For more than 40 years, nephrologists have classified diminished kidney function as two distinct syndromes — acute and chronic kidney failure. Whereas chronic kidney disease was recognized in the 19th century, acute renal dysfunction became evident during the London Blitz of World War II, with the realization that crush injuries could cause dramatic but often reversible cessation of renal function. The disease states and stages of both acute and chronic renal syndromes are delineated according to the serum creatinine concentration or the glomerular filtration rate (GFR), functional markers that were identified in the early 20th century.1 Advanced renal impairment in both syndromes is treated with dialysis.
During the past decade, separate conceptual models for chronic kidney disease2 and acute kidney injury3 were developed to facilitate organized approaches to clinical research and trials. However, recent epidemiologic and mechanistic studies suggest that the two syndromes are not distinct entities but rather are closely interconnected — chronic kidney disease is a risk factor for acute kidney injury, acute kidney injury is a risk factor for the development of chronic kidney disease, and both acute kidney injury and chronic kidney disease are risk factors for cardiovascular disease (Fig. 1).4
Figure 1. Acute Kidney Injury and Chronic Kidney Disease as an Interconnected Syndrome.
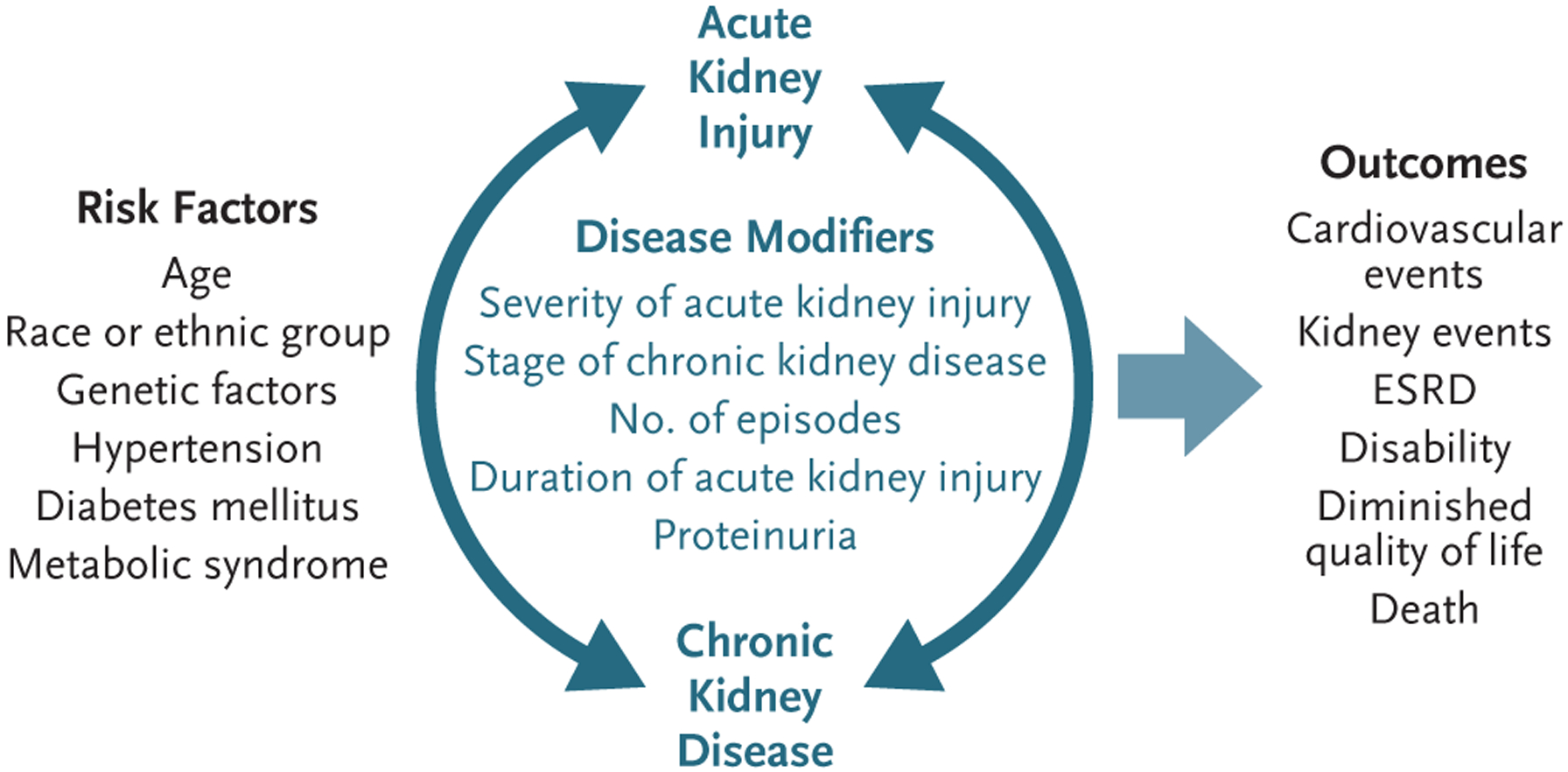
Acute kidney injury and chronic kidney disease share common risk factors and disease modifiers. When acute kidney injury occurs without preexisting kidney disease, chronic kidney disease still may develop. Conversely, the presence of chronic kidney disease is an important risk factor for the development of acute kidney injury. Either acute kidney injury or chronic kidney disease (and presumably their combination) is associated with an increased risk of death and may result in complications such as cardiovascular disease, progressive decreases in kidney function, diminished quality of life, and the development and progression of disability. ESRD denotes end-stage renal disease.
DEFINITION OF ACUTE KIDNEY INJURY
