Abstract
Fungal pathogens are notorious for causing chronic and latent infections, but the mechanism by which they evade the immune response is poorly understood. A major limitation in the study of chronic fungal infection has been the lack of suitable animal models where the infection is controlled and yet persists. Pulmonary Cryptococcus neoformans infection in rats results in a diffuse pneumonitis that resolves without dissemination or scarring except for the persistence of interstitial and subpleural granulomas that harbor viable cryptococci inside macrophages and epithelioid cells. Infected rats are asymptomatic but remain infected for as long as 18 months after inoculation with C. neoformans. Containment of infection is associated with granuloma formation that can be partially abrogated by glucocorticoid administration. Using this model, we identified several features associated with persistent infection in the rat lung, including (i) localization of C. neoformans to discrete, well-organized granulomas; (ii) intracellular persistence of C. neoformans within macrophages and epithelioid cells; (iii) reduced inducible nitric oxide synthase expression by granulomas harboring C. neoformans; and (iv) reduced antibody responses to cryptococcal polysaccharide. The results show that maintenance of persistent infection is associated with downregulation of both cellular and humoral immune responses.
Cryptococcus neoformans is a fungal pathogen that causes meningoencephalitis in immunocompromised individuals. Infection is believed to be acquired through the respiratory tract, although the precise relationship between pulmonary and central nervous system infection is not understood. Several lines of evidence suggest that C. neoformans causes persistent, primary lung infection in immunocompetent individuals that is similar to infections caused by Mycobacterium tuberculosis and Histoplasma capsulatum. Primary cryptococcal infection is likely to be associated with few or minimal symptoms, but it may disseminate in the context of an acquired immunodeficiency, such as AIDS, or corticosteroid therapy. Persistent, pulmonary cryptococcosis has been described in humans. In one study, approximately 47% of individuals with pulmonary cryptococcosis had abnormal radiographic findings for at least 3 months before diagnosis, and an additional 17% of patients had radiographic findings for more than 18 months before diagnosis (2). Primary cryptococcal pneumonia has also been described as an incidental finding of autopsy studies of immunocompetent individuals. In these cases, infection was associated with small subpleural granulomas containing C. neoformans (16). A primary cryptococcal complex consisting of circumscribed granulomas with hilar lymphadenitis without calcifications has also been described (24).
Current animal models are inadequate for studying the pathogenesis of persistent cryptococcosis. The two species that have been most extensively studied are mice and rabbits. Mice are extremely susceptible to pulmonary infection, which is invariably associated with dissemination and high mortality (9). Rabbits are highly resistant to infection and require immunosuppression for the establishment of infection (22). Neither species is suitable for the study of cryptococcal persistence and the development of a latent infection model where an initial infection is contained, persists, and then is amenable to reactivation.
In previous studies, we have shown that intratracheal inoculation of rats with C. neoformans produces a local pulmonary infection that shares many of the histopathological and serological features of pulmonary infection in immunocompetent humans (13). Rats infected with C. neoformans mount a brisk granulomatous response associated with increased inducible nitric oxide synthase (NOS2) expression and reduction in pulmonary fungal burden (12). In this study, we extend these findings and show that while the rat inflammatory response contains pulmonary cryptococcal infection, the organism persists intracellularly within macrophages and epithelioid cells and that infection can be reactivated by the administration of glucocorticoids, a known risk factor for cryptococcal disease in humans. We further demonstrate that persistence of cryptococcal infection is associated with modulation of both humoral and cellular inflammatory responses. To our knowledge, this is the first animal model for a latent infection of a human pathogenic fungus.
MATERIALS AND METHODS
Rat infection.
Male Fisher rats (National Cancer Institute, Frederick, Md.) weighing 250 to 300 grams were anesthetized by exposure to methoxyflurane (Mallinckrodt Veterinary, Mundelein, Ill.) and infected intratracheally with 107 organisms as described earlier (13).
To examine the effects of immunosuppression relative to the stage of the infection, two experiments were done in which dexamethasone phosphate (1.5 mg/liter) (Sigma, St. Louis, Mo.) was added to the drinking water of infected animals. Assuming the average water intake of a rat is 10 ml for every 100 g (15), the daily dexamethasone dose was calculated to be 0.15 mg/kg. For one group of animals (n = 3), dexamethasone treatment was initiated 1 week after infection and was continued for 5 weeks. Dexamethasone was given at 1 week of infection because previous experiments showed that the inflammatory response of rats to pulmonary challenge has not fully matured by this time (13). For a second group (n = 4), dexamethasone treatment was initiated 11 months after infection and continued for 7 weeks. To prevent Pneumocystis carinii pneumonia, trimethoprim-sulfamethoxazole (250 mg of the trimethoprim component per liter) was added to the drinking water of dexamethasone-treated rats. Assuming the average water intake of a rat is 10 ml for every 100 g (15), the daily trimethoprim dose was calculated to be 25 mg/kg. This dose is significantly lower than that shown to cause leukopenia in rats (25). The age-matched controls, four uninfected rats, were housed in identical conditions as the experimental group for 1.5 years. One control rat developed polyarteritis nodosa and was excluded from the study.
