Abstract
The breakthrough of induced pluripotent stem cell (iPSC) technology has raised the possibility that patient-specific iPSCs may become a renewable source of autologous cells for cell therapy without the concern of immune rejection. However, the immunogenicity of allogeneic hiPSC-derived cells is not well understood. Using a humanized mouse model (denoted Hu-mice) reconstituted with a functional human immune system, we demonstrate that most teratomas formed by autologous integration-free hiPSCs exhibit local infiltration of antigen-specific T cells and associated tissue necrosis, indicating immune rejection of certain hiPSC-derived cells. In this context, autologous hiPSC-derived smooth muscle cells (SMCs) appear to be highly immunogenic, while autologous hiPSC-derived retinal pigment epithelial (RPE) cells are immune tolerated even in non-ocular locations. This differential immunogenicity is due in part to abnormal expression of immunogenic antigens in hiPSC-derived SMCs but not in hiPSC-derived RPEs. These findings support the feasibility of developing hiPSC-derived RPEs for treating macular degeneration.
Graphical Abstract
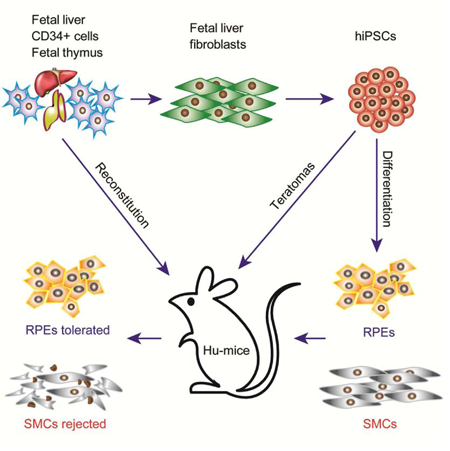
Introduction
The recent breakthrough in the generation of induced pluripotent stem cells (iPSCs) by reprogramming somatic cells with defined factors has raised the hope that iPSCs, which are identical to human embryonic stem cells (hESCs) in the context of pluripotency, could become a renewable source of autologous cells for transplantation into human patients (Lewitzky and Yamanaka, 2007). In addition, the disease-specific iPSCs could provide the unique opportunity to develop the much needed disease models in drug discovery. While it has been assumed that the immune rejection problem challenging hESCs could be mitigated by the development of patient-specific hiPSCs without the concern of immune rejection (Park et al., 2008; Takahashi et al., 2007; Yu et al., 2007), recent studies have shown that certain cell types derived from mouse iPSCs such as cardiomyocytes are immunogenic in syngeneic recipients, and other immunogenic cell types such as endothelial cells are immune tolerated by expressing high levels of immune suppressive cytokines such as IL-10 (Araki et al., 2013; de Almeida et al., 2014; Zhao et al., 2011). Therefore, as an integral part of the effort to develop hiPSCs into human cell therapy, it is important to evaluate the immunogenicity of hiPSC-derived cells in the context of an autologous human immune system. To address this bottleneck, we established humanized mouse models that are efficiently reconstituted with both T and B cells as well as macrophages and dendritic cells required for antigen presentation (Figure S1A). As we recently published (Rong et al., 2014), these Hu-mice can mount vigorous immune rejection of allogeneic cells derived from hESCs (Figure S1B, C, D, E). Therefore, the Hu-mice provide a unique opportunity to examine the immunogenicity of autologous cells derived from hiPSCs.
Results
Teratomas formed by hiPSCs are immunogenic to autologous human T cells
To generate autologous integration-free hiPSC, fibroblasts were derived from the same human fetal liver used to reconstitute the human immune system in Hu-mice (Figure 1A). These cells were reprogrammed into integration-free hiPSCs with either the episomal approaches we previously described (Zhao et al., 2011) or developed by Yamanaka group (Okita et al., 2011). The hiPSCs are positive for alkaline phosphatase, express pluripotency markers to the same levels as hESCs, have normal karyotypes and can form teratomas containing cells derived from each of the three germ layers in immunodeficient mice, confirming their pluripotency (Figure S2A–D). In addition, Southern blotting analysis with probes covering the entire episomal vector further confirmed that hiPSCs are integration-free (Figure S2E). These integration-free hiPSCs were transplanted subcutaneously into Hu-mice to form teratomas, allowing the simultaneous evaluation of the immunogenicity of various cell types differentiated from hiPSCs.