Until 2004, acute renal failure was generally defined, with some variations, as a sudden increase in the serum creatinine concentration, often accompanied by decreased urine output.3 A time-honored differential diagnosis included prerenal azotemia, postrenal urinary tract obstruction, and intrinsic renal diseases (usually categorized anatomically), including renal vascular disease, glomerulonephritis, acute interstitial nephritis, and acute tubular necrosis.3,5 Recently developed consensus functional definitions on the basis of specific changes in the serum creatinine concentration and urine volume now complement anatomical approaches to diagnosis.3,6 These definitions have standardized the study of acute kidney injury,6 allowing the classification of patients into subgroups with graded outcomes (Table 1). However, these definitions do not incorporate in the diagnostic schema the importance of the various causes of acute kidney injury (e.g., prerenal azotemia vs. intrinsic acute kidney injury).3 Potential biomarkers to predict risk, diagnosis, prognosis, and therapeutic response in patients with acute kidney injury are being identified and evaluated in clinical studies. Despite some progress, the promise of biomarkers that can be used to classify and stratify patients according to risk has not yet been achieved.3,4
Table 1.
Classifications of Acute Kidney Injury and Chronic Kidney Disease.*
| Stage | Assessments | |
|---|---|---|
| Acute kidney injury | Serum Creatinine Concentration | Urine Output |
| 1 | 1.5–1.9× baseline or ≥0.3 mg/dl above baseline | <0.5 ml/kg/hr for 6–12 hr |
| 2 | 2.0–2.9× baseline | <0.5 ml/kg/hr for >12 hr |
| 3 | ≥3.0× baseline, ≥4.0 mg/dl, or initiation of renal-replacement therapy | <0.3 ml/kg/hr for ≥24 hr or anuria for ≥12 hr |
| Chronic kidney disease | Definition | GFR |
| ml/min/1.73 m 2 | ||
| 1 | Kidney damage with normal GFR | ≥90 |
| 2 | Kidney damage with mild decrease in GFR | 60–89 |
| 3A | Mild-to-moderate decrease in GFR | 45–59 |
| 3B | Moderate-to-severe decrease in GFR | 30–44 |
| 4 | Severe decrease in GFR | 15–29 |
| 5 | End-stage renal disease | <15 |
The stage of acute kidney injury is determined according to the Kidney Disease Improving Global Outcomes Acute Kidney Injury Classification,6 and that of chronic kidney disease according to the glomerular filtration rate (GFR).2 Kidney damage is usually detected by means of an increased urinary albumin-to-creatinine ratio. To convert the values for creatinine to micromoles per liter, multiply by 88.4.
