Abstract
Some Escherichia coli strains isolated from intestinal or extraintestinal infections in pigs produce cytotoxic necrotizing factor 1 (CNF1). In order to analyze the role of CNF1 in the pathogenesis of porcine colibacillosis, newborn colostrum-deprived germfree piglets were orally inoculated with a wild-type CNF1-producing strain (M623) or with an isogenic cnf1 mutant (M623ΔCNF1). The two isogenic strains induced a high mortality with similar lung and serosal inflammatory lesions, indicating that both strains were pathogenic in these piglets. Bacterial counts in various organs of inoculated piglets revealed an intestinal predisposition of M623 and M623ΔCNF1 strains for the cecum and colon. Extraintestinal organs (lungs, liver, spleen, and kidney) were also colonized by both strains. Similar colonization of intestinal and extraintestinal tissues in animals inoculated with either strain was observed, except in the ileum, where M623 showed a higher colonization than M623ΔCNF1. Intestinal (ileum and colon), extraintestinal (lung and kidney), and immune (mesenteric lymph nodes and spleen) tissues were sampled at 1 day postinoculation and analyzed for cytokine expression by a reverse transcriptase PCR technique. Inoculation with E. coli M623 induced an enhanced expression of inflammatory cytokines (interleukin-1α [IL-1α], tumor necrosis factor α, and IL-12p40) in the intestinal organs compared to uninoculated piglets or piglets inoculated with nonpathogenic intestinal E. coli 862B, which is also able to colonize the intestinal tract. There was little difference in cytokine transcript levels in the intestinal and extraintestinal organs in piglets inoculated with E. coli strains M623 or M623ΔCNF1, except in the ileum, where IL-1α and IL-8 mRNA levels correlated with bacterial colonization. Expression of regulatory cytokines (gamma interferon and IL-4) was weak in immune tissues from piglets inoculated with M623 or M623ΔCNF1. Taken together, our data indicate that the CNF1-producing strain, M623, is pathogenic and induces inflammatory cytokine expression in germfree, colostrum-deprived piglets. Nevertheless, in this model, the CNF1 toxin does not appear to be a major factor for pathogenicity or cytokine response, as demonstrated by the use of an isogenic cnf1 mutant.
Escherichia coli is a normal inhabitant of the intestinal tract but certain strains cause disease. Pathogenic E. coli belong to a restricted number of pathotypes defined by the presence of virulence factors which determine the host specificity and type of disease produced by these pathotypes (43, 62). The virulence mechanisms of E. coli strains are complex and only partially understood. They include the ability to colonize mucosal surfaces, invade extraintestinal tissues, survive and multiply in body fluids with low concentrations of available iron (58), and escape phagocytosis and intracellular killing by phagocytes (46). E. coli strains and/or their products modulate host cytokine responses (67). These cytokines, together with other inflammatory mediators are involved in the induction, persistence, or elimination of microbial infection (29, 70).
The production of cytokines during bacterial infection has been extensively studied in human septic shock (50). In this model, the release of endotoxin-lipopolysaccharide (LPS) triggers the synthesis of inflammatory cytokines such as tumor necrosis factor (TNF), interleukin-1 (IL-1), and IL-6. These cytokines induce many changes which result in the failure of the major organs and rapid death of the patient (50). In addition to LPS, other bacterial components have the capacity to induce cytokine production (for a review, see reference 72). Specific examples of pathogenic E. coli virulence factors that influence cytokine production include alpha-hemolysin, at nontoxic concentrations, which inhibits the production of TNF, IL-6, and IL-1β by human peripheral blood cells (38); an as-yet-unknown protein from enteropathogenic E. coli (EPEC) that inhibits IL-2, IL-4, IL-5, and gamma interferon (IFN-γ) expression by peripheral and mucosal mononuclear cells (37, 40); and Shiga-like toxin, which induces inflammatory cytokine production by murine macrophages (66). Adhesion to or invasion of epithelial cell monolayers by uropathogenic E. coli or EPEC also leads to the production of cytokines (19, 30, 59). Indeed, P fimbriae, which mediate attachment of uropathogenic E. coli to epithelial cells, enhance the host inflammatory response to infection and increase virulence (10, 31). Similarly, EPEC stimulate intestinal epithelial cell lines to produce IL-8 through the activation of NF-κB (55).
Among the putative virulence factors produced by E. coli, cytotoxic necrotizing factors (CNFs) are produced by strains involved in diarrhea and septicemia in humans and in domestic animals (4, 6, 12). Necrotizing E. coli producing CNF1 have also been isolated from piglets with diarrhea and with edema disease (27) and have been associated clinically with lesions of polyserositis and septicemia in young pigs (22). CNF toxins are lethal when administrated intravenously to mice or sheep and are dermatonecrotic when inoculated into the rabbit skin (13–15). In addition, experimental oral inoculation of neonatal calves and pigs has shown that CNF-positive E. coli causes septicemia and enteritis (57, 73). S. Clément, B. Martineau-Doizé, I. P. Oswald, E. Oswald, M. Odin, and J. M. Fairbrother (submitted for publication) have also examined the dynamics of infection of CNF1-producing E. coli in experimentally inoculated conventional piglets of various ages and immune or weaning states. They demonstrated that CNF1-producing E. coli colonizes predominantly the large intestine and disseminates to mesenteric lymph nodes and internal organs, particularly in colostrum-deprived piglets. CNF1 and CNF2 are 110- to 115-kDa monomeric toxins that covalently interact with Rho (24, 48), resulting in its activation through the deamidation of a glutamine residue (25, 56). This activation of Rho GTPases results in polymerization of F actin, increased formation of stress fibers and multinucleation of cells (6, 23, 48). In addition to being implicated in the regulation of cytoskeletal structure, the Rho family of small GTP-binding proteins is also involved in the gene transcription and activation of the NF-κB (41). Since this nuclear transcription factor plays a major role in the transcriptional regulation of many acute phase proteins and inflammatory cytokines (1, 2), we could anticipate that CNF induces cytokine synthesis.
Even if several lines of evidence implicate CNF1 and CNF2 in the pathogenesis of colibacillosis, their exact role still needs to be determined. Indeed, CNF1-producing E. coli strains often express other virulence factors (17, 34) and have also been found in the intestine of healthy piglets (27). Moreover, inactivation of the cnf1 gene in diarrhea-associated E. coli did not lead to a decrease in diarrhea and inflammation in a rabbit intestinal ligated loop model (20).