Figure 1.
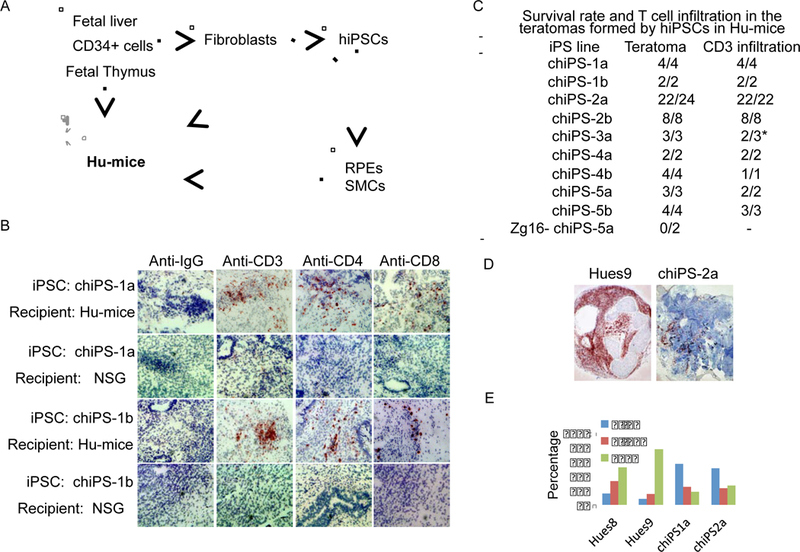
Cells within teratomas formed by hiPSCs can be immunogenic in Hu-mice reconstituted with autologous immune system. (A) Strategy to study the immunogenicity of hiPSC derivatives. Human fetal liver/thymus and autologous CD34+ fetal liver cells were transplanted into NSG mice to generate Humice. Fibroblasts were isolated from the same fetal liver and reprogrammed into autologous integration-free hiPSCs, which were either directly transplanted into the Hu-mice or differentiated into mature cell types before transplanting into Hu-mice. (B) T cell infiltration was detected in the majority of teratomas formed by hiPSCs in Hu-mice with autologous human immune system. T cells are revealed by dark brown color. (C) The summary of teratomas formation and T cell infiltration into teratomas after subcutaneous implantation of 9 independent hiPSC lines reprogrammed from 5 different donors as well as Zg16-hiPSCs in Hu-mice reconstituted with corresponding autologous immune system. (D) Low magnification (4x) images show the apparent difference in T cell infiltration between hESC-derived teratoma and autologous iPSC-derived teratoma. (E) Frequency of T cells infiltrating into each teratoma. Sections of teratomas formed by Hues8, Hues9, chiPS1a and chiPS2a in Hu-mice were stained with antiCD3 antibody. Five teratomas from each line were analyzed. At least 50 frames (20x) from each teratomas were randomly chosen for analysis. The numbers of T cells detected were categorized into three groups: 0–3, 4–19 or ≥20 T cells per frame.
Serial section analysis of the teratomas formed by hiPSCs in Hu-mice reconstituted with autologous immune system indicated that the majority of these teratomas contained regions that were infiltrated with human CD4+ and CD8+ T cells and exhibited tissue necrosis (Figure 1B, C; Figure S1K). However, the immunogenicity of autologous hiPSC-derived teratomas in Hu-mice appeared to be much weaker than allogeneic hESC-derived teratomas that were extensively infiltrated with T cells (Figure 1D,E). In this context, after examining sequential sections of one teratoma formed by autologous hiPSCs in Hu-mice, we did not detect any T cell infiltration (Figure S1L). Therefore, certain, but not all, cells derived from hiPSCs are immunogenic to the autologous human immune system.