EPIDEMIOLOGIC EVIDENCE FOR INTERCONNECTION OF THE TWO SYNDROMES
New consensus definitions have facilitated the understanding of the epidemiologic factors associated with acute kidney injury, focusing on prevalence, short-term outcomes, and long-term outcomes.3,4,7 Estimates of the prevalence of acute kidney injury ranged widely before classification systems for acute kidney injury were adopted (from 1 to 26%)3–5 owing to differences in definitions (based on billing codes from the International Classification of Diseases, 9th Revision; the patient’s need for dialysis; or the evaluation of specific high-risk groups, such as patients in critical care or surgical units or those with sepsis).8–10 Nonetheless, large epidemiologic studies confirm that the incidence of acute kidney injury in the general hospitalized-patient population is increasing, perhaps more than doubling.11,12 Multiple risk factors for acute kidney injury are now known to include advanced age, diabetes mellitus, and black race (Fig. 1).11–13 Similar risk factors have been identified for chronic kidney disease.2 However, the most important risk factor for acute kidney injury is preexisting chronic kidney disease, which increases risk by as much as 10 times, as compared with the absence of chronic kidney disease.11,12,14
In-hospital mortality is extremely high among patients with acute kidney injury, with death often assumed to occur primarily in patients requiring dialysis.5 However, observational studies have shown links between small increases in the serum creatinine concentration and nonlinear increases in the risk of adverse short-term and long-term outcomes among patients with or without chronic kidney disease.4,15,16 For patients who recovered renal function after acute kidney injury, the common belief was that long-term outcomes were benign.4,17 However, since 2008, multiple observational studies have shown a strong reproducible association between acute kidney injury, including mild cases, and the subsequent development of chronic kidney disease.4,11,18 Such observational studies have consistently shown that a substantial proportion of patients with acute kidney injury, even those without previous kidney disease, often recover some degree of renal function but then have progression to advanced stages of chronic kidney disease.4,11,13,18 Observational studies have shown that acute kidney injury leads to new chronic kidney disease, the progression of existing chronic kidney disease, an increased long-term risk of end-stage renal disease (ESRD), and excess mortality.4,11,18,19
Acute kidney injury may lead to chronic kidney disease regardless of the cause of the acute injury. For example, an observational study showed an association between the diagnosis of preeclampsia and the later development of ESRD.20 In a study involving hospitalized Medicare beneficiaries, acute kidney injury was associated with a risk of ESRD that was 13 times as high as the risk among patients without acute kidney injury, and the risk of ESRD was 40 times as high if the patients had both acute kidney injury and preexisting chronic kidney disease.11 In another study, after adjustment for potential confounders such as the presence of diabetes and a low estimated GFR at baseline, acute kidney injury necessitating dialysis was independently associated with a risk of stage 4 or 5 chronic kidney disease that was 28 times as high, and a risk of death that was more than twice as high, as the risks among hospitalized patients who did not require dialysis.21 In a Canadian study involving patients with acute kidney injury who required in-hospital dialysis and survived without dialysis for at least 30 days after discharge, the risk of ESRD was 3 times as high as the risk in a matched cohort, but there was no increased risk of death.19
Amdur et al. examined long-term outcomes of acute tubular necrosis, a severe form of acute kidney injury diagnosed by means of a characteristic urinalysis and clinical course or by the presence of necrotic or regenerating tubule cells on biopsy.22 Patients with acute tubular necrosis without preexisting chronic kidney disease had more rapid progression to stage 4 chronic kidney disease than did patients with acute kidney injury in whom acute tubular necrosis was not diagnosed. Patients with either chronic kidney disease or acute tubular necrosis had higher mortality than did a matched sample of seriously ill hospitalized patients without acute kidney injury.
