Abstract
Background
Solid organ and haematopoietic stem cell transplant recipients are more vulnerable to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) than non-transplant recipients due to immunosuppression, and may pose a continued transmission risk, especially within hospital settings. Detailed case reports including symptoms, viral load and infectiousness, defined by the presence of replication-competent viruses in culture, provide an opportunity to examine the relationship between clinical course, burden and contagiousness, and provide guidance on release from isolation.
Objectives
To investigate the relationship between serial SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) cycle threshold (Ct) value or cycle of quantification value, or other measures of viral burden and the likelihood and duration of the presence of infectious virus based on viral culture, including the influence of age, sex, underlying pathologies, degree of immunosuppression, and/or vaccination on this relationship, in transplant recipients.
Methods
LitCovid, medRxiv, Google Scholar and the World Health Organization COVID-19 database were searched from 1st November 2019 to 26th October 2022. Studies reporting relevant data (results from serial RT-PCR testing and viral culture data from the same respiratory samples) for transplant recipients with SARS-CoV-2 infection were included in this systematic review: Methodological quality was assessed using five criteria, and the data were synthesized narratively and graphically.
Results
Thirteen case reports and case series reporting on 41 transplant recipients (22 renal, five cardiac, one bone marrow, two liver, one bilateral lung and 10 blood stem cell) were included in this review. A relationship was observed between proxies of viral burden and likelihood of shedding replication-competent SARS-CoV-2. Three individuals shed replication-competent viruses for >100 days after symptom onset. Lack of standardization of testing and reporting platforms precludes establishing a definitive viral burden cut-off. However, the majority of transplant recipients stopped shedding replication-competent viruses when the Ct value was >30 despite differences across platforms.
Conclusions
Viral burden is a reasonable proxy for infectivity when considered within the context of the clinical status of each patient. Standardized study design and reporting are essential to standardize guidance based on an increasing evidence base.
Keywords: COVID-19, SARS-CoV-2, Transmission, Organ transplants, Viral culture, Polymerase chain reaction, Viral load, Cycle threshold calibration, Infectivity
Introduction
Haematopoietic stem cell transplant and solid organ transplant recipients have significant immunosuppression, affecting both cellular and humoral immunity, and less favourable outcomes with severe acute respiratory virus syndrome 2 (SARS-CoV-2) infection due to immunosuppression and/or pre-existing comorbidities [1]. Immunosuppression associated with transplantation places patients at risk for prolonged carriage and shedding of several respiratory viruses [2]. However, identification of respiratory viral shedding, by reverse transcriptase polymerase chain reaction (RT-PCR), depending on the testing platform does not necessarily correlate with the presence of replication-competent virus [3]. Accordingly, this systematic review investigated RT-PCR testing and viral culture of SARS-CoV-2, focusing on patients receiving solid organ or haematopoietic stem cell transplants following a published protocol [4].
The research questions were:
-
1.
What is the correlation between serial SARS-CoV-2 RT-PCR cycle threshold (Ct) value or cycle of quantification value (Cq), or other measures of viral burden and the likelihood of producing replication-competent virus among transplant recipients?
-
2.
What is the likelihood and duration of the presence of infectious virus based on viral culture among transplant recipients with SARS-CoV-2 infection?
-
3.
What is the influence of age, sex, underlying pathologies and degree of immunosuppression on infectiousness of SARS-CoV-2?
-
4.
What is the relationship of vaccination status with infectiousness of SARS-CoV-2?
This review included studies reporting serial Ct values from sequential RT-PCR testing or other measures of viral burden, such as RNA gene copies of respiratory samples (from nasopharyngeal or throat specimens), along with viral culture data on the same samples from patients with SARS-CoV-2 infection who were about to receive a transplant or who were post-transplant.
Methods
Search strategy
LitCovid, medRxiv, Google Scholar and the World Health Organization COVID-19 database were searched from 1st November 2019 until 26th October 2022. No language restrictions were applied.
The literature search terms were: (coronavirus OR covid-19 OR SARS-CoV-2) AND (immunosuppressed OR immunocompromised OR transplant OR immunosuppression OR ‘immune deficient’ OR HIV) AND (CPE OR ‘cytopathic effect’ OR ‘viral culture’ OR ‘virus culture’ OR vero OR ‘virus replication’ OR ‘viral replication’ OR ‘cell culture’ or ‘viral load’ OR ‘viral threshold’ OR ‘log copies’ OR ‘cycle threshold’). The search strategy is available in Supplementary File 2 (see online supplementary material).
Screening
Four reviewers (JB, SM, ER, ES) screened titles and abstracts independently to identify studies for full-text screening. Subsequently, full-text screening was performed in duplicate, and disagreements were resolved with assistance from a fifth reviewer (TJ).
Inclusion criteria
This review included studies reporting serial Ct values from sequential RT-PCR testing, or RNA gene copies of respiratory samples (nasopharyngeal, throat, sputum, bronchoalveolar lavage, endotracheal tube secretions), and viral culture data from the same samples from patients with SARS-CoV-2 infection who were about to receive a transplant or were post-transplant. Primary studies were included provided that they reported sufficient information to extract quantitative data on PCR testing and viral culture for each included individual. Studies that included transplant and non-transplant recipients were included if the results could be examined separately. Studies that reported in poster or abstract form alone were excluded. Reviews were excluded, but the reference lists of reviews were screened for potential relevant primary studies, and the bibliographies of included primary studies were hand-searched for possible studies for inclusion.
