Abstract
Background:
Rheumatic heart disease and its impact on cardiac health is still a concern in developing countries. Percutaneous trans-mitral commissurotomy (PTMC) is the standard of care in managing severe rheumatic mitral stenosis (MS). This article reports a single-center, 10-year real-world experience in Qatar.
Methods:
In this retrospective study, we reviewed all the patients who underwent PTMC in Qatar between January 1, 2012, and January 1, 2022. Periprocedural data were collected at baseline, postprocedural, 1 year, and during the last follow-up. The primary outcome was procedural success (improvement in valve area by 50%, final valve area >1.5 cm2, and freedom from > moderate mitral regurgitation, stroke, or pericardial effusion). Safety endpoints were freedom from death, periprocedural cardiogenic shock and cardiac arrest, stroke urgent mitral valve replacement (MVR), or pericardiocentesis. Long-term outcomes included the requirement of redo PTMC or MVR, in addition to rehospitalization due to arrhythmias, heart failure, or stroke.
Results:
Sixty-five patients were included in the review (age 42 ± 10, female 38 [58.5%]). Sixty-two patients (95.4%) had a successful procedure. One patient developed a hemorrhagic pericardial tamponade and cardiogenic shock, for which he underwent pericardiocentesis and emergency aortic root repair. One patient developed acute stroke 8 h after the procedure, and one patient had tamponade resolved with emergency pericardiocentesis. Two patients required MVR after 1 and 4 years, respectively.
Conclusion:
PTMC is the mainstay of rheumatic MS management in patients with suitable anatomy as most patients have excellent outcomes with long-term freedom from surgery, which has been the case in our single-center experience.
Keywords: Mitral stenosis, percutaneous trans-mitral commissurotomy, rheumatic heart disease, structural heart disease
INTRODUCTION
Rheumatic heart disease is the predominant cause of severe mitral stenosis (MS). It is highly prevalent in developing countries and among the female gender. Most of the patients are young, in their third decade of life, and present with noncalcified commissural fusion, unlike elderly patients who tend to have valvular calcification on presentation and other cardiovascular comorbidities.[1] The mainstay of managing severe rheumatic MS is the percutaneous transvenous balloon mitral commissurotomy (PTMC) in patients with suitable anatomy.[2]
Over the past three decades, and since its introduction in 1983, PTMC has played an integral role in managing severe rheumatic MS. Inoue et al. have reported the initial use of the PTMC in five patients. They have described the procedure in detail and the short and long-term outcomes.[3] In 1990, Abascal et al. studied and performed the procedure in 130 patients to predict the successful outcomes of the PTMC. Eighty-four percent of the population with an echocardiographic score of 8 or less (n = 73) reported having good outcomes, defined by a final mitral valve area of >1.5 cm2 or an increase in the area by >25%. While 58% of the population who had an echocardiographic of >8 (n = 57) had suboptimal outcomes.[4] Two years later, the short-term outcomes were studied on a large scale in a multicenter study consisting of 738 patients, which demonstrated significant short-term hemodynamic and clinical outcomes.[5] In the same time frame, the long-term outcomes predictors of the PTMC have been studied, and the echocardiographic scores, New York Heart Association (NYHA), and left ventricular end-diastolic pressure were reported as independent predictors for the long-term outcomes.[6]
PTMC has evolved rapidly, and currently, it is the standard of management and the preferred procedure for rheumatic MS as implemented in the current guidelines.[2,7] There is no consensus or statistics describing the prevalence of using the PTMC for rheumatic MS in every region; however, it is likely to decrease in developed countries and increase the developing countries due to the current prevalence of rheumatic fever and its consequences in both regions.
In Qatar, the population is heterogeneous, and most of the noncitizens are from areas of high burden of rheumatic heart disease, particularly in Asia and Africa.[8] In this cohort, we aim to reflect on our nationwide PTMC experience in Qatar. We report the demographic characteristics of our patients, the mitral valve structural parameters, and the procedural and clinical outcomes.
METHODS
Study setting
The study was carried out in the heart hospital (HH), the only specialized tertiary cardiac center in Qatar, and a member of Hamad Medical Corporation (HMC). Approval was obtained from the institutional review board to conduct this study.