T cell response to specific cell types within autologous teratomas
To identify the potential immunogenic tissues inside the teratoma, we analyzed the histology of a large number of teratomas infiltrated with T cells. We found that the autologous hiPSC-derived Desmin+ smooth muscle cells (SMCs) were frequently surrounded by infiltrating T cells in the teratomas (Figure 2A, C). In contrast, the majority of autologous hiPSC-derived retinal pigment epithelium (RPE) cells within the teratomas were not surrounded by T cells (Figure 2B, C).
Figure 2.
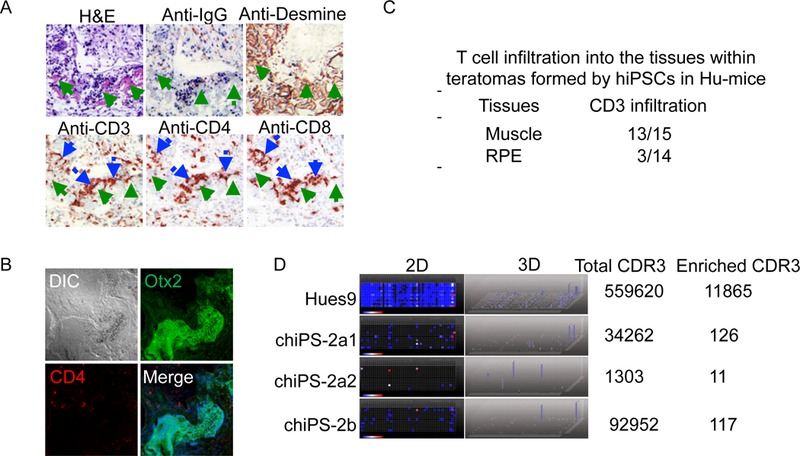
Characterization of the immune responses to the teratomas formed by hiPSCs in Hu-mice reconstituted with autologous immune system. (A) Smooth muscle cells (SMCs) within the teratomas formed by hiPSCs in Hu-mice are frequently infiltrated with T cells (Green arrow: smooth muscle cells; Blue arrow: T cells). (B) The retinal pigment epithelium (RPE) cells within the teratomas formed by hiPSCs are immune tolerated. (C) Summary of the T cell infiltration into SMCs and RPEs within the teratomas formed by hiPSCs in Hu-mice reconstituted with autologous immune system. (D) TCR repertoire distributions in 4 teratomas formed by Hues9 and two independent hiPSC lines (chiPS-2a and chiPS-2b) in Hu-mice. 2D: 2 dimension map; Dot: different TCR, the change of color from blue to red represent the enrichment of the specific TCR; 3D: 3 dimension map; blue column: enrichment of the specific TCR. The enrichment of several monoclonal TCR in hiPSC-derived teratomas suggests the antigen specific T cell response to cells within the teratomas formed by autologous iPSCs.
To further characterize the T cell responses to the teratomas formed by autologous hiPSCs, we performed deep sequencing to analyze the T cell receptor (TCR) repertoire of the T cells that had infiltrated into the teratomas formed by either allogeneic hESC or autologous hiPSC (Figure 2D). Intriguingly, in contrast to the highly polyclonal nature of the TCR repertoire of T cells infiltrated into the allogeneic hESC-derived teratomas, the TCR repertoire of T cells infiltrating into the teratomas formed by autologous hiPSCs in Hu-mice were oligoclonal, indicating antigen-specific T cell responses to the autologous hiPSC derived cells within the teratomas.