Exclusion criteria
Studies that used post-mortem samples alone or non-respiratory samples alone were excluded from this review. In addition, studies of non-transplant recipients or those not attempting viral cultures were excluded.
Data extraction
One reviewer (ER) extracted data, and this was checked independently by a second reviewer (ES). Disagreements were arbitrated by a third reviewer (TJ). Data were extracted on study type and study characteristics, including population, setting, sampling and laboratory methods, clinical information, prescribed treatments, vaccination status, laboratory findings and clinical outcomes. Where data were only available in figures or charts, these were estimated by two reviewers (ES, SM) and cross-checked by another reviewer (CH). For three studies, clarification was sought from the corresponding authors.
Quality assessment
The assessment of bias was based on the updated QUADAS-2 criteria for assessing the diagnostic accuracy of studies [5]. The following five criteria were developed based on patient identification and reporting, timing, and index and reference tests:
-
1.
Were the criteria for diagnosing a case reported clearly and appropriate?
-
2.
Was the reporting of patient/population characteristics, including clinical symptoms, treatments with degree of immunosuppression and outcomes, adequate?
-
3.
Was the study period, including follow-up, sufficient for adequate assessment of any potential relationship between viral burden measures and likelihood of producing replication-competent virus and the rise in neutralizing antibodies? Sufficient was defined as more than one observation.
-
4.
Were the methods used to obtain RT-PCR results replicable, generalizable and appropriate? It was considered that each study should establish the relationship between their Ct values and the target gene copy number, using internal standards.
-
5.
Were the methods used to obtain viral culture results replicable and appropriate? It was considered that the methods used should, at a minimum, include a description of specimen sampling and management, preparation, media and cell line used, exclusion of contamination or co-infection (use of controls and appropriate antibacterials and antimycotics in the cell culture and use of gene sequencing if available), and results of inspection of culture.
One reviewer (ES) assessed the quality of reporting, and this was verified independently by a second reviewer (ER). Disagreements were resolved through discussion. If agreement could not be reached, a third reviewer (TJ) arbitrated.
Data reporting and pooling
Study flow was reported according to the PRISMA reporting standards (see Supplementary File 3 [6]. Study characteristics, including age, sex, clinical symptoms, treatments and events in the participants, were reported in tabular form. Data on disease burden measures and viral culture were reported in tabular form. For studies reporting more than one patient participant, data were extracted related to each participant if available. Medians, interquartile ranges (IQR) and outliers for viral culture results in relation to symptom duration were plotted, and individual study plots of viral culture results and Ct values were produced to day 120.
It was not possible to meta-analyse the data on PCR cycle counts/RNA log copies and viral culture due to a lack of detailed information on laboratory practices and assays, the absence of internal controls in some studies, and heterogeneous sampling. Therefore, the studies were reviewed narratively, and, where possible, the results were presented graphically within the limitations noted. The relationship between Ct values, days since symptom onset and likelihood of shedding replication-competent virus was analysed by presenting the data on a scatter plot.
Results
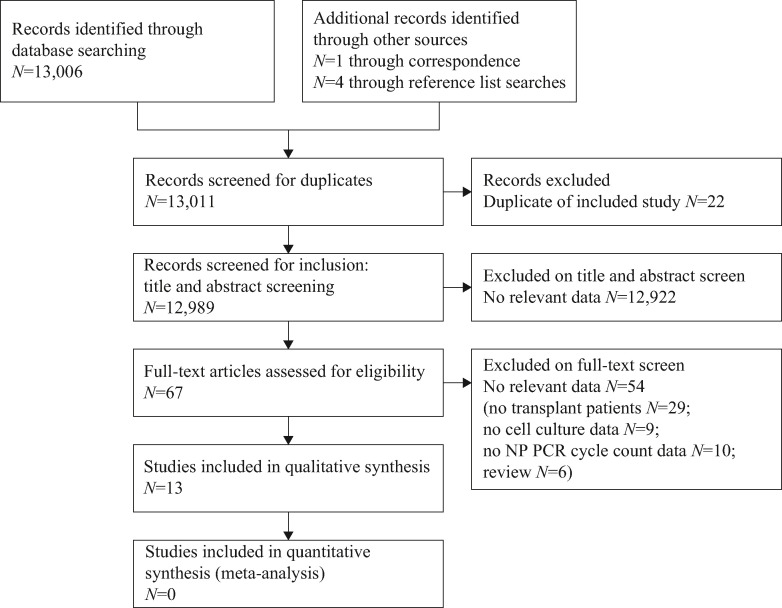
The literature search identified 12,989 titles for screening (Figure 1 ). Of these, 67 underwent full-text review. A total of 54 studies were excluded after full-text analysis; the reasons for exclusion are reported in Supplementary File 1 (see online supplementary material).
Figure 1.