Several findings suggest that acute kidney injury not only is directly linked to the progression of chronic kidney disease but causes chronic kidney disease as well. First, the increased severity of acute kidney injury is associated with the development of chronic kidney disease.13,18,23 Second, multiple episodes of acute kidney injury predict the development of chronic kidney disease.24 Third, chronic kidney disease has been reported in children who had acute kidney injury who did not have coexisting conditions such as hypertension, diabetes, or cardiovascular disease.25 Finally, acute kidney injury is independently associated with outcomes of chronic kidney disease, after accounting for risk factors for chronic kidney disease, such as diabetes and hypertension, which strengthens the case for primary pathogenic links between the conditions rather than associations due to confounding.4,11–13,19,21–23
Much is unknown regarding the long-term course of acute kidney injury, such as the roles of age group (neonatal, pediatric, or elderly), the clinical setting in which acute kidney injury occurs (outpatient, intensive care unit, or general ward), the cause of acute kidney injury (ischemia, nephrotoxin, or sepsis), specific renal diseases (e.g., acute interstitial nephritis and renal vascular disease), and the severity of initial and ongoing injury (prerenal azotemia vs. acute kidney injury vs. acute kidney injury necessitating dialysis) in determining clinically meaningful outcomes. For example, older patients are particularly susceptible to both the development of sepsis and acute kidney injury.11,26,27 Although sepsis is an important common etiologic factor in the development of acute kidney injury, relatively little is known about the long-term course of patients who have acute kidney injury after sepsis and survive.9,28
ACUTE KIDNEY INJURY, CHRONIC KIDNEY DISEASE, AND CARDIOVASCULAR DISEASE
In addition to being associated with chronic kidney disease, acute kidney injury is linked to the development and treatment of cardiovascular disease (Fig. 1).29 A strong association between chronic kidney disease and an increased risk of cardiovascular events is well documented.30,31 Patients who survive an episode of acute kidney injury are also at risk for major adverse cardiovascular events, as well as for progression to chronic kidney disease, regardless of whether there is underlying cardiovascular disease.4,29 Patients with acute kidney injury after coronary angiography are at risk for hospitalization for cardiovascular causes, myocardial infarction, and vessel reocclusion; the severity of acute kidney injury has been associated with hospitalization for heart failure.29,32 Acute kidney injury is associated with higher rates of death or subsequent hospitalization for stroke, heart failure, or myocardial infarction than the rates associated with previous myocardial infarction.33 The increased risk of cardiovascular events after an episode of acute kidney injury may be mediated by the intervening development of chronic kidney disease, but the sequelae of acute kidney injury may directly increase the risk of cardiovascular disease by means of inflammatory or other pathways.33,34
PROGRESSION OF CHRONIC KIDNEY DISEASE AFTER ACUTE KIDNEY INJURY
Although the mechanisms underlying progression of renal dysfunction in humans are incompletely understood, studies in animals delineate a number of causal pathways — including maladaptive repair, disordered regeneration, or both — that may potentiate ongoing organ dysfunction.4,27 Baldwin and colleagues in a series of studies first proposed that the progression of chronic kidney disease might occur by means of processes independent of the initiating acute pathologic disorder or injury.35 Cascading mechanisms associated with progressive injury include the effects of systemic and intrarenal hypertension and glomerular hyperfiltration, tubular hypertrophy and atrophy, tubulointerstitial fibrosis, progressive glomerular sclerosis, arteriosclerosis, genetic susceptibility, and the disordered humoral responses that are associated with chronic kidney disease.4,27,36
Some pathologic processes (evaluated primarily in animal models) that persist after acute kidney injury are similar to those that have been thought to cause the progression of chronic kidney disease. Endothelial injury, as part of tubulointerstitial damage, and vascular dropout may engender vicious cycles of tissue hypoxia and ischemia, in turn affecting renal cellular function.37 The combination of vascular insufficiency, glomerular hypertension, and interstitial fibrosis is a pernicious set of self-reinforcing mechanisms that perpetuates injury, dampens repair, and causes progressive tissue damage (Fig. 2).4,27 Other factors initiated by acute kidney injury include dysregulated apoptosis; abnormal cellular responses including those of epithelial cells, pericytes, myofibroblasts, and infiltrating immune and bone marrow cells; failed differentiation and sustained proinflammatory profibrotic signaling; progressive capillary loss; perturbation of normal cell-cycle mechanisms; and epigenetic changes within renal epithelial and interstitial cells (Fig. 2).27,38–42 Fibrosis may continue after acute kidney injury resolves, presumably because fibrogenic cells do not return to their resting state38,40,43 owing in part to epigenetic factors.38 Some of these maladaptive processes are exacerbated by high-sodium and high-protein diets.27,44 However, the role of individual cell types in kidneys, during ongoing stages of injury or recovery, in the complex in vivo microenvironment in humans is not completely understood.42,45
Figure 2. Pathophysiological Features of Acute Kidney Injury Leading to Chronic Kidney Disease.