In the present study, we orally inoculated germfree colostrum-deprived newborn piglets with either E. coli M623, a wild-type CNF1-producing strain, an isogenic cnf1 derivative of M623, or a nonpathogenic E. coli strain. Our aim was to investigate the pathogenicity of a CNF1-producing strain in this model and to elucidate the role of CNF1 in bacterial pathogenicity and host cytokine response. In addition, we analyzed in vivo cytokine levels by reverse transcriptase PCR (RT-PCR) in the tissues of piglets. Herein, we demonstrated that M623 is pathogenic in germfree piglets and induces an inflammatory cytokine response in intestinal organs. Nevertheless, there were few differences at the level of pathogenicity, colonization, and cytokine levels elicited by M623 or its isogenic cnf1 mutant M623ΔCNF1. Overall, our results suggest that CNF1 is not essential for pathogenicity, nor does it greatly influence the induction of host inflammatory cytokines by strain M623 in experimentally inoculated germfree piglets.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains and plasmids used throughout this study are summarized in Table 1. Wild-type E. coli 862B (O115:K−) is nonpathogenic and serum sensitive and was isolated from the intestinal contents of a pig (21, 45). E. coli M623 (O2:K+), isolated from the intestine of a pig with enteritis (C. Wray, Central Veterinary Laboratory, Surrey, England) was used for inoculation studies. This strain is serum resistant and produces cytotoxin CNF1, alpha-hemolysin, and P and S fimbriae (17) but does not produce cytolethal distending toxin. Cosmid 2CO2 (also called cosmid 10) contains the cnf1 and hly gene clusters from uropathogenic strain E. coli J96 and was kindly supplied by D. E. Berg (64). Cloning vector pBluescript KSII+ was obtained from Stratagene (La Jolla, Calif.) and cloning vector pILL570 was kindly supplied by A. Labigne (39). Plasmid pKNG101 is a positive selection suicide vector containing strAB, sacBR and a pir-dependent R6K replicon (35). Plasmids were maintained in laboratory strain DH5α (54), except for suicide plasmids (pKNG101 and derivatives), which were maintained in SM10λpir (32). Bacteria were isolated on Luria-Bertani (LB) agar and cultured in LB broth. Prior to piglet inoculation, strains were grown in tryptic soy broth (TSB). Media were supplemented with appropriate antibiotics at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; streptomycin, 50 μg/ml; and spectinomycin, 100 μg/ml.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description or genotype | Source or reference |
|---|---|---|
| E. coli | ||
| M623 | Isolated from a pig with enteritis; O2, K+ | C. Wraya |
| M623ΔCNF1 | cnf1 mutant obtained by allelic exchange | This study |
| 862B | Isolated from the intestinal contents of pigs; O115, K−, F165− | 21 |
| DH5α | F− (Φ80dlacZΔM15) thi-1 endA1 relA1 hsdR17(rK−mK+) Δ(lacIZYA-argF)U169 recA1 gyrA96 (Nalr) supE44 | 54 |
| SM10λpir | thi-1 thr leuB6 tonA lacY1 supE44 λpir (R6K) recA::RP4-2 Tc::Mu (Kmr) | 32 |
| Plasmids and cosmids | ||
| pBluescript IIKS+ | Cloning vector, Apr | Stratagene |
| pILL570 | Cloning vector, Spr | 39 |
| 2CO2 | Insert coding for cnf1 and hly from E. coli J96 | 64 |
| pKNG101 | Suicide cloning vector, strAB sacBR pir-dependent R6K replicon | 35 |
| pSB315 | 978-bp fragment coding for aphT in pBluescript | 26 |
Central Veterinary Laboratory, Surrey, England.
Recombinant DNA, genetic techniques, and nonpolar mutations in cnf1.
Routine recombinant DNA techniques were performed by using standard procedures (54). A 13-kb EcoRI fragment from 2CO2 was cloned into pILL570. A 4.1-kb EcoRI/BamHI fragment bearing only cnf1 was subcloned into pBluescript KSII+. The aphT gene devoid of its transcription terminator was retrieved from pSB315 (26) by BamHI restriction and cloned at the BglII site within cnf1. The ApaI fragment bearing cnf1 disrupted by aphT was cloned into pKNG101 vector, resulting in pKNGcnf::aphT. Suicide plasmid pKNGcnf::aphT was introduced into strain M623 by conjugation. Mutants that had undergone allelic exchange leading to the replacement of the wild-type locus with the locus disrupted by aphT were selected on LB plates without NaCl and containing 5% sucrose and kanamycin, as previously described (35). Mutations were confirmed by Southern blots and cytotoxic assay as previously described (15).
Experimental inoculation of piglets.
Piglets were delivered from four specific-pathogen-free Yorkshire hybrid gilts by Caesarian delivery. Piglets were immediately passed through an iodine bath, placed in germfree isolators, and fed condensed milk ad libitum, as previously described (45). We confirmed the absence of bacteria in the feces of piglets in isolators prior to inoculation. At 2 days of age, piglets received 10 ml of 1.2% NaHCO3 through an intragastric tube to neutralize gastric acid. Piglets, chosen at random, were then similarly intubated with 1 ml of 0.9 to 2.1 × 109 CFU in 19 ml of TSB of the wild-type parent strain M623 (n = 12) or with its isogenic cnf1 derivative M623ΔCNF1 (n = 14) or with the nonpathogenic strain 862B (n = 2). Piglets were examined for mortality for up to 7 days postinoculation. An additional group of three uninoculated piglets served as controls for the determination of baseline cytokine mRNA levels.
Necropsy procedure.
Piglets were killed by an intracardiac injection of Euthanyl Forte (sodium pentobarbital at 540 mg/ml diluted in 0.20 ml of propylene glycol; Pharmacie, Faculté de Médecine Vétérinaire, Saint-Hyacinthe, Québec, Canada) at 1 or 7 days postinoculation or if moribund.
Tissues were sampled from the lung, liver, spleen, kidney, duodenum, jejunum, ileum, cecum, colon, and mesenteric lymph nodes draining the corresponding jejunal and ileal segments of euthanized animals. These samples were consistently taken from the same area from respective organs in all animals. Lung samples were obtained from nonconsolidated areas. Portions of each sample were used immediately for bacteriological and histopathological examination. Another portion of each tissue was frozen in 1 ml of Trizol (Gibco-BRL, Burlington, Ontario, Canada) for RNA extraction and analysis of cytokine gene expression.
Bacteriological counts.
Tissues were evaluated quantitatively for the presence of E. coli. Samples were weighed and suspended in 2 ml of phosphate-buffered saline (PBS), homogenized at 5,000 rpm by using a Cat homogenizer x120 (PolyScience, Niles, Ill.), and 10-fold serially diluted in sterile PBS. Dilutions were plated on tryptic soy agar for the parental strain and on the same medium with kanamycin (50 μg/ml) for the mutant strain by using a Spiral Plater System Model C (Meyer Service and Supply Ltd., Long Sault, Ontario, Canada) as recommended by the manufacturer. After overnight incubation at 37°C, bacterial counts were determined. Several colonies from each individual were confirmed as being the infecting strain by PCR and agglutination tests.
Histopathology.
Tissue samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin and phloxin saffron for examination by light microscopy. Bacterial localization in intestinal and extraintestinal tissues was determined by immunocytochemistry. Sections were stained by Vector red (Vector Laboratories, Burlington, Ontario, Canada) as already described (52) by using rabbit polyclonal anti-O2 serogroup serum which was prepared as previously described (47).
RNA extraction.
Samples from each organ, maintained in Trizol at −80°C, were homogenized by using a Cat homogenizer. Total RNA was extracted as recommended by the manufacturer. The RNA was resuspended in 50 to 500 μl of ultrapure water containing 0.02% (wt/vol) diethyl pyrocarbonate (Sigma, St. Quentin Fallavier, France) and 1 mM EDTA. Total RNA was quantified by using a spectrophotometer at an optical density of 260 nm (OD260), and the purity was assessed by determining the OD260/OD280 ratio. All of the samples had an OD260/OD280 ratio above 1.8.
RT-PCR detection of cytokine mRNA and densitometric quantification of PCR products.
An RT-PCR procedure was performed as previously described (18). Briefly, 1 μg of RNA was reverse transcribed (Superscript II RNase H−; Life Technologies, Eragny, France) and then amplified (Taq DNA polymerase; Promega, Charbonnières, France). Primer sequences and the number of PCR cycles chosen for each cytokine are listed in Table 2. Amplified DNA was analyzed after electrophoresis on 1.2% TBE (Tris-borate-EDTA) agarose gels, which were stained with ethidium bromide and photographed with Polaroid 665 film. The level of each PCR product was quantified by densitometry by using Image Aquisition and Whole Band Analyzer software (Bioimage) on a Sun Sparc Station 5 (Cadrus, Ramonville St-Agne, France) as previously described (18). To compare the relative cytokine mRNA expression levels among samples, the values are presented as the ratio of the band intensity of the cytokine-specific RT-PCR product over that of the corresponding constitutively expressed “housekeeping” gene, cyclophilin.