Organism.
C. neoformans 24067, a serotype D strain, was obtained from the American Type Culture Collection (Manassas, Va.). Serotype D strains are pathogenic in humans and represent the majority of isolates in certain geographic regions such as northern Europe. Organisms were grown in Sabouraud's dextrose broth for 2 days at 30°C and washed three times in 0.02 M phosphate-buffered saline (PBS). To ensure the accuracy of the inoculum, C. neoformans colonies were counted after the infecting dose was diluted, plated on Sabouraud's dextrose agar, and incubated at 30°C for 3 days.
Fungal burden.
At 1.5 (n = 3), 6 (n = 5), 12.5 (n = 4), and 18 (n = 3) months after infection, rats were killed by lethal injection of sodium pentobarbital (Abbott Laboratories, Chicago, Ill.). Dexamethasone-treated rats were killed at 1.5 (n = 3) and 12.5 (n = 3) months after infection. At the time of death, blood was withdrawn through the inferior vena cava and the lungs, spleens, kidneys, and brains were removed. For all organs other than the lungs, a small portion (ca. 25%) of the organ was removed and placed in 10% buffered formalin for histopathologic studies. For the lungs, the entire right lung was placed in formalin. The remainder of each organ was homogenized in sterile PBS, a 100-μl aliquot was plated on Sabouraud's dextrose agar, and cultures were counted after 3 days of incubation at 30°C. One immunocompetent rat and one dexamethasone-treated rat died before being killed for organ harvest. Both rats that died suffered from leukemia with infiltration of the lungs and were excluded from the study, although both rats were infected with C. neoformans. These rats were excluded from the study since it is likely that the leukemia had significant effects on the host response to cryptococcal infection.
C. neoformans polysaccharide serum antigen levels.
Serum samples were treated with proteinase K (Boehringer-Mannheim, Indianapolis, Ind.) at a concentration of 10 μg/ml for 20 min and heated to 100°C for 15 min to destroy the residual enzyme activity. Glucuronylxylomannan (GXM) levels were determined on proteinase-treated serum by capture enzyme-linked immunosorbent assay (ELISA) as described elsewhere (3).
Immunohistochemistry.
Immunohistochemistry for GXM, and inducible NOS2 was performed as described previously (12). Briefly, deparaffinized sections were incubated in 0.1% hydrogen peroxide for 30 min and then incubated with 5% goat serum. Primary antibodies to GXM (21) and NOS2 (Transduction Laboratories, Lexington, Ky.) were applied at dilutions of 1:500 and 1:200, respectively. Peroxidase-conjugated isotype-specific antibody was applied, and color was developed with diaminobenzidine (Dako, Carpinteria, Calif.). For all immunohistochemical studies, negative controls consisting of the omission of the primary antibody and staining of uninfected tissues were done. As positive controls, sections obtained from previous experiments (0.5 and 0.8 months after infection) were stained for GXM and NOS2 (12). Double-label staining for GXM and NOS2 were done sequentially by using peroxidase- and alkaline phosphatase-conjugated secondary antibodies as described elsewhere (12).
ELISA for antibodies to cryptococcal polysaccharide, cryptococcal protein, and tetanus toxoid.
Polystyrene microtiter 96-well plates (Corning, Corning, N.Y.) were coated with either cryptococcal polysaccharide (1 μg/ml), cryptococcal protein extract (10 μg/ml), or tetanus toxoid (2.5 μg/ml) (CalBiochem, La Jolla, Calif.). Cryptococcal polysaccharide, composed of 90% GXM, was isolated from ATCC strain 24067 as described by others (8). Protein was isolated from whole-cell extracts of the nonencapsulated ATCC strain 52817 as described elsewhere (14). For polysaccharide serology, plates were blocked with 1% bovine serum albumin (BSA) and 0.5% horse serum in Tris-buffered saline (TBS). For protein and tetanus toxoid serology, plates were blocked with 1% BSA in TBS. Serum was diluted serially and incubated for 2 h or overnight at 4°C. Immunoglobulin G (IgG) was detected with alkaline phosphatase-labeled goat anti-rat IgG (Southern Biotechnology Associates, Birmingham, Ala.), and color was developed with p-nitrophenyl phosphate disodium salt (Southern Biotechnology). The titer was defined as the lowest dilution giving an absorbance reading of two times more than the background level at 405 nm.
Vaccination studies.
Rats were infected with C. neoformans 12 months prior to vaccination. Infected rats (n = 3) and control, noninfected rats (n = 5) were given three intramuscular (i.m.) doses of a GXM-tetanus toxoid conjugate vaccine (GXM-TT) at 3- to 5-week intervals. The dose of GXM-TT was 2.5 μg of the GXM component per rat. Previous studies have shown that a dose of 25 to 50 μg of the GXM component is capable of eliciting GXM-specific antibody responses in humans (28). The GXM-TT vaccine was a gift from R. Schneerson and J. Robbins (National Institutes of Health [NIH]) and has been described elsewhere (7). Another group of chronically infected rats (n = 3) was given 3 i.m. doses (50 μl) of diphtheria-tetanus toxoid vaccine (dT; Connaught, Swiftwater, Pa.) at the same intervals. Serial determinations of antibody titers to GXM and tetanus toxoid were determined by ELISA.
