TGF-β signaling plays key roles in cancer progression. Most carcinoma cells have inactivated their epithelial antiproliferative response but benefit from increased TGF-β expression and autocrine TGF-β signaling through effects on gene expression, release of immunosuppressive cytokines and epithelial plasticity, which enables invasion and dissemination, increased stem cell properties and cancer drug resistance. TGF-β released by cancer cells, stromal fibroblasts and other cells in the tumor micro-environment further promotes cancer progression by shaping the architecture of the tumor and suppressing anti-tumor activities of immune cells, thus generating an immunosuppressive environment that prevents or attenuates the efficacies of cancer immunotherapies. Repression of TGF-β signaling is therefore seen as a prerequisite and major avenue to enhance the efficacies of current and forthcoming immuno-oncological therapies, also when the tumor cells themselves are not TGF-β responsive. We discuss approaches to TGF-β signaling blockade, their use in cancer immunotherapies, and possible adverse effects.
Since its discovery as a secreted factor that induces reversible phenotypic transformation of select fibroblast cell lines1–3, the roles of TGF-β have been studied in tumorigenesis, particularly carcinomas. These studies were encouraged by three sets of observations: (1) TGF-β expression in tumor cell lines and tissues is often increased when compared to normal cells or healthy tissue4,5, (2) the growth inhibitory response to TGF-β seen in normal epithelial cells is often impaired in carcinomas6,7, and (3) the TGF-β response of epithelial and carcinoma cells confers attenuation of epithelial characteristics and increased cell migration and invasion, which contribute to cancer progression8,9. Yet, tumorigenesis depends on the tumor cells’ ability to generate and condition a tumor micro-environment (TME) containing diverse stromal cell types that enables cancer cells to thrive, and promote cancer progression10–20. Most if not all cells of the TME have the potential to respond to TGF-β, although their responses depend on the cell type, are highly contextual, and show considerable signaling heterogeneity. Consequently, understanding the roles of TGF-β in cancer progression requires a full appreciation of the complex interplay of TGF-β responses in cancer cells and non-cancer cells of the tumor. That such insights might enable new therapeutic approaches has become apparent with the success of anti-cancer therapies based on immune checkpoint blockade, and observations that increased TGF-β signaling in the TME correlates with poor overall survival and resistance to immunotherapeutic blockade of PD-L1 or its receptor, PD-121-24. Complementing previous reviews25–27, this critical review aims to convey insight into the roles of TGF-β signaling in carcinomas and their TME, including immune cells, and addresses expected outcomes of targeting TGF-β signaling in cancer progression to enhance and benefit immunotherapy.
Carcinomas and their tumor micro-environment
As epithelial cells proliferate, initiate tumor formation, and transform into carcinoma cells, their ability to establish tumors and the behavior of those tumors depend on conditioning a micro-environment conducive for cancer progression. The tumor and TME evolve coordinately with reciprocal signaling between epithelial and TME cells10,11. An early tumor-promoting microenvironment may develop in response to insults, or chemical or radiation-induced damage11,28–30. Paracrine signals from tumor cells with activated oncogenes or lost tumor suppressor genes are likely to continue to condition the TME and help instruct the behavior of its cell populations. Conversely, signals from distinct TME cell populations instruct the behavior of tumor cells while also defining the behavior and functions of each other through a web of instructive and inhibitory autocrine and paracrine interactions. Many interactions occur locally through cell-cell interactions, or between cells and extracellular matrices, including contacts between T and NK cell receptors and peptide-MHC complexes31–33. Most cytokines, chemokines and differentiation factors, including TGF-β, also act primarily locally.
Dependent on tumor type, grade and stage, carcinoma cells might represent a minority of tumor cells, with the bulk of the tumor consisting of stromal cells including fibroblasts, myeloid and lymphoid cells, as well as pericytes and endothelial cells (Figure 1). In most human carcinomas, the majority of tumor cells retain epithelial characteristics, including epithelial cell junctions and gene expression signatures, whereas some cells, mostly at invasive fronts, attenuate these characteristics through a transdifferentiation process, named epithelial-mesenchymal transition (EMT)34–37.
Figure 1:
Cell type heterogeneity within the TME. This schematic diagram illustrates the irregular distribution of cancer cells within the tumor with extensive interactions with stromal fibroblasts and immune cells, and interspersion of blood vessels and lymphatic vessels. The cell types that have been discussed are shown in this cartoon format.
For Figure 1, please refer to Figure 1 of Derynck, R., Turley, S.J., and Akhurst, R.J. (2021) TGFβ biology in cancer progression and immunotherapy. Nature Reviews in Clinical Oncology, 18, 9–34
Stromal fibroblasts and fibroblast-like cells, referred to as carcinoma-associated fibroblasts (CAFs), play key roles in providing a beneficial tumor stroma, are interspersed between populations of tumor cells, and thus are well positioned to communicate with carcinoma cells (Figure 1). CAFs are thought to originate from fibroblasts that were activated in response to inflammatory and tumor-derived signals, and further expand through cell proliferation38–40. However, CAFs may additionally originate from epithelial and endothelial cells through EMT, thought to occur in response to cytokines, most prominently but not exclusively TGF-β35,41,42. CAFs support tumor growth and progression through secreted or membrane-associated signals that benefit the carcinoma cells, and deposition of extracellular matrix (ECM) proteins, which confer instructive signals, and retain and thus store secreted chemokines and cytokines, including TGF-β40,43. The ECM network of the TME helps define tumor architecture. Tumor growth depends on extensive vascularization to transport oxygen and nutrient into the tumor mass, thus allowing both the tumor cells and non-malignant stromal cells to thrive18,44–47. The vascular capillary system, elaborated by endothelial cells and pericytes that organize into vessels, supply blood, oxygen and nutrients to the TME, facilitates entry of immune and other cell types into the tumor13–17, whereas the lymphatic system enables exit of immune cells, antigens, interstitial fluid, live and dying tumor cells, and particles from the tumor into tumor-draining lymph nodes18,19.