PRISMA flow chart of study screening for inclusion. NP, nasopharyngeal; PCR, polymerase chain reaction.
The characteristics of the 13 included studies are shown in Table S1 (see online supplementary material). In total, they reported data for 41 transplant recipients (eight females and 25 males); sex was not reported for eight patients in one study [7]. The studies were performed in nine countries: Austria [8], Brazil [9], Canada [10], Denmark [11], France [12,13], Germany [[14], [15], [16]], Saudi Arabia [17], the USA [7,18] and the UK [19]. The ages of participants ranged from 26 to 77 years.
Thirty-nine patients were infected with SARS-CoV-2 post-transplant. Twenty-two patients in four studies had undergone kidney transplantation [12,16,17,19], five patients in four studies had undergone cardiac transplantation [10,13,14,17], one patient had undergone bone marrow transplantation for multiple myeloma [11], one patient had undergone liver transplantation [11], and 10 patients in three studies had undergone haematopoietic cell transplantation [7,18,19]. Two patients were infected with SARS-CoV-2 and subsequently underwent transplantation: one underwent liver transplantation [15], and one underwent bilateral lung transplantation after SARS-CoV-2 infection that affected the lungs severely [8].
Typically, patients received a mixture of antivirals, antibiotics, convalescent plasma and immunosuppressants, as reported in Table S1 (see online supplementary material). Anti-SARS-CoV-2 vaccination status was not reported for any of the included patients. The clinical course of COVID-19 varied widely amongst the included patients, from mild COVID-19-related symptoms to severe pneumonia and lung failure; no deaths were reported specifically for this group, although one study reported the deaths of four patients within 30 days of diagnosis [7]. Prescribed treatments reflected the variation in severity.
Quality assessment
Table I shows the quality of studies included in this review, based on five criteria. Four studies met all five criteria [9,10,13,16]. Follow-up was judged to be adequate in all but one study, which was a diagnostic test comparison [19]. In nine studies, the reporting of patient characteristics was sufficiently comprehensive [[7], [8], [9], [10], [11], [12],[14], [15], [16]], and clinical information was not available for two studies [17,19]. A case definition was missing or unclear in four studies [7,8,14,17], and methods for RT-PCR testing were unclear in three studies [7,8,14]. The methods used for viral culture were unclear in four studies [8,14,15,17], and one study reported using a cell line that has not typically been used to demonstrate SARS-CoV-2 growth (Buffalo green monkey kidney cell line) [12].
Table I.
Quality of included studies
| Study ID | Were the criteria for diagnosing a case clearly reported and appropriate? | Was the reporting of patient/population characteristics adequate? | Was the study period, including follow-up, sufficient? | Were the methods used to obtain RT-PCR results replicable and appropriate? | Were the methods used to obtain viral culture results replicable and appropriate? |
|---|---|---|---|---|---|
| Alshukairi et al. [17] | Uncleara | Nob | Yes | Yes | Unclear |
| Aydillo et al. [7] | Unclear | Yes | Yes | Unclear | Yes |
| Benotmane et al. [12] | Yes | Yes | Yes | Yes | Noc |
| Decker et al. [14] | No | Yes | Yes | Unclear | Unclear |
| Han et al. [18] | Yes | Yes | Yes | Yes | Unclear |
| Lang et al. [8] | Yes | Yes | Yes | Unclear | Unclear |
| Mendes-Correa et al. [9] | Yes | Yes | Yes | Yes | Yes |
| Niess et al. [15] | Yes | Yes | Yes | Yes | Unclear |
| Niyonkuru et al. [11] | Noa | Yes | Yes | Yes | Yes |
| Pickering et al. [19] | Yes | Unclear | Unclear | Yes | Yes |
| Rajakumar et al. [10] | Yes | Yes | Yes | Yes | Yes |
| Tarhini et al. [13] | Yes | Yes | Yes | Yes | Yes |
| Weigang et al. [16] | Yes | Yes | Yes | Yes | Yes |
RT-PCR, reverse transcription polymerase chain reaction.
Case definition unclear, article reported positive RT-PCR, but cycle threshold cut-off was not reported.
Data on clinical symptoms lacking.
The cell line used was not one that is demonstrated to support the growth of severe acute respiratory syndrome coronavirus-2.
Results of the studies
Details of patient characteristics, clinical course, treatments, PCR and viral culture for transplant recipients in each individual study are reported in Tables S1 and S2 (see online supplementary material). Study results are also presented in Figure 2 (SARS-CoV-2 culture results in transplant recipients from day of symptom onset), Figure 3 (duration of infectivity as indicated by viral culture and corresponding PCR cycle counts/log copies among transplant recipients) and Figure 4 (relationship between Ct value and symptom onset).
Figure 2.
Severe acute respiratory syndrome coronavirus-2 culture results in transplant recipients from days of symptom onset. Culture positive (N=68 samples): median 18 [interquartile range (IQR) 8–29] days, range 1–169 days, mean 37.1 days. Culture negative (N=116 samples): median 40 (IQR 21–60) days, range 1–218 days, mean 45.2 days.
