Abstract
Background
Biologic disease-modifying antirheumatic drugs (bDMARDs) have changed the treatment of juvenile idiopathic arthritis (JIA) patients notably, as bDMARDs enable substantially more patients to achieve remission. When sustained remission is achieved, tapering or even discontinuation of the bDMARD is advocated, to reduce side effects and costs. However, when and how to discontinue bDMARD therapy and what happens afterwards, is less known.
Objectives
With this scoping review we aim to collect available data in current literature on relapse rate, time to relapse (TTR) and possible flare associated variables (such as time spent in remission and method of discontinuation) after discontinuing bDMARDs in non-systemic JIA patients.
Methods
We performed a literature search until July 2022 using the Pubmed database. All original studies reporting on bDMARD discontinuation in non-systemic JIA patients were eligible. Data on patient- and study characteristics, the applied discontinuation strategy, relapse rates and time to relapse were extracted in a standardized template.
Results
Of the 680 records screened, 28 articles were included in this review with 456 non-systemic JIA patients who tapered and/or stopped bDMARD therapy. Relapse rate after discontinuation of bDMARDs, either abruptly or following tapering, were 40–48%, 36.8–45.0% and 60–78% at 6, 8 and 12 months respectively. Total relapse rate ranged from 26.3% to 100%, with mean time to relapse (TTR) of 2 to 8.4 months, median TTR 3 to 10 months. All studies stated a good response after restart of therapy after flare.
JIA subtype, type of bDMARD, concomitant methotrexate use, treatment duration, tapering method, age, sex, and time in remission could not conclusively be related to relapse rate or TTR. However, some studies reported a positive correlation between flare and antinuclear antibodies positivity, younger age at disease onset, male sex, disease duration and delayed remission, which were not confirmed in other studies.
Conclusion
Flares seem to be common after bDMARD discontinuation, but little is known about which factors influence these flares in JIA patients. Follow up after discontinuation with careful registration of patient variables, information about tapering methods and flare rates are required to better guide tapering and/or stopping of bDMARDs in JIA patients in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12969-022-00769-5.
Keywords: Juvenile idiopathic arthritis, Biologic therapy, Biologic disease-modifying antirheumatic drugs, Tapering, Relapse, Flare
Introduction
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease in childhood and an important cause of short- and long-term disability [1]. Biologic disease-modifying antirheumatic drugs (bDMARDs) are proven effective in JIA patients and have been successfully implemented in the standard treatment regime [2–5]. Still bDMARDs are costly and come with (dose-dependent) side effects [2, 6–8]. Reducing or stopping bDMARDs (when disease activity is low) might therefore be a valuable strategy to reduce side effects (in particular infections) and costs. Dose reduction in adult population of rheumatoid arthritis (RA) patients has been proven to be successful, yet discontinuation is not recommended, as many adult RA patient flare after discontinuation [9–13]. However, some JIA patients may recover spontaneously, with studies reporting over 50% of patients being in clinical remission off medication, 30 years after disease onset. Children therefore have more favorable outcomes than RA patients, this suggests that discontinuation might be a viable option in JIA patients [14, 15]. However, the evidence about the success rate of discontinuation bDMARDs in JIA is not summarized yet. Furthermore when, how and in whom to discontinue bDMARDs in JIA is not known.
The aim of this review was to conduct a scoping review of all available evidence on relapse rates and relapse associated variables after bDMARD discontinuation in children with non-systemic JIA.
Methods
This review was guided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews Checklist, checklist and additional information can be found in supplement 1 [16].
To better guide bDMARD discontinuation in children with non-systemic JIA, the following key questions were formulated;
What is the relapse rate and time to relapse after discontinuation of a bDMARD?
- Which factors are associated with flares after treatment discontinuation;
- Are there differences in flare rate between age, type of bDMARD or JIA subgroups?
- Does disease severity (e.g., time in remission, treatment or disease duration, time to remission) affect flare rate or flare severity after bDMARD discontinuation?
- What is the difference between flare rate/flare severity when patients are tapered before bDMARD discontinuation compared to abrupt discontinuation?
- Does the use of multiple bDMARDs or concomitant therapy affect flare rate or flare severity, and when concomitant therapy is used, is there a preference in stopping one before the other?
Search strategy and eligibility of the studies
Eligible articles were identified by a systematic search of PubMed/MEDLINE database. The initial search was performed in April 2021, and was repeated in July 2022 to include articles published between April 2021 and July 2022. Different MeSH terms and synonyms for JIA, bDMARD, Abatacept, Adalimumab, Etanercept, Golimumab, Infliximab, and Tocilizumab were used. The complete list of used search terms and complete search strategy are set out in supplement 2.
Titles and abstracts were independently screened by two researchers. Any discrepancies were resolved by consensus agreement between the two investigators. After reading title and abstract, original articles written in English, Dutch, French or German reporting on bDMARD discontinuation in non-systemic JIA patients were selected. Review papers, guidelines, case reports and studies focusing on systemic onset JIA (soJIA), uveitis and psoriasis were excluded. Subsequently, after reading the full text, studies lacking data on previously mentioned key questions, studies with overlapping data and studies of which no full text article was available were excluded. Using references mentioned in relevant articles, a further manual search for additional articles was conducted.
Data selection
Data charting was primarily performed by one researcher. When data was ambiguous, interpretation of this data was verified by a second researcher. Data regarding study design, number of patients undergoing bDMARD discontinuation, mode of discontinuation, medication use, disease course, flare rate and response to therapy after flare were extracted from each eligible study. We defined patients as having flared when the study described the patient as having flared, relapsed, when therapy was restarted or when the patient was no longer defined as being in remission by the study. Additionally, patient demographics of the relevant study groups were noted, if provided. Statements about possible correlations to flare were obtained from text or extracted from presented tables or supplements when possible. After the outcome measures of the eligible studies were tabulated, they were analyzed with a purely descriptive approach.