The TME contains diverse types of innate and adaptive immune cells (Figure 1); these include monocytes and macrophages, myeloid-derived suppressor cells (MDSCs), neutrophils and other granulocytes, dendritic cells (DCs), conventional and non-conventional T cells, B cells and NK cells, all of which are thought to contribute to or control cancer progression. Among these, T cells and NK cells exert cytotoxic anti-tumor effects48,49, DCs present tumor antigens to T cells50–54, and macrophages and neutrophils remove cells and cell debris by phagocytosis55. Local cytokines in the TME, including TGF-β, direct the behavior of these cell types, often resulting in functional changes that convert them to support cancer progression rather than direct tumor destruction. IgA-producing plasmablasts derived from B lymphocytes can contribute to tumor progression, and also their activities are controlled by TGF-β56. Among the T cell populations that, together with NK cells and B cells, control cancer progression, are CD8+ T cells whose differentiation and anti-tumor killer cell activity can be repressed by several cell types, including MDSCs57 and regulatory T (Treg) cells58. TGF-β promotes Treg cell differentiation59,60 and enables Treg cells to suppress adaptive and innate immune responses61–64.
Fundamentals of TGF-β biology with relevance to cancer
To understand the roles of TGF-β in cancer, we introduce some fundamentals of TGF-β biology that are more extensively summarized elsewhere65. The mammalian genome encodes three TGF-βs, TGF-β1, -β2 and -β3, which act as disulfide-linked dimers. Each gene encodes a precursor protein with a short amino-terminal signal peptide required for secretion and a carboxy-terminal 112 amino acid, mature TGF-β polypeptide. These two domains are linked by a long pro-segment. During secretion, the pro-segments are cleaved from the mature polypeptides, yet remain associated with mature TGF-β to act as chaperones. Consequently, the mature TGF-β dimer is secreted in “latent” complex with two copies of the non-covalently associated pro-segment, often called “latency-associated polypeptide” (LAP), that prevent TGF-β binding to its cell surface receptors (Figure 2A).
Figure 2:
Latent TGF-β activation and initiation of TGF-β signaling through receptors and Smads. The TGF-β1 dimer is expressed in complex with its pro-segments (a) that confer latency and associate with either LTBP1 that promotes the latent complex localization within ECM, or GARP (or related molecules) that localizes the latent complex at the cell surface (B). Activation of latent TGF-β1 complexes involves non-covalent association of TGF-β1’s prosegment through an RGD sequence (circle) with one of several integrin β chains, and likely requires physical tension and proteases to release the active TGF-β1 dimer from the latent complex (C). Active TGF-β1 binds heteromeric type II-type I TGF-β receptor (RII-RI) complexes at the cell surface (D). When associated with clathrin in nascent endosomes, ligand-activated receptor complexes activate Smad2 and Smad3 that combine with Smad4 to form trimeric complexes that translocate into the nucleus. These Smad complexes cooperate with high affinity DNA binding transcription factors and coregulators to activate or repress genes. TGF-β also activates non-Smad signaling pathways from distinct receptor complexes in caveolar compartments. TGF-β-induced Erk MAPK pathway activation and Akt signaling may initiate from the same receptor complexes. However, TGF-β-induced Akt activation was also shown in different cells to require TRAF6 and not require the TβRI kinase activity, suggesting distinct receptor complexes, as shown. TGF-β-induced p38 MAPK and JNK activation initiate from similar or distinct receptor complexes in cholesterol-rich lipid raft compartments.
For Figure 2, please refer to Figure 2 of Derynck, R., Turley, S.J., and Akhurst, R.J. (2021) TGFβ biology in cancer progression and immunotherapy. Nature Reviews in Clinical Oncology, 18, 9–34
Since TGF-β1, purified from platelets, was biochemically seen to primarily exist as homodimer66, it is assumed that TGF-β1, TGF-β2 and TGF-β3 are always expressed and act as homodimers. This notion is reinforced by the commercial availability of TGF-β homodimers that are used to evaluate effects of TGF-βs; however, the isolation of natural TGF-β1:β2 heterodimers67 raises the possibility that TGF-β heterodimers, e.g. TGF-β1:β3, may also be naturally expressed in tumors. Carcinoma cells express TGF-β1, although its expression is heterogeneous and dynamic, and may occur with or without TGF-β2 or -β321,41,68. MDSCs, type II macrophages and stromal CAFs are also predominant sources of TGF-β1, and CAFs and poorly differentiated spindle cell carcinomas additionally express TGF-β341,69,70. Hematopoietic cells, including immune cells, predominantly express TGF-β1, thus making TGF-β1 the predominant but not the only TGF-β actor in the TME71.