TABLE 2.
Oligonucleotides designed in this study to specifically detect porcine cytokine and cyclophilin cDNA
| Gene specificity | Primera | Oligonucleotide sequences (5′–3′) | No. of cycles |
|---|---|---|---|
| IL-1α | S | TGCCAGCTATGAGCCACTTCC | 40 |
| AS | TGACGGGTCTCGAATGATGCT | ||
| IL-4 | S | TACCAGCAACTTCGTCCAC | 45 |
| AS | ATCGTCTTTAGCCTTTCCAA | ||
| IL-6 | S | ATGAACTCCCTCTCCACAAGC | 45 |
| AS | TGGCTTTGTCTGGATTCTTTC | ||
| IL-8 | S | TTTCTGCAGCTCTCTGTGAGG | 40 |
| AS | CTGCTGTTGTTGTTGCTTCTC | ||
| IL-12p40 | S | GATGCTGGCCAGTACACC | 40 |
| AS | TCCAGCACGACCTCAATG | ||
| IFN-γ | S | GTTTTTCTGGCTCTTACTGC | 45 |
| AS | CTTCCGCTTTCTTAGGTTAG | ||
| TNF-α | S | GATGGCAGAGAGGAGGTTGAC | 38 |
| AS | ATCGGCCCCCAGAAGGAAGAG | ||
| Cyclophilin | S | TAACCCCACCGTCTTCTT | 29 |
| AS | TGCCATCCAACCACTCAG |
S, sense primer; AS, antisense primer.
Statistical analysis.
Student's t test and/or analysis of variance were used to analyze bacterial counts and cytokine production. P values of <0.05 were considered significant. Macroscopic lesions were analyzed by use of the χ2 test. χ2 values of <3.84 were not considered significant.
RESULTS
Pathogenicity of E. coli M623 in piglets.
We first investigated the pathogenicity of E. coli M623 in germfree, colostrum-deprived piglets inoculated by the oral route. Of 12 piglets, 2 demonstrated respiratory distress and died suddenly at less than 12 h postinoculation. At 24 h postinoculation, six inoculated piglets were randomly chosen and euthanized for necropsy; of the four remaining piglets, only one survived up to 7 days postinoculation. Table 3 summarizes the macroscopic lesions noted in the 10 piglets necropsied after death or after euthanasia. Most of the piglets inoculated with M623 demonstrated congestion of the lung, and half of these piglets presented intestinal congestion. Fluid and fibrin were observed in the thoracic, pericardial, and/or peritoneal cavities of all piglets examined at more than 24 h postinoculation but rarely in piglets examined at 24 h postinoculation. Microscopically, changes in piglets examined at 24 h postinoculation were minimal, the most important being inflammatory changes in the lung. These changes were characterized by a multifocal septal leukocytic infiltration composed of a mixed population of neutrophils and mononuclear cells and by an occasional fibrinous to leukocytic alveolitis. Bronchioalveolar necrosis was observed in one piglet (Table 4). Of note, no significant lesions were observed in uninoculated controls or piglets inoculated with strain 862B (data not shown).
TABLE 3.
Macroscopic lesions in germfree piglets inoculated with isogenic E. coli strains expressing or not expressing CNF1 toxin
| Macroscopic-lesion type(s) | % Piglets with lesionsa inoculated with:
|
|||
|---|---|---|---|---|
| M623 at:
|
M623ΔCNF1 at:
|
|||
| 24 h p.i.b(n = 6) | >24 h p.i.c(n = 4) | 24 h p.i.b(n = 7) | >24 h p.i.c(n = 4) | |
| Pulmonary congestion | 66 | 100 | 29 | 75 |
| Pulmonary consolidation | 17 | 25 | 0 | 25 |
| Thoracic, pericardial, and/or peritoneal fluid | 17 | 100 | 14 | 50 |
| Thoracic, pericardial, and/or peritoneal fibrin | 0 | 100 | 0 | 50 |
| Intestinal congestion | 50 | 50 | 29 | 25 |
| Mesenteric lymph node congestion | 17 | 25 | 29 | 0 |
Piglets were inoculated with M623 or M623ΔCNF1 E. coli strains.
Piglets were euthanized and necropsied at 24 h postinoculation (p.i.).
Immediately after death or after euthanasia, animals were necropsied at >24 h postinoculation.
TABLE 4.
Microscopic findings in germfree piglets inoculated with isogenic E. coli strains expressing or not expressing CNF1 toxin and then euthanized 24 h postinoculation
| Microscopic finding(s) | % Piglets with lesionsa inoculated with:
|
|
|---|---|---|
| M623 (n = 6) | M623ΔCNF1 (n = 7) | |
| Pulmonary interstitial inflammation | 66 | 14 |
| Pulmonary alveolar inflammation | 33 | 14 |
| Bronchio-alveolar necrosis | 17 | 0 |
| Renal congestion and/or hemorrhage | 50 | 57 |
| Intestinal congestion and/or hemorrhage | 50 | 50b |
Piglets were inoculated with M623 or M623ΔCNF1 E. coli strains.
The analysis of one piglet could not be realized.
We examined the ability of strain M623 to colonize intestinal and extraintestinal organs as demonstrated by bacterial counts in different tissues of the piglets euthanized at 24 h postinoculation. At this time, M623 colonized the intestinal tract, mostly the large intestine and to a lesser extent the small intestine (Table 5). Bacteria had translocated to the mesenteric lymph nodes and disseminated to the lungs, liver, spleen, and kidney. Bacteria persisted in all organs until 7 days postinoculation. As already described (45), the control nonpathogenic strain 862B, which did not induce death in piglets, also colonized the intestines and was recovered from the mesenteric lymph nodes and extraintestinal organs at levels similar to strain M623 at 24 h postinoculation. This strain colonized the intestines and was recovered from the mesenteric lymph nodes but not from other extraintestinal organs at 7 days postinoculation (data not shown).
TABLE 5.
Quantitative bacterial counts in tissues of piglets necropsied at 1 day postinoculation with E. coli M623 and M623ΔCNF1
| Organ(s) | Bacterial count (log10 CFU/g)a
|
Difference between groupsb (P) | |
|---|---|---|---|
| M623 (n = 6) | M623ΔCNF1 (n = 7) | ||
| Intestine | |||
| Duodenum | 6.10 ± 0.47 | 5.58 ± 0.70 | 0.56 |
| Jejunum | 4.74 ± 0.67 | 5.42 ± 0.66 | 0.49 |
| Ileum | 7.92 ± 0.45 | 5.45 ± 0.84 | 0.03c |
| Cecum | 9.09 ± 0.21 | 9.41 ± 0.27 | 0.38 |
| Colon | 8.36 ± 0.33 | 8.69 ± 0.38 | 0.54 |
| Intestinal lymph node | |||
| Jejunal | 5.21 ± 0.64 | 5.44 ± 0.53 | 0.78 |
| Ileal | 4.06 ± 1.01 | 5.36 ± 0.30 | 0.21 |
| Spleen | 4.50 ± 0.57 | 4.65 ± 0.29 | 0.81 |
| Lungs | 3.95 ± 0.74 | 3.83 ± 0.24 | 0.86 |
| Liver | 2.95 ± 0.86 | 2.81 ± 0.77 | 0.90 |
| Kidney | 3.78 ± 0.62 | 3.53 ± 0.22 | 0.69 |
Data for each organ represents the geometric mean count ± the SEM from a group of six or seven pigs.