Differential immunogenicity of SMCs and RPEs derived from autologous hiPSCs
To further evaluate the immunogenicity of hiPSC-derived RPE cells, we differentiated the autologous hiPSCs into RPE cells in vitro with a high efficiency protocol as previously described (Krohne et al., 2012). As a positive control, allogeneic hESCs were also differentiated into RPE cells. HiPSC-derived RPEs were tightly coupled, positive for Mitf and Bestrophin, exhibited vectorial transport of water and ions, phagocytosed photoreceptor outer segments (POS), could be incorporated into diseased retinas in a polarized monolayer, and rescue photoreceptors in an animal model (RCS rats) of RPE-mediated retinal degeneration (Figure S3A–G). Furthermore, the global gene expression analysis demonstrated high similarity between hiPSC-derived RPEs and the human primary RPEs (Figure 4A, B). These findings validated the identity and function of hiPSC-derived RPEs. The autologous hiPSC-derived RPEs and the control allogeneic hESC-derived RPEs were transplanted into the eye of Hu-mice. Since the eye is considered an immune privileged site, and thus, could mask the immunogenicity of the hiPSC-derived RPE cells, we also implanted these RPEs into the hindleg skeletal muscle of Hu-mice. In support of this concept, even the allogeneic hESC-derived RPE cells survived in the eye of Hu-mice without apparent immune rejection. When injected into the skeletal muscle of Hu-mice, the allogeneic hESC-derived RPEs were infiltrated with T cells, consistent with T-dependent immune rejection (Figure 3A,C,D,G). In contrast, autologous hiPSC-derived RPEs implanted in the muscle of the same Hu-mice were mostly protected from T cells (Figure 3B,C,D,G). Therefore, we conclude that hiPSC-derived RPEs have low immunogenicity.
Figure 4.
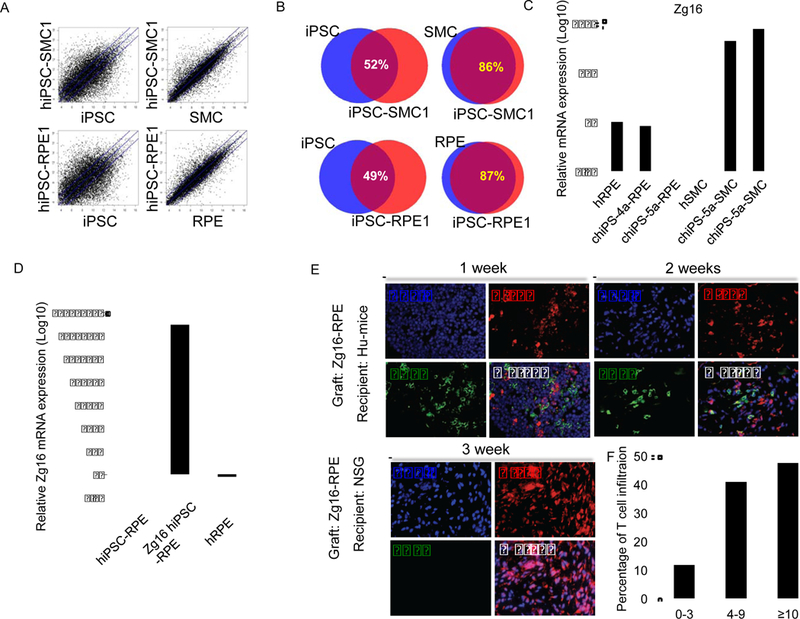
Abnormal expression of immunogenic antigens contributes to the differential immunogenicity of hiPSC-derived SMCs and RPEs. (A) The global gene expression was profiled by oligonucleotide DNA microarrays and compared between hiPSCs and hiPSC-derived SMCs (hiPSC-SMCs), human primary SMCs and hiPSC-SMCs, hiPSCs and hiPSC-derived RPEs (hiPSC-RPEs), human primary RPEs and hiPSCRPEs. The blue lines indicate the diagonal and 3-fold changes between the two samples. (B) The gene expression similarity between various samples. The overexpression of the Zg16 gene in hiPSC-SMCs (C) and RPEs differentiated from Zg16-hiPSCs (D) was confirmed by qPCR. (E) The RPE cells derived from Zg16-hiPSCs are immunogenic in Hu-mice with autologous immune system. The Zg16-RPE grafts were extensively infiltrated by T cells and mostly rejected two weeks after transplantation into the hindleg muscle of Hu-mice with autologous immune system. As a control, the same batch of Zg16-RPEs injected into the hindleg muscle of NSG mice survived three weeks after transplantation. (F) Frequency of T cell infiltration into the grafts of RPEs derived from Zg16-hiPSC in Hu-mice as quantified as Figure 2G
Figure 3.