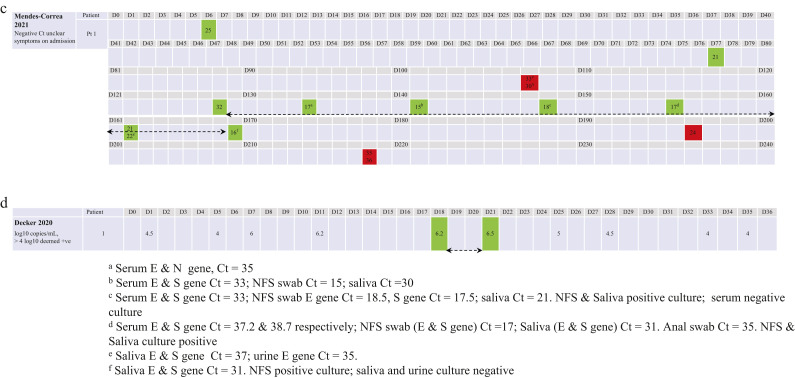
Figure 3.
Duration of infectivity as indicated by viral culture and corresponding reverse transcription polymerase chain reaction (RT-PCR) cycle count/log copies among transplant recipients. (a) Alshukairi et al., Benotmane et al., Rajakumar et al., Niyonkuru et al. and Pickering et al. (days 1–42). (b) Han et al. (days 1–86), Aydillo et al. (days 1–90), Tarhini et al. and Weigang et al. (days 1–120). (c) Mendes-Correa et al. (days 1–218). Red indicates negative culture, green indicates positive culture, and the number in each red/green box indicates the RT-PCR cycle threshold (Ct). (d) Duration of infectivity and log10 copies/mL among transplant recipients. Decker et al. (days 1–36). Green indicates positive culture, and the number in each green box indicates log copies/mL. NFS, nasopharyngeal swab.
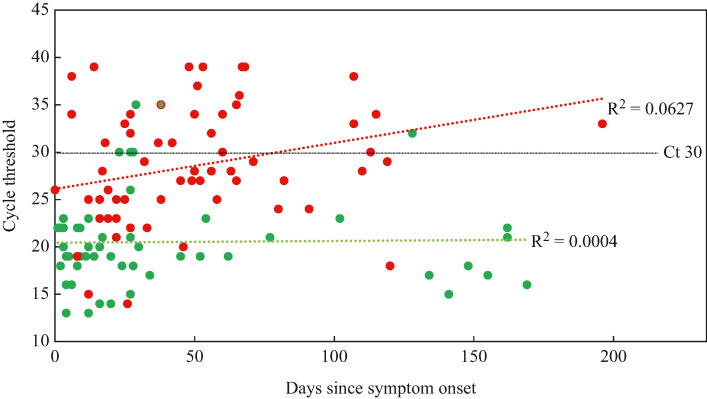
Figure 4.
Relationship between cycle threshold (Ct), time since symptom onset (days) and likelihood of shedding replication-competent virus. Red indicates negative culture, green indicates positive culture.
The clinical course of infection was highly variable. The time between transplant receipt and COVID-19 infection varied from days to years [7,10]. Sampling schedules varied between studies, with no regular timetable of testing taking place, so results for PCR and viral culture are available for different time points in a patient's clinical course and with different gaps in time between samples being taken.
Figure 2 shows that the median interval between symptom onset and identification of a positive viral culture was 18 days (IQR 8–29; range 1–169 days, based on 68 cultures performed). The median interval for a negative culture was 40 days and the mean was 40.2 days (IQR 21–60; range 1–218 days, based on 116 cultures performed).
Alshukairi et al. (Figure 3 a) reported no positive viral cultures among samples collected from cardiac/renal transplant recipients between 9 and 26 days after COVID-19 symptom onset [17]. Aydillo et al. (Figure 3b) reported repeated viral culture tests for eight stem cell transplant recipients, with fluctuations in and out of shedding replication-competent virus [7]. Decker et al. (Figure 3d) reported that the samples from a heart transplant recipient showed a positive viral culture on days 18 and 21 [14]. Han et al. (Figure 3b) reported that COVID-19 symptoms fluctuated over several weeks in a young male adult transplant recipient, with a positive viral culture 54 days post-symptom onset [18].
Mendes-Correa et al. (Figure 3c) described a case whose symptoms led to three hospital admissions over several months, with nasopharyngeal swabs positive on RT-PCR over 163 days; viral cultures of these swabs were positive in nine of 12 tests, with the latest positive culture found 169 days after COVID-19 symptom onset [9]. Positive viral cultures were found in nasopharyngeal swabs from two transplant recipients reported by Niyonkuru et al. (Figure 3a), indicating a duration of infectiousness of approximately 2 weeks [11]. Pickering et al. (Figure 3a) reported data for a renal transplant recipient with severe COVID-19 whose respiratory samples tested positive by RT-PCR for 12 days, and with a positive viral culture for the day 3 sample but no later samples [19].
Rajakumar et al. (Figure 3a) described two cardiac transplant recipients: viral culture found replication-competent virus in samples from one patient on day 16; and in samples from the other patient on day 4 and repeatedly up to day 27, after which all viral cultures were negative [10].