The architecture of normal kidney tissue is compared with that of injured tissue after an episode of acute kidney injury. Injured tissue shows alteration of tissue architecture and cell structure, including changes in the brush border. A variety of pathologic processes are initiated in injured and regenerating cells, including premature cell-cycle arrest, activation of myofibroblasts and fibrocytes, recruitment of various infiltrating immune and bone marrow cells to the site of injury, vascular dropout, and fibrosis. The change in tissue architecture leads to altered anatomical relationships between structures and a tissue microenvironment that promotes additional fibrosis and vascular dropout. Specific subpopulations of cells such as macrophages and T cells, differentially recruited into injured kidney tissues, may determine whether organ responses are ameliorative or maladaptive. The factors mediating recruitment, the populations of cells in human tissues that will have different responses leading to different long-term outcomes, and the interaction of recruited cells to determine outcomes are still incompletely understood. The inset shows renal tubular epithelial cells after an episode of acute kidney injury. Representative examples from various experimental studies in animals are listed in the inset. The fate of the cell, as well as the microenvironment and organ, depends on the balance between the results of repair and regenerative pathways, including apoptosis, dedifferentiation, and proinflammatory and antiinflammatory, epigenetic, and profibrotic changes. These processes may occur differentially in heterogeneous cell sets in the kidney microenvironment. Specific macrophage and T-cell subsets, as well as certain cytokines and immunoreactants, may be associated with either injury or repair. The chronic dysregulation of these factors over time and their net interactions are likely to determine the extent of fibrotic responses and organ function. BMP-7 denotes bone morphogenetic protein 7, TGF-β transforming growth factor β, and Tregs regulatory T cells.
The course of renal disease after an episode of acute kidney injury is determined by the extent of the decrement in GFR, the reversibility of the injury, and the temporal balance among effective and maladaptive repair and regenerative mechanisms.4,27 Repetitive insults may cause worsened long-term outcomes.24,46,47 Cell-cycle mechanisms and mediators such as heme oxygenase 1, hypoxia-inducible factors, vascular endothelial growth factor, and transforming growth factor β1 have been identified as protective factors in acute kidney injury, but some also may contribute to the development of chronic kidney disease.47–50 The chronic dysregulation of such factors and the complex interactions between their expression and counterregulation over time may determine the character and extent of fibrotic responses27,38,40,42,50 (Fig. 2). The roles of these factors, as well as the roles of vasoactive mediators and mediators of progressive fibrosis such as transforming growth factor β, are only beginning to be delineated in animal models. These mechanisms all have the capacity to interact, synergistically accelerating loss of function. Many of the mechanisms implicated in the development and progression of chronic kidney disease and cardiovascular disease after an episode of acute kidney injury may be targeted by conventional therapies.26,27,50
CHRONIC KIDNEY DISEASE AS A RISK FACTOR FOR ACUTE KIDNEY INJURY
The clinical consequences of acute kidney injury superimposed on chronic kidney disease, or acute-on-chronic renal failure, have been recognized but poorly evaluated.51 Patients with chronic kidney disease may be at risk for the development of transient decreases in renal function consistent with acute kidney injury. The mechanisms by which these decreases occur include failure of autoregulation, abnormal vasodilatation, susceptibility to antihypertensive agents, and side effects of medication (with drugs such as diuretic agents, antihypertensive agents, including inhibitors of the renin–angiotensin–aldosterone system, and nephrotoxins). Age-related physiologic changes, including the decline of GFR that occurs in most people who are older than 50 years of age and the loss of renal reserve, may also place older patients at risk for acute kidney injury.4,26,52
Congestive heart failure, which is often present both in patients with acute kidney injury and in those with chronic kidney disease, may be associated with acute changes in renal function. The heart undergoes progressive molecular alterations similar to those that occur in the kidney after injury.4,27,53 The roles of normal and abnormal physiological responses in patients with acute kidney injury and chronic kidney disease, including unanticipated results of conventional therapies, in mediating long-term adaptive and maladaptive outcomes in humans is largely unknown but must be evaluated. In animal models, chronic kidney disease dramatically increases the severity of sepsis and sepsis-induced acute kidney injury by altering the pathophysiological pathways and drug targets (e.g., vascular endothelial growth factor and the high-mobility group box 1 protein).54