Study design and population
The study is a retrospective observational study that included all patients who underwent PTMC in the HH between January 1, 2012, and January 1, 2022. The study reports all the patients’ relevant data, which consists of the basic demographic features, a detailed past cardiac history, mitral valve morphology, and hemodynamics. The postprocedural mitral valve hemodynamics, history of hospitalization, complications, and need for other interventions were all reported.
Data collection procedures
The baseline characteristics of the study participants and the parameters of interest were collected from the HMC electronic medical records system (Cerner®) mainly by reviewing the physician's notes, diagnostics, procedural notes, and laboratory values done during the admission. The mitral valve area was calculated retrospectively using the pressure half-time method (PHT) and transesophageal echocardiogram (TEE) derived mitral valve planimetry in accordance with most recent guidelines.[9,10] The mean diastolic mitral valve gradients were reported by echocardiography and by retrospectively reevaluating the direct invasive left atrial pressure and left ventricular pressure tracings. Pre- and postprocedural mitral regurgitation (MR) was graded using the most current guidelines.[9,10] Pulmonary pressure was estimated using right ventricular systolic pressure (RVSP) and obtained invasively pre- and postPTMC using fluid-filled catheters.
Outcome measures and follow-up
The primary outcome is to report our local procedural success, defined as improvement in valve area by >50% with final valve area >1.5 cm2 and freedom from complications or > moderate MR. We also reported safety endpoints including freedom from death, periprocedural cardiogenic shock and cardiac arrest, stroke urgent mitral valve replacement (MVR), or pericardiocentesis. Long-term outcomes included the requirement of redo PTMC or MVR, in addition to rehospitalization due to arrhythmias, heart failure, or stroke.
Secondary long-term endpoints of death, mitral valve restenosis, need for repeat PTMC or MVR, and presence of > moderate MR were also reported. The last clinical encounter at HH was considered the last day of follow-up for the patient.
Percutaneous trans-mitral commissurotomy procedure
The procedure was performed using an INOUE balloon (Toray, Japan). The choice of balloon size (26, 28, 30 mm) depended on the height of the patient. Access was obtained in the right femoral vein for right heart catheterization and PTMC, and femoral or radial artery for left heart catheterization. Baseline right and left heart catheterization were performed using fluid-filled catheters in all cases. Cardiac output was measured using the assumed Fick method. Then, the transseptal puncture was obtained using biplane fluoroscopy guidance in the early phase of experience or transesophageal echocardiography in the later phase (the year 2020) of experience. Once access to the left atrium (LA) was obtained, a balloon commissurotomy was performed. PTMC was considered to be successful if echocardiographic derived valve area ≥1.5 cm2 was achieved with no more than moderate MR. The right and left heart catheterization were repeated postvalvuloplasty.
Statistical analyses
The continuous data were described with the mean and the standard deviation for normal distribution and median with interquartile range (IQR) for skewed distribution, whereas the categorical data were described as a percentage. Data were analyzed using SPSS; Statistical Package for Social Sciences Version 7, year 2013 United States statistical package. The pre- and post-PTMC echocardiographic parameters were compared using the independent paired sample t-test. The statistical significance is indicated by 95% confidence interval and P < 0.05.
RESULTS
The baseline characteristics were described in Table 1. Sixty-five patients underwent PTMC. Of these, 58.5% were female. Asian descent was predominant, followed by Middle Eastern and African descent. Regarding the cardiac history, 10 patients had previous PTMC for severe MS before the index procedure, 18 patients had atrial fibrillation, and five had a previous history of cerebrovascular events. The NYHA classification before the procedure was predominantly classes 2 and 3 that improved to classes 1 and 2 as shown in Figure 1.
Table 1.