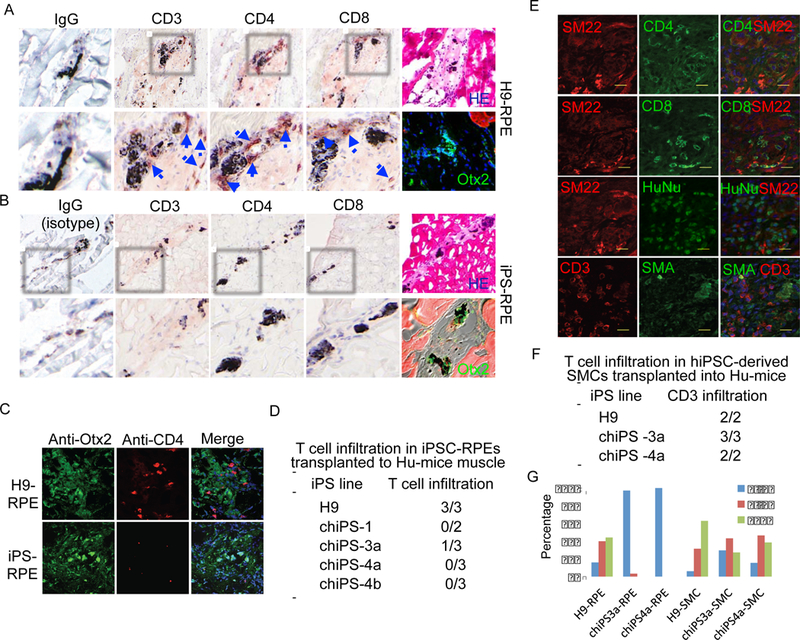
The immunogenicity of RPE cells and SMCs differentiated from hiPSCs in Hu-mice reconstituted with autologous immune system. (A) The RPEs differentiated from H9 hESCs were infiltrated with T cells five weeks after being transplanted into the hindleg muscle of the Hu-mice. Blue arrow indicates the infiltrated T cells. H&E staining of H9 derived RPE cells transplanted into the hindleg muscle of the Humice. Otx2 staining shows the well differentiated RPE cells (Green: Otx2; Blue: DAPI) transplanted into the hindleg muscle of Hu-mice. (B) The hiPSC-derived RPEs are not infiltrated with T cells five weeks after transplanting into the hindleg muscle of the Hu-mice reconstituted with autologous immune system. H&E staining of hiPSC derived RPEs transplanted into the hindleg muscle of Hu-mice. Otx2 staining shows hiPSC-derived RPEs, Red: mouse muscle. (C) The co-staining of anti-Otx2 and anti-CD4 antibodies confirmed the status of T cell infiltration into the allogenic hESC-derived and autologous hiPSC-derived RPEs transplanted into the Hu-mice. Green: Otx2; Red: CD4; Blue: DAPI. (D) Summary of T cell infiltration into RPE cells differentiated from four independent hiPSCs reprogrammed from three donors after transplanting into Hu-mice reconstituted with autologous human immune system. (E) SMCs differentiated from hiPSCs in vitro were immunogenic in Hu-mice reconstituted with autologous immune system. hiPSC-derived SMCs were transplanted into hindleg muscle of Hu-mice reconstituted with autologous human immune system. Five weeks later, the transplants were harvested and stained with indicated antibodies, showing infiltration of T cells into the SMC grafts. SM22 and SMA, smooth muscle markers; HuNu, human nuclei. Cell nuclei were counterstained with DAPI. (F) Summary of T cell infiltration into SMCs differentiated from two independent hiPSCs reprogrammed from two donors after transplanting into Hu-mice reconstituted with autologous human immune system. (G) Frequency of T cell infiltration into the grafts of hiPSC-RPEs and hiPSC-SMCs in Hu-mice with autologous immune system. Sections of grafts were stained with anti-CD3 antibody. Three sections from each graft were analyzed. At least 10 frames (20x) from each slide were randomly chosen for analysis. The numbers of T cells detected were categorized into three groups: 0–3, 4–9 or ≥10 T cells per frame.
