β-GAL) to determine how the response to this reporter antigen is affected by a simultaneous response to L. major. L. major is known to induce strongly polarized Th1 or Th2 responses in resistant or susceptible mouse strains, respectively (reviewed in references 4, 20, 24, 25, 32). Our results suggest that (i) β-GAL-specific T cells were primed in susceptible BALB/c mice following infection with L. major-βGAL (Fig. 2), (ii) these cells were not primed by soluble β-GAL released from L. major-βGAL that were lysed when injected into mice (Fig. 3) (although this does not exclude the possibility that later in infection, soluble β-GAL would be released from phagocytic cells degrading L. major-βGAL), and (iii) β-GAL-specific T cells were capable of secreting large quantities of IFN-γ when restimulated with β-GAL in vitro (Fig. 4).
It is interesting that BALB/c Mφs were able to present β-GAL to responding T cells in vitro following infection with L. major-βGAL and activation with IFN-γ plus LPS (Fig. 5). This suggests that Mφs in BALB/c mice also present β-GAL in vivo when the cells become infected with L. major-βGAL. The data of Fig. 5 also suggest that efficient presentation of β-GAL does not occur unless BALB/c Mφs are activated. The reason activation is required is unknown. Since the location of β-GAL in L. major-βGAL parasites is exclusively intracellular, some destruction of L. major-βGAL by BALB/c Mφs (by, for example, activation with IFN-γ) may be required before the cells can effectively present β-GAL. Taken as a whole, these observations suggest that priming of β-GAL-specific T cells would not occur in BALB/c mice infected with L. major-βGAL unless antigen-presenting cells in the animals are activated to kill L. major-βGAL, thus releasing β-GAL for antigen processing and presentation. The literature supports this contention. Although there is rapid multiplication of L. major in the first week of infection in both resistant and susceptible mice, the rate of multiplication of the parasite in BALB/c mice slows considerably beyond the second week of infection (34), and the second week of infection is the time by which β-GAL-specific T cells could be recovered from BALB/c mice (Fig. 2 and 4). Moreover, treating BALB/c mice with a neutralizing anti-IFN-γ antibody worsens an infection with L. major and results in rapid dissemination of the parasite in BALB/c mice, which suggests that IFN-γ activates cells infected with L. major to kill the parasite in vivo (3). Finally, work by Wolfram et al. (35) directly demonstrated that Mφs were unable to present amastigote cysteine proteinase antigens of L. mexicana to T cells unless the Mφs were activated to kill the parasite intracellularly.
Other investigators have examined whether an ongoing Th2-skewed immune response can influence the immune response to an unrelated antigen. For example, Kullberg et al. (17) showed that the response to sperm whale myoglobin was more Th2-like in mice infected with Schistosoma mansoni as compared to that in uninfected control mice. In addition, Barral-Netto et al. (1) and Doherty et al. (9) showed that an ongoing Th2-biased response in mice exacerbated infection with either Leishmania amazonensis or L. major, respectively. Curiously, Doherty et al. (9) showed that even though infection with L. major was exacerbated, the mice still produced Th1 cytokines when lymphoid tissue was restimulated with leishmanial antigens in vitro. This result is in agreement with the results presented here (Fig. 4).
Genetically engineered Leishmania organisms have been used by others to study antigen processing in Mφs infected with L. major (16) and to study the importance of cytotoxic T lymphocytes in the resistance of mice to infection with L. mexicana (21). In this study, we used these transfection systems to express β-GAL in L. major. We then used the resulting parasite (L. major-βGAL) to study immunoregulation in mice infected with L. major-βGAL. The advantage of this approach is that it allows one to study the effect of L. major infection on an unrelated antigen (β-GAL) without having to coinfect mice with another pathogen. Compared to the L. major-βGAL system, coinfecting mice with two pathogens introduces unnecessary complications which might confound the interpretation of results. A second advantage of the L. major-βGAL system is that since β-GAL is exclusively intracellular in L. major-βGAL, L. major and its reporter antigen, β-GAL, should be targeted to the same phagolysosome of phagocytic cells in mice infected with L. major-βGAL. Therefore, the ability to genetically manipulate Leishmania offers a powerful tool to address matters as diverse as mechanisms of immunoregulation in mice infected with the parasite to the development of auxotrophic gene knockout parasites that can be used as a platform for the development of safe live vaccines for leishmaniasis (33).
ACKNOWLEDGMENTS
This work was supported by NIH grants AI 29955 (R.G.T.) and 29646 (S.M.B.).
We thank Lucia Borges for providing the pXG-NEO- and pXG-βGAL-transfected L. major organisms and Monica Estay for excellent technical assistance.