Baseline characteristics of patients who underwent percutaneous transvenous mitral commissurotomy (n=65)
| Characteristic | n (%) |
|---|---|
| Age (years) | 42±10 |
| Gender | |
| Male | 27 (41.5) |
| Female | 38 (58.5) |
| Region of origin | |
| Asia | 31 (47.7) |
| Middle east | 24 (36.9) |
| Africa | 9 (13.8) |
| North America | 1 (1.5) |
| Weight (kg) | 75±18 |
| Height (cm) | 162±9 |
| Medical history | |
| Prior PTMC | 10 (15.4) |
| Hypertension | 6 (9.2) |
| Diabetes mellitus | 6 (9.2) |
| Stroke/TIA | 5 (7.7) |
| Atrial fibrillation | |
| Paroxysmal | 11 (16.9) |
| Permanent | 6 (9.2) |
| Persistent | 1 (1.5) |
| Cardiomyopathy | 1 (1.5) |
| NYHA class | |
| 1 | 12 (18.5) |
| 2 | 34 (52.3) |
| 3 | 17 (26.2) |
| 4 | 2 (3.1) |
| Ejection fraction | 53±11 |
PTMC: Percutaneous transvenous mitral commissurotomy, TIA: Transient ischemic attack, NYHA: New York Heart Association
Figure 1.
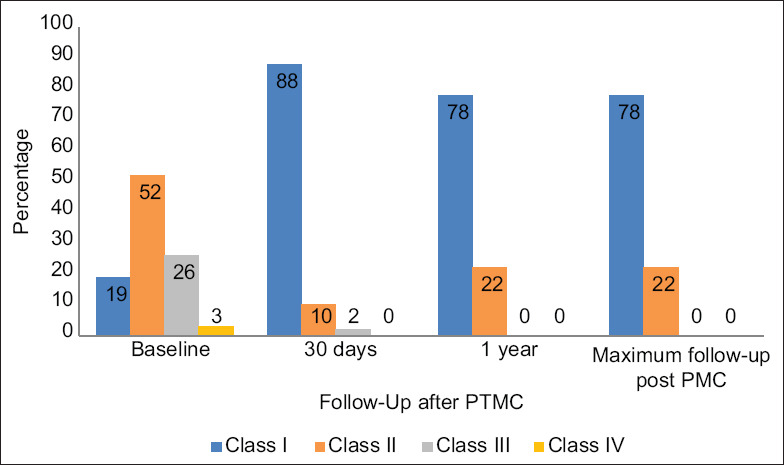
NYHA class after PTMC (n = 65). PTMC: Percutaneous transvenous mitral commissurotomy, NYHA: New York Heart Association
The noninvasive hemodynamics were shown in Table 2. The preprocedural mean MV gradient was found to be 15.5 ± 7.9. The MV area by PHT and planimetry were 1.1 ± 0.28 and 1.1 ± 0.27, respectively. The RVSP was 45 ± 17.13. About 30.8% of the patients had mild MR.
Table 2.
Baseline transthoracic echocardiographic parameters before percutaneous transvenous mitral commissurotomy (n=65)
| Characteristic | Mean±SD |
|---|---|
| LVIDs (cm) | 3.3±0.39 |
| LVIDd (cm) | 4.8±0.44 |
| Mitral valve gradient | 14.5±7.90 |
| Mitral valve area PHT | 1.1±0.28 |
| Mitral valve area planimetry | 1.1±0.27 |
| RVSP | 45±17.13 |
| Wilkin score* | |
| 4 | 6 (9.2) |
| 5 | 8 (12.3) |
| 6 | 9 (13.8) |
| 7 | 17 (26.2) |
| 8 | 7 (10.8) |
| 9 | 5 (7.7) |
| Mitral regurgitation grade* | |
| Normal | 32 (54) |
| Grade 1 | 23 (39) |
| Grade 2 | 4 (7) |
| Grade 3 | 0 |
| Grade 4 | 0 |
*Reported as frequency (%). LVIDd: Left ventricular internal dimension at end-diastole, LVIDs: Left ventricular internal dimension end-systolic, RVSP: Right ventricular systolic pressure, PHT: Pressure half-time, SD: Standard deviation
The RVSP in the right heart catheterization was 20.6 ± 5.76, and the diastolic LA to left ventricle gradient was 13.6 ± 6.80, as described in Table 3.
Table 3.