Statistical analysis.
Lung fungal burdens were compared by t test, and antibody titers were compared by the Kruskall-Wallis test. P values of less than 0.05 were considered significant. The localization of NOS2 expression between areas of confluent inflammation and small interstitial granulomas at 1.5 months was compared by counting the number of NOS2-positive foci in these different areas for three rats and by applying the χ2 test modified for a single variable.
RESULTS
Pilot study.
Prior to the initiation of these studies, we conducted a small pilot experiment to determine whether rats could be infected for prolonged periods of time with C. neoformans. For this study, rats (n = 2) were endotracheally infected with C. neoformans and killed at 22 months of infection. Neither of these rats demonstrated any clinical signs of persistent infection. Nevertheless, histopathological examination of the lung tissue from these rats revealed the presence of C. neoformans organisms within small intraparenchymal granulomas. On the basis of the results from the pilot study, a significantly larger experiment was designed to investigate the long-term outcome and consequences of pulmonary C. neoformans infection in rats.
Pulmonary and extrapulmonary fungal burden.
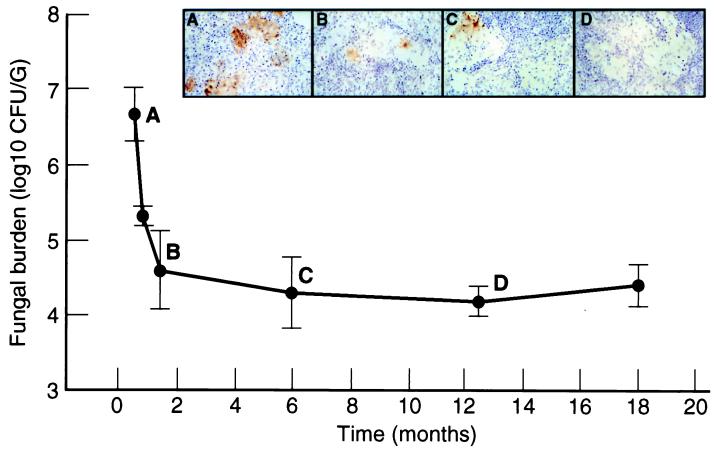
The average lung fungal burden of immunocompetent, infected rats remained relatively constant over the 1.5-year interval of infection (see Fig. 2). Rats that received dexamethasone treatment 1 week after inoculation had a large (∼3 log10-fold) increase in lung fungal burden at 1.5 months of infection relative to immunocompetent controls. Rats that received dexamethasone 11 months after infection had an ∼0.5 log10-fold increase in lung fungal burden (4.75 ± 0.13 log10 CFU/g) at 12.5 months of infection compared with immunocompetent controls (4.22 ± 0.21 log10 CFU/g) (P = 0.013).
FIG. 2.
NOS2 expression in persistent pulmonary infection. The graph demonstrates lung fungal burden (mean CFU/gram of tissue) at 0.5, 0.8, 1.5, 6, 12.5, and 18 months after infection. Results for rats at 0.5 and 0.8 months after infection were previously described and are presented here for comparison (12). These numbers were plotted in the same graph though they originated from a different experiment because the CFUs are very consistent in this system. Representative NOS2 staining (brown) within granulomas at 0.5 (A), 1.5 (B), 6 (C), and 12.5 (D) months are shown in the panels above. All magnifications, ×70. The lung sections from 0.5 and 0.8 months of infection were obtained from a previous experiment and were used as a positive control for these experiments (12).
Despite pulmonary infection, little or no associated extrapulmonary dissemination was present in immunocompetent rats. At 1.5 and 12.5 months after infection, C. neoformans was not isolated from the spleen, kidney or brain. At 6 months, two of five rats had C. neoformans colonies isolated from their spleens with an average fungal burden of 2.46 ± 0.01 log10 CFU/g. One of these rats also had a single C. neoformans colony isolated from the kidney. At 18 months after infection, one rat had a single C. neoformans colony isolated from the brain.
Dexamethasone-treated rats killed 1.5 months after infection had substantially more extrapulmonary involvement than immunocompetent rats. All three rats in this group had brain infection with an average fungal burden of 2.18 ± 1.1 log10 CFU/g. In two of three rats in this group, C. neoformans was also isolated from the spleen and kidney with average fungal burdens (log10 CFU/g) of 4.62 ± 0.22 and 2.53 ± 0.07, respectively. Dexamethasone-treated rats killed 12.5 months after infection had low levels of extrapulmonary involvement. Two rats had spleen infection with an average fungal burden of 2.76 ± 0.21 log10 CFU/g. One rat in this group had brain and kidney involvement with fungal burdens (log10 CFU/g) of 2.57 and 2.76, respectively.
Serum GXM levels.
Serum GXM was not detectable (<0.1 μg/ml) any time after infection in immunocompetent rats (Table 1). Among dexamethasone-treated rats, two of three rats had detectable serum GXM levels at 1.5 months, with an average level of 2.49 ± 1 μg/ml. GXM was not detected in the sera of dexamethasone-treated rats killed 12.5 months after infection.