It would be useful to have tissue and other samples from well-characterized patients with various types of acute kidney injury to test key hypotheses regarding the role of specific gene expression and translation, as well as epigenetic changes, in acute kidney injury.38,43 Furthermore, the role of the uremic milieu in the entwined pathogenesis of acute kidney injury and chronic kidney disease remains elusive.
UNANSWERED QUESTIONS
The long-term outcomes of acute kidney injury have not been well documented. Numerous biases regarding methods of selection and ascertainment have hampered approaches in observational studies that have used administrative databases.4 Patient-level data suggest that the trajectories of chronic kidney disease may be influenced by episodes of acute kidney injury but in a complex manner that renders modeling difficult.55 The Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) study is prospectively evaluating long-term outcomes in hospitalized patients, with or without chronic kidney disease, after an episode of acute kidney injury, to determine the natural history of acute kidney injury and delineate the risk factors for progression and for the development of complications, including cardiovascular disease, and the associations between putative biomarkers and long-term outcomes.56
Observational data suggest that few patients with acute kidney injury receive follow-up assessments by generalists, cardiologists, or nephrologists after hospital discharge.14 Patients with acute kidney injury should have periodic assessment of renal function and the urinary albumin-to-creatinine ratio to assess prognosis and outcome after discharge.
The appropriate treatment for patients who survive an episode of acute kidney injury, regardless of whether they have chronic kidney disease, is unclear. Reasonable therapeutic approaches to patients who do not have preexisting kidney disease but do have evidence of renal injury include, first, “do no harm,” by avoiding nephrotoxic medications, including nonsteroidal antiinflammatory drugs and radiocontrast agents. In addition, we need to determine the appropriate treatment for important risk factors for chronic kidney disease such as diabetes and hypertension. The preventive use of inhibitors of the renin–angiotensin–aldosterone system, low-sodium diets, or both should be evaluated in such patients.2–4 However, we do not know whether these therapeutic approaches ameliorate or worsen outcomes in patients with acute kidney injury or in those with combinations of acute kidney injury and chronic kidney disease. Patients who have had acute-on-chronic episodes of acute kidney injury and chronic kidney disease should be followed by primary care physicians as well as nephrologists to ensure the highest standards of care.4,22,51
Results from previous trials of dialysis and pharmacologic therapies in patients with severe acute kidney injury that have focused on diverse vascular, regeneration, and inflammatory targets have not generated useful, clinically meaningful therapies.3 Intertwined molecular pathways are involved in repair, regeneration, and maladaptive responses, which may culminate in fibrosis. Studies in animals regarding the pathophysiological features of acute kidney injury and potential therapies generally have not considered the multiplicity of responses simultaneously in experimental evaluations. Signals that are protective in early stages may promote damage or impede repair later in the course of acute kidney injury,39,47,48,50 suggesting the need for therapies that act at the correct location at the right time. Current studies are evaluating the protective effects of antiinflammatory drugs, small interfering RNAs, remote ischemic preconditioning (i.e., a brief period of ischemia in distant tissues, followed by a short interval of reperfusion), and mesenchymal stem cells.57
New approaches that are currently being developed may allow the selective targeting of cellular therapy, increasing the locally active renal dose without increasing systemic side effects, to allow even the rescue of established acute kidney injury. Such exquisite control, however, may be quite difficult to achieve with discrete single-agent therapies and may require broad-acting agents targeting final common pathways. Unfortunately, the set of mediators of cellular processes of these complex responses to injury are not yet available for clinical trials. During the coming decade, translational scientists and clinical-trial investigators will be challenged to determine the dose and timing of drug intervention and the clinical characteristics of patients who will have a response to such therapies.