Student's t tests were realized to compare bacterial counts from tissues of animals inoculated with either of the two strains.
P < 0.05 (all other values were not significant).
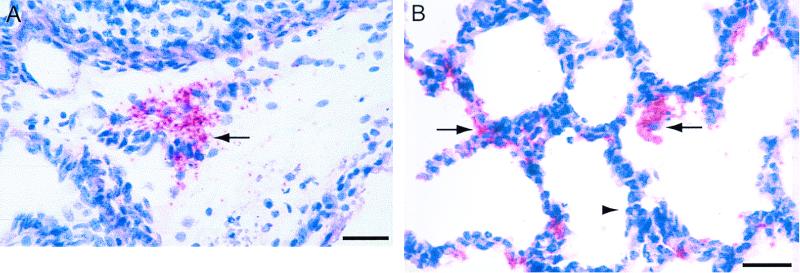
Bacterial distribution in tissues was assessed by immunocytochemistry by using Vector red (52). In the digestive tract, most bacteria were found on the luminal aspect, occasionally in close contact with the mucosa. Bacterial colonization was also observed in the serosa and to a lesser extent in the submucosa. Bacteria were also found extraintestinally in the mesenteric lymph nodes (Fig. 1A), lung (Fig. 1B), kidneys, and, when present, the mesentery. In the lung, bacteria were found in the interlobular and alveolar septa, lining the alveoli or within the alveolar lumen, associated with macrophages. Renal colonization was characterized by the presence of O2-positive rods in the interstitium and occasionally in the tubular lumen. Bacteria were scattered throughout the mesentery.
FIG. 1.
In situ visualization of bacteria in tissues by immunohistochemistry with an anti-O2 serum by Vector red staining. Piglets were inoculated with M623 and were euthanized 24 h postinoculation. (A) Clusters of bacteria are found extracellularly close to the hilus of mesenteric lymph node (arrow). Bar, 25 μm. (B) Bacteria are observed along the pulmonary epithelium (arrow facing right) and are occasionally found in the alveolar lumen, associated or not with alveolar macrophages (arrow facing left). A focal septal leukocytic infiltration composed of a mixed population of neutrophils and mononuclear cells can be seen (arrowhead). Bar, 10 μm.
Role of cnf1 on these effects.
In order to understand the role of CNF1 in pig colibacillosis, a derivative of strain M623 unable to produce CNF1 (M623ΔCNF1) was constructed by allelic exchange. As expected, bacterial lysates from M623ΔCNF1 did not induce stress fibers and multinucleation in cultured HeLa cells, in contrast to lysates obtained from the wild-type M623 strain (data not shown).
We then examined the pathogenicity of the cnf1 mutant in orally inoculated piglets. Of 14 inoculated piglets, 3 demonstrated severe respiratory distress and died at between 14 and 24 h postinoculation. At 24 h postinoculation, seven piglets inoculated with M623ΔCNF1 were randomly chosen and euthanized for necropsy. Overall, strain M623ΔCNF1 tended to be slightly less pathogenic than M623. Two of four piglets inoculated with M623ΔCNF1 survived for up to 7 days postinoculation compared to only one of four piglets inoculated with M623. Piglets inoculated with either strain demonstrated similar macroscopic lesions on necropsy at 24 h postinoculation (Table 3). However, pulmonary inflammatory changes were observed significantly (P = 0.05) more often in piglets inoculated with strain M623 than in those inoculated with the CNF1 mutant at 24 h postinoculation (Table 4). The bronchioalveolar necrosis, although only observed in one M623 inoculated piglet, was not observed at all in piglets inoculated with the CNF1 mutant. Finally, thoracic, pericardial, and/or peritoneal fluid and fibrin were observed more frequently in piglets inoculated with M623 than in those inoculated with the CNF1 mutant, at 36 h or more following inoculation (Table 3).
M623ΔCNF1 colonized the examined tissues, except for the ileum, to the same extent as strain M623 (Table 5). Indeed, about five times more CFU per gram were recovered from the ileum of piglets inoculated with strain M623 than from piglets inoculated with strain M623ΔCNF1. Bacteria persisted in all organs until 7 days postinoculation, and no difference in bacterial persistence was observed for the surviving piglets belonging to either the M623ΔCNF1 or M623 inoculated groups (data not shown).
E. coli M623 and M623ΔCNF1 induce an enhanced production of inflammatory cytokines in the intestine.
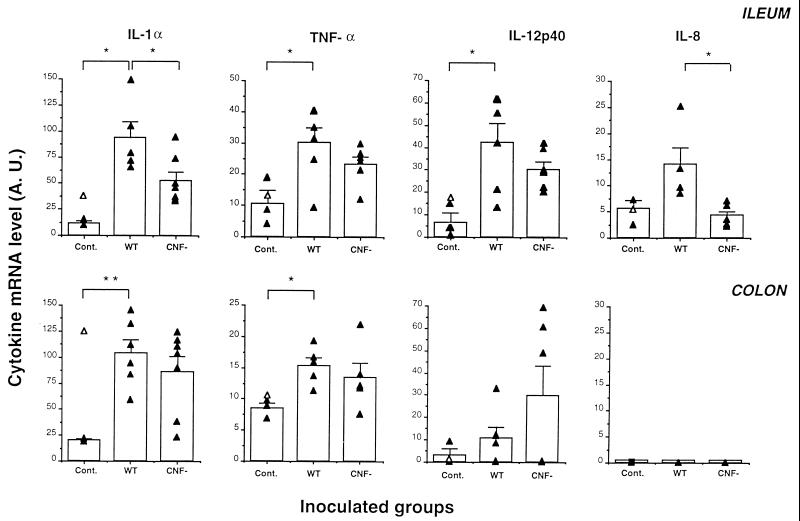
The ability of E. coli M623 and M623ΔCNF1 to induce inflammatory cytokines such as IL-1α, IL-6, IL-8, IL-12p40, and TNF-α at the transcriptional level was then compared in piglets necropsied at 24 h postinoculation. Cytokine mRNA expression was measured by semiquantitative RT-PCR in samples from various organs. Since piglets were inoculated by the oral route, we first investigated the mRNA expression of inflammatory cytokines in the small intestine (ileum) and in the large intestine (colon). In the ileum, IL-1α, TNF-α, and IL-12p40 mRNA levels were significantly higher in piglets inoculated with M623 than in control piglets (Fig. 2). On the other hand, IL-8 mRNA production was only slightly higher in the ileum of M623-inoculated piglets than in uninoculated controls. In the colon, IL-1α and TNF-α mRNA levels for M623-inoculated piglets were significantly greater than for control piglets, whereas IL-12p40 mRNA production was only weakly enhanced. IL-8 mRNA was not detected in the colon of any of the piglets. The expression of IL-6 was not detected in the two intestinal organs investigated. Expression of the different cytokines by a piglet inoculated with the strain 862B was similar to that observed in uninoculated piglets, except in the case of IL-1α in the colon (Fig. 2).
FIG. 2.
Inflammatory cytokine production in intestinal samples from inoculated piglets. Piglets were inoculated with M623 (wild type [WT]) or with its isogenic cnf derivative (M623ΔCNF1) (CNF−) and were euthanized 1 day postinoculation. Untreated piglets were used as controls (Cont.). Tissues from the ileum and colon were sampled and homogenized. Total RNA was then isolated and assayed for expression of inflammatory cytokine (IL-1α, TNF-α, IL-12p40, and IL-8) and the cyclophilin housekeeping genes by RT-PCR. Quantification of these cytokine mRNAs from the ileum and the colon in different animals (closed triangles) and the mean (± the standard error of the mean [SEM]) of these results for each group are shown (vertical bars). Opened triangles in the controls represent the values of piglets inoculated with nonpathogenic strain 862B. Student's t tests were performed to compare M623-inoculated piglets with control ones or M623ΔCNF1-inoculated animals. ∗, P < 0.05; ∗∗, P < 0.01.