Right heart catheterization during percutaneous transvenous mitral commissurotomy (n=65)
| Characteristic | Mean±SD |
|---|---|
| PCWP | 20.6±5.76 |
| LA pressure | 22.3±6.02 |
| Diastolic LA to LV pressure gradient | 13.6±6.80 |
| Diastolic LA to LV pressure gradient postballooning | 6.3±6.60 |
| LA pressure postballooning | 13.9±5.31 |
| Inoue-balloon inflation diameter (mm)* | |
| 26 | 24 (45) |
| 28 | 24 (45) |
| 30 | 5 (10) |
| Inoue-balloon inflation attempts* | |
| 1 | 7 (14) |
| 2 | 20 (41) |
| 3 | 15 (31) |
| 4 | 5 (10) |
| 5 | 1 (2) |
| 6 | 1 (2) |
*Reported as frequency (%). PCWP: Pulmonary capillary wedge pressure, LA: Left atrial, LV: Left ventricular, SD: Standard deviation
Postprocedural TTE parameters showed a significant improvement in the mitral valve area and RVSP that persisted at 1-year follow-up and maximum follow-up (reported as a median and IQR) as demonstrated in Table 4. Seven patients (11%) had > moderate MR at 1 year.
Table 4.
Transthoracic echocardiographic parameters after percutaneous transvenous mitral commissurotomy (n=65)
| Characteristic | Baseline | 1 day post-PTMC | P* | 30 days post-PTMC | P* | 1 year post-PTMC | P* | Maximum follow-up post-PTMC | P* |
|---|---|---|---|---|---|---|---|---|---|
| LVIDs (cm) | 3.3±0.39 | - | - | 3.3±0.44 | 0.975 | 3.4±0.39 | 0.427 | 3.4±0.66 | 0.466 |
| LVIDd (cm) | 4.8±0.44 | - | - | 4.9±0.35 | 0.143 | 5.0±0.42 | 0.067 | 4.8±0.60 | 0.603 |
| Mitral valve gradient (mmHg) | 14.5±7.90 | - | - | 5.2±2.09 | <0.001 | 5.2±4.03 | <0.001 | 5.0±2.64 | <0.001 |
| Mitral valve area PHT (cm2) | 1.1±0.28 | 1.7±0.33 | <0.001 | 1.7±0.37 | <0.001 | 1.8±0.39 | <0.001 | 1.8±0.40 | <0.001 |
| Mitral valve area planimetry (cm2) | 1.1±0.27 | 1.8±0.39 | <0.001 | 1.7±0.31 | <0.001 | 1.8±0.37 | <0.001 | 1.8±0.39 | <0.001 |
| RVSP (mmHg) | 45±17.13 | 34.2±9.01 | <0.001 | 36.1±8.40 | <0.001 | 34.6±8.83 | <0.001 | 34.4±7.05 | 0.004 |
*P-value calculated using paired sample t-test comparing baseline echocardiographic parameters with the echocardiographic parameters post-PTMC at different intervals. LVIDd: Left ventricular internal dimension at end-diastole, LVIDs: Left ventricular internal dimension end-systolic, RVSP: Right ventricular systolic pressure, PHT: Pressure half-time, PTMC: Percutaneous transvenous mitral commissurotomy
Procedural success and acute complications
There was no procedural-related mortality, and the procedure was successful in 62 patients; one patient developed hemorrhagic cardiac tamponade due to a tear at the back of the aorta from transseptal puncture that was managed successfully with pericardiocentesis, sternotomy, and suturing with no long-term squeal. Another patient developed tamponade after PTMC procedure that was resolved with urgent pericardiocentesis. Both of these cases were performed in the early phase without transesophageal guidance. One patient developed transient ischemic attack and was found to have a minor stroke on the magnetic resonance imaging without neurological sequelae.
Long-term follow-up
The median time of maximum follow-up was 4 (IQR: 2.5) years. There were no reported deaths during follow-up. Five patients were hospitalized for atrial fibrillation with rapid ventricular response. Four patients were admitted with acute decompensated heart failure; two of whom underwent successful MVR.
DISCUSSION
In this report of single-center PTMC experience of the mixed population in Qatar, we made several important observations: (1) procedural success is high (>95%) in carefully selected patients with suitable MV anatomy, (2) pericardial effusion is most common complication which occurred in two patients in the early phase before utilization of TEE guidance, (3) Only 3% needed mitral valve reintervention over maximum 4.5 (IQR: 2.5) years of follow-up.