REFERENCES
- 1.Barral-Netto M, da Silva J S, Barral A, Reed S. Up-regulation of T helper 2 and down-regulation of T helper 1 cytokines during murine retrovirus-induced immunodeficiency syndrome enhances susceptibility of a resistant mouse strain to Leishmania amazonensis. Am J Pathol. 1995;146:635–642. [PMC free article] [PubMed] [Google Scholar]
- 2.Behin R, Mauel J, Sordat B. Leishmania tropica: pathogenicity and in vitro macrophage function in strains of inbred mice. Exp Parasitol. 1979;48:81–93. doi: 10.1016/0014-4894(79)90057-2. [DOI] [PubMed] [Google Scholar]
- 3.Belosevic M, Finbloom D S, van der Meide P, Slayter M V, Nacy C A. Administration of monoclonal anti IFN-γ antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–274. [PubMed] [Google Scholar]
- 4.Bogdan C, Gessner A, Rollinghoff M. Cytokines in leishmaniasis: a complex network of stimulatory and inhibitory interactions. Immunobiology. 1993;189:356–396. doi: 10.1016/S0171-2985(11)80366-9. [DOI] [PubMed] [Google Scholar]
- 5.Chakkalath H R, Titus R G. Leishmania major-parasitized macrophages augment Th2-type T cell activation. J Immunol. 1994;153:4378–4387. [PubMed] [Google Scholar]
- 6.Chakkalath H R, Theodos C M, Markowitz J S, Grusby M J, Glimcher L H, Titus R G. Class II major histocompatibility complex-deficient mice initially control an infection with Leishmania major but succumb to the disease. J Infect Dis. 1995;171:1302–1308. doi: 10.1093/infdis/171.5.1302. [DOI] [PubMed] [Google Scholar]
- 7.Cruz A, Beverley S M. Gene replacement in parasitic protozoa. Nature. 1990;348:171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- 8.Cruz A K, Titus R, Beverley S M. Plasticity in chromosome number and testing of essential genes in Leishmania by targeting. Proc Natl Acad Sci USA. 1993;90:1599–1603. doi: 10.1073/pnas.90.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty T M, Morse III H C, Coffman R L. Modulation of specific T cell responses by concurrent infection with Leishmania major and LP-BM5 murine leukemia viruses. Int Immunol. 1995;7:131–138. doi: 10.1093/intimm/7.1.131. [DOI] [PubMed] [Google Scholar]
- 10.Furth U, Solioz N, Louis J A. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993;150:1857–1864. [PubMed] [Google Scholar]
- 11.Glaser T A, Wells S J, Spithill T W, Pettitt J M, Humphris D C, Mukkada A J. Leishmania major and L. donovani: a method for rapid purification of amastigotes. Exp Parasitol. 1990;71:343–345. doi: 10.1016/0014-4894(90)90039-f. [DOI] [PubMed] [Google Scholar]
- 12.Ha D S, Schwarz J K, Turco S J, Beverley S M. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 13.Hall L R, Titus R G. Sand fly vector saliva selectively modulates macrophage functions that inhibit killing of Leishmania major and nitric oxide production. J Immunol. 1995;155:3501–3506. [PubMed] [Google Scholar]
- 14.Hill J O, North R J, Collins F M. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect Immun. 1983;39:1087–1094. doi: 10.1128/iai.39.3.1087-1094.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapler G M, Coburn C M, Beverley S M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaye P M, Coburn C, McCrossan M, Beverley S M. Antigens targeted to the Leishmania phagolysosome are processed for CD4+ T cell recognition. Eur J Immunol. 1993;23:2311–2319. doi: 10.1002/eji.1830230939. [DOI] [PubMed] [Google Scholar]
- 17.Kullberg M C, Pearce E J, Hieny S E, Sher A, Berzofsky J A. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992;148:3264–3270. [PubMed] [Google Scholar]
- 18.LeBowitz J H, Coburn C M, McMahon-Pratt D, Beverley S M. Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc Natl Acad Sci USA. 1990;87:9736–9740. doi: 10.1073/pnas.87.24.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBowitz J H, Coburn C M, Beverley S M. Simultaneous transient expression assays of the trypanosomatid parasite Leishmania using beta-galactosidase and beta-glucuronidase as reporter enzymes. Gene. 1991;103:119–123. doi: 10.1016/0378-1119(91)90402-w. [DOI] [PubMed] [Google Scholar]
- 20.Liew F Y, O'Donnell C A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 21.Lopez J A, LeBowitz J H, Beverley S M, Rammensee H-G, Overath P. Leishmania mexicana promastigotes induce cytotoxic T lymphocytes in vivo that do not recognize infected macrophages. Eur J Immunol. 1993;23:217–223. doi: 10.1002/eji.1830230134. [DOI] [PubMed] [Google Scholar]
- 22.Marchand M, Daoud S, Titus R G, Louis J, Boon T. Variants with reduced virulence derived from Leishmania major after mutagen treatment. Parasite Immunol. 1987;9:81–92. doi: 10.1111/j.1365-3024.1987.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 24.Reed S G, Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 25.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 26.Sacks D L, Perkins P V. Identification of an infective stage of Leishmania promastigotes. Science (Washington, DC) 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 27.Scharton T M, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankar A H, Morin P, Titus R G. Leishmania major: differential resistance to infection in C57BL/6 (high interferon-α/β) and congenic B6.C-H28c (low interferon-α/β) mice. Exp Parasitol. 1996;84:136–143. doi: 10.1006/expr.1996.0099. [DOI] [PubMed] [Google Scholar]
- 29.Shankar A H, Titus R G. Leishmania-major-specific, CD4+, major histocompatibility complex class II-restricted T cells derived in vitro from lymphoid tissues of naive mice. J Exp Med. 1993;178:101–111. doi: 10.1084/jem.178.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar A H, Titus R G. T cell and non-T cell compartments can independently determine resistance to Leishmania major. J Exp Med. 1995;181:845–855. doi: 10.1084/jem.181.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Titus R G, Kelso A, Louis J A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984;55:157–165. [PMC free article] [PubMed] [Google Scholar]
- 32.Titus R G, Theodos C M, Shankar A, Hall L R. Interactions between Leishmania major and macrophages. Immunol Ser. 1994;60:437–459. [PubMed] [Google Scholar]
- 33.Titus R G, Gueiros-Filho F, de Freitas L A R, Beverley S M. Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci USA. 1995;92:10267–10271. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titus R G, Marchand M, Boon T, Louis J. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 35.Wolfram M, Ilg T, Mottram J C, Overath P. Antigen presentation by Leishmania mexicana-infected macrophages: activation of helper T cells specific for amastigote cysteine proteinases requires intracellular killing of the parasites. Eur J Immunol. 1995;25:1094–1100. doi: 10.1002/eji.1830250435. [DOI] [PubMed] [Google Scholar]