For each patient, viral culture was negative in samples with Ct values >25. Within the samples showing positive viral cultures, the PCR results showed that the Ct value for the N gene was lower than the Ct value for the E gene by an average of 5.4. A cardiac transplant recipient described by Tarhini et al. tested culture positive with a Ct value of 23 on day 103 (Figure 3b); all other viral cultures were negative from samples with Ct values of 18–>40 [13].
Weigang et al. described a kidney transplant recipeint who experienced three hospital admissions [16]. During the first admission (day 0 to day 72), 19 RT-PCR tests were performed, and viral cultures were performed alongside, showing eight of 19 positive cultures (Ct values ranging from 15 to 25) and 11 of 19 negative (Ct values from 25 to 30). The patient was culture positive again on day 105 (Ct value=23). After re-admission on day 140, the patient was still RT-PCR positive, but was viral culture negative; he was treated for 10 days (days 141–149) with remdesivir. Subsequently, negative RT-PCR tests until day 189 and negative cultures suggested that the infection had resolved (Figure 3b).
The data from the study by Benotmane et al. appear to be outliers (Figure 3a), as five positive viral results were reported from samples with Ct values ≥30 [12]. However, the cell line used in this study has not been demonstrated to readily support SARS-CoV-2 growth. In all other studies, despite the use of a minimum of 10 different PCR platforms and different culture techniques, viral cultures were unsuccessful in samples with Ct values >30.
Figure 3a–d, of all the viral culture data points available from the included studies, shows the wide range of duration of COVID-19 disease course across these 41 transplant recipients, and suggests a correlation between viral burden (measured as log copies or Cq/Ct) and probable infectiousness as indicated by observing a positive viral culture. The data are too few and heterogeneous to allow combination to make a summary assessment, but a general trend is observable in the figures showing that most samples with Ct values <30 do generate a positive viral culture, whilst many samples with Ct values > 30 are unlikely to generate a positive viral culture. The viral load estimates appear to be related to the administration of courses of antiviral treatment including remdesivir [Figure 3a (Ct/Cq) and 3b (log copies)].
Prolonged shedding of replication-competent virus was associated with alternating increases and decreases in viral burden over time, which in some cases may be up to approximately 100 days [13,16]. Figure 4 shows a scatter plot of Ct value vs time since symptom onset, and indicates whether positive or negative viral cultures were obtained using these samples; this clearly displays a trend that higher Ct value samples were less likely to produce a positive viral culture, and lower Ct value samples were more likely to produce a positive viral culture.
The magnitude and robustness of the correlation is difficult to assess because laboratory methods differed; it was not possible to pool the data to produce a summary cut-off value for infectiousness due to the variations in testing platforms and varying time windows for sampling from patients (see Figure 2 and Table S2, see online supplementary material).
Discussion
This review included 13 studies that used viral culture and RT-PCR testing among 41 transplant recipients with immunosuppressive treatment who experienced COVID-19 infection. In response to Research Question 1, the evidence indicates a relationship between indicators of viral burden (Ct, Cq or RNA log copies) and probable infectiousness as indicated by the presence of replication-competent virus. The presence of replication-competent virus reflects the highest grade of evidence supporting the capability for forward transmission of SARS-CoV-2 [20,21], and suggests that some transplant recipients remain potentially infectious over a period of months.
Gaps in the data remain, with variable methods and reporting, and it has not been possible to establish summary estimates of the relationship. The data show a long-term rise and fall of viral burden associated with the likelihood of infectiousness that appears to be a sequential pattern of a vacillating state of infectiousness in some transplant recipients. Replication-competent virus was most commonly observed in samples with Ct values <25; one study was an exception to this, reporting viable virus at a Ct value >30, but the use of a cell line not typically used for SARS-CoV-2 isolation makes interpretation unclear [12]. The duration of viral RNA shedding was variable, with the longest duration reported at 169 days [9].
The findings of this review suggest that a Ct value ≥30 indicates low likelihood of the presence of replication-competent virus, consistent with findings from a recent review on fomite transmission of SARS-CoV-2 [22]. This replicable observation suggests that a Ct value of 30, regardless of the PCR testing platform used, may be a useful and reasonable proxy to rule out infectious SARS-CoV-2, as there is a consistent correlation between a rising Ct value and the likelihood of isolating replication-competent virus. Such a value would be useful to guide clinicians managing these difficult patients, particularly if there were repeated values in this range. At Ct values <30, clinicians may choose to repeat nasopharyngeal or throat swabs to assess the direction of the Ct values to allow a more dynamic assessment which, taken in conjunction with the clinical status, may facilitate decision making for isolation or antiviral treatment considerations.
It was not possible to address the influence of age, sex, underlying pathologies and degree of immunosuppression on infectiousness (Research Question 3): at present, the heterogeneity and limited amount of available data preclude answering this question. The authors were also unable to answer Research Question 4 on the effect of vaccination status on infectiousness, because no studies reported the vaccination status of the transplant recipeints. Immunizations, and differing virus variants, may mean that early studies are less applicable to current practice; however, no evidence is available to evaluate this.
Variability in the clinical course of SARS-CoV-2 infection among transplant recipients has been reported previously, including observed prolonged viral shedding [23]. Antiviral drugs may impact on these observations, especially symptoms and viral burden [24].