Results
Search results
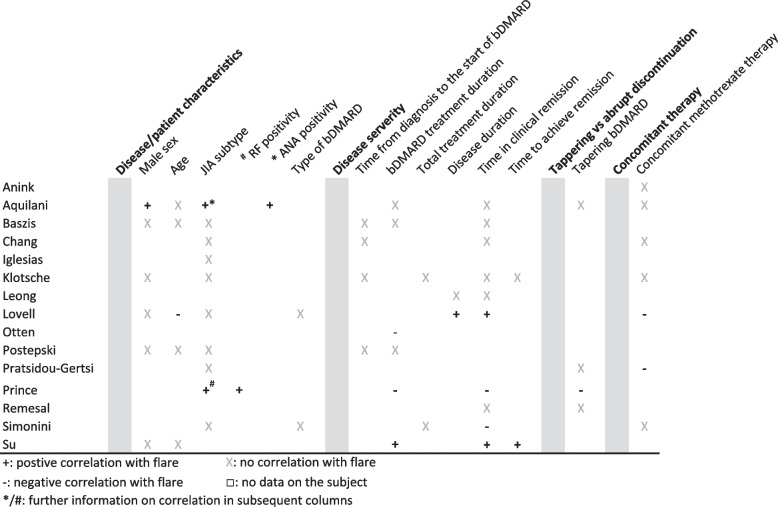
Literature screening identified a total of 680 articles. Of these 28 articles, published in 12 different journals from 2008 to 2021, were included after full text analysis, as depicted in Fig. 1. The number of prospective and retrospective studies were equally split. Three studies used either a specific selection of patients from the BiKeR registry [17] or a combination of registries including the BikeR registry, so some overlap in data between these studies could not be excluded [18–20]. Etanercept (ETN) was the most frequently studied bDMARD. Flare rate after bDMARD discontinuation was a primary outcome measure in 12 articles. Of the remaining articles, flare rate or flare associated variables were mentioned in text or figures, but were not the primary outcome. Fourteen articles (456 patients) reported on non-systemic JIA patients discontinuing bDMARDs, whereas 14 articles (730 patients) included both systemic and non-systemic JIA patients, without adequate discrimination between the two subgroups by the authors. Therefore, these outcome variables may also contain results of patients with soJIA. However, their absolute contribution to the study group was deemed to be low (Table 2). Relapse rates are displayed for both groups separately (non-systemic JIA (Table 1) and mixed systemic/non-systemic JIA (Table 2). The percentage of soJIA patients are also shown in the table. Figure 2 shows the correlations of variables with flares as stated in the different articles.
Fig. 1.
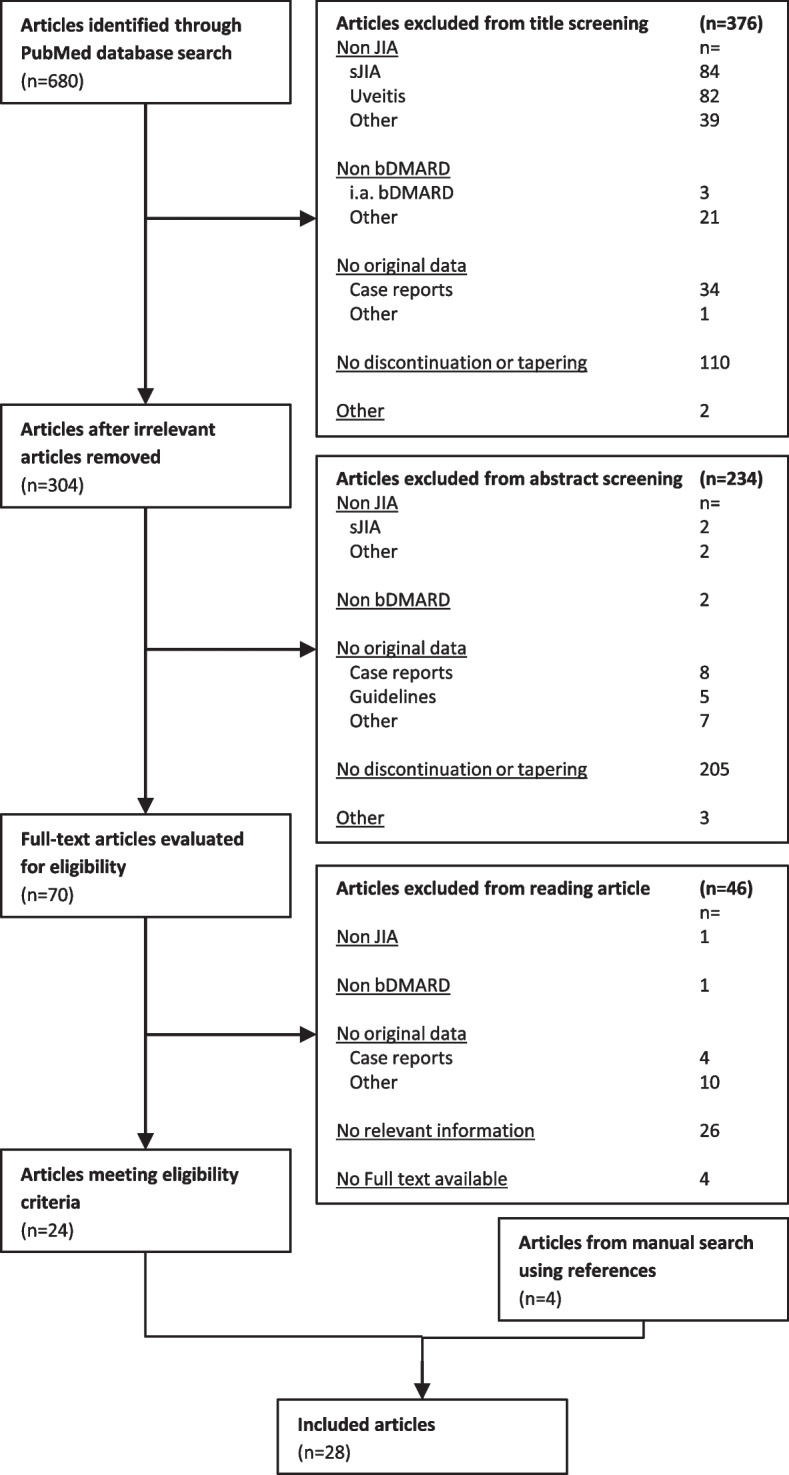
Article selection
Table 2.