Rheumatic heart disease is the leading cause of MS in the developing world, and it is estimated to be accounting for 79% of MS etiologies.[11,12] The PTMC has been proven to effectively and safely manage severe MS, and it has become the standard of care.[7,13,14,15] Besides the improvement of the immediate and early symptoms, around 80% of the patients became symptom-free at 10 years, and about 40% continued to be asymptomatic at 20 years.[16,17,18] In patients with unfavorable mitral valve anatomy, who have moderate or severe MR or failed the PTMC, the outcomes tend to be better by valve replacement.[13,19] In our carefully selected population with suitable MV anatomy, 78% of patients had NYHA class 1 symptoms or less at long-term follow-up which is similar to what has been reported in larger registries.
The procedural complications have been reported to be significantly increased by the first decade of this century which partially could be related to choosing patients with less suitable MV anatomy for the procedure.[20] Although mortality is uncommon in PTMC, it has been reported to be 1% and is mostly related to the patient's comorbidities, including cardiovascular and cerebrovascular diseases or advanced age.[21] The most common complications are MR, cardiac tamponade, perforation, and cerebrovascular accidents.[5,21] Of these, acute MR and cardiac tamponade are the most common cases of emergent surgery.[22] In our patient population, we noticed few complications mainly pericardial effusion and bleeding in the early phase of procedural experience without the use of TEE guidance and one case of self-limiting stroke during hospitalization.
To the best of our knowledge, this article is the largest experience in a single center in the region. There was no mortality over 10 years. Two patients developed cardiac tamponade of whom one required emergency surgery due to tearing of the aorta, and no other patients required emergency intervention. Two patents required MVR after 1 and 4 years, respectively.
This report is limited by the retrospective observational nature of the study, limited number of patients, and limited duration of follow-up. Moreover, the single-center experience might not reflect similar procedural experiences in the region or in other populations. However, the population in Qatar is quite heterogeneous with a significant number of patients of Asian and African descents.
CONCLUSION
PTMC is the mainstay of treatment for carefully selected rheumatic heart disease patients with suitable mitral valve anatomy, and it is associated with high procedural success, low procedural complication, and high chance of freedom from reintervention.
Ethical approval
Ethical approval is obtained for this analysis from the institutional review board.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank all the HH and catheterization laboratory staff for their immense efforts in taking care of our patients.
PTMC: 10 years of experience.
REFERENCES
- 1.Shah SN, Sharma S. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [Last accessed on 2022 Apr 16]. Mitral stenosis. Available from: http://www.ncbi.nlm.nih.gov/books/NBK430742/ [Google Scholar]
- 2.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 3.Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:394–402. [PubMed] [Google Scholar]
- 4.Abascal VM, Wilkins GT, O’Shea JP, Choong CY, Palacios IF, Thomas JD, et al. Prediction of successful outcome in 130 patients undergoing percutaneous balloon mitral valvotomy. Circulation. 1990;82:448–56. doi: 10.1161/01.cir.82.2.448. [DOI] [PubMed] [Google Scholar]
- 5.Multicenter experience with balloon mitral commissurotomy. NHLBI Balloon Valvuloplasty Registry Report on immediate and 30-day follow-up results. The National Heart, Lung, and Blood Institute Balloon Valvuloplasty Registry Participants. Circulation. 1992;85:448–61. doi: 10.1161/01.cir.85.2.448. [DOI] [PubMed] [Google Scholar]
- 6.Cohen DJ, Kuntz RE, Gordon SP, Piana RN, Safian RD, McKay RG, et al. Predictors of long-term outcome after percutaneous balloon mitral valvuloplasty. N Engl J Med. 1992;327:1329–35. doi: 10.1056/NEJM199211053271901. [DOI] [PubMed] [Google Scholar]
- 7.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 8.Population of Qatar by Nationality in 2019. Priya D Souza Communications. 2019. [Last accessed on 2022 Apr 16]. Available from: http://priyadsouza.com/population-of-qatar-by-nationality-in-2017 .