TABLE 1.
Serological and histopathological features of persistent C. neoformans pulmonary infection
| Time after infection (mo) | na | Anti-GXM titerb | Anti-C. neoformans protein titerb | Serum GXM levelc | Organism locationd | Inflammatione | NOS2f |
|---|---|---|---|---|---|---|---|
| Control | 3 | 0.6 | 1.0 | NDg | NAh | ND | ND |
| 1.5 | 3 | 0.6 | 2.6 ± 0.6 | ND | I | +++ | ++ |
| 1.5 (dex)i | 3 | 1.1 ± 0.9 | 1.2 ± 0.3 | 2.5 ± 1.0 | E > I | + | + |
| 6 | 5 | 1.8 ± 0.7 | 2.0 ± 0.3 | ND | I | ++ | + |
| 12.5 | 4 | 0.9 ± 0.2 | 2.1 ± 0.2 | ND | I | + | + |
| 12.5 (dex) | 3 | 0.9 ± 0.5 | 1.6 ± 0.3 | ND | I = E | + | ND |
| 18 | 3 | 1.2 ± 1.0 | 2.4 ± 0.5 | ND | I | + | +/− |
Number of rats per group.
Mean titer (1/log10 ± 1 SD) as determined by ELISA.
Mean serum GXM level in micrograms per milliliter ± 1 SD.
I, intracellular; E, extracellular.
Inflammation was graded on an arbitrary scale from “+” to “++++,” with “++++” representing the greatest amount of inflammation.
NOS2 expression was scored on an arbitrary scale from “+/−” to “++++,” with “++++” representing maximal NOS2 expression.
ND, not detected.
NA, not available.
dex, dexamethasone-treated rats.
Serum antibodies to cryptococcal polysaccharide and cryptococcal protein.
Serum IgG reactive to cryptococcal polysaccharide was not detected at 1.5 months of infection in immunocompetent rats. After this time, average IgG anti-GXM titers (1/log10) for immunocompetent rats were: 1.8 ± 0.7, 0.9 ± 0.2, and 1.2 ± 1.0 at 6, 12.5, and 18 months, respectively. Average IgG anti-GXM titers for dexamethasone-treated rats were 1.1 ± 0.9 and 0.9 ± 0.5 1/log10 at 1.5 and 12.5 months, respectively (Table 1). Serum IgG to cryptococcal proteins was present in all infected rats at all times. The mean IgG (1/log10) anti-C. neoformans protein titers for immunocompetent rats were: 2.6 ± 0.6, 2.0 ± 0.3, 2.1 ± 0.2, and 2.4 ± 0.5 at 1.5, 6, 12.5, and 18 months, respectively. Titers to cryptococcal protein were decreased by 0.5 to 1 log10 in dexamethasone-treated rats compared with immunocompetent rats killed at the same time (Table 1).
Histopathology.
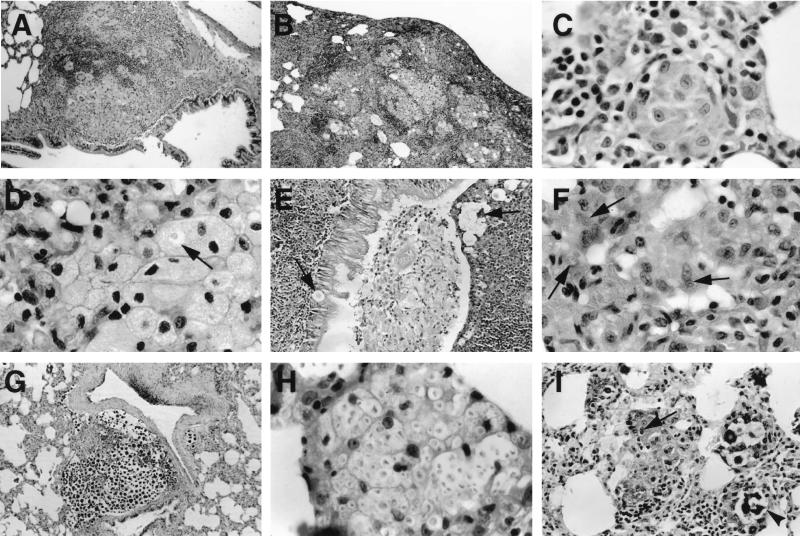
The pathology of long-term cryptococcal infection in immunocompetent rats was characterized by involvement of the bronchial, bronchiolar, subpleural, and interstitial regions (Fig. 1A, B, and C). Both routine hematoxylin and eosin staining and GXM immunohistochemistry demonstrated that the majority of organisms and GXM were intracellular and localized within vacuolated macrophages and epithelioid cells (Table 1). Granulomatous inflammation consisting of lymphocytes, epithelioid cells, Langhan's giant cells, and macrophages was present, along with lymphocyte perivascular cuffing (Fig. 1A). Vacuolated macrophages with distended cell membranes, some of which contained C. neoformans and GXM were prominent in areas of confluent inflammation (Fig. 1D). In some regions, organisms, macrophages, polymorphonuclear leukocytes, and cellular debris disrupted the respiratory epithelium of bronchioles and partially filled the bronchiolar lumen (Fig. 1E). Over the course of infection, there was a decrease in the size and number of areas of inflammation, as well as the extent of GXM reactivity within the lung. By 18 months of infection, the inflammation was reduced to small granulomas within the interstitium and subpleural spaces (Fig. 1F). Minimal fibrosis was present, but some bronchiolar ectasia was noted.