We need new therapeutic approaches to acute kidney injury, evidence from well-designed, controlled prevention trials, and trials in specific patient populations. Attention to the stage, duration, and clinical setting of acute kidney injury in conjunction with coexisting conditions is essential for future interventional research and clinical observation studies. Developing proteomic and metabolomic approaches to diagnosis and prognosis, improving the phenotyping of patients with acute kidney injury (including complex clinical, environmental, and pharmacologic interactions), determining genetic links with disease susceptibility and outcomes, and assessing, characterizing, and validating fit-for-purpose biomarkers and functional stress tests in observational and clinical trials may enhance studies and aid the translation of basic science results to bedside therapeutic approaches in order to improve outcomes in patients with acute kidney injury in the presence or absence of chronic kidney disease.
Acknowledgments
The views expressed in this article do not necessarily reflect those of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institutes of Health, the Department of Health and Human Services, or the U.S. government.
Dr. Chawla reports receiving fees for serving on steering committees from AbbVie and AM-Pharma, fees for serving as an adjudicator for a clinical end-point study from Alere, fees for device development from Covidien, Gambro, Bard Medical, and NxStage Medical, fees for serving as a principal investigator for trials sponsored by Astute Medical, fees for clinical-trial development and planning from Ikaria, and grant support from Eli Lilly. He also reports providing expert testimony in a case of a patient with severe rhabdomyolysis and in a case of a patient who died after intubation. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Ms. Roberta Albert of the NIDDK for assistance in the preparation of the original drafts of the figures and several colleagues for providing critical reviews of an earlier version of the manuscript.
REFERENCES
- 1.McMahon GM, Waikar SS. Biomarkers in nephrology: Core Curriculum 2013. Am J Kidney Dis 2013;62:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levey AS, Coresh J. Chronic kidney disease. Lancet 2012;379:165–80. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012;380:756–66. [DOI] [PubMed] [Google Scholar]
- 4.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012;82:516–24. [DOI] [PubMed] [Google Scholar]
- 5.Star RA. Treatment of acute renal failure. Kidney Int 1998;54:1817–31. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [Google Scholar]
- 7.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol 2010;21:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology 2009; 110:505–15. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SD, Kimmel PL. Long-term sequelae of acute kidney injury in the ICU. Curr Opin Crit Care 2012;18:623–8. [DOI] [PubMed] [Google Scholar]
- 10.Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 2002;62:986–96. [DOI] [PubMed] [Google Scholar]
- 11.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 2009;20:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 2006;17:1135–42. [DOI] [PubMed] [Google Scholar]
- 13.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 2011;79:1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Renal Data System. USRDS 2007 annual data report. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 2007. (http://www.usrds.org/atlas07.aspx). [Google Scholar]
- 15.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 16.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR. The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta-analysis. Am J Kidney Dis 2007;50:712–20. [DOI] [PubMed] [Google Scholar]
- 17.Liaño F, Felipe C, Tenorio MT, et al. Long-term outcome of acute tubular necrosis: a contribution to its natural history. Kidney Int 2007;71:679–86. [DOI] [PubMed] [Google Scholar]
- 18.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009;302:1179–85. [Erratum, JAMA 2009;302:1532.] [DOI] [PubMed] [Google Scholar]
- 20.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med 2008;359:800–9. [DOI] [PubMed] [Google Scholar]
- 21.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 2009;76:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int 2009;76:1089–97. [DOI] [PubMed] [Google Scholar]
- 23.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 2011;171:226–33. [Erratum, Arch Intern Med 2011;171:1919.] [DOI] [PubMed] [Google Scholar]