One well-designed study on immunosuppressed patients, which was not included in this review as disaggregated data solely for transplant recipients were not fully available, supports these conclusions [25]. While this review was limited to transplant recipients, evidence suggests that similar prolonged viral cultures are found in immunosuppressed cancer patients. The authors plan to perform a further review in this group, analysing the type of cancer and the impact of immunotherapy on viral culture findings.
The transplant recipient population is of particular importance. Clinicians need guidance regarding when to release the patient from quarantine or isolation, given the heavy burden of immunosuppression. This review has aimed to narrow the uncertainty and offer some general guidance regarding when patients are unlikely to be shedding replication-competent virus, but clinical assessment of each patient must inform that decision because each patient and setting is different.
The strengths of this review are that it followed a published protocol, exhaustive literature searches were undertaken, data extraction and quality assessment were double checked, and there was a high level of input of clinical and epidemiological expertise to deliberate the findings. It was possible to include data from an additional eight transplant recipients after correspondence with the study authors [7]. Limitations include the small number of studies with viral culture and serial viral load estimates among transplant recipients, high variability in study design and reporting, and the inability to pool results due to the well-known variability in sensitivity across assays [26]. Some of the data were extracted directly from figures in published papers, and these estimates may have been inaccurate.
Case series are conventionally considered low in the evidence hierarchy, as they may entail inherent bias in the selection of study participants and therefore have limited generalizability; however, in this review, they were essential to provide the detailed reports needed for this unusual patient group. The case reports included here comprise some of the most detailed longitudinal reports of this patient group for whom data are needed. The evidence base is limited, however, by heterogeneous design and reporting within the studies, with different observation windows for reporting of viral burden and culturability or clinical characteristics of patients. Vaccinations and differing virus variants may mean that early studies are less applicable to current practice; however, no evidence is available to evaluate this.
In addition to providing appropriate care for the individual patient, ongoing transmission of SARS-CoV-2 is a concern, and immunosuppressed individuals may pose a challenge by experiencing prolonged carriage of the virus that could lead to forward transmission. Based on the findings of this review, the following general guidance is offered to clinicians.
Physicians who are experienced with these immunosuppressed patient populations should work with public health and infection prevention and control to direct their isolation and quarantine requirements in the community and the healthcare setting, respectively. Infectious patients with immunosuppressive treatment following solid organ or stem cell transplantation should be isolated until at least two consecutive respiratory specimens collected ≥24 h apart demonstrate a rising Ct value (i.e. indicating diminishing viral burden) in conjunction with assessment of their clinical status. After discharge, they should be followed up closely for chronic or vacillating SARS-CoV-2 infection for several weeks to months, depending on the individual clinical scenario.
Standardization of methods is needed when obtaining data; each laboratory should use consistently applied platforms with suitable internal standards to calibrate the relationship between Ct value and genome copy in these patient populations.
Publication of the results of case series or other longitudinal studies should be reported in a standardized format to avoid loss of data. It is suggested that observation windows should be within a short range of 3–7 days during the acute periods post-transplantation, and during periods of rejection when higher doses of immunosuppressants are employed, depending on clinical circumstances. Each observation window should include a summary of symptoms and interventions, report the Ct value and, for samples with Ct values <30, report attempts at viral culture if available. Descriptions of patients should include past medical histories and details of treatments received. Observed drug interactions should be highlighted. Reasons for admission, discharge and changes in isolation should be reported clearly. To investigate the duration of viral shedding, studies should report the time between the first positive viral culture and the first negative viral culture.
With additional data gathering and standardization of methods, it will be possible for transplant physicians and infection prevention and control personnel to develop evidence-based approaches to deal with these patients for the benefit of the patients, their families and the community at large.
Finally, differences in viral persistence and replication between transplant recipients and the general population demonstrate once again the protective value of an intact and fully functional immune system.
Acknowledgements
The authors wish to acknowledge the contributions of Drs Mini Kamboj and Jeroen van Kampen who provided additional data from their studies and helped with the progression of this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2022.11.018.
Author contributions
TJ, CH and JC designed the study. JB performed the literature searches. JB, TJ, SM, ER and ES screened the studies for eligibility and performed data extraction. Additional expertise on clinical and laboratory issues was given by DE, JC, SM and ER. CH generated the data figures. All authors contributed to interpreting and writing up the results and conclusions.
Conflict of interest statement
TJ's competing interests are accessible at: https://restoringtrials.org/competing-interests-tom-jefferson.
CJH holds grant funding from the National Institute for Health Research (NIHR), NIHR School of Primary Care Research, NIHR BRC Oxford and the World Health Organization (WHO) for a series of Living rapid reviews on the modes of transmission of SARS-CoV-2 (WHO Registration No. 2020/1077093). He has received financial remuneration from an asbestos case and given legal advice on mesh and hormone pregnancy test cases. He has received expenses and fees for his media work including occasional payments from BBC Radio 4 Inside Health and The Spectator. He receives expenses for teaching evidence-based medicine and is also paid for his general practice work in NHS out of hours (contract Oxford Health NHS Foundation Trust). He has also received income from the publication of a series of toolkit books, and for appraising treatment recommendations in non-NHS settings. He is the Director of CEBM and is an NIHR Senior Investigator.