FIG. 1.
Histopathology of persistent infection. Peribronchiolar infection (A) (magnification, ×82.5), subpleural infection (B) (magnification, ×82.5), and interstitial infection (C) (magnification, ×825). (D) Vacuolated macrophages some of which contain C. neoformans (arrow) (magnification, ×825) are also shown. C. neoformans and associated inflammatory cells disrupt the respiratory epithelium (6 months). (E) C. neoformans and cellular debris are present in the bronchiolar lumen which is lined by hypertrophic respiratory epithelium (magnification, ×165). (F) An interstitial granuloma at 18 months of infection can be seen. Arrows point to intracellular C. neoformans (mucin staining; magnification, ×825). (G) Large cluster of extracellular C. neoformans cells with minimal inflammation in the lung of a rat that received dexamethasone 1 week after infection (mucin staining; magnification, ×165). (H) Macrophages containing many C. neoformans organisms in the lung of the same rat (magnification, ×825). (I) In rats treated with dexamethasone at 11.5 months after infection, both intracellular (arrow) and extracellular (arrowhead) C. neoformans organisms are present (mucin staining; magnification, ×330). All staining was done with hematoxylin and eosin unless stated otherwise.
The lung pathology of immunosuppressed rats varied with the timing of dexamethasone administration. The lungs of rats that received dexamethasone 1 week after infection and were killed at 1.5 months exhibited less inflammation and contained more C. neoformans and GXM than the lungs of immunocompetent rats at 1.5 months. Inflammation consisted mostly of clusters of macrophages with few lymphocytes, although occasional small granulomas were present. No perivascular lymphocyte cuffing was noted. Organisms were found in large extracellular collections within the parenchyma and air spaces, including the bronchioles and alveoli (Table 1; Fig. 1G). Macrophages containing large numbers of C. neoformans, some of which were budding, were seen (Fig. 1H). The lungs of rats that received dexamethasone 11 months after infection exhibited occasional small granulomas and loose collections of macrophages. C. neoformans were detected within epithelioid cells and macrophages, as well as in extracellular collections within the alveoli and bronchioles (Fig. 1I).
NOS2 immunohistochemistry.
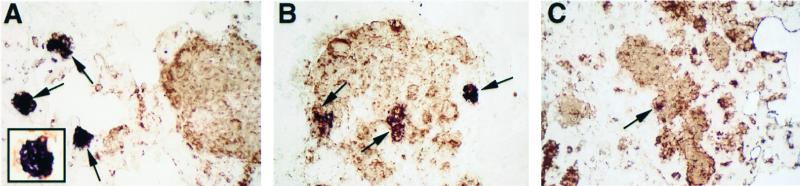
NOS2 staining localized primarily to epithelioid cells and occasional macrophages within the interstitium. In the lungs of immunocompetent rats, NOS2 staining within granulomas decreased over the course of infection (Fig. 2). This was manifested as a decrease in both the number of granulomas that stained positive for NOS2 and the size of NOS2 reactive foci within granulomas. At 1.5 months, NOS2 staining was limited to approximately 25% of granulomas, while at 6 and 12.5 months NOS2 staining was weak and present in fewer than 10% of the granulomas. NOS2 staining at 18 months of infection was detected only in rare epithelioid cells within granulomas. NOS2 staining in the lungs of dexamethasone-treated rats, 1.5 months after infection, was markedly reduced compared to immunocompetent rats at 1.5 months (not shown). NOS2 staining was not detected in the lungs of dexamethasone-treated rats at 12.5 months after infection (not shown). Double labeling for NOS2 and cryptococcal polysaccharide, revealed colocalization of NOS2 and GXM within epithelioid cells of some granulomas (Fig. 3). At 1.5 months of infection, small, discrete collections of GXM were more likely to be NOS2 positive than regions of the lung that contained confluent areas of GXM (Fig. 3A). A total of 52 NOS2 reactive foci were observed, with 38 of these foci localizing to discrete, small granulomas and 14 of these foci localizing to areas of confluent, granulomatous inflammation (P < 0.001). Within these areas of confluent inflammation and extensive GXM immunoreactivity, NOS2 staining was restricted to small clusters of cells (Fig. 3B and C). Hence, there appeared to be an inverse relationship between the extent of GXM reactivity in tissue and NOS2 staining.
FIG. 3.
Double staining for NOS2 (blue) and GXM (brown). (A) Small interstitial granulomas (1.5 months after infection) containing GXM demonstrate NOS2 staining, whereas areas containing large amounts of GXM demonstrate little or no NOS2 staining. The inset in panel A shows interstitial granuloma at high magnification. (B) Area of confluent inflammation containing large amounts of GXM show small foci of NOS2 reactivity (1.5 months after infection). (C) Granulomas at 6 months contain GXM but demonstrate minimal NOS2 staining. All magnifications, ×175.