When cytokine mRNA expression was compared in intestinal samples from M623- and M623ΔCNF1-inoculated piglets, a higher expression was observed in animals inoculated with the wild-type strain (Fig. 2). However, these differences were only significant for IL-1α and IL-8 in the ileum. Cytokine expression was also assayed in two extraintestinal organs: the lungs and kidney. No significant differences were observed between animals inoculated with either M623 or M623ΔCNF1 except for an increase in TNF-α expression in the lungs of piglets inoculated with M623ΔCNF1 (Table 6).
TABLE 6.
Cytokine mRNA levels in the lungs and the kidney of infected piglets
| Organ(s) | Cytokine | Cytokine mRNA levelsa
|
Difference between groupsb (P) | |
|---|---|---|---|---|
| M623 | M623ΔCNF1 | |||
| Lungs | IL-1α | 62.9 ± 7.1 | 69.4 ± 8.1 | 0.59 |
| TNF-α | 12.7 ± 1.3 | 53.0 ± 16.1 | 0.05c | |
| IL-6 | 2.9 ± 2.9 | 4.5 ± 3.1 | 0.72 | |
| IL-12p40 | 32.9 ± 6.6 | 18.9 ± 4.0 | 0.10 | |
| IL-8 | 2.0 ± 2.0 | 10.6 ± 4.1 | 0.12 | |
| Kidney | IL-1α | 147.8 ± 32.0 | 107.5 ± 7.0 | 0.17 |
| TNF-α | 54.5 ± 14.0 | 47.6 ± 12.3 | 0.72 | |
| IL-6 | 14.5 ± 9.3 | 7.5 ± 3.6 | 0.42 | |
| IL-12p40 | 54.8 ± 22.5 | 37.5 ± 22.1 | 0.61 | |
| IL-8 | 15.4 ± 15.4 | 3.5 ± 3.5 | 0.35 | |
Total RNA was isolated from lungs and kidney and assayed for expression of inflammatory cytokine (IL-1α, TNF-α, IL-6, IL-12p40, and IL-8) and cyclophilin genes by RT-PCR. Cytokine mRNA is normalized to the housekeeping gene and expressed in arbitrary units. Data for each organ represents the mean ± the SEM from a group of six M623- or seven M623ΔCNF1-inoculated piglets.
Student's t tests were realized to compare bacterial counts from tissues of animals inoculated with either of the two strains.
P < 0.05 (all other values were not significant).
Expression of Th1 and Th2 cytokines by M623 and M623ΔCNF1.
In order to determine whether the immune response elicited by M623 and M623ΔCNF1 was of the Th1 or Th2 type, we examined the production of a Th1 (IFN-γ) and a Th2 (IL-4) cytokine, by RT-PCR, in two immune organs (the spleen and the intestinal lymph nodes). As we only observed a weak production of these cytokines, probably due to the immaturity of the immune system of the piglets, we determined the frequency of detection of these two cytokines from controls and from piglets inoculated with M623 or M623ΔCNF1 (Fig. 3). Control animals did not express these two cytokines in their intestinal lymph nodes. In inoculated animals, IFN-γ was not expressed in the spleen and was expressed in only a low proportion of the lymph nodes. By contrast, IL-4 was expressed in a much higher proportion (up to 100%) of spleen and intestinal lymph node samples from inoculated animals, suggesting that these piglets display a Th2 response.
FIG. 3.
Regulatory cytokine gene expression in immune organs from inoculated piglets. IL-4 and IFN-γ mRNA production by spleen and intestinal lymph nodes from uninoculated controls (Cont., □) or from M623 (M623,  )- and M623ΔCNF1 (CNF−, ■)-inoculated piglets. Frequencies of detection of IL-4 and IFN-γ mRNAs are expressed as the percentage of positive samples observed by RT-PCR assays. Student's t tests were performed to compare M623-inoculated piglets with control ones or with M623ΔCNF1-inoculated animals. ∗, P < 0.05; ND, not done.
)- and M623ΔCNF1 (CNF−, ■)-inoculated piglets. Frequencies of detection of IL-4 and IFN-γ mRNAs are expressed as the percentage of positive samples observed by RT-PCR assays. Student's t tests were performed to compare M623-inoculated piglets with control ones or with M623ΔCNF1-inoculated animals. ∗, P < 0.05; ND, not done.
DISCUSSION
In this study, we demonstrated the pathogenicity of the CNF1-producing E. coli strain M623 in colostrum-deprived germfree piglets. This strain induced pulmonary interstitial and exudative inflammation and subsequently typical lesions of polyserositis which are observed in natural cases of E. coli septicemia in the pig (22, 46). E. coli isolates from such cases are often CNF1 positive. Rapid death (>24 h postinoculation) was observed in two piglets inoculated with the CNF1-producing E. coli strain and three piglets inoculated with the CNF1 mutant. These piglets demonstrated nonspecific lesions of pulmonary and intestinal congestion macroscopically, which may have been due to endotoxic shock rather than to trauma or injury due to the inoculation, since no evidence of inoculum aspiration was observed during the removal of the stomach tube and this phenomenon has not been observed in piglets similarly inoculated with other septicemic or nonpathogenic E. coli (45). Only one piglet examined at 24 h postinoculation with M623 demonstrated a pulmonary lesion, i.e., bronchoalveolar necrosis, which could have been related to the inoculation procedure with secondary aspiration of remaining bacteria during extubation. The bacteria not present at the site of inflammation may have already been eliminated by the inflammation. Hence we feel that, in general, this route was not a contributing factor in bacterial colonization of the lung.
Strain M623 colonizes intestinal and extraintestinal organs from at least 1 day postinoculation and persists up to 7 days postinoculation (Table 5). The colonization is predominantly in the intestine and particularly in the cecum and colon. These results are in agreement with the fact that the gastrointestinal tract would act as a reservoir for bacteria that can cause extraintestinal infections (63). Bacteria probably pass through the epithelial cells lining the intestines and are carried in the lymph to the mesenteric lymph node and possibly to the systemic complex (3), allowing bacterial establishment in extraintestinal organs. Of note, as already demonstrated (45), the nonpathogenic strain 862B was also able to colonize the intestine and to translocate into the mesenteric lymph nodes in colostrum-deprived germfree piglets. However, this strain did not persist in other extraintestinal organs nor did it induce any lesions or mortality. Hence, strain M623 possesses additional virulence attributes which may not be required for translocation from the intestine but which permit bacteria to persist and induce lesions in extraintestinal sites, at least in this model. In conventional, 2-day-old colostrum-deprived piglets (S. Clément, submitted), we also found that M623 was able to translocate to the draining lymph nodes and to the extraintestinal organs. However, in this model the strain did not induce any lesions or mortality. The difference in the pathogenesis observed in the two cases is probably due to the absence of intestinal barrier in germfree piglets. Indeed, newborn germfree piglets lack the ability to transfer maternal regulatory factors, both antigens and immunoglobulins, which have been shown to inhibit bacterial adherence to receptors on the intestinal epithelial cells and to neutralize the activity of the cytotoxins produced by E. coli (68). Using other CNF1-producing strains and higher doses of bacteria, Wray et al. (73) observed bacterial colonization of the intestine and extraintestinal organs, diarrhea, and respiratory signs in inoculated piglets, although clinical signs appeared to vary widely depending on the bacterial strain used and even within a group of animals given the same strain.