Latent complexes of the TGF-β1 homodimer, often have one of the dimeric pro-segments covalently linked to a fibrillin-like latent TGF-β binding protein 1 (LTBP1) that enables their deposition in the ECM43 (Figure 2B). Secretion of such complexes does not result in release of soluble active TGF-β, but in localized deposition of inactive complexes in proximity to TGF-β expressing cells. As a consequence, subsequent activation is required to release mature TGF-β that can bind and activate TGF-β receptors on cells, in close proximity43. Alternatively, the pro-segments in latent TGF-β1 complexes also associate with transmembrane proteins that belong to a subset of LRRC proteins and allow for cell-associated retention of latent TGF-β1. Indeed, latent TGF-β1 associates with LRRC32, also known as GARP (“glycoprotein-A repetitions predominant”), at the surface of Treg cells, activated B cells, mesenchymal stromal cells and platelets, thus enabling GARP to control retention of latent TGF-β complexes72,73 (Figure 2B). GARP is also expressed by endothelial cells, fibroblasts and megakaryocytes74, raising the possibility for an even broader role for GARP in TGF-β1 latency or distinct functions with no direct link to TGF-β activation. The GARP-related LRRC33 may play a similar role for other cell types, including myeloid cells, since it also associates with latent TGF-β1, and consequently also controls TGF-β1 latency and activation75,76. Other closely related LRRC proteins, such as LRRC15, which is expressed by stromal fibroblasts in advanced stage cancers77,78, may play similar roles in cell-associated TGF-β retention.
Since many, if not all, cell types of the tumor deposit or retain latent TGF-β complexes locally, and have variable degrees of responsiveness to TGF-β, the TGF-β activation mechanisms and tumor architecture of cancers are key determinants of TGF-β actions in the TME. How latent TGF-β1 complexes, with or without LTBP1, are activated has been extensively studied43; however, no studies elucidate the activation of TGF-β2 or -β3 homodimers or TGF-β heterodimers. Together, many findings highlight (1) key roles of structural interactions between the TGF-β1 prosegment and selected integrins in maintaining TGF-β1 latency and TGF-β1 activation, (2) contributions of proteases, often metalloproteases, in degrading the prosegments and associated proteins, thus leading to release of active TGF-β1, and (3) cell type- and context-dependent diversity of the activation mechanisms as determinants of the spatiotemporal control of TGF-β actions in the TME43. While structural deformation of the integrin-prosegment interaction can release TGF-β1 from latency79, physiological TGF-β1 activation scenarios are likely to combine molecular deformation of this interface with metalloprotease activities43 (Figure 2C). Which integrin is involved in TGF-β1 latency and activation depends on cell type and context, and often involves heterotypic cell interactions. Thus αvβ6 and αvβ1 mediate TGF-β1 activation at the surface of epithelial cells and fibroblasts80,81, respectively, whereas αvβ8 is required for activation of GARP-bound TGF-β1 at the surface of Treg cells81,82, and endothelial cells during retinal and neurovascular development83,84. TGF-β1 and αvβ8 also associate with LRRC33 at the surface of microglial cells75, enabling LRRC33 to control TGF-β signaling. The expression of αvβ8 by carcinoma cells suggests that this integrin controls TGF-β1 activation on or adjacent to malignant cells85. Thus, targeted interference with the integrin-prosegment interface represents an approach to selectively inhibit or attenuate TGF-β1 activation in some cell populations. The diversity of local TGF-β1 activation mechanisms may help explain differences in the roles of TGF-β in the TME, and in susceptibility to TGF-β inhibition, depending on the architectural organization of the TME.
Following activation, TGF-β binds a tetrameric combination of two types of transmembrane kinases, type I and type II receptors, that are able to phosphorylate serine, threonine and tyrosine65 (Figure 2D). TGF-β binding to these receptor complexes activates the receptors, and, consequently, Smad2 and Smad3 through C-terminal serine phosphorylation by the type I receptor kinases. These effector Smads, in combination with Smad4, then translocate into the nuclei, where they combine with DNA sequence-specific transcription factors and coregulators at regulatory gene sequences, and thus activate or repress target gene transcription in response to TGF-β65,86,87 (Figure 2D). Detection of C-terminally phosphorylated Smad2 and/or Smad3 is indicative of TGF-β/Smad signaling, yet may also result from their activation in response to other TGF-β family members, such as activins, myostatin and GDFs65,88. While Smad signaling uniquely defines “canonical” signaling for TGF-β family proteins, TGF-β also activates non-Smad signaling pathways, including the PI3K-Akt-mTOR pathway, Erk MAPK and p38 MAPK signaling, and Rho GTPases65,89 (Figure 2D). These pathways, however, are not diagnostic of TGF-β signaling, since they are strongly activated by receptor tyrosine kinases90.
The number of TGF-β receptors at the cell surface is low, especially when compared to receptor tyrosine kinases65. Unlike receptor tyrosine kinases, the bulk of TGF-β receptors are retained intracellularly, not available for TGF-β binding but ready for transport to the cell surface91. Cells have a remarkable ability to rapidly upregulate TGF-β responsiveness by inducing receptor transport to the cell surface, thus enhancing the availability of receptors for TGF-β binding91,92. Additionally, ubiquitylases and de-ubiquitylases extensively control the stability and degradation of TGF-β receptors88,93, whereas activation of the metalloprotease TACE, e.g. in response to inflammatory stimuli or growth factors, induces ectodomain cleavage of one of the two TGF-β receptors, thus attenuating TGF-β responsiveness. Consequently, metalloprotease inhibition can prevent TACE activity, thus enhancing TGF-β responsiveness and signaling94.