Antibody responsiveness.
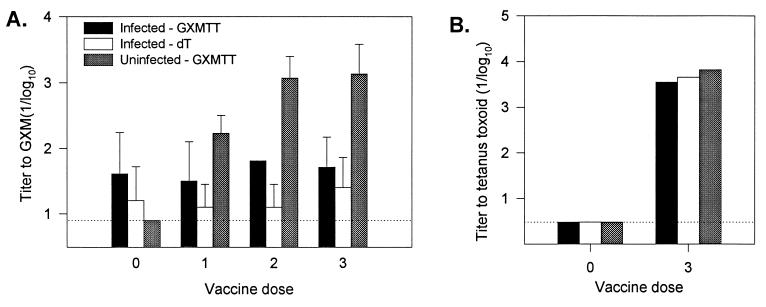
Persistent infection was confirmed in rats receiving vaccine by CFU determinations after completion of the vaccination schedule. Furthermore, histopathological examination of lungs from these rats revealed discrete, C. neoformans containing granulomas in a pattern similar to that observed in our earlier experiments. To explore the effects of persistent cryptococcal infection on the ability of rats to develop an antibody response to GXM, infected and noninfected rats were vaccinated with GXM-TT, and antibody titers were determined (Fig. 4). As a control, one group of infected rats was vaccinated with dT. Prior to vaccination, low titers of antibodies to GXM were detected in infected rats. After vaccination, antibody titers against GXM increased significantly in noninfected, control rats but not in infected rats. Both noninfected and infected animals exhibited a significant increase in antibody titers against tetanus toxoid after vaccination with GXM-TT.
FIG. 4.
GXM and tetanus toxoid antibody titers. Mean antibody titers (1/log10 ± 1 standard deviation [SD]) to GXM (A) and tetanus toxoid (B) in uninfected and infected rats after various doses of vaccine (GXM-TT and dT). The P value for GXM titers for infected versus uninfected rats receiving GXM-TT after 3 doses of vaccine was 0.025. After three doses of tetanus toxoid, at least one rat in each group had a titer greater than the maximal limit detectable by the ELISA, so the SD was not calculated. The dotted line represents the minimal limit of detection.
DISCUSSION
In previous studies, we demonstrated that intratracheal infection of rats elicits an exuberant granulomatous response that is associated with a reduction in fungal burden. Our current studies demonstrate that despite this initial containment that C. neoformans can persist within the lung. Inoculation of C. neoformans into the rat trachea resulted in a diffuse pneumonitis that resolved without scarring, except for the persistence of small subpleural and interstitial granulomas. These granulomas are very similar to those described by Haugen and Baker in human infection (16). Acute and persistent infection in rats produced no obvious clinical symptoms. Macrophages were intimately involved with C. neoformans at all stages of infection, and the administration of corticosteroid exacerbated infection. In this regard, the rat model of pulmonary cryptococcosis has striking similarities to human pulmonary infection.
The mechanism(s) by which C. neoformans persists in tissue despite the host inflammatory response is poorly understood. T-cell-mediated macrophage activation and granuloma formation are clearly important in limiting the growth of C. neoformans (13). During the course of persistent pulmonary infection in the rat, C. neoformans was primarily localized to the intracellular spaces of epithelioid cells and vacuolated macrophages. Since there was no significant change in CFU with time after 2 months and >99% of all yeast cells were inside either macrophages or epithelioid cells, we conclude that C. neoformans has the capacity for long-term survival within macrophages. In dexamethasone-treated rats, C. neoformans were predominantly in the extracellular spaces, suggesting that reactivation is accompanied by a transition from intracellular to extracellular location. The mechanism of and precise location of intracellular persistence is not known. Recent electron microscopy studies by Feldmesser et al. have shown intracellular replication of C. neoformans and a progressive increase in the amount of cryptococcal polysaccharide within phagolysosomes of pulmonary, murine macrophages during experimental C. neoformans infection (10). Accumulation of polysaccharide was associated with alteration of the phagolysosomal membrane and, ultimately, the destruction of the macrophage. It is possible that the vacuolation appreciated in the present study represents at least in part the intracellular collection of cryptococcal polysaccharide within vacuoles and is associated with altered cell function that may in turn promote cryptococcal persistence.