Several studies have investigated the action of CNF toxin in vitro. Based on these studies, the effect of CNF appears to be very different depending on the model used. For example, CNF1 increases intestinal permeability in Caco-2 cells (28), whereas it does not affect tight junction permeability in T84 monolayer (33). Similarly, this toxin induced a phagocytic behavior in human epithelial HEp-2 cells (23) but downmodulated integrin activation-dependent phagocytosis in human monocytes (5). In addition, CNF1 does not seem to have any effect on the ability of hemolytic E. coli to damage human bladder cell monolayers in vitro (34), but CNF1 effaced cell microvilli and decreased transepithelial migration of polymorphonuclear leukocytes on polarized T84 epithelial intestinal cell monolayers (33).
In the present study we investigated the in vivo role of CNF1 in the pathogenicity of colibacillosis. CNF1-positive E. coli are associated clinically with lesions of polyserositis and septicemia in young pigs (22). Initial experiments carried out in conventional piglets of different ages (10-day-old weaned or unweaned and 2-day-old colostrum fed or colostrum deprived) in an attempt to reproduce these lesions were not able to demonstrate any pathogenicity for E. coli M623 (Clément et al., submitted). Indeed, even in the presence of bacterial colonization of the intestine and dissemination to mesenteric lymph nodes and internal organs, we did not observe any mortality or significant lesions. Hence, we chose the more sensitive colostrum-deprived, germfree piglet, with which we have had considerable experience in the study of the pathogenesis of porcine E. coli septicemia (45), as our model for the study of the pathogenesis of M623 infection. We constructed a CNF1-isogenic mutant of a wild-type pathogenic E. coli strain, and we orally inoculated germfree neonatal piglets with either the M623 parental strain or the M623ΔCNF1 mutant strain. We hypothesized that inactivation of cnf1 would decrease the ability of E. coli to colonize the intestine and/or to translocate to and cause lesions in internal organs in swine. However, inactivation of cnf1 did not reduce the pathogenicity of the wild-type M623 strain except for a weaker colonization of the ileum (Table 5), a slight decrease in mortality, and a slight decrease in inflammatory response in the lungs and serosal surfaces. Our results confirm and extend those of Elliot et al. (20), who used CNF1 mutants in a rabbit model of intestinal ligated loops and did not observe a significant effect on the onset, duration, or severity of diarrhea.
The inability to find significant differences in the pathogenicity of the M623 and the M623ΔCNF1 can be interpreted in several different ways. First, CNF1 may play no role in bacterial pathogenicity and the fact that CNF-positive strains are pathogenic may result solely from a genetic linkage of the cnf1 gene with other virulence factors genes, such as those encoding alpha-hemolysin (hly) and P-related adhesin (prs) located on the same pathogenicity island on strain J96 (64) as well as strain M623 (E. Oswald, unpublished data). Second, our germfree piglet model may be insufficiently sensitive for the detection of the effect of CNF1. Moxley et al. found that inactivation of hemolysin did not reduce the incidence of septicemia in gnotobiotic piglets inoculated with isogenic enterotoxigenic E. coli strains after oral inoculation (42). Similarly, expression of heat-stable enterotoxin STb by adherent E. coli is not sufficient to cause severe diarrhea in neonatal pigs (7). Finally, other virulence factors may obscure the effects of CNF1. Indeed, strain M623 produces P and S fimbriae and hemolysin, which may contribute to the development of infection (17). In light of the results presented here, we tend to favor the last two hypotheses. Indeed, necrotoxigenic E. coli strains could be considered as pathogens of an opportunistic nature (Clément et al., submitted), and we have demonstrated that in vitro CNF potentiates the effect of another toxin, CDT (S. Pérès, F. Daigle, N. Ghichemerre, O. Marchés, F. Hérault, J. De Rycke, and E. Oswald, submitted for publication). Thus, using another infectious model, such as an immunocompromised piglet and/or E. coli strains that express other virulence factors, we would expect to increase the slight differences observed in the present study between isogenic strains expressing or not CNF1 toxin, in terms of mortality, inflammatory lesions, and cytokine response.
Cytokines are important in the regulation of the immune response and in the control of inflammation, but they can also contribute to immunopathological changes in the host after bacterial infection. In the current study, we investigated the cytokine response in both the intestinal tract and the immune tissues. In the spleen and intestinal draining lymph nodes, we particularly investigated the Th1-Th2 balance by measuring IFN-γ and IL-4. These two cytokines were weakly expressed, probably due to the age of the animals whose lymphoid organs were not totally developed. However, in inoculated animals the higher frequency of IL-4 mRNA detection in the lymphoid organs compared to that of IFN-γ argue in favor of a Th2 response following E. coli inoculation. This is in agreement with the shift toward the Th2-cell-type response observed in volunteers given a low dose of E. coli endotoxin (74) and may reflect the fact that E. coli, as do other extracellular bacteria, stimulates a stronger humoral than cellular immune response.
We also determined the cytokine response in intestinal tissues from control animals and from piglets inoculated with M623, M623ΔCNF1, or 862B strains. In contrast to control uninoculated or 862B inoculated animals, piglets inoculated with M623 or M623ΔCNF1 strains produced increased levels of mRNA encoding for inflammatory cytokines in their intestinal tract. Bacterial LPS did not seem to trigger this local synthesis of cytokines since strain 862B, which colonizes the intestine to the same extent as the two other strains, did not induce any inflammatory response in the ileum or in the colon (Fig. 2). Inoculation with either of the pathogenic strains M623 and M623ΔCNF1 induces an inflammatory response in the intestinal tract, as measured by the production of IL-1α and TNF-α (Fig. 2). These cytokines play a major role in the course of bacterial infection and in sepsis (16), and local induction of these inflammatory cytokines has been detected in murine models of pyelonephritis (36, 53) and epididymitis (65) induced by E. coli. Recombinant IL-1 and TNF have also been shown to increase the colonization of EPEC in the rabbit small bowel (71). Thus, the local induction of these inflammatory cytokines during oral inoculation by pathogenic E. coli may create a microenvironment that facilitates their own colonization of the intestine. Surprisingly, we did not find any increase in IL-6 and IL-8 mRNA levels in the intestine of inoculated piglets, although these cytokines have been detected in clinical patients or in experimental animals inoculated with E. coli (11, 61).
Similar responses were observed in piglets inoculated with either M623 or M623ΔCNF1 E. coli strains, except in the ileum where animals inoculated with the mutant strain displayed lower inflammatory cytokine transcript levels than animals inoculated with the parental strain (Fig. 2). In the ileum, the bacterial colonization also differed between the two groups of animals (Table 5). We believe that the lower cytokine production reflects the lower bacterial colonization of this organ and is not directly related to a putative effect of CNF1 on NF-κB (1, 2). This hypothesis is substantiated by the observation of Capo and coworkers (5), who did not find any specific inflammatory cytokine production in macrophages stimulated by purified CNF1 toxin. However, we cannot exclude an alternative explanation, i.e., that higher levels of IL-1 and TNF-α expression in M623-inoculated piglets compared to M623ΔCNF1-inoculated ones could facilitate bacterial colonization of the wild-type E. coli strain, as has already been demonstrated for EPEC (71).
Possible sources of the inflammatory cytokines induced by M623 strains include macrophages (9, 44), as well as dendritic cells (8, 69), and epithelial cells (19, 30, 60). The latter cells which line the intestine are continuously in contact with bacteria and their products and are known to play an active role in the mucosal immune system (19, 30). Of note, most of the inflammatory cytokine mRNAs that we investigated were detected by RT-PCR, although at low levels, in samples from control piglets (Fig. 2). This has been described in other studies and may reflect the dynamic nature of immune regulation even in the absence of microbial invasion (49, 51).