TGF-β signaling defines the phenotype and behavior of carcinoma cells.
While resting epithelial cells show very low, if any, TGF-β expression in vivo, hyperplasia and neoplasia confer increased expression of TGF-β15,29 that can suppress the growth of benign or low-grade cancers, or stimulate cancer progression of malignant and metastatic tumors41,95 (Figure 3). The TGFB1 gene is transcriptionally activated by AP-1 or E2F transcription factors in response to activities of oncogenic proteins such as mutant Ras, growth factors and/or tumor promoters96,97. TGF-β receptor expression is transcriptionally enhanced concomitantly with hyperplasia98, and Akt activation promotes transport of intracellular TGF-β receptors to the cell surface, thus enhancing TGF-β responsiveness65,91. Increased glucose uptake, a hallmark of carcinoma cells, and hyperglycemia induce Akt activation and thus promote an increase in cell surface TGF-β receptors and TGF-β responsiveness92. Enhanced Akt activation also results from mutations, e.g. PTEN inactivation, or increased signaling of IGF-1 receptors or other receptor tyrosine kinases, which is common in carcinomas and promotes cell proliferation or survival99,100. We therefore surmise that most carcinoma cells have increased sensitivity to autocrine or localized TGF-β signaling, when compared to normal epithelial cells.
Figure 3:
Epithelial plasticity responses of carcinoma cells to autocrine and paracrine TGF-β most commonly lead to partial EMT, in which gene expression patterns are extensively reprogrammed and cells convert from an apical-basal polarity toward front-rear polarity. These EMT-associated changes enable directed cell migration and invasion, which are prerequisites for cancer cell dissemination. Concomitant with the EMT-associated changes, cancer cells are enabled to acquire stem cell characteristics and increase their resistance to cancer drugs, and express and release immununosuppressive ligands, while also showing increased protection against senescence and decreasing DNA repair, thus enhancing genomic instability.
For Figure 3, please refer to Figure 3 of Derynck, R., Turley, S.J., and Akhurst, R.J. (2021) TGFβ biology in cancer progression and immunotherapy. Nature Reviews in Clinical Oncology, 18, 9–34
In addition to a plethora of changes in gene expression, TGF-β induces or represses expression of microRNAs101,102 and enables Smad-mediated control of the maturation of microRNAs65,103, with each microRNA regulating translation of many target gene transcripts. TGF-β/Smad signaling also directs extensive changes in mRNA splicing, thus generating different protein isoforms104,105. Many autocrine TGF-β-induced responses control differentiation and behavior of carcinoma cells, while others affect cell metabolism and additional activities of direct relevance to the carcinoma cells and/or their TME. Among these, TGF-β induces ECM protein expression, and induces or represses the expression of proteases and protease inhibitors106,107. These responses to autocrine TGF-β signaling allow carcinoma cells to contribute to the composition of the ECM, which serves as a reservoir of latent TGF-β and controls its activation (Figure 3). The ECM in turn helps direct the differentiation and behavior of different cell populations, and contributes to mechanical properties of tumors, with an ensuing increase in intratumoral interstitial fluid pressure that contributes to poor accessibility of cancer drugs in the tumor108. Overall, though, how TGF-β responsiveness of carcinoma cells affects the TME has been minimally characterized.
TGF-β inhibits cell proliferation in normal epithelial cells by inhibiting cell cycle progression through G1109-111, which is seen as a tumor suppressor pathway that is incompatible with malignant cell transformation and cancer progression111,112. However, carcinoma cells develop strategies to inactivate this growth inhibitory response110,111. Colorectal and other carcinomas with microsatellite instability (MSI) commonly have inactivating mutations in TGFBR2, which encodes the TGF-β type II receptor, TβRII, thus rendering cells unresponsive to TGF-β. Such microsatellite-unstable colon carcinomas have a lower capacity for dissemination than their more common MSI-negative counterparts113. Genetic loss of TGFBR2 also occurs at very low frequency in other tumor types, such as head and neck squamous cell carcinoma (SCC)114, and genetic inactivation of the TGFBR1 gene, encoding the TGF-β type I receptor TβRI, also known as ALK5, is also seen in gastric and several other carcinomas115,116. While TGFBR2 mutations should affect all TGF-β responses, it is unclear whether this holds for TGFBR1 mutations.
Mutations in the gene encoding Smad3, the major effector of TGF-β signaling, are infrequent. However, deletions in the gene encoding Smad4, the co-Smad for all effector Smads, are frequent in pancreatic ductal adenocarcinomas (PDAC) and other gastro-intestinal tract cancers117, but do not necessarily inactivate TGF-β/Smad signaling118,119. In PDAC, SMAD4 inactivation is a late event in cancer progression that may selectively inhibit TGF-β-induced growth inhibition, and, by extrapolation to loss of Tgfbr2120, might change the profile of cytokines and chemokines released from tumor cells. By some accounts, loss-of-function TGF-β receptor or Smad mutations occur in the majority of head and neck SCCs121, and are frequent in other carcinomas116. TGFBR1 mutations in SCCs are unlikely to drive cancer progression, since they are subclonal, and loss of heterozygosity is rare121. However, this may illustrate the role of tumor heterogeneity in cancer progression, whereby tumor cells with TGF-β receptor or Smad mutations interact with those that do not, to cooperatively promote tumor outgrowth.