Macrophage activation and the production of reactive nitrogen intermediates, including nitric oxide (NO), appear to play a critical role in the host response to C. neoformans infection. Chemically generated NO inhibits C. neoformans growth in vitro (1). Furthermore, inhibition of NO synthase by the administration of arginine analogs inhibits the anticryptococcal activity of murine macrophages in vitro (29) and exacerbates experimental cryptococcal infection in mice (20). Hence, reduced NOS2 expression could play a role in promoting persistent cryptococcal infection. In this study, we demonstrate that the granulomas of long-term infection expressed low levels of NOS2. Furthermore, there was an apparent decrease in NOS2 expression over the course of infection despite a constant level of fungal burden. This contrasts with the high levels NOS2 expressed at 14 days of infection that we previously observed in association with an almost 2 log10-fold reduction in fungal burden (see Table 1 and Fig. 2) (12). These findings are consistent with those of Rossi et al. who observed decreased nitrite production by peritoneal macrophages after 14 days of infection in rats infected systemically with C. neoformans (23). We also observed regional differences in NOS2 expression among granulomas of long-term infection, suggesting that NOS2 expression is regulated on a local level. Areas of confluent inflammation that contained large amounts of cryptococcal polysaccharide exhibited little to no NOS2 expression. The mechanism of reduced NOS2 expression is unclear but is presumably related to the local cytokine milieu. Various cytokines, including interleukin-10 (IL-10) and transforming growth factor β (TGF-β), are known to downregulate NOS2 expression by macrophages (5, 27). Furthermore, C. neoformans polysaccharide can enhance IL-10 production by macrophages in vitro, and TGF-β expression has been detected in the granulomas of rats with pulmonary cryptococcosis (12, 26). Alternatively, inhibition of NOS2 expression by a mechanism that requires direct contact of C. neoformans with macrophages in vitro has been described and could be responsible for the observed decrease in NOS2 expression (18). Future studies directed at restoring NOS2 expression by immunomodulation may provide important insights into the mechanism and role of decreased NOS2 expression in persistent infection.
Persistent C. neoformans infection in the rat was accompanied by increased antibody titers to both cryptococcal proteins and GXM, although titers against cryptococcal proteins were higher than those against GXM. Increased titers against GXM and protein antigens in the sera of human immunodeficiency virus-negative individuals without a history of cryptococcosis has recently been described, suggesting that asymptomatic C. neoformans infection of immunocompetent individuals is common (4, 6). Our findings support these conclusions; however, direct comparisons between antibody titers in these studies are problematic secondary to differences in methodology. Administration of the GXM-TT conjugate vaccine where the GXM component behaves as a T-cell-dependent antigen did not significantly increase the anti-GXM titers in chronically infected rats. The decreased antibody responsiveness to GXM was specific and is similar to that reported in humans after natural infection with C. neoformans and in experiments involving vaccination with mice (17, 19). This phenomenon may have important ramifications on the efficacy of the vaccine in immunocompetent humans who are asymptomatically infected with C. neoformans.
In the rat model, pulmonary infection was exacerbated by the administration of corticosteroids. Corticosteroid therapy has multiple effects on the inflammatory response and is a recognized risk factor for the development of cryptococcosis in humans. Cortisone treatment of rats has been shown to decrease the capsular specific antibody response of rats immunized with capsular polysaccharide in complete Freund's adjuvant (11). In our experiments, a more striking effect was observed with respect to decreases in antibody titers against cryptococcal proteins. Administration of corticosteroids to rats early in infection partially abrogated the strong granulomatous inflammation and was associated with increased lung fungal burden, a change in organism localization from a primarily intracellular to an extracellular location, decreased inflammation, decreased NOS2 expression, and extrapulmonary dissemination. Corticosteroid treatment of rats infected for 11 months produced smaller effects with respect to increases in fungal burden and extrapulmonary dissemination. Similar results were described by Gadebusch and Gikas, who observed that cortisone treatment exacerbated pulmonary cryptococcal infection in rats as long as the interval between challenge and cortisone treatment did not exceed 4 weeks (11). These results suggest that the late stages of infection may be relatively resistant to the effects of corticosteroid immunosuppression and that the different immune mechanisms may be responsible for the control of early and late infections. It is likely that reactivation of latent infection will require the use of additional immunosuppressive agents. This observation may have a human parallel, since cryptococcosis is relatively rare among individuals on corticosteroid therapy despite serological evidence that human exposure is common (4).
In summary, we describe a model of long-term C. neoformans infection in rats that has striking similarities to the course and histopathology of human infection. Long-term containment of infection was dependent on granulomatous inflammation. However, persistence of infection in specific lung sites was associated with downregulation of both cellular and humoral responses and intracellular residence of yeasts in macrophages. The model described here should be useful for dissecting host and pathogen variables that contribute to persistent infection and represents a significant advance over existing models of cryptococcal infection. Pulmonary cryptococcosis in the rat provides a system for evaluating therapeutic and immunomodulatory interventions to eradicate chronic infections. Our results strongly suggest that measures to eliminate latent infection may require interventions to enhance macrophage antifungal activity.
ACKNOWLEDGMENTS
D.L.G. is supported by NIH grant A.I.O. 1300. S.C.L. is supported by NIH grants AI44641 and MH55477. A.C. is supported by NIH grants RO1-AI22774, AI13342, and HL59842 and by the Burroughs Wellcome Trust.
We thank Linda Johnson for her assistance in analyzing rat histopathology.