In conclusion, our results showed that the CNF1-producing strain M623 is pathogenic in germfree piglets and specifically induces the production of inflammatory cytokines. The CNF1 toxin does not seem to be the essential factor of virulence since the isogenic mutant is also pathogenic.
ACKNOWLEDGMENTS
Sylvie Fournout and Charles M. Dozois were supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada grant GPG0181728 and by an INRA postdoctoral fellowship, respectively. This work was supported in part by NSERC of Canada grant GPG0181728, by a grant from the European Community program FAIR (number 1335), and by programme prioritaire “Génomes et Fonctions” (INRA) grant.
REFERENCES
- 1.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P J, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 3.Berg R D, Garlington A W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco J, Alonso M P, Gonzalez E A, Blanco M, Garabal J I. Virulence factors of bacteraemic Escherichia coli with particular reference to production of cytotoxic necrotizing factor (CNF) by P-fimbriate strains. J Med Microbiol. 1990;31:175–183. doi: 10.1099/00222615-31-3-175. [DOI] [PubMed] [Google Scholar]
- 5.Capo C, Meconi S, Sanguedolce M-V, Bardin N, Flatau G, Boquet P, Mege J-L. Effect of cytotoxic necrotizing factor-1 on actin cytoskeleton in human monocytes: role in the regulation of integrin-dependent phagocytosis. J Immunol. 1998;161:4301–4308. [PubMed] [Google Scholar]
- 6.Caprioli A, Falbo V, Roda L G, Ruggeri F M, Zona C. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect Immun. 1983;39:1300–1306. doi: 10.1128/iai.39.3.1300-1306.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey T A, Herring C J, Schneider R A, Bosworth B T, Whipp S C. Expression of heat-stable enterotoxin STb by adherent Escherichia coli is not sufficient to cause severe diarrhea in neonatal pigs. Infect Immun. 1998;66:1270–1272. doi: 10.1128/iai.66.3.1270-1272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavaillon J M. Cytokines and macrophages. Biomed Pharmacother. 1994;48:445–453. doi: 10.1016/0753-3322(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 10.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalrymple S A, Slattery R, Aud D M, Krishna M, Lucian L A, Murray R. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect Immun. 1996;64:3231–3235. doi: 10.1128/iai.64.8.3231-3235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Rycke J, Guillot J F, Boivin R. Cytotoxins in non-enterotoxigenic strains of Escherichia coli isolated from feces of diarrheic calves. Vet Microbiol. 1987;15:137–150. doi: 10.1016/0378-1135(87)90139-8. [DOI] [PubMed] [Google Scholar]
- 13.De Rycke J, Plassiart G. Toxic effects for lambs of cytotoxic necrotizing factor from Escherichia coli. Res Vet Sci. 1990;49:349–354. [PubMed] [Google Scholar]
- 14.De Rycke J, Gonzalez E A, Blanco J, Oswald E, Blanco M, Boivin R. Evidence for two types of cytotoxic necrotizing factor in human and animal clinical isolates of Escherichia coli. J Clin Microbiol. 1990;28:694–699. doi: 10.1128/jcm.28.4.694-699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Rycke J, Mazars P, Nougayrède J P, Tasca C, Boury M, Hérault F, Valette A, Oswald E. Mitotic block and delayed lethality in HeLa epithelial cells exposed to Escherichia coli BM2-1 producing cytotoxic necrotizing factor type 1. Infect Immun. 1996;64:1694–1705. doi: 10.1128/iai.64.5.1694-1705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello C A. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and treatment of the septic shock syndrome. J Infect Dis. 1991;163:1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- 17.Dozois C M, Clément S, Desautels C, Oswald E, Fairbrother J M. Expression of P, S, and F1C adhesins by cytotoxic necrotizing factor 1-producing Escherichia coli from septicemic and diarrheic pigs. FEMS Microbiol Lett. 1997;152:307–312. doi: 10.1111/j.1574-6968.1997.tb10444.x. [DOI] [PubMed] [Google Scholar]
- 18.Dozois C M, Oswald E, Gautier N, Serthelon J-P, Fairbrother J M, Oswald I P. A reverse transcription-polymerase chain reaction method to analyze porcine cytokine gene expression. Vet Immunol Immunopathol. 1997;58:287–300. doi: 10.1016/s0165-2427(97)00039-1. [DOI] [PubMed] [Google Scholar]
- 19.Eckmann L, Kagnoff M F, Fierer J. Intestinal epithelial cells as watchdogs for the natural immune system. Trends Microbiol. 1995;3:118–120. doi: 10.1016/s0966-842x(00)88894-0. [DOI] [PubMed] [Google Scholar]
- 20.Elliott S J, Srinivas S, Albert M J, Alam K, Robins-Browne R M, Gunzburg S T, Mee B J, Chang B J. Characterization of the roles of hemolysin and other toxins in enteropathy caused by alpha-hemolytic Escherichia coli linked to human diarrhea. Infect Immun. 1998;66:2040–2051. doi: 10.1128/iai.66.5.2040-2051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairbrother J M, Broes A, Jacques M, Larivière S. Pathogenicity of Escherichia coli O115:K"V165" strains isolated from pigs with diarrhea. Am J Vet Res. 1989;50:1029–1036. [PubMed] [Google Scholar]
- 22.Fairbrother J M, Ngeleka M. Extraintestinal Escherichia coli infections in pigs. In: Gyles C L, editor. Escherichia coli in domestic animals and man. Oxford, England: CAB International; 1994. pp. 221–237. [Google Scholar]
- 23.Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanié L, Oswald E, Boquet P. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 24.Fiorentini C, Donelli G, Matarrese P, Fabbri A, Paradisi S, Boquet P. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 26.Galàn J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garabal J I, Gonzalez E A, Vazquez F, Blanco J, Blanco M. Toxigenic Escherichia coli in Spanish piggeries from 1986 to 1991. Vet Microbiol. 1995;47:17–25. doi: 10.1016/0378-1135(95)00107-l. [DOI] [PubMed] [Google Scholar]
- 28.Gerhard R, Schmidt G, Hofmann F, Aktories K. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect Immun. 1998;66:5125–5131. doi: 10.1128/iai.66.11.5125-5131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heath A W. Cytokines and infection. Curr Opin Immunol. 1990;2:380–384. doi: 10.1016/0952-7915(89)90145-3. [DOI] [PubMed] [Google Scholar]
- 30.Hedges S R, Agace W W, Svanborg C. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 1995;3:266–270. doi: 10.1016/s0966-842x(00)88941-6. [DOI] [PubMed] [Google Scholar]
- 31.Hedlund M, Svensson M, Nilsson A, Duan R D, Svanborg C. Role of the ceramide-signaling pathway in cytokine responses to P-fimbriated Escherichia coli. J Exp Med. 1996;183:1037–1044. doi: 10.1084/jem.183.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrero M, De Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofman P, Flatau G, Selva E, Gauthier M, Le Negrate G, Fiorentini C, Rossi B, Boquet P. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect Immun. 1998;66:2494–2500. doi: 10.1128/iai.66.6.2494-2500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Island M D, Cui X, Foxman B, Marrs C F, Stamm W E, Stapleton A E, Warren J W. Cytotoxicity of hemolytic, cytotoxic necrotizing factor 1-positive and -negative Escherichia coli to human T24 bladder cells. Infect Immun. 1998;66:3384–3389. doi: 10.1128/iai.66.7.3384-3389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 36.Khalil A, Brauner A, Bakhiet M, Burman L G, Jaremko G, Wretlind B, Tullus K. Cytokine gene expression during experimental Escherichia coli pyelonephritis in mice. J Urol. 1997;158:1576–1580. [PubMed] [Google Scholar]