DE holds grant funding from the Canadian Institutes for Health Research and Li Ka Shing Institute of Virology relating to the development of COVID-19 vaccines, as well as the Canadian Natural Science and Engineering Research Council concerning COVID-19 aerosol transmission. He is a recipient of WHO and Province of Alberta funding, which supports the provision of BSL3-based SARS-CoV-2 culture services to regional investigators. He also holds public and private sector contract funding relating to the development of poxvirus-based COVID-19 vaccines, SARS-CoV-2 inactivation technologies, and serum neutralization testing.
JMC holds grants from the Canadian Institutes for Health Research on acute and primary care preparedness for COVID-19 in Alberta, Canada and was the primary local investigator for a Staphylococcus aureus vaccine study funded by Pfizer, for which all funding was provided solely to the University of Calgary. He is co-investigator on a WHO-funded study using integrated human factors and ethnographic approaches to identify and scale innovative infection prevention and control guidance implementation supports in primary care with a focus on low-resource settings and the use of drone aerial systems to deliver medical supplies and personal protective equipment to remote First Nations communities during the COVID-19 pandemic. He also received support from the Centers for Disease Control and Prevention to attend an Infection Control Think Tank Meeting. He is a member and Chair of the WHO Infection Prevention and Control Research and Development Expert Group for COVID-19, and a member of the WHO Health Emergencies Programme Ad-hoc COVID-19 IPC Guidance Development Group, both of which provide multi-disciplinary advice to WHO and for which no funding is received and from which no funding recommendations are made for any WHO contracts or grants. He is also a member of the Cochrane Acute Respiratory Infections Working Group.
JB is a major shareholder in the Trip Database search engine (www.tripdatabase.com) as well as being an employee. In relation to this work, Trip has worked with a large number of organizations over the years, none of which have any links with this work. The main current projects are with AXA and SARS-CoV-2 (WHO Registration 2020/1077093–0). JB is part of the review group carrying out rapid reviews for Collateral Global. He worked on a Living rapid literature review on the modes of transmission of SARS-CoV-2 and a scoping review of systematic reviews and meta-analyses of interventions designed to improve vaccination uptake (WHO Registration No. 2021/1138353–0).
ECR was a member of the European Federation of Neurological Societies/European Academy of Neurology Scientist Panel, Subcommittee of Infectious Diseases from 2013 to 2017. Since 2021, she has been a member of the International Parkinson and Movement Disorder Society Multiple System Atrophy Study Group, the Mild Cognitive Impairment in Parkinson Disease Study Group, and the Infection Related Movement Disorders Study Group. She was an external expert and sometimes rapporteur for COST proposals (2013, 2016, 2017, 2018, 2019) for neurology projects. She is a Scientific Officer for the Romanian National Council for Scientific Research.
AP holds grants from NIHR School for Primary Care Research.
SM has worked as a pharmacist for the Italian National Health System since 2002, and has been a member of one of the three institutional review boards of Emilia-Romagna Region (Comitato Etico Area Vasta Emilia Centro) since 2018.
IJO and EAS have no conflicts of interest to disclose.
Funding source
This work was supported by NIHR School for Primary Care Research (Project 569) and the University of Calgary. The views expressed are those of the authors and not necessarily those of NIHR or the Department of Health and Social Care.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jering K.S., McGrath M.M., McCausland F.R., Claggett B., Cunningham J.W., Solomon S.D. Excess mortality in solid organ transplant recipients hospitalized with COVID-19: a large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant. 2022;36 doi: 10.1111/ctr.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen K.M., Bates B.A., Rashidi E.S., Olex A.L., Mannon R.B., Patel R.C., et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022;4:e33–e41. doi: 10.1016/S2665-9913(21)00325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández-Ruiz M., Aguado J.M. Severe acute respiratory syndrome coronavirus 2 infection in the stem cell transplant recipient – clinical spectrum and outcome. Curr Opin Infect Dis. 2021;34:654–662. doi: 10.1097/QCO.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jefferson T., Spencer E.A., Maltoni S., Brassey J., Onakpoya I.J., Rosca E.R., et al. Viral cultures, PCR cycle threshold values and viral load estimation for COVID-19 infectious potential assessment in transplant patients: systematic review – Protocol Version 30 December 2021. medRxiv. 2021;12 [Google Scholar]