Also, non-mutational mechanisms impair the growth inhibitory response to TGF-β. In advanced tumors, TGFBR2 or SMAD2 genes are frequently silenced by histone and DNA methylation122,123. Additionally, increased inhibitory Smad6 or Smad7 expression in various carcinomas, including PDAC, attenuates Smad signaling124,125, whereas increased expression of the Smad corepressors c-Ski or SnoN, observed in some carcinomas, selectively impairs Smad-mediated target gene transcription87,125. Highly active PI3K-Akt signaling prevents FoxO nuclear import, and thus impairs Smad-mediated induction of p21Cip1 expression111, and increased expression of the c-Myc-binding protein Miz represses TGF-β-induced expression of cdk inhibitors111. Such scenarios enable carcinoma cells to overcome the tumor suppressive activity of TGF-β signaling, while maintaining TGF-β responses that benefit cancer progression.
TGF-β promotes differentiation plasticity, stem cell properties and cancer drug resistance.
An increasingly appreciated response to TGF-β signaling in normal and transformed epithelial cells is the epithelial plasticity response, which represses epithelial characteristics and promotes transdifferentiation toward a mesenchymal phenotype (Figure 3). This EMT process results in deconstruction of epithelial cell-cell junctions and reorientation of apical-basal polarity toward a front-rear polarity, elongation of cells, and increased motility that enables directed migration and invasion through ECM. Accompanying these phenotypic and behavioral changes are extensive changes in gene reprogramming and mRNA splicing34–36,126,127. The ability of TGF-β to promote epithelial plasticity and EMT results primarily from activation of TβRI, enabling Smad3/4-mediated activation of genes that encode master EMT transcription factors, such as Snail1 and Snail2/Slug, and ZEB1 and ZEB2. These in turn cooperate with Smad3/4 complexes to repress epithelial and activate mesenchymal genes128. Additionally, TGF-β signaling through another type I receptor, ACVR1/ALK2, which activates Smad1 and Smad5, is also required for TGF-β-induced EMT129. Added to extensive gene expression changes are abundant changes in miRNA expression and mRNA splicing that alter the diversity of protein isoform expression37,102,128,130. Some changes in gene expression are seen as diagnostic for decreased epithelial character and the EMT process127,128,131. Since Smads control transcription through cooperation with high-affinity DNA-binding transcription factors65, TGF-β-induced EMT requires cooperation with other pathways, most prominently Wnt and MAPK signaling128,132, and mTOR signaling downstream from Akt is required for progression and completion of EMT128,132. In human carcinomas, extensive changes that include an elongated phenotype and mesenchymal gene expression suggestive of full EMT are rarely seen41,42. Most commonly, human carcinoma cells acquire an intermediate, partial EMT, i.e. a hybrid epithelial/mesenchymal state that allows for coexistence of epithelial and mesenchymal characteristics37,127,133–135. Furthermore, gradations in EMT are often apparent, with leading cells at the tumor periphery having more pronounced EMT than those that follow them37,127,133,134, reminiscent of collective cell migration, when groups of cells move together directionally135,136. Decreased adhesion and increased motility and invasion enable carcinoma cell dissemination and thus provide carcinoma cells their metastatic potential37,135.
In a variety of carcinomas, EMT increases the number of carcinoma cells with stem cell properties, i.e. cancer stem cells (CSCs)34,37,127,137. Their “stemness” is defined by an ability to initiate tumor formation in vivo, but is more conveniently scored by expression of cell surface markers, exclusion of certain dyes and drugs, and generation of multicellular spheres from single cells34,37,127,137,138. Under the influence of TME factors, particularly TGF-β9,37,139, epithelial cells acquire CSC-like phenotypes, providing a dynamic balance of interconversion between a small population of CSCs with EMT properties and a much larger population of differentiated carcinoma cells37,140. How, at the cellular and molecular levels, stem cell properties relate to EMT is unclear, but EMT is thought to be required for and enables stemness34,127,140,141 A linear relationship may not exist between progression along the EMT spectrum and stem cell properties, a notion that is supported by the observation that in mammary carcinoma cells partial EMT or stabilized EMT correlates with more pronounced stemness characteristics than full, reversible EMT36,134,142.
Of further therapeutic relevance, EMT enables carcinoma cells to exert local immunosuppression143,144. EMT confers repression of major histocompatibility complex class I (MHCI) antigen presentation by tumor cells, which is required for recognition by CD8+ T cells, and thus indirectly suppresses cytotoxic activities of CD8+ T cells and cytolytic activities of NK cells141,143–145. TGF-β-induced EMT also suppresses the expression of other components of the antigen processing and presentation machinery that is required for cancer cell recognition and killing by cytotoxic T lymphocytes145. Local immunosuppression induced by EMT also correlates with increased expression of immunosuppressive cytokines and chemokines141,143,144. Among these, increased TGF-β1 release and activation141 may play a key role since TGF-β promotes Treg cell differentiation and represses tumor cell killing by CD8+ T cells. EMT also confers increased cancer cell expression of PD-L1, the ligand for the immune-inhibitory PD-1 receptor on CD8+ T lymphocytes141,146. These and other observations predict that EMT allows for escape from antitumor immunity, and decreases susceptibility to immunotherapy37,143,146. Accordingly, in mice, breast carcinoma cells with mesenchymal characteristics show resistance to checkpoint blockades, and those that remain largely epithelial are more susceptible34,141,143. On the other hand, triple-negative breast cancer in humans, which are marked by the mesenchymal appearance of cancer cells, show susceptibility to anti-PD-L1 checkpoint inhibition147,148.