Relapse rate in mixed systemic/non-systemic JIA patients after withdrawal of bDMARD
| Main author | Baszis | Remesal | Postępski | Simonini | Romano | Vidqvist | Pratsidou-Gertsi & Trachana | Anink | Otten | Su | Tynjälä | Klotsche |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [ref] | [32] | [33] | [34] | [35] | [36] | [37] | [38, 39] | [40] | [41] | [42] | [43] | [20] |
| n= | 136 | 26 | 39 | 135 | 27 | 19 | 13 | 18 | 39 | 30 | 27 | 209 |
| % of soJIA patients in withdrawl-group | unknown | 8 | 18 | 9 | unknown | unknown | 27e | unknown | unknown | 10 | unknown | unknown |
| % of soJIA patients in entire studya | 12 | NA | NA | NA | 17 | 1 | NA | 28 | 18 | NA | 6 | 6 |
| Medication type | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept |
| Adalimumab | Adalimumab | Adalimumab | Adalimumab | Infliximab | ||||||||
| Infliximab | Infliximab | Infliximab | Infliximab | |||||||||
| d | ||||||||||||
| Time in inactive disease, months | at least 6c | at least 6 | at least 6 | unknown | at least 6 | unknown | unknown | at least 6 | unknown | unknown | ||
| mean (range) | 21.3 (4-42) | |||||||||||
| median (range) | 6.1 (0-67.9) | |||||||||||
| Follow up duration, months | b | unknown | unknown | at least 12 | unknown | |||||||
| mean (range) | 21 (5-44.5) | 25.4 (16-60) | 6 (3-109) | (10-78) | 13.4f | 26 | 46.8g | |||||
| % relapse after withdrawal | 68% | 69.2% | 69.2% | 75.6% | 48.1% | unknown | 84.6% | 77.8% | 38.5% | 56.7% | 55.6% | 77% |
| Time to relapse, months | ||||||||||||
| mean (range) | 5.8 (0.6-15.9) | 14.2 (1-60) | 18 (0-108) | |||||||||
| median (range) | 6.6 (2.4-28.6) | 3 (1-15) | 9 (1-69) | |||||||||
| % relapse after 3 months | 25% | |||||||||||
| % relapse after 6 months | 50% | 50% | 38.5% | |||||||||
| % relapse after 12 months | 68% | 61% | 68.9% | |||||||||
NA not applicable
aIn case the portion of sJIA patients in the withdrawl study was not known
bfollow up was > 12 months in 71% of patients
c24 out of 26 were in clinical remission on medication
das well as anakinra (in 7 patients), rituximab and abatacept (both in 1 patient)
eIn 2 patients JIA subtype is unknown [43]
fIQ 5.3 - 24.7
gSD 3.5 years
Table 1.
Relapse rate in non-systemic JIA patients after withdrawal of bDMARD
| Main author | Anink | Giménez- Roca | Lovell | Leong | Aquilani | Chang | Hissink Muller | Prince | Foeldvari | Iglesias | Windschall | Southwood | Otten | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [Ref] | [18] | [21] | [22] | [23] | [24] | [25] | [26] | [27] | [28] | [29] | [19] | [30] | [31] | ||
| n= | 26 | 6 | 106 | 20 | 110 | 25e | 17f | 7g | 19 | 14 | 22 | 18 | 24 | 5 | 6 |
| Medication type | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | Etanercept | ||
| Adalimumab | Adalimumab | Adalimumab | Adalimumab | Adalimumab | |||||||||||
| Infliximab | Infliximab | Infliximab | Infliximab | ||||||||||||
| Time in inactive disease, months | at least 6 | 12 | at least 6 | at least 6 | unknown | unknown | at least 18j | unknown | unknownk | ||||||
| mean (range) | 5.8 (3-6) | 8 (0-35) | 12.2h | ||||||||||||
| median (range) | 14.6c | ||||||||||||||
| Follow up duration, months | 6 | 7 | 8 | 8 | 12 | at least 6 | unknown | unknown | unknown | unknown | unknown | unknown | unknown | ||
| % relapse after withdrawal | 46.2% | 100% | 36.8% | 45% | 60% | 78%e | 76%f | 78%g | 26.3% | 57.1% | 59.1% | 77.80% | 50% | 60% | 83.3% |
| Time to relapse, months | |||||||||||||||
| mean (range) | 7a | 3.0i | 8.4 (4-72) | 2 (0,7-10) | |||||||||||
| median (range) | 8.3b | 4.3d | 3 (3-7.5) | 2,6 (0,4-27) | 7 | 10 (5-34) | |||||||||
| % relapse after 6 months | 46.2% | 40% | 47%e | 48%f | 31%g | ||||||||||
| % relapse after 7 months | 100% | ||||||||||||||
| % relapse after 8 months | 36.8% | 45% | |||||||||||||
| % relapse after 12 months | 60% | 78%e | 76%f | 78%g | |||||||||||
aSEM 0.32
bmedian CI (7.8-8.6)
cIQR (11.1-21.3)
dIQR 2.5 -6.4
eTNFi +MTX; TNFi withdrawl first
fTNFi + MTX; TNFi MTX withdrawl first
gTNFi monotherapy
hSD 7.2 hSD 2.0
iSD 11,3
jIn 23 of the 24 patients time of inactive disease was longer than 18 months
kTreatment duration 22 months (13-55)
Fig. 2.
Flare associated variables
How many flares are seen after discontinuation of a bDMARD?
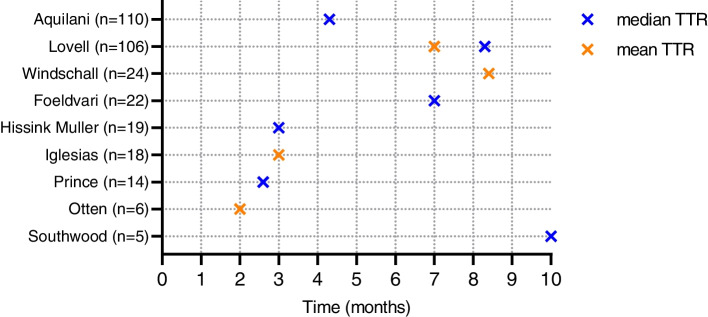
Table 1 and the figure in supplement 3 display the relapse rate of the non-systemic group. Relapse rate at 6 and 12 months were 40–48%, and 60–78% respectively [18, 24, 25]. One study reported a relapse rate of 100% at 7 months based on a small study population of 6 patients [21]. Total relapse rate, at different follow-up durations, ranged from 26.3% to 100%, with mean time to relapse (TTR) of 2 to 8.4 months and median TTR of 3 to 10 months [18, 19, 21–31] as shown in Fig. 3.
Fig. 3.
Time to relapse (TTR) in non systemic JIA patients after withdrawl of bDMARDs
As shown in Table 2 relapse rates in the group in which soJIA could not be excluded were comparable to rates in the non-systemic JIA group, with relapse rates at 6, 12 and 48 months of 38.5–50%, 61–63.9% and 77% [20, 32–35]. Total relapse rate ranged from 38.5% to 84.6% with a mean TTR of 5.8 to 18 months, and median TTR of 3 to 9 months [32–42].