- 9.Recommended Standards for the Performance of Transesophageal Echocardiographic Screening for Structural Heart Intervention. [Last accessed on 2022 May 26]. Avai lable from: https://www.asecho.org/guideline/recommended-standards-for-the-performance-of-transesophagealechocardiographic-screening-for-structural-heart-intervention . [DOI] [PubMed]
- 10.Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. [Last accessed on 2022 May 26]. Available from: https://www.asecho.org/guideline/echocardiographic-assessmentof-valve-stenosis-eae-ase-recommendations-for-clinicalpractice/
- 11.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 12.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group a streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 13.Ben Farhat M, Ayari M, Maatouk F, Betbout F, Gamra H, Jarra M, et al. Percutaneous balloon versus surgical closed and open mitral commissurotomy: Seven-year follow-up results of a randomized trial. Circulation. 1998;97:245–50. doi: 10.1161/01.cir.97.3.245. [DOI] [PubMed] [Google Scholar]
- 14.Reyes VP, Raju BS, Wynne J, Stephenson LW, Raju R, Fromm BS, et al. Percutaneous balloon valvuloplasty compared with open surgical commissurotomy for mitral stenosis. N Engl J Med. 1994;331:961–7. doi: 10.1056/NEJM199410133311501. [DOI] [PubMed] [Google Scholar]
- 15.Arora R, Nair M, Kalra GS, Nigam M, Khalilullah M. Immediate and long-term results of balloon and surgical closed mitral valvotomy: A randomized comparative study. Am Heart J. 1993;125:1091–4. doi: 10.1016/0002-8703(93)90118-s. [DOI] [PubMed] [Google Scholar]
- 16.Neumayer U, Schmidt HK, Fassbender D, Mannebach H, Bogunovic N, Horstkotte D. Early (three-month) results of percutaneous mitral valvotomy with the Inoue balloon in 1,123 consecutive patients comparing various age groups. Am J Cardiol. 2002;90:190–3. doi: 10.1016/s0002-9149(02)02452-9. [DOI] [PubMed] [Google Scholar]
- 17.Bouleti C, Iung B, Laouénan C, Himbert D, Brochet E, Messika-Zeitoun D, et al. Late results of percutaneous mitral commissurotomy up to 20 years: Development and validation of a risk score predicting late functional results from a series of 912 patients. Circulation. 2012;125:2119–27. doi: 10.1161/CIRCULATIONAHA.111.055905. [DOI] [PubMed] [Google Scholar]
- 18.Meneguz-Moreno RA, Costa JR, Jr, Gomes NL, Braga SL, Ramos AI, Meneghelo Z, et al. Very long term follow-up after percutaneous balloon mitral valvuloplasty. JACC Cardiovasc Interv. 2018;11:1945–52. doi: 10.1016/j.jcin.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Song H, Kang DH, Kim JH, Park KM, Song JM, Choi KJ, et al. Percutaneous mitral valvuloplasty versus surgical treatment in mitral stenosis with severe tricuspid regurgitation. Circulation. 2007;116(11 Suppl):I246–50. doi: 10.1161/CIRCULATIONAHA.107.678151. [DOI] [PubMed] [Google Scholar]
- 20.Badheka AO, Shah N, Ghatak A, Patel NJ, Chothani A, Mehta K, et al. Balloon mitral valvuloplasty in the United States: A 13-year perspective. Am J Med. 2014;127:1126.e1–12. doi: 10.1016/j.amjmed.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Complications and mortality of percutaneous balloon mitral commissurotomy. A Report from the National Heart, Lung, and Blood Institute Balloon Valvuloplasty Registry. Circulation. 1992;85:2014–24. doi: 10.1161/01.cir.85.6.2014. [DOI] [PubMed] [Google Scholar]
- 22.Varma PK, Theodore S, Neema PK, Ramachandran P, Sivadasanpillai H, Nair KK, et al. Emergency surgery after percutaneous transmitral commissurotomy: Operative versus echocardiographic findings, mechanisms of complications, and outcomes. J Thorac Cardiovasc Surg. 2005;130:772–6. doi: 10.1016/j.jtcvs.2005.04.021. [DOI] [PubMed] [Google Scholar]