EMT also correlates with increased resistance of carcinoma cells to chemotherapies149–152. The underlying basis of this correlation needs to be further explored, although cancer drug resistance correlates with stem cell-like properties149,153–155, and cancer drug resistance and stem cell properties are coordinately repressed by mTOR inhibition142. Additionally, EMT in carcinoma cells is accompanied by changes in genomic stability and DNA repair. Although divergent observations are reported156,157, TGF-β treatment and EMT-associated CSC properties correlate with a decreased ability of carcinoma cells to repair double-stranded DNA breaks and increased genomic instability158,159. The resulting accumulation of genomic changes may contribute to heritable genotypic diversity that contributes to tumor evolution during cancer progression.
Considering the roles of TGF-β sensitivity and autocrine TGF-β signaling in defining the behavior of carcinoma cells (Figure 3), one may speculate how therapeutic inhibition of TGF-β signaling might directly affect TGF-β-responsive cancer cells. Since TGF-β signaling promotes TGF-β1 expression, TGF-β inhibition should decrease the de novo synthesis, release and activation of TGF-β1. Furthermore, considering its role in driving epithelial plasticity and EMT-associated changes, TGF-β inhibition should enhance the epithelial characteristics of cancer cells, and decrease their dissemination and tumor re-initiation capacity. Moreover, localized EMT-associated immunosuppression, cancer drug resistance, and increased genomic instability, might be repressed by TGF-β inhibition, thus impairing long-term cancer progression. However, TGF-β inhibition is not expected to lead to cancer cell death or inhibition of tumor cell proliferation. Ultimately, however, these effects on tumor cells need to be conceptualized in the context of anti-tumor immunity driven by TGF-β inhibition.
Diverse roles of TGF-β in innate immune cell populations in cancer
Early in tumor development, the immune system exerts surveillance, destroying genetically or epigenetically abnormal cells through actions of NK cells, macrophages and intra-epithelial T cells. Mutational activation of a single or multiple oncogenes is, however, insufficient for immune eradication of an oncogene-activated cell, reflected by the existence of oncogenic mutations at a high rate within normal tissues160. Nevertheless, immunosurveillance-mediated destruction of cells with dominant neoantigens shapes the tumor’s antigenic repertoire161. Concomitantly, increased TGF-β1 expression and activation, seen as initial events in tumorigenesis, are induced by insults29, and expose immune cells, pre-neoplastic and neoplastic tumor cells to epithelial cell-derived TGF-β from the earliest stages of tumorigenesis. At some critical point during tumor progression, cancer cells prevail over immune surveillance, which allows the tumor to progress towards full-blown malignancy161. Once established, the tumor comprises a mix of immune cell types including monocytes, macrophages, DCs, granulocytes, T cells, B cells, NK cells and various subsets thereof, with considerable heterogeneity within each tumor and between tumor types. Innate and adaptive immune cells interact with each other as well as the tumor and its microenvironment. Several types of innate and adaptive immune cells respond to TGF-β that can be released by cancer cells, stromal cells and immune cells themselves, resulting in an immunosuppressive TME (Figure 4).
Figure 4:
Actions of TGF-β and TGF-β inhibition on immune cell properties. Stimulatory (black arrows) and inhibitory (red lines) activities of TGF-β on distinct cells of the immune system. Blue arrows represent activities of pharmacological and/or genetic inhibition of TGF-β signaling on specific cell types. The boxes highlight major effects of TGF-β on distinct cell types.
For Figure 4, please refer to Figure 4 of Derynck, R., Turley, S.J., and Akhurst, R.J. (2021) TGFβ biology in cancer progression and immunotherapy. Nature Reviews in Clinical Oncology, 18, 9–34
Among the innate immune armory, NK cells exhibit tumor suppressive functions during early and late stages of anti-cancer immunity. Ligation of activating receptors, such as NKG2D, by MHC class I-related (MIC)-A or MICB proteins on target cancer cells can incite activation, cytokine production, degranulation and cytotoxic potential in NK cells162. Notably, TGF-β1 represses NKG2D expression directly and through induction of miR-183 that targets the expression of an NKG2D adaptor protein, and suppresses MICA and MICB expression by tumor cells163–167. As in other immune cell types, TGF-β/Smad signaling represses interferon-γ (IFN-γ) expression that supports myeloid cell and CD8+ T cell proliferation and differentiation168.