All studies reported a good response to restarting therapy after a flare, with most studies mentioning a prompt return to inactive disease. However the definition of a good response and the therapy started after flare were not always well defined [20, 26, 27, 29, 31, 33, 34, 38, 43].
Are there differences between age, bDMARD or JIA subgroups?
General patient characteristics
The impact of sex and age on successful discontinuation was contradictory. Where Aquilani reported more flares in men than women (n = 110, p = 0.02), this was not confirmed by Lovell or Klotsche [20, 22, 24]. With respect to age, the results again were inconclusive. Lovell found an association between younger age and an increased chance of flares, whereas Aquilani did not find age as a discriminatory factor [22, 24]. Other studies stated no significant correlation between age or sex, and flares [32, 34, 42].
JIA subtype
Most studies found no correlation between JIA subtype and relapse rate or TTR [20, 22, 25, 29, 32, 34, 35, 38], however two studies described higher relapse rate in ANA or Rheumatoid factor (RF) positive patients. In one of them, a significant difference in flare rate was seen between the ANA positive and negative JIA groups, noting 48 out of 71 patients flared versus 18 out of 39 patients respectively (p = 0.047). Flares were never uveitis-only [24]. The other study stated RF to be negatively related with sustained remission, however this study included no more than 2 RF positive patients [27].
bDMARD
Only two studies reported on the relation between different bDMARDs and flares concluding that the type of biologic treatment was not a predictor of long-lasting remission [22, 35].
Does disease severity (e.g., treatment or disease duration, time in remission, time to achieve remission) affect flare rate or flare severity?
Time from diagnosis to start of bDMARD treatment, treatment duration and disease duration
Time from diagnosis to the start of bDMARD treatment [20, 25, 32, 34] and total treatment duration [20, 35] were not related to relapse rate or TTR. Some studies reported contradictory results regarding bDMARD treatment duration such as Prince and Otten reporting on a (trend towards) higher flare rate after a shorter duration of ETN treatment (2.1 vs. 3.5 years p = 0.21 and 2.4 vs. 3.8 years p = 0.03 resp.) whereas Su showing the opposite (15.8 vs 6.1 months p = 0.0006) [27, 41, 42]. A few other studies found no difference in flare rate at all, when comparing duration of bDMARD therapy [20, 24, 32, 34]. Shorter total disease duration was linked to fewer flares in Lovell (n = 105, Hazard Ratio (HR) 1.12 p < 0.01), but not in Leong (n = 39) [22, 23].
Time in clinical remission
Contradictory data was also found regarding the relationship between time in clinical remission before discontinuation and relapse rate/TTR. Most articles report no association between time in clinical remission and flare [20, 23–25, 32, 33]. Lovel found longer time in remission to be a predictor of more frequent flares with a HR 1.16 (p = 0.04), as did Su who observed a longer period of clinical inactive disease in their relapse group (8.4 vs. 4.2 months, p = 0.046) [22, 42]. Yet Simonini found a clinical remission of longer than 2 years to be linked to a reduction in flares (p < 0.002) [35]. The same link between long clinical remission and number of flares was seen by Prince, however their data was heavily skewed by a disproportionately long clinical remission in there soJIA subgroup. Therefore, conclusions regarding non-soJIA patients could not reliably be made on the basis of this specific study [27].
Time to achieve remission
Su noted delayed remission to be a risk factor for flare [42]. The duration from starting ETN to achieving remission was 8 months in the non-relapsing group vs. 14.9 months in the relapsing group, resulting in a HR of 1.12 (p = 0.0004) when remission is delayed by one month. This is in contrast to Klotsche who found response to ETN in the first 6 months not to be related to reoccurrence of active disease after ETN discontinuation [20].
Does tapering before discontinuation affect flare rate or flare severity, when compared to abrupt discontinuation?
Prince found 4 out of 5 non-systemic JIA patients flared after abrupt discontinuation versus 4 out of 9 in whom ETN was tapered before stopping [27]. Other studies reported no differences in relapse rate and TTR between tapered and abrupt discontinuation groups [24, 33, 38]. No data was found on the correlation between the tapering method (e.g., dose reduction or interval prolongation) and relapses.
Two studies did not identify loss of effectiveness after halving the standard dose of ETN from 0.4 mg/kg to 0.2 mg/kg, showing a low relapse rate after tapering. However, bDMARDs were not completely stopped in these studies [44, 45].
Does the use of multiple bDMARDs or concomitant therapy affect flare rate or flare severity, and when concomitant therapy is used is there a preference in stopping one before the other?
No data was found on the use of multiple bDMARDs in relation to flares.
Concomitant methotrexate (MTX) therapy did not affect the number of flares after discontinuation of TNF inhibitor (TNFi) therapy in most studies [18, 22, 24, 35]. Nonetheless, Lovell reported a decreased risk of flare in concomitant MTX users when compared to non MTX users with a HR of 11.6 (95%CI 1.20, 112.78), but this was seen only in a subgroup who discontinued adalimumab (ADA), not in other bDMARD users [22]. Similarly, Pratsidou-Gertsi found a shorter TTR when MTX was continued after ETN discontinuation (8 vs 2.5 months, p = 0.04, n = 11) [38].
Chang compared the relapse rate of JIA patients on TNFi-MTX combination therapy after discontinuation of TNFi or MTX while continuing the other, and found a higher relapse rate after TNFi discontinuation when compared to MTX discontinuation (47%, 78% vs 16% and 19% at 6 and 12 months, P < 0.0005). When subsequently TNFi was discontinued in the latter group, relapse rates were consistent with relapse rates of the early discontinuation of TNFi (48% and 76% at 6 and 12 months). Therefore, relapse rate after TNFi discontinuation seemed to be unaffected by MTX use [25].
Discussion
This review summarizes the current knowledge on relapse rate, time to relapse and possible flare associated variables after discontinuing bDMARDs in non-systemic JIA patients. Sixty to 78% of patients flared within one year after discontinuation, mean time to relapse was 2 to 8.4 months. None of the possible flare associated variables could definitively be linked to flares. Studies reporting a correlation were opposed with multiple studies finding no correlation, or correlations contradicting each other altogether. Comparison of data was further complicated by studies not displaying their data on possible flare associated variables in a verifiable and uniform manner.