- 24.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 2011;6:2567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein SL. Acute kidney injury in children and its potential consequences in adulthood. Blood Purif 2012;33:131–7. [DOI] [PubMed] [Google Scholar]
- 26.Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. J Am Soc Nephrol 2011;22:28–38. [DOI] [PubMed] [Google Scholar]
- 27.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 2010;298:F1078–F1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol 2011;22: 999–1006. [DOI] [PubMed] [Google Scholar]
- 29.James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 2011;123:409–16. [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–305. [DOI] [PubMed] [Google Scholar]
- 31.Chang A, Kramer H. Should eGFR and albuminuria be added to the Framingham risk score? Chronic kidney disease and cardiovascular disease risk prediction. Nephron Clin Pract 2011;119:c171–c177. [DOI] [PubMed] [Google Scholar]
- 32.Tsagalis G, Akrivos T, Alevizaki M, et al. Renal dysfunction in acute stroke: an independent predictor of long-term all combined vascular events and overall mortality. Nephrol Dial Transplant 2009;24:194–200. [DOI] [PubMed] [Google Scholar]
- 33.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. The association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol 2014;9:448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS. Messengers without borders: mediators of systemic inflammatory response in AKI. J Am Soc Nephrol 2013;24:529–36. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin DS. Poststreptococcal glomerulonephritis: a progressive disease? Am J Med 1977;62:1–11. [DOI] [PubMed] [Google Scholar]
- 36.Hostetter TH. Progression of renal disease and renal hypertrophy. Annu Rev Physiol 1995;57:263–78. [DOI] [PubMed] [Google Scholar]
- 37.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 2001; 281:F887–F899. [DOI] [PubMed] [Google Scholar]
- 38.Bechtel W, McGoohan S, Zeisberg EM, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 2010;16:544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol 2013; 229:221–31. [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 2010;16:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens 2011;20:297–305. [DOI] [PubMed] [Google Scholar]
- 42.Eddy AA. The origin of scar-forming kidney myofibroblasts. Nat Med 2013;19: 964–6. [DOI] [PubMed] [Google Scholar]
- 43.Bonventre JV, Basile D, Liu KD, et al. AKI: a path forward. Clin J Am Soc Nephrol 2013;8:1606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure pre-disposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 2007; 293:F269–F278. [DOI] [PubMed] [Google Scholar]
- 45.Wick G, Grundtman C, Mayerl C, et al. The immunology of fibrosis. Annu Rev Immunol 2013;31:107–35. [DOI] [PubMed] [Google Scholar]
- 46.Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 2012;82:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nath KA, Croatt AJ, Haggard JJ, Grande JP. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int 2000;57:2423–33. [DOI] [PubMed] [Google Scholar]
- 48.Geng H, Lan R, Wang G, et al. Inhibition of autoregulated TGFbeta signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am J Pathol 2009;174:1291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson AC, Becker K, Zager RA. Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury. Am J Physiol Renal Physiol 2010;299:F426–F435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1α and TGF-β/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol 2011;300:F898–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palevsky PM. Chronic-on-acute kidney injury. Kidney Int 2012;81:430–1. [DOI] [PubMed] [Google Scholar]
- 52.Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med 2002;347:1256–61. [DOI] [PubMed] [Google Scholar]
- 53.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest 2007;117:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leelahavanichkul A, Huang Y, Hu X, et al. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int 2011;80:1198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 2012;59:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Go AS, Parikh CR, Ikizler TA, et al. The Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) study: design and methods. BMC Nephrol 2010;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarjou A, Sanders PW, Mehta RL, Agarwal A. Enabling innovative translational research in acute kidney injury. Clin Transl Sci 2012;5:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]