The studies of teratoma formed by hiPSCs in Hu-mice suggest that SMCs derived from hiPSCs might be immunogenic to the autologous immune system. To further investigate the immunogenicity of SMCs differentiated from autologous hiPSCs, we efficiently derived SMCs from hiPSCs, using established protocols (Huang et al., 2006). The hiPSC-derived SMCs expressed SMC-specific markers and were associated with vascular networks in vitro and in vivo, validating their functionality as SMCs (Figure S4H–K). In addition, the profile of the global gene expression showed high similarity between hiPSC-derived SMCs and primary human SMCs, further confirming the identity of hiPSC-derived SMCs. The hiPSCderived SMCs were transplanted into the skeletal muscle of Hu-mice reconstituted with autologous human immune system. When analyzed by serial sections, the hiPSC-derived SMC grafts were uniformly infiltrated with T cells, confirming that hiPSC-derived SMCs are immunogenic to autologous human immune system (Figure 3E,F,G).
Misexpression of immunogenic antigens induces T cell responses to hiPSC-derived SMCs
The overexpression of IL-10 has been shown to induce immune tolerance of mouse iPSC-derived endothelial cells in syngeneic recipients (de Almeida et al., 2014). However, the immune tolerance of hiPSC derived RPEs is not due to the lack of HLA expression nor the overexpression of immune suppressive cytokines such as IL-10 (Figure S3). To understand the mechanism underlying the differential immunogenicity of hiPSC-derived RPEs and SMCs, we screened these cells for the expression of potential immunogenic antigens. While the global gene expression of hiPSC-derived SMCs and RPEs exhibited close to 90% similarity to their primary counterparts, we found that the expression of Zg16, an immunogenic antigen, was greatly increased in hiPSC-derived SMCs but not in hiPSC-derived RPEs (Figure 4C). In addition, another immunogenic antigen Hormad1 was also overexpressed in the hiPSCderived SMCs (Figure S4G). To determine whether the abnormal expression of immunogenic antigens induces the immunogenicity of hiPSC-derived cells, we ectopically expressed Zg16 in iPSCs (Zg16-hiPSCs). This blocked the teratoma formation of Zg16-hiPSCs in Hu-mice with autologous immune system, suggesting that Zg16-hiPSCs are immune rejected (Figure 1C). More importantly, in contrast to hiPSCRPEs that are immune protected from autologous immune system, RPEs differentiated from Zg16-hiPSCs expressed Zg16 and were immune rejected by autologous immune system (Figure 4D–E). Together with previous findings that the abnormal expression of these immunogenic antigens (Zg16 and Hormad1) can directly induce antigen-specific T cell responses to mouse iPSC-derived cells transplanted in syngeneic mice (Zhao et al., 2011), these data support the notion that the abnormal expression of immunogenic antigens in hiPSC-derived SMCs induces their immunogenicity to autologous human immune system.
Discussion
Using inbred mouse strains, recent studies have revealed the immunogenicity of the cells derived from mouse iPSCs (Araki et al., 2013; de Almeida et al., 2014; Zhao et al., 2011). While the debate on the levels of immunogenicity of cells derived from mouse iPSCs in syngeneic recipients is ongoing, these mouse studies support the notion that certain cells such as cardiomyocytes derived from mouse iPSCs in vitro are immunogenic in syngeneic recipients (Araki et al., 2013; de Almeida et al., 2014; Guha et al., 2013; Zhao et al., 2011). Therefore, to achieve the potential of hiPSCs in human cell therapy, it is important to evaluate the immunogenicity of the cells differentiated from hiPSCs. We demonstrated that certain hiPSC-derived cells such as RPE cells are much less immunogenic than other hiPSC-derived cell types such as SMCs. The mechanisms underlying the differential immunogenicity can be very complex, depending on the intrinsic immune characteristics of the cell-types and the impact of epigenetic abnormality of hiPSCs on differentiated cell types as previously noted (Bar-Nur et al., 2011; Bock et al., 2011; Kim et al., 2010; Kim et al., 2011; Polo et al., 2010; Ruiz et al., 2012). In support of this notion, we demonstrate that the abnormal expression of immunogenic antigens in some hiPSC-derived cells but not in others contributes to the differential immunogenicity. Consistent with this finding, immunogenic antigens are also overexpressed during the differentiation of mouse iPSCs but not during the differentiation of mouse ESCs, leading to antigen-specific T cell responses to iPSC-derived cells in syngeneic recipients (Araki et al., 2013; de Almeida et al., 2014; Zhao et al., 2011). While SMCs differentiated from hiPSCs exhibit functionalities and global gene expression profiles highly similar to normal human counterparts, the finding that the immunogenic antigens expressed in hiPSC-derived SMCs are not expressed by normal human SMCs suggests that further improvement of the robustness of the differentiation process of hiPSCs could help to reduce the immunogenicity of hiPSC-derived cells. Finally, our findings of the lack of immunogenicity of hiPSC-derived RPEs support the feasibility of developing hiPSC-derived RPE cells for treating atrophic age related macular degeneration.