A former review by Halyabar et al. also looked at treatment withdrawal in JIA but did not focus specifically on the non-systemic JIA group [46]. As natural flare rates in soJIA are known to be significantly lower compared to other JIA subgroups [47], it is important to look at these groups separately in relation to relapse rates following discontinuation of bDMARDs.
In the adult RA population relapse rates have also been studied. In this population, like in JIA, the treatment goal is to achieve remission soon after onset of RA, followed by the most optimal treatment that results in the lowest possible disease activity, the least adverse events and the lowest costs. This is achieved by tapering and when possible discontinuation of medication. In this adult population tapering of TNFi to a more optimal dose has been shown to be safe and feasible when low disease activity or remission is reached [48, 49]. Furthermore, disease activity guided dose tapering seemed to be non-inferior to continuation of full dose TNFi [50, 51]. This is in line with the studies of Cai and Mori reporting no loss of effectiveness after tapering ETN in a JIA population [44, 45]. Still tapering can be accompanied by (temporary) flaring. Fautrel et al. found a relapse rate of 77% after 18 months in RA patients tapering ADA and/or ETN every 3 months followed by discontinuation, compared to 47% in RA patient continuing ADA and/or ETN in a standard dose [52]. Unfortunately, similar to our findings in the JIA population, no markers for successful tapering have been found [53] and even a multi-biomarker score could not predict successful tapering in adults [54]. Nevertheless, protocolised tapering of TNFi seemed to be cost effective in the RA population [55]. Studies have shown that discontinuation without prior tapering is inferior to full dose continuation in the adult RA population [48]. It is therefore recommended to taper medication when low disease activity is reached in RA patients, thereby identifying a more optimal dose, as well as identifying patients who are able to discontinue their TNFi [11]. It is not yet clear if this recommendation is applicable to the JIA population. One could argue that a more favourable disease course of JIA in general justifies a more liberal tapering policy.
When interpreting the data in this review there are some considerations to keep in mind. First, flares occur frequently in JIA patients in inactive disease, even when medication is not stopped. Guzman collected data of 1146 JIA patients in inactive disease (receiving different forms of therapy) and found 42.5% of patients developing a flare within one year of achieving inactive disease, with 26.6% of patients developing a significant flare, requiring treatment intensification [47]. Therefore, some of the flares reported in the included studies in this review could also be due to the natural course of the disease and not directly related to stopping of medication.
Another aspect that one should consider, is the likely occurrence of selection bias: JIA patients receiving bDMARDs are probably of a more severe subclass and more flares can be expected in this subclass, either in general or after stoppage. This is again illustrated by Guzman, who showed that bDMARD use was associated with an increased risk of flare (HR 1.65).
This was especially true in the earlier years of bDMARD use, when these drugs were preserved for the most severe patients failing all other therapies. Since treatment strategies changed, bDMARDs are given earlier in the disease course and to less severely affected patients, which will likely result in fewer flares. Earlier studies in this review may therefore report higher flare rates than one would find today.
In addition to publication date, different discontinuation and treatment policies between studies should be kept in mind when interpreting data. For example, time in clinical remission could be longer because of cautious discontinuation policies in general, or as a result of a decision to withhold discontinuation in a specific patient due to severity of the disease in this patient. These different reasons for a longer time in clinical remission could very well explain the contradictory statements of correlation of time in clinical remission and flare made by Prince, Simonini, Su and Lovell [22, 27, 35, 42]. Likewise, selection bias could be an explanation for other correlations found in other flare associated variables. Health professionals could have been more inclined to prescribe concomitant MTX to more severe JIA patients and consequently, more flares could have been found by Lovell or Pratsidou-Gertsi [22, 38]. Unfortunately, the articles did not present enough information to verify these potential explanations.
The most important limitation of this review is that more than half of the selected studies did not select flare rate as their primary outcome. Therefore data was not always sufficiently powered and statements on flare associated variables could not always be verified numerically. Furthermore, data regarding patients lost to follow up was missing in these studies. Additionally, even though we excluded soJIA in order to make the study group more homogeneous, JIA still is a heterogenous disease and general statements are therefore difficult to make. Finally, the follow up in the studies rarely exceeds one year, as only Klotsche reported flare rates up until 48 months after discontinuation [20]. Long-term results of discontinuation are therefore still unclear. Despite these limitations the presented data in this review give an adequate representation of the current JIA population as data regarding relapse rates is comparable among the different studies.
Future studies are needed to show if delayed remission is indeed a risk factor for development of flares post bDMARD stoppage, as suggested by Su et al. [42]. A large multicentred cohort evaluating bDMARD discontinuation in JIA patients, using the same definitions for inactive disease, could provide more insight into this topic and might identify other possible flare related variables. We furthermore encourage future studies to display finding in a verifiable manner using uniform definitions and with a separate analysis of soJIA and non-systemic JIA subgroups.
In addition to patient characteristics and variables, biomarkers might also be helpful in predicting successful discontinuation [18, 23, 56]. Furthermore, ultrasound guided discontinuation is suggested as a mode to reduce flares after discontinuation, however studies outcomes are conflicting [57–60]. In summary, still more research needs to be conducted before a biomarker/ultrasound-based discontinuation strategy will be ready to be implemented.
In conclusion, this review showed that 60 to 78% of non-systemic JIA patients flare within one year after discontinuation, with a mean time to relapse of 2 to 8.4 months. Flares could not be reliably predicted by any predetermined variable at this point in time, mainly due to a lack of sufficient studies that primarily focussed on relapse rates and associated variables. This is the first study summarizing data on relapse rate and associated variables in non-systemic JIA patients after withdrawal of bDMARDs. Our current overview highlights the importance of future research and identifies several focus points for these studies. However this review shows that discontinuation of bDMARDs is feasible in JIA patients in general, and can be of assistance in daily practise in informing JIA patients and their parents on discontinuation of biologic therapy.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- bDMARDs
Biologic disease-modifying antirheumatic drugs
- JIA
Juvenile idiopathic arthritis
- soJIA
Systemic onset JIA
- TTR
Time to relapse
- RA
Rheumatoid arthritis
- ETN
Etanercept
- ANA
Antinuclear Antibodies
- RF
Rheumatoid factor
- MTX
Methotrexate
- TNFi
Tumor Necrosis Factor Inhibitor
- HR
Hazard Ratio
Authors’ contributions
JG conducted the literature search, article selection, data selection and data charting, and was a major contributor in writing the manuscript. ES conducted the literature search and article selection. All authors read and approved the final manuscript.