TGF-β additionally affects myeloid cell types within the tumor, including MDSCs, macrophages and neutrophils. These cells normally accumulate at sites of infection and wounding, where they contribute to elimination of infectious agents and resolution of inflammation. They accumulate early during tumor outgrowth in response to chemokines and cytokines, including TGF-β, that are produced by tumor cells and in response to tumor promotion. While macrophages and neutrophils evolved to eliminate infectious agents and damaged cells, they adopt a tumor-promoting type II phenotype during cancer progression in response to factors released by the tumor and its microenvironment169, and in response to TGF-β170–173. Within the tumor, the phenotypes of MDSCs overlap with those of monocytes and immature neutrophils. MDSCs are highly plastic in their migratory behavior and phenotype, suppress NK cell activities174, and contribute to metastatic effects of TGF-β57,174. TGF-β represses antigen presentation, skews cytokine production and promotes immunosuppressive activity in DCs and other myeloid cells175–177. TGF-β also suppresses reactive oxygen species and NO production, which is required for the destructive properties of macrophages178,179. TGF-β signaling in myeloid cells of the pre-metastatic niche is essential to breast cancer metastasis, and Tgfbr2 inactivation within the myeloid compartment dramatically reduces lung colonization by mammary tumor cells180.
DCs specialize in antigen processing and presentation, and function as liaisons between the innate and adaptive arms of the immune system. They normally patrol tissues, an example being the Langerhans DCs in the epidermis, which depend on TGF-β signaling, initiated by paracrine TGF-β activation by keratinocytes181. Multiple types of DCs, including the CD103+ subset, are detected in tumors54,182. DCs internalize soluble and particulate antigens, including tumor cell fragments, and transport their antigenic cargo to draining lymph nodes through lymphatic vessels. Although DCs constitute only a small fraction of immune cells of the tumor, they are critical to an adaptive immune response54,182. En route to lymph nodes, DCs process tumor antigens into peptide ligands for presentation to CD4+ and CD8+ T cells183. TGF-β suppresses antigen presentation by DCs, thus impairing T-cell-mediated anti-tumor responses183, and may suppress DC cell migration184,185. Additionally, intestinal DCs can induce Treg cell differentiation through activation of TGF-β, possibly involving cell-intrinsic expression of integrin αvβ8186,187.
Control of intratumoral T cell-mediated cytotoxicity by TGF-β
Immune-mediated tumor eradication requires cytotoxic CD8+ T cells, except in tumors with lost or reduced HLA/MHC expression, in which case NK cells may serve this role188. Additionally, memory CD8+ T cells provide long-term immunity against key tumor epitopes189. Accordingly, activation of CD8+ T cells is a major focus of cancer immunology, and in the design of effective immunotherapies, illustrated by the engineering of adoptive CD8+ T cell therapies190, chimeric antigen receptor-expressing CD8+ T cells (CAR-T cells) of different functionalities191–193, and checkpoint blockade inhibitors that boost the tumoricidal activity of CD8+ T cells194–196. The consecutive steps leading to CD8+ T cell-mediated tumor cell killing, including T cell activation, expansion, differentiation and infiltration, are all regulated by immune and non-immune cell types, including myeloid cells, Treg cells, endothelial cells, fibroblasts and tumor cells. Each of these cell types are themselves TGF-β-responsive and support or control cytolytic T cell activity, and thus help define the outcome of tumor rejection. Furthermore, TGF-β impinges on key steps of the T cell response to tumor, including T cell activation, proliferation, differentiation and migration, in both the TME and tumor-draining lymph nodes. TGF-β represses CD8+ T cell-mediated anti-tumor immunity, through direct effects on the CD8+ T cells, and effects on accessory cells that govern CD8+ T cell function (Figure 4). Key roles of TGF-β1 in suppressing the adaptive immune system are apparent from the lymphoproliferative autoimmune phenotype of Tgfb1−/− mice197–199, and similar phenotypes following selective ablation of TGF-β signaling components within CD4+ and CD8+ T cells200.
Activation of CD8+ T cells involves signals provided by APCs; T cell receptor (TCR)-mediated recognition of peptide-MHCI complexes at the surface of any cell, including tumor cells, and interaction of the T cell-restricted co-activating receptor, CD28, with one of its APC-expressed ligands, CD80 (B7.1) or CD86 (B7.2) are both required. This dual APC-dependent ligation mechanism initiates TCR signaling to drive T cell activation, proliferation and differentiation into cytotoxic CD8+ T cells with antigen-specific tumoricidal potential201. TGF-β signaling suppresses processes that lead to CD8+ T cell activation and maturation, including, in APCs, expression of components of the antigen processing and presentation machinery, such as HLA/MHC175,183,202. Furthermore, TGF-β signaling in T cells activates SHP1 expression, which represses protein tyrosine kinase activities required for TCR signaling203,204. In addition, TGF-β inhibits CD8+ T cell proliferation, as shown in mice with T cell-specific inactivation of TGF-β signaling that develop multifocal lymphoproliferative inflammation similar to Tgfb1−/− mice200. CD8+ T cell proliferation additionally requires interleukin (IL)-2 expression and responsiveness, and TGF-β suppresses the expression of IL-2 and its receptor by effector and memory T cells205. Finally, TGF-β signaling represses expression of IFN-γ and genes for cytolytic machinery components or transcription mediators that orchestrate their expression in activated CD8+ T cells206,207.