EXPERIMENTAL PROCEDURES
Reconstitution of humanized mice
The NOD/SCID/γ null (NSG) mice were purchased from the Jackson Laboratories at 7 to 10 weeks of age. Human fetal thymus and liver tissues at age 17 to 20 weeks were obtained from Advanced Bioscience Resource (Alameda, CA, USA). All protocols used for human tissues and animal manipulation have been approved by University of California San Diego Research Committees on human subjects and research animal care.
Humanized mice were generated as previously described (Lan et al., 2006). Briefly, the NSG mice were first treated with sub-lethal irradiation (2 Gy), and human fetal thymus and liver pieces (1mm3) were transplanted under the kidney capsule of the irradiated NSG mice. The human CD34+ cells were simultaneously purified from the same human fetal liver using a MACS separation system with antihuman CD34+ antibody (Miltenyi Biotec, Auburn, CA, USA), and 1 to 5×105 human CD34+ cells were intravenously transfused into the same mice.
Generation of integration-free hiPSCs
The fibroblasts derived from human fetal liver were reprogrammed into hiPSCs with episomal approaches as previously described (Okita et al., 2011; Zhao et al., 2011) After puromycin selection, the transfected cells were plated on feeder layer, and the hiPSC colonies were mechanically picked, dissected into small pieces, and plated onto the feeder layer in 24-well plate. Southern blotting analysis or real time PCR was used to confirm that the hiPSCs are integration-free as described.
Histological analysis
Tissues were harvested and frozen in OCT (for immunohistochemical and fluorescent staining), or fixed in 10% buffered formalin and embedded in paraffin (for hematoxylin and eosin staining). Antibodies include polyclonal rabbit anti–human CD3 antibody (DAKO, Glostrup, Denmark), mouse anti–human CD4 mAb (RPA-T4, BD Pharmingen), mouse anti–human CD8 mAb (RPA-T8, BD Pharmingen), mouse anti– human CD123 mAb (6H6; eBioscience, San Diego, CA)
TCR repertoire analysis of infiltrating T cells
Total RNA was extracted from explanted grafts using Qiagen RNA extraction kits (Qiagen). TCRβ repertoires were amplified and sequenced using Illumina MiSeq by iRepertoire Inc (Huntsville). Data analysis was performed using the website provided by iRepertoire Inc (http://www.irepertoire.com).
Differentiation of hiPSC/hESCs into RPEs and SMCs in vitro
The differentiation of hiPSC/hESCs into RPEs and subsequent expansion were performed as previously described (Krohne et al., 2012). Briefly, hiPSC/hESCs were maintained in serum-free differentiation media consisting of Knockout DMEM (Invitrogen), 2mM glutamine (Invitrogen), 0.1mM NEAA (Invitrogen), 0.1mM ß-mercaptoethanol (Sigma-Aldrich), 100U/ml penicillin (Invitrogen), 100µg/ml streptomycin (Invitrogen), and 20% Knockout Serum Replacement (Invitrogen). Pigmented colonies began appearing after about 6 weeks. Once they reached adequate size, the colonies were manually excised and placed onto Matrigel (Becton-Dickinson) coated plates. Only very low-passage cells (P1-P3) were used in these experiments.
To express Zg16 in the hiPSC-derived RPEs, the human Zg16 cDNA was inserted downstream of the CAG promoter of an episomal vector we used for iPSC reprogramming (Zhao et al., 2011), and electroporated into human iPSC lines. The empty vector was used as a control. The hiPSC lines with stable Zg16 expression were differentiated into Zg16-expressing RPEs.