- 37.Klapproth J-M, Donnenberg M S, Abraham J M, James S P. Products of enteropathogenic E. coli inhibit lymphokine production by gastrointestinal lymphocytes. Am J Physiol. 1996;271:G841–847. doi: 10.1152/ajpgi.1996.271.5.G841. [DOI] [PubMed] [Google Scholar]
- 38.König B, König W. Induction and suppression of cytokine release (tumor necrosis factor-α, interleukin-6, interleukin-1β) by Escherichia coli pathogenicity factors (adhesions, α-haemolysin) Immunology. 1993;78:526–533. [PMC free article] [PubMed] [Google Scholar]
- 39.Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991;173:1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malstrom C, James S. Inhibition of murine splenic and mucosal lymphocyte function by enteric bacterial products. Infect Immun. 1998;66:3120–3127. doi: 10.1128/iai.66.7.3120-3127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montaner S, Perona R, Saniger L, Lacal J C. Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- 42.Moxley R A, Berberov E M, Francis D H, Xing J, Moayeri M, Welch R A, Baker D R, Barletta R G. Pathogenicity of an enteroxigenic Escherichia coli hemolysin (hlyA) mutant in gnotobiotic piglets. Infect Immun. 1998;66:5031–5035. doi: 10.1128/iai.66.10.5031-5035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muhldorfer I, Hacker J. Genetics aspects of Escherichia coli virulence. Microb Pathog. 1994;16:171–181. doi: 10.1006/mpat.1994.1018. [DOI] [PubMed] [Google Scholar]
- 44.Murch S H. Local and systemic effects of macrophage cytokines in intestinal inflammation. Nutrition. 1998;14:780–783. doi: 10.1016/s0899-9007(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 45.Ngeleka M, Jacques M, Martineau-Doize B, Daigle F, Harel J, Fairbrother J M. Pathogenicity of an Escherichia coli O115:K"V165" mutant negative for F165(1) fimbriae in septicemia of gnotobiotic pigs. Infect Immun. 1993;61:836–843. doi: 10.1128/iai.61.3.836-843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngeleka M, Martineau-Doize M, Fairbrother J M. Septicemia-inducing Escherichia coli O115:K"V165"F165(1) resists killing by porcine polymorphonuclear leukocytes in vitro: role of F165(1) fimbriae and K"V165" O-antigen capsule. Infect Immun. 1994;62:398–404. doi: 10.1128/iai.62.2.398-404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ørskov F, Ørskov I. Serotyping of Escherichia coli. Methods Microbiol. 1984;14:43–112. [Google Scholar]
- 48.Oswald E, Sugai M, Labigne A, Wu H C, Fiorentini C, Boquet P, O'Brien A D. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc Natl Acad Sci USA. 1994;91:3814–3818. doi: 10.1073/pnas.91.9.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oswald I P, Dozois C M, Petit J F, Lemaire G. IL-12 synthesis is a required step in trehalose dimycolate-induced activation of mouse peritoneal macrophages. Infect Immun. 1997;65:1364–1369. doi: 10.1128/iai.65.4.1364-1369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parrillo J E. Pathogenetic mechanisms of septic shock. N Eng J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 51.Pepin M, Seow H F, Corner L, Rothel J S, Hodgson A L, Wood P R. Cytokine gene expression in sheep following experimental infection with various strains of Corynebacterium pseudotuberculosis differing in virulence. Vet Res. 1997;28:149–163. [PubMed] [Google Scholar]
- 52.Rogers D G, Andersen A A, Hunsaker B D. Lung and nasal lesions caused by a swine chlamydial isolate in gnotobiotic pigs. J Vet Diagn Investig. 1996;8:45–55. doi: 10.1177/104063879600800108. [DOI] [PubMed] [Google Scholar]
- 53.Rugo H S, O'Hanley P, Bishop A G, Pearce M K, Abrams J S, Howard M, O'Garra A. Local cytokine production in a murine model of Escherichia coli pyelonephritis. J Clin Investig. 1992;89:1032–1039. doi: 10.1172/JCI115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 55.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 57.Smith H W. Observations on Escherichia coli infection in calves. In: Rutter J M, editor. Proceedings of the first seminar on “pathology” in the CEC program of coordination of research in beef production. Brussels, Belgium: Commission of the European Communities; 1975. pp. 47–59. [Google Scholar]
- 58.Smith H. Virulence determinants of Escherichia coli: present knowledge and questions. Can J Microbiol. 1992;38:747–752. doi: 10.1139/m92-121. [DOI] [PubMed] [Google Scholar]
- 59.Stadnyk A W. Cytokine production by epithelial cells. FASEB J. 1994;8:1041–1047. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 60.Stadnyk A W, Sisson G R, Waterhouse C C. IL-1 alpha is constitutively expressed in the rat intestinal epithelial cell line IEC-6. Exp Cell Res. 1995;220:298–303. doi: 10.1006/excr.1995.1319. [DOI] [PubMed] [Google Scholar]
- 61.Steiner T S, Lima A A, Nataro J P, Guerrant R L. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 62.Sussman M. Escherichia coli: mechanisms of virulence. Cambridge, United Kingdom: Cambridge University Press; 1997. [Google Scholar]
- 63.Svanborg-Eden C, Andersson B, Aniansson G, Jodal U, Lomberg H, Linder H, de Man P. Bacterial adherence in urinary and respiratory tract infection. Kansenshogaku Zasshi. 1988;62(Suppl.):136–148. [PubMed] [Google Scholar]
- 64.Swenson D L, Bukanov N O, Berg D E, Welch R A. Two pathogenicity islands in uropathogenic Escherichia coli J96: cosmid cloning and sample sequencing. Infect Immun. 1996;64:3736–3743. doi: 10.1128/iai.64.9.3736-3743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka K, Fujisawa M, Arakawa S, Kamidono S. Local expression of cytokine messenger RNA in rat model of Escherichia coli epididymitis. J Urol. 1995;154:2179–2184. [PubMed] [Google Scholar]
- 66.Tesh V L, Ramegowda B, Samuel J E. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine peritoneal macrophages. Infect Immun. 1994;62:5085–5094. doi: 10.1128/iai.62.11.5085-5094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tesh V L. Virulence of enterohemorrhagic Escherichia coli: role of molecular crosstalk. Trends Microbiol. 1998;6:228–233. doi: 10.1016/s0966-842x(98)01282-7. [DOI] [PubMed] [Google Scholar]
- 68.van der Waaij D. The role of the microbial flora in the development, maintenance, and modulation of the immune function. Adv Physiol Sci. 1980;29:443–477. [Google Scholar]
- 69.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 70.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wanke C A, Bistrian B. Recombinant human tumor necrosis factor and recombinant murine interleukin-1 alter the binding of Escherichia coli to intestine, mucin glycoprotein, and the HT29-C1 intestinal cell line. Nutrition. 1997;13:959–964. doi: 10.1016/s0899-9007(97)00337-7. [DOI] [PubMed] [Google Scholar]
- 72.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wray C, Piercy D W, Carroll P J, Cooley W A. Experimental infection of neonatal pigs with CNF toxin-producing strains of Escherichia coli. Res Vet Sci. 1993;54:290–298. doi: 10.1016/0034-5288(93)90125-y. [DOI] [PubMed] [Google Scholar]
- 74.Zimmer S, Pollard V, Marshall G D, Garofalo R P, Taber D, Prough D, Herndon D N. Effects of endotoxin on the Th1/Th2 response in humans. J Burn Care Rehabil. 1996;17:491–496. [PubMed] [Google Scholar]