Funding
No funds were received to complete this study.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369(9563):767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 2.Lovell DJ, Ruperto N, Mouy R, Paz E, Rubio-Perez N, Silva CA, et al. Long-term safety, efficacy, and quality of life in patients with juvenile idiopathic arthritis treated with intravenous abatacept for up to seven years. Arthritis Rheumatol. 2015;67(10):2759–2770. doi: 10.1002/art.39234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovell DJ, Reiff A, Jones OY, Schneider R, Nocton J, Stein LD, et al. Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2006;54(6):1987–1994. doi: 10.1002/art.21885. [DOI] [PubMed] [Google Scholar]
- 4.Tynjala P, Lahdenne P, Vahasalo P, Kautiainen H, Honkanen V. Impact of anti-TNF treatment on growth in severe juvenile idiopathic arthritis. Ann Rheum Dis. 2006;65(8):1044–1049. doi: 10.1136/ard.2005.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horneff G, Burgos-Vargas R, Constantin T, Foeldvari I, Vojinovic J, Chasnyk VG, et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis. 2014;73(6):1114–1122. doi: 10.1136/annrheumdis-2012-203046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis. 2015;74(6):1110–1117. doi: 10.1136/annrheumdis-2014-205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannini EH, Ilowite NT, Lovell DJ, Wallace CA, Rabinovich CE, Reiff A, et al. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(9):2794–2804. doi: 10.1002/art.24777. [DOI] [PubMed] [Google Scholar]
- 8.Hashkes PJ, Uziel Y, Laxer RM. The safety profile of biologic therapies for juvenile idiopathic arthritis. Nat Rev Rheumatol. 2010;6(10):561–571. doi: 10.1038/nrrheum.2010.142. [DOI] [PubMed] [Google Scholar]
- 9.Dierckx S, Sokolova T, Lauwerys BR, Avramovska A, de Bellefon LM, Toukap AN, et al. Tapering of biological antirheumatic drugs in rheumatoid arthritis patients is achievable and cost-effective in daily clinical practice: data from the Brussels UCLouvain RA Cohort. Arthritis Res Ther. 2020;22(1):96. doi: 10.1186/s13075-020-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouman CA, van Herwaarden N, van den Hoogen FH, Fransen J, van Vollenhoven RF, Bijlsma JW, et al. Long-term outcomes after disease activity-guided dose reduction of TNF inhibition in rheumatoid arthritis: 3-year data of the DRESS study - a randomised controlled pragmatic non-inferiority strategy trial. Ann Rheum Dis. 2017;76(10):1716–1722. doi: 10.1136/annrheumdis-2017-211169. [DOI] [PubMed] [Google Scholar]
- 11.Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi T, Genovese MC, Haraoui B, Li Z, Xie L, Klar R, et al. Dose reduction of baricitinib in patients with rheumatoid arthritis achieving sustained disease control: results of a prospective study. Ann Rheum Dis. 2019;78(2):171–178. doi: 10.1136/annrheumdis-2018-213271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Hirata S, Saleem B, Emery P. Discontinuation of biologics in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2013;31(4 Suppl 78):S22–S27. [PubMed] [Google Scholar]
- 14.Selvaag AM, Aulie HA, Lilleby V, Flato B. Disease progression into adulthood and predictors of long-term active disease in juvenile idiopathic arthritis. Ann Rheum Dis. 2016;75(1):190–195. doi: 10.1136/annrheumdis-2014-206034. [DOI] [PubMed] [Google Scholar]
- 15.Minden K. Adult outcomes of patients with juvenile idiopathic arthritis. Horm Res. 2009;72(Suppl 1):20–25. doi: 10.1159/000229759. [DOI] [PubMed] [Google Scholar]
- 16.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 17.BIKER Register. 2022.
- 18.Anink J, Van Suijlekom-Smit LW, Otten MH, Prince FH, van Rossum MA, Dolman KM, et al. MRP8/14 serum levels as a predictor of response to starting and stopping anti-TNF treatment in juvenile idiopathic arthritis. Arthritis Res Ther. 2015;17:200. doi: 10.1186/s13075-015-0723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Windschall D, Horneff G. Safety and efficacy of etanercept and adalimumab in children aged 2 to 4 years with juvenile idiopathic arthritis. Clin Rheumatol. 2016;35(12):2925–2931. doi: 10.1007/s10067-016-3439-y. [DOI] [PubMed] [Google Scholar]
- 20.Klotsche J, Klein A, Niewerth M, Hoff P, Windschall D, Foeldvari I, et al. Re-treatment with etanercept is as effective as the initial firstline treatment in patients with juvenile idiopathic arthritis. Arthritis Res Ther. 2021;23(1):118. doi: 10.1186/s13075-021-02492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gimenez-Roca C, Iglesias E, Torrente-Segarra V, Bou R, Sanchez-Manubens J, Calzada-Hernandez J, et al. Efficacy and safety of TNF-alpha antagonists in children with juvenile idiopathic arthritis who started treatment under 4 years of age. Rheumatol Int. 2015;35(2):323–326. doi: 10.1007/s00296-014-3103-2. [DOI] [PubMed] [Google Scholar]
- 22.Lovell DJ, Johnson AL, Huang B, Gottlieb BS, Morris PW, Kimura Y, et al. Risk, timing, and predictors of disease flare after discontinuation of anti-tumor necrosis factor therapy in children with polyarticular forms of juvenile idiopathic arthritis with clinically inactive disease. Arthritis Rheumatol. 2018;70(9):1508–1518. doi: 10.1002/art.40509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong JY, Chen P, Yeo JG, Ally F, Chua C, Nur Hazirah S, et al. Immunome perturbation is present in patients with juvenile idiopathic arthritis who are in remission and will relapse upon anti-TNFalpha withdrawal. Ann Rheum Dis. 2019;78(12):1712–1721. doi: 10.1136/annrheumdis-2019-216059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aquilani A, Marafon DP, Marasco E, Nicolai R, Messia V, Perfetti F, et al. Predictors of flare following etanercept withdrawal in patients with rheumatoid factor-negative juvenile idiopathic arthritis who reached remission while taking medication. J Rheumatol. 2018;45(7):956–961. doi: 10.3899/jrheum.170794. [DOI] [PubMed] [Google Scholar]