REFERENCES
- 1.Alspaugh J A, Granger D L. Inhibition of Cryptococcus neoformans replication by nitrogen oxide supports the role of these molecules as effectors of macrophage-mediated cystostasis. Infect Immun. 1991;59:2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell G D. Primary pulmonary cryptococcosis. Am Rev Respir Dis. 1966;94:236–243. doi: 10.1164/arrd.1966.94.2.236. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall A, Mukherjee J, Scharff M D. Monoclonal antibody ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Goldman D L, Doering T L, Pirofski L-A, Casadevall A. The antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect Immun. 1999;67:2218–2224. doi: 10.1128/iai.67.5.2218-2224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunha F Q, Moncada S, Liew F Y. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–1159. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- 6.DeShaw M, Pirofski L-A. Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clin Exp Immunol. 1995;99:425–432. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devi S J N, Schneerson R, Egan W, Ulrich T J, Bryla D, Robbins J B, Bennett J E. Cryptococcus neoformans serotype A glucoronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991;59:3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dromer F, Salamero J, Contrepois A, Carbon C, Yeni P. Production, characterization, and antibody specificity of a mouse monoclonal antibody reactive with Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987;55:742–748. doi: 10.1128/iai.55.3.742-748.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 10.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans: a facultative intracellular pathogen. Clin Infect Dis. 1998;27:982. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadebusch H H, Gikas P W. The effect of cortisone upon experimental pulmonary cryptococcosis. Am Rev Respir Dis. 1965;92:64–74. [Google Scholar]
- 12.Goldman D, Cho Y, Zhao M-L, Casadevall A, Lee S C. Expression of inducible nitric oxide synthase in rat pulmonary Cryptococcus neoformans granulomas. Am J Pathol. 1996;148:1275–1282. [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman D, Lee S C, Casadevall A. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect Immun. 1994;62:4755–4761. doi: 10.1128/iai.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman D L, Fries B C, Franzot S P, Montella L, Casadevall A. Phenotypic switching in the human pathogenic fungus Cryptococcus neoformans is associated with changes in virulence and pulmonary inflammatory response in rodents. Proc Natl Acad Sci USA. 1998;95:14967–14972. doi: 10.1073/pnas.95.25.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harkness J E, Wagner J E. The biology and medicine of rabbits and rodents. Philadelphia, Pa: Lea & Febiger; 1983. [Google Scholar]
- 16.Haugen R K, Baker R D. The pulmonary lesions in cryptococcosis with special reference to subpleural nodules. Am J Clin Pathol. 1954;24:1381–1390. doi: 10.1093/ajcp/24.12.1381. [DOI] [PubMed] [Google Scholar]
- 17.Henderson D K, Kan V L, Bennett J E. Tolerance to cryptococcal polysaccharide in cured cryptococcosis patients: failure of antibody secretion in vitro. Clin Exp Immunol. 1986;65:639–646. [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami K, Zhang T, Qureshi M H, Saito A. Cryptococcus neoformans inhibits nitric oxide production by murine peritoneal macrophages stimulated with interferon-gamma and lipopolysaccharide. Cell Immunol. 1997;180:47–54. doi: 10.1006/cimm.1997.1166. [DOI] [PubMed] [Google Scholar]
- 19.Kozel T R, Gulley W F, Cazin J J. Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. Infect Immun. 1977;18:701–707. doi: 10.1128/iai.18.3.701-707.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovchik J A, Lyons C R, Lipscomb M F. A role for gamma interferon-induced nitric oxide in pulmonary clearance of Cryptococcus neoformans. Am J Respir Cell Mol Biol. 1995;13:116–124. doi: 10.1165/ajrcmb.13.1.7598935. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee J, Scharff M D, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfect J R, Lang S D R, Durack D T. Chronic cryptococcal meningitis. Am J Pathol. 1980;101:177–193. [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi G R, Cervi L A, Sastre D A, Masih D T. Lack of involvement of nitric oxide in the macrophage-mediated inhibition of spleem cell proliferation during cryptococcosis. Clin Immunol Immunopathol. 1998;86:16–26. doi: 10.1006/clin.1997.4459. [DOI] [PubMed] [Google Scholar]
- 24.Salyer W R, Salyer D C, Baker R D. Primary complex of Cryptococcus and pulmonary lymph nodes. J Infect Dis. 1974;130:74–77. doi: 10.1093/infdis/130.1.74. [DOI] [PubMed] [Google Scholar]
- 25.Udall V. Toxicology of sulphonamide-trimethoprim combinations. Postgrad Med J. 1969;45:42–45. [PubMed] [Google Scholar]
- 26.Vecchiarelli A, Retini C, Monari C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vodovotz Y, Bogdan C, Paik C, Xie Q, Nathan F. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor-β. J Exp Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson P R, Bennett J E, Polis M A, Robbins J B, Schneerson R. Immunogenicity and safety of a conjugate glucuronoxylomannan-tetanus conjugate vaccine in volunteers. Clin Infect Dis. 1993;17:540. [Google Scholar]
- 29.Yoshida K, Akaike T, Doi T, Sato K, Ijiri S, Suga M, Ando M, Maeda H. Pronounced enhancement of NO-dependent antimicrobial activity by a NO oxidizing agent, imidazolineoxyl N-oxide. Infect Immun. 1993;61:3552–3555. doi: 10.1128/iai.61.8.3552-3555.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]