- 5.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 6.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aydillo T., Gonzalez-Reiche A.S., Aslam S., van de Guchte A., Khan Z., Obla A., et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang C., Jaksch P., Hoda M.A., Lang G., Staudinger T., Tschernko E., et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020;8:1057–1060. doi: 10.1016/S2213-2600(20)30361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendes-Correa M.C., Ghilardi F., Salomão M.C., Villas-Boas L.S., de Paula A.V., Tozetto-Mendoza T.R., et al. SARS-CoV-2 shedding, infectivity and evolution in an immunocompromised adult patient. medRxiv. 2021;6 doi: 10.1590/S1678-9946202466028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajakumar I., Isaac D.L., Fine N.M., Clarke B., Ward L.P., Malott R.J., et al. Extensive environmental contamination and prolonged severe acute respiratory coronavirus-2 (SARS CoV-2) viability in immunosuppressed recent heart transplant recipients with clinical and virologic benefit with remdesivir. Infect Control Hosp Epidemiol. 2022;43:817–819. doi: 10.1017/ice.2021.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niyonkuru M., Pedersen R.M., Assing K., Andersen T.E., Skov M.N., Johansen I.S., et al. Prolonged viral shedding of SARS-CoV-2 in two immunocompromised patients, a case report. BMC Infect Dis. 2021;21:743. doi: 10.1186/s12879-021-06429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benotmane I., Risch S., Doderer-Lang C., Caillard S., Fafi-Kremer S. Long-term shedding of viable SARS-CoV-2 in kidney transplant recipients with COVID-19. Am J Transplant. 2021;21:2871–2875. doi: 10.1111/ajt.16636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarhini H., Recoing A., Bridier-Nahmias A., Rahi M., Lambert C., Martres P., et al. Long-term severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis. 2021;223:1522–1527. doi: 10.1093/infdis/jiab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decker A., Welzel M., Laubner K., Grundmann S., Kochs G., Panning M., et al. Prolonged SARS-CoV-2 shedding and mild course of COVID-19 in a patient after recent heart transplantation. Am J Transplant. 2020;20:3239–3245. doi: 10.1111/ajt.16133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niess H., Börner N., Muenchhoff M., Khatamzas E., Stangl M., Graf A., et al. Liver transplantation in a patient after COVID-19 – rapid loss of antibodies and prolonged viral RNA shedding. Am J Transplant. 2021;21:1629–1632. doi: 10.1111/ajt.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigang S., Fuchs J., Zimmer G., Schnepf D., Kern L., Beer J., et al. Within-host evolution of SARS-CoV-2 in an immunosuppressed COVID-19 patient as a source of immune escape variants. Nat Commun. 2021;12:6405. doi: 10.1038/s41467-021-26602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alshukairi A.N., Tolah A.M., Dada A., Al-Tawfiq J.A., Almagharbi R.S., Saeedi M.F., et al. Test-based de-isolation in COVID-19 immunocompromised patients: cycle threshold value versus SARS-CoV-2 viral culture. Int J Infect Dis. 2021;108:112–115. doi: 10.1016/j.ijid.2021.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han A., Rodriguez T.E., Beck E.T., Relich R.F., Udeoji D.U., Petrak R., et al. Persistent SARS-CoV-2 infectivity greater than 50 days in a case series of allogeneic peripheral blood stem cell transplant recipients. Curr Probl Cancer Case Rep. 2021;3 doi: 10.1016/j.cpccr.2021.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering S., Batra R., Merrick B., Snell L.B., Nebbia G., Douthwaite S., et al. Comparative performance of SARS-CoV-2 lateral flow antigen tests and association with detection of infectious virus in clinical specimens: a single-centre laboratory evaluation study. Lancet Microbe. 2021;2:e461–e471. doi: 10.1016/S2666-5247(21)00143-9. Erratum in: Lancet Microbe 2021;2:e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferson T., Spencer E.A., Brassey J., Onakpoya I.J., Rosca E.C., Plüddemann A., et al. Transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) from pre and asymptomatic infected individuals: a systematic review. Clin Microbiol Infect. 2022;28:178–189. doi: 10.1016/j.cmi.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferson T., Heneghan C.J., Spencer E., Brassey J., Plüddemann A., Onakpoya I., et al. A hierarchical framework for assessing transmission causality of respiratory viruses. Viruses. 2022;14:1605. doi: 10.3390/v14081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onakpoya I.J., Heneghan C.J., Spencer E.A., Brassey J., Rosca E.C., Maltoni S., et al. Viral cultures for assessing fomite transmission of SARS-CoV-2: a systematic review and meta-analysis. J Hosp Infect. 2022;130:63–94. doi: 10.1016/j.jhin.2022.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinelli T., Ferreira V.H., Ierullo M., Ku T., Lilly L., Kim S.J., et al. Prospective clinical, virologic, and immunologic assessment of COVID-19 in transplant recipients. Transplantation. 2021;105:2175–2183. doi: 10.1097/TP.0000000000003860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton C.S., Huntley K., Berenger B.M., Bristow M., Evans D.H., Fonseca K., et al. Prolonged SARS-CoV-2 infection following rituximab treatment: clinical course and response to therapeutic interventions correlated with quantitative viral cultures and cycle threshold values. Antimicrob Resist Infect Control. 2022;11:28. doi: 10.1186/s13756-022-01067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Kampen J.J.A., van de Vijver D.A.M.C., Fraaij P.L.A., Haagmans B.L., Lamers M.M., Okba N., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12:267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce E.A., Mills M.G., Sampoleo R., Perchetti G.A., Huang M.L., Despres H.W., et al. Predicting infectivity: comparing four PCR-based assays to detect culturable SARS-CoV-2 in clinical samples. EMBO Mol Med. 2022;14 doi: 10.15252/emmm.202115290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.