- 25.Chang CY, Meyer RM, Reiff AO. Impact of medication withdrawal method on flare-free survival in patients with juvenile idiopathic arthritis on combination therapy. Arthritis Care Res (Hoboken) 2015;67(5):658–666. doi: 10.1002/acr.22477. [DOI] [PubMed] [Google Scholar]
- 26.Hissink Muller P, Brinkman DMC, Schonenberg-Meinema D, van den Bosch WB, Koopman-Keemink Y, Brederije ICJ, et al. Treat to target (drug-free) inactive disease in DMARD-naive juvenile idiopathic arthritis: 24-month clinical outcomes of a three-armed randomised trial. Ann Rheum Dis. 2019;78(1):51–59. doi: 10.1136/annrheumdis-2018-213902. [DOI] [PubMed] [Google Scholar]
- 27.Prince FH, Twilt M, Simon SC, van Rossum MA, Armbrust W, Hoppenreijs EP, et al. When and how to stop etanercept after successful treatment of patients with juvenile idiopathic arthritis. Ann Rheum Dis. 2009;68(7):1228–1229. doi: 10.1136/ard.2008.101030. [DOI] [PubMed] [Google Scholar]
- 28.Foeldvari I, Constantin T, Vojinovic J, Horneff G, Chasnyk V, Dehoorne J, et al. Etanercept treatment for extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis, or psoriatic arthritis: 6-year efficacy and safety data from an open-label trial. Arthritis Res Ther. 2019;21(1):125. doi: 10.1186/s13075-019-1916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iglesias E, Torrente-Segarra V, Bou R, Ricart S, Gonzalez MI, Sanchez J, et al. Non-systemic juvenile idiopathic arthritis outcome after reaching clinical remission with anti-TNF-alpha therapy: a clinical practice observational study of patients who discontinued treatment. Rheumatol Int. 2014;34(8):1053–1057. doi: 10.1007/s00296-013-2884-z. [DOI] [PubMed] [Google Scholar]
- 30.Southwood TR, Foster HE, Davidson JE, Hyrich KL, Cotter CB, Wedderburn LR, et al. Duration of etanercept treatment and reasons for discontinuation in a cohort of juvenile idiopathic arthritis patients. Rheumatology (Oxford) 2011;50(1):189–195. doi: 10.1093/rheumatology/keq308. [DOI] [PubMed] [Google Scholar]
- 31.Otten MH, Prince FH, Ten Cate R, van Rossum MA, Twilt M, Hoppenreijs EP, et al. Tumour necrosis factor (TNF)-blocking agents in juvenile psoriatic arthritis: are they effective? Ann Rheum Dis. 2011;70(2):337–340. doi: 10.1136/ard.2010.135731. [DOI] [PubMed] [Google Scholar]
- 32.Baszis K, Garbutt J, Toib D, Mao J, King A, White A, et al. Clinical outcomes after withdrawal of anti-tumor necrosis factor alpha therapy in patients with juvenile idiopathic arthritis: a twelve-year experience. Arthritis Rheum. 2011;63(10):3163–3168. doi: 10.1002/art.30502. [DOI] [PubMed] [Google Scholar]
- 33.Remesal A, DEI J, Merino R, Garcia-Consuegra J. Discontinuation of etanercept after successful treatment in patients with juvenile idiopathic arthritis. J Rheumatol. 2010;37(9):1970–1. doi: 10.3899/jrheum.100219. [DOI] [PubMed] [Google Scholar]
- 34.Postepski J, Kobusinska K, Olesinska E, Osinska V, Opoka-Winiarska V. Clinical remission in juvenile idiopathic arthritis after termination of etanercept. Rheumatol Int. 2013;33(10):2657–2660. doi: 10.1007/s00296-012-2468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonini G, Ferrara G, Pontikaki I, Scoccimarro E, Giani T, Taddio A, et al. Flares after withdrawal of biologic therapies in juvenile idiopathic arthritis: clinical and laboratory correlates of remission duration. Arthritis Care Res (Hoboken) 2018;70(7):1046–1051. doi: 10.1002/acr.23435. [DOI] [PubMed] [Google Scholar]
- 36.Romano M, Pontikaki I, Gattinara M, Ardoino I, Donati C, Boracchi P, et al. Drug survival and reasons for discontinuation of the first course of biological therapy in 301 juvenile idiopathic arthritis patients. Reumatismo. 2014;65(6):278–285. doi: 10.4081/reumatismo.2013.682. [DOI] [PubMed] [Google Scholar]
- 37.Vidqvist KL, Malin M, Varjolahti-Lehtinen T, Korpela MM. Disease activity of idiopathic juvenile arthritis continues through adolescence despite the use of biologic therapies. Rheumatology (Oxford) 2013;52(11):1999–2003. doi: 10.1093/rheumatology/ket256. [DOI] [PubMed] [Google Scholar]
- 38.Pratsidou-Gertsi P, Trachana M, Pardalos G, Kanakoudi-Tsakalidou F. A follow-up study of patients with juvenile idiopathic arthritis who discontinued etanercept due to disease remission. Clin Exp Rheumatol. 2010;28(6):919–922. [PubMed] [Google Scholar]
- 39.Trachana M, Pratsidou-Gertsi P, Badouraki M, Haidich AB, Pardalos G. Achievement of clinical remission in patients with juvenile idiopathic arthritis under a 2–10-year Etanercept exposure. Clin Rheumatol. 2013;32(8):1191–1197. doi: 10.1007/s10067-013-2261-z. [DOI] [PubMed] [Google Scholar]
- 40.Anink J, Prince FH, Dijkstra M, Otten MH, Twilt M, ten Cate R, et al. Long-term quality of life and functional outcome of patients with juvenile idiopathic arthritis in the biologic era: a longitudinal follow-up study in the Dutch Arthritis and Biologicals in Children Register. Rheumatology (Oxford) 2015;54(11):1964–1969. doi: 10.1093/rheumatology/kev195. [DOI] [PubMed] [Google Scholar]
- 41.Otten MH, Prince FH, Armbrust W, ten Cate R, Hoppenreijs EP, Twilt M, et al. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA. 2011;306(21):2340–2347. doi: 10.1001/jama.2011.1671. [DOI] [PubMed] [Google Scholar]
- 42.Su Y, Yang YH, Chiang BL. Treatment response to etanercept in methotrexate refractory juvenile idiopathic arthritis: an analysis of predictors and long-term outcomes. Clin Rheumatol. 2017;36(9):1997–2004. doi: 10.1007/s10067-017-3682-x. [DOI] [PubMed] [Google Scholar]