Subretinal injections
Subretinal injections were performed as described previously (Krohne et al., 2012). Briefly, the eyes from deeply anaesthetized mice were pierced with a sharp 30-gauge needle (Becton-Dickinson) just below the limbus. A blunt-tipped 33 gauge Hamilton syringe (Hamilton) was used to deliver roughly 100,000 cells in suspension into the subretinal space using a transscleral route through the diametrically opposed retina.
Supplementary Material
Acknowledgment
We thank UCSD Cancer Center pathologic core for help with histologic analysis and Ms. Shengyun Zhu for technical support. This work was supported by a grant from National Natural Science Foundation of China (81430032), grants from Chinese Ministry of Science and Technology (2013CB966900, 2013ZX10002008-002), the Strategic Priority Research Program of the Chinese Academy of Sciences to T.Z (XDA01040108), grants to M.F. from the California Institute of Regenerative Medicine (TR1-01219) and the National Eye Institute of the National Institutes of Health (EY11254), and Early Translational Awards from California Institute for Regenerative Medicine to Y.X. (TR1-01277).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
The authors declare no competing financial interests.
Reference
- Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, et al. (2013). Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 494, 100–104. [DOI] [PubMed] [Google Scholar]
- Bar-Nur O, Russ HA, Efrat S, and Benvenisty N (2011). Epigenetic Memory and Preferential Lineage-Specific Differentiation in Induced Pluripotent Stem Cells Derived from Human Pancreatic Islet Beta Cells. Cell Stem Cell 9, 17–23. [DOI] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH, et al. (2011). Reference Maps of Human ES and iPS Cell Variation Enable HighThroughput Characterization of Pluripotent Cell Lines. Cell 144, 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida PE, Meyer EH, Kooreman NG, Diecke S, Dey D, Sanchez-Freire V, Hu S, Ebert A, Odegaard J, Mordwinkin NM, et al. (2014). Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat Commun 5:3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, and Boyd AS (2013). Lack of Immune Response to Differentiated Cells Derived from Syngeneic Induced Pluripotent Stem Cells. Cell Stem Cell 12(4):407–12. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhao X, Chen L, Xu C, Yao X, Lu Y, Dai L, and Zhang M (2006). Differentiation of human embryonic stem cells into smooth muscle cells in adherent monolayer culture. Biochemical and Biophysical Research Communications 351, 321–327. [DOI] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LIR, et al. (2010). Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Hongguang H, Loh Y-H, Aryee MJ, Lensch MW, et al. (2011). Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotech 29, 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne T, Westenskow P, Kurihara T, Friedlander D, Lehmann M, Dorsey A, Li W, Zhu S, Schultz A, Wang J, et al. (2012). Generation of retinal pigment epithelial cells from small molecules and OCT4reprogrammed human induced pluripotent stem cells. Paediatr Int Child Health 1, 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Tonomura N, Shimizu A, Wang S, and Yang Y-G (2006). Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108, 487–492. [DOI] [PubMed] [Google Scholar]
- Lewitzky M, and Yamanaka S (2007). Reprogramming somatic cells towards pluripotency by defined factors. Current Opinion in Biotechnology 18, 467–473. [DOI] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K. -i., et al. (2011). A more efficient method to generate integration-free human iPS cells. Nat Meth 8, 409–412. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, and Daley GQ (2008). Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141146. [DOI] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al. (2010). Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotech 28, 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z, Wang M, Hu Z, Stradner M, Zhu S, Kong H, Yi H, Goldrath A, Yang Y-G, Xu Y, et al. (2014). An Effective Approach to Prevent Immune Rejection of Human ESC-Derived Allografts. Cell Stem Cell 14, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Diep D, Gore A, Panopoulos AD, Montserrat N, Plongthongkum N, Kumar S, Fung H-L, Giorgetti A, Bilic J, et al. (2012). Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proceedings of the National Academy of Sciences 109, 16196–16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, and Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang Z-N, Rong Z, and Xu Y (2011). Immunogenicity of induced pluripotent stem cells. Nature 474, 212–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