- 43.Tynjala P, Vahasalo P, Honkanen V, Lahdenne P. Drug survival of the first and second course of anti-tumour necrosis factor agents in juvenile idiopathic arthritis. Ann Rheum Dis. 2009;68(4):552–557. doi: 10.1136/ard.2007.087130. [DOI] [PubMed] [Google Scholar]
- 44.Mori M, Takei S, Imagawa T, Imanaka H, Nerome Y, Kurosawa R, et al. Etanercept in the treatment of disease-modifying anti-rheumatic drug (DMARD)-refractory polyarticular course juvenile idiopathic arthritis: experience from Japanese clinical trials. Mod Rheumatol. 2011;21(6):572–578. doi: 10.3109/s10165-011-0450-7. [DOI] [PubMed] [Google Scholar]
- 45.Cai Y, Liu X, Zhang W, Xu J, Cao L. Clinical trial of etanercept tapering in juvenile idiopathic arthritis during remission. Rheumatol Int. 2013;33(9):2277–2282. doi: 10.1007/s00296-012-2642-7. [DOI] [PubMed] [Google Scholar]
- 46.Halyabar O, Mehta J, Ringold S, Rumsey DG, Horton DB. Treatment withdrawal following remission in juvenile idiopathic arthritis: a systematic review of the literature. Paediatr Drugs. 2019;21(6):469–492. doi: 10.1007/s40272-019-00362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzman J, Oen K, Huber AM, Watanabe Duffy K, Boire G, Shiff N, et al. The risk and nature of flares in juvenile idiopathic arthritis: results from the ReACCh-Out cohort. Ann Rheum Dis. 2016;75(6):1092–1098. doi: 10.1136/annrheumdis-2014-207164. [DOI] [PubMed] [Google Scholar]
- 48.van Herwaarden N, den Broeder AA, Jacobs W, van der Maas A, Bijlsma JW, van Vollenhoven RF, et al. Down-titration and discontinuation strategies of tumor necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database Syst Rev. 2014;9:CD010455. [DOI] [PubMed]
- 49.Verhoef LM, Tweehuysen L, Hulscher ME, Fautrel B, den Broeder AA. bDMARD dose reduction in rheumatoid arthritis: a narrative review with systematic literature search. Rheumatol Ther. 2017;4(1):1–24. doi: 10.1007/s40744-017-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Herwaarden N, van der Maas A, Minten MJ, van den Hoogen FH, Kievit W, van Vollenhoven RF, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial. BMJ. 2015;350:h1389. doi: 10.1136/bmj.h1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.den Broeder AA, van Herwaarden N, van der Maas A, van den Hoogen FH, Bijlsma JW, van Vollenhoven RF, et al. Dose REduction strategy of subcutaneous TNF inhibitors in rheumatoid arthritis: design of a pragmatic randomised non inferiority trial, the DRESS study. BMC Musculoskelet Disord. 2013;14:299. doi: 10.1186/1471-2474-14-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fautrel B, Pham T, Alfaiate T, Gandjbakhch F, Foltz V, Morel J, et al. Step-down strategy of spacing TNF-blocker injections for established rheumatoid arthritis in remission: results of the multicentre non-inferiority randomised open-label controlled trial (STRASS: Spacing of TNF-blocker injections in Rheumatoid ArthritiS Study) Ann Rheum Dis. 2016;75(1):59–67. doi: 10.1136/annrheumdis-2014-206696. [DOI] [PubMed] [Google Scholar]
- 53.Tweehuysen L, van den Ende CH, Beeren FM, Been EM, van den Hoogen FH, den Broeder AA. Little Evidence for usefulness of biomarkers for predicting successful dose reduction or discontinuation of a biologic agent in rheumatoid arthritis: a systematic review. Arthritis Rheumatol. 2017;69(2):301–308. doi: 10.1002/art.39946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouman CAM, van der Maas A, van Herwaarden N, Sasso EH, van den Hoogen FHJ, den Broeder AA. A multi-biomarker score measuring disease activity in rheumatoid arthritis patients tapering adalimumab or etanercept: predictive value for clinical and radiographic outcomes. Rheumatology (Oxford) 2017;56(6):973–980. doi: 10.1093/rheumatology/kex003. [DOI] [PubMed] [Google Scholar]
- 55.den Broeder N, Bouman CAM, Kievit W, van Herwaarden N, van den Hoogen FHJ, van Vollenhoven RF, et al. Three-year cost-effectiveness analysis of the DRESS study: protocolised tapering is key. Ann Rheum Dis. 2019;78(1):141–142. doi: 10.1136/annrheumdis-2018-213547. [DOI] [PubMed] [Google Scholar]
- 56.Mor-Vaknin N, Rivas M, Legendre M, Mohan S, Yuanfan Y, Mau T, et al. High Levels of DEK autoantibodies in Sera of patients with polyarticular juvenile idiopathic arthritis and with early disease flares following cessation of anti-tumor necrosis factor therapy. Arthritis Rheumatol. 2018;70(4):594–605. doi: 10.1002/art.40404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miotto ESVB, Mitraud SAV, Furtado RNV, Natour J, Len CA, Terreri M. Patients with juvenile idiopathic arthritis in clinical remission with positive power Doppler signal in joint ultrasonography have an increased rate of clinical flare: a prospective study. Pediatr Rheumatol Online J. 2017;15(1):80. doi: 10.1186/s12969-017-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Lucia O, Ravagnani V, Pregnolato F, Hila A, Pontikaki I, Gattinara M, et al. Baseline ultrasound examination as possible predictor of relapse in patients affected by juvenile idiopathic arthritis (JIA) Ann Rheum Dis. 2018;77(10):1426–1431. doi: 10.1136/annrheumdis-2017-211696. [DOI] [PubMed] [Google Scholar]
- 59.Nieto-Gonzalez JC, Rodriguez A, Gamir-Gamir ML, Boteanu A, Lopez-Robledillo JC, Garulo DC, et al. Can ultrasound-detected subclinical synovitis be an indicator of flare recurrence in juvenile idiopathic arthritis remission patients on tapered TNFi? Clin Exp Rheumatol. 2019;37(4):705–712. [PubMed] [Google Scholar]
- 60.Magni-Manzoni S, Scire CA, Ravelli A, Klersy C, Rossi S, Muratore V, et al. Ultrasound-detected synovial abnormalities are frequent in clinically inactive juvenile idiopathic arthritis, but do not predict a flare of synovitis. Ann Rheum Dis. 2013;72(2):223–228. doi: 10.1136/annrheumdis-2011-201264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.