With a focus on anti-tumor immunity by cytotoxic CD8+ T cells, CD4+ T lymphocytes and their roles in tumor rejection have received less attention. However, renewed interest in CD4+ T cells has arisen from studies of CAR-T cells208–210, and growing evidence that TGF-β blockade targets CD4+ CAR-T cells in anti-cancer therapy211. CD4+ T cells, activated by MHCII-presented peptides on the surface of APCs, are classically associated with their helper function for CD8+ T cells and other immune cells. For example, release of Th1 and Th2 cytokines by activated CD4+ T helper (Th) cells supports tumor cell killing by cytotoxic T cells, macrophages and granulocytes212. However, tumor rejection that depends on CD4+ Th cells and NK cells but not on CD8+ T cell activity has been observed213–215. Indeed, CD4+ T cells can adopt tumor-reactive cytotoxic activity that contributes to tumor eradication, as shown in mice with inactivated TGF-β signaling in CD4+ T cells, leading to NK- and cytotoxic T cell-like phenotypes216. Notably, CD4+ CAR-T cells are more effective cytolytic effectors than CD8+ CAR-T cells, demonstrating CD28-dependent granzyme and perforin-mediated cytotoxicity208. They are also less susceptible to exhaustion following TCR activation209. Opposing the activities of CD4+ Th cells, CD4+ Treg cells repress the functional differentiation and cytolytic activities of CD8+ T cells, and anti-tumor activities of myeloid cells212.
TGF-β orchestrates the development and plasticity of CD4+ T cells. In much the same way as cytotoxic CD8+ T cells are suppressed, TGF-β suppresses CD4+ Th cell proliferation and expression of master transcription factors that drive TNF-α and IFN-γ expression217. Furthermore, TGF-β skews the differentiation of CD4+ Th1 cells along alternative pathways, dependent on the cytokine context, e.g. supporting differentiation of Th2 and Th17 cells in the presence of IL-4, and IL-6 and IL-1β, respectively217. Importantly, TGF-β in the presence of IL-2 promotes functional differentiation and survival of CD4+ Treg cells. In naïve CD4+ T cells, TGF-β/Smad3 signaling activates Foxp3, which encodes a master transcription factor that directs differentiation into activated CD4+Foxp3+CD25+ Treg cells60. These then functionally repress, through various activities, including TGF-β1 activation82,145, the tumor-killing activity of CD8+ T cells 58,63,82,145,218,219, suppress differentiation of NK and DCs220, and contribute to macrophage and neutrophil polarization towards a regenerative rather than inflammatory cell state58,63,82,145,172,173,218,219. As single cell analyses reveal extensive phenotype heterogeneity among Treg cells221, it will be important to differentiate the effects of TGF-β and its inhibitors on distinct Treg cell sub-populations.
Taken together, TGF-β signaling exerts profound immunosuppressive activities on key cell types that orchestrate innate and adaptive immunity, thereby attenuating the intrinsic tumoricidal potential of immune cells within the TME. Inhibition of TGF-β signaling is therefore expected to enhance the anti-tumor responses of both myeloid and T cells.
TGF-β signaling and stromal fibroblasts
In addition to immune cells, the TME comprises a large CAF population with subsets of fibroblasts of diverse phenotypes77,222–226. In primary and metastatic tumors, cancer cells reside adjacent to these stromal cells, and tumor cells play key roles in their recruitment, differentiation and behavior. In turn, CAFs define and support the properties, compartmentalization and behavior of cancer cells. Additionally, they can help define the immunosuppressive environment, through effects on immune cell activation and impeding infiltration of immune cells into tumors227–231. The interplay between cancer cells, stromal fibroblasts and immune cells drives and defines cancer progression.
Fibroblasts play central roles in connective tissue by maintaining tissue homeostasis and enabling wound healing. In healthy adult tissues, they show a “resting” phenotype with low levels of proliferation and metabolism. Upon tissue injury or in response to inflammation, they are “activated”, with enhanced proliferation and protein synthesis, and are more metabolically active. They can further differentiate into myofibroblasts that express α-smooth muscle actin232. CAFs show characteristics of activated fibroblasts and/or myofibroblasts, consistent with the chronic inflammation and mechanical tension in tumors12,233,234. Depending on the tumor type, fibroblasts can represent a sizable component of primary tumors and metastatic lesions that proportionally expands with increased tumor size.
Stromal fibroblasts are key determinants of the architectural and functional organization of the tumor, in part through abundant secretion of ECM proteins and protease-mediated remodeling of the ECM. Indeed, activated fibroblasts and myofibroblasts abundantly secrete various ECM proteins, and matrix metalloproteases, cathepsins and other ECM remodeling enzymes77,224,235,236. They also express lysyl oxidases such as LOXL2, which crosslinks collagen fibers yet also promotes TGF-β signaling in CAFs237,238. Additionally, the markedly adhesive and contractile myofibroblasts sense and incite mechanical tension and tissue rigidity239. The ECM deposition and remodeling by stromal fibroblasts and contractility of myofibroblasts define the mechanical properties and stiffness of the tumor, and position stromal fibroblasts as determinants of therapeutic efficacy. Finally, CAFs secrete a plethora of soluble and ECM-associated factors, including cytokines, chemokines and growth factors that promote tumor growth and cancer cell dissemination40,240. Some of these act directly on tumor cells, while others act on immune and endothelial cells, thus influencing trafficking and functions of myeloid and T cells, or act through autocrine and paracrine mechanisms on the stromal cell populations241. Conversely, in a PDAC mouse model, depletion of proliferative α-smooth muscle actin expressing cells, which may comprise a mixture of CAFs and other stromal cells, increased the ratio of Treg and effector T cells in late stage tumors, and extended survival following treatment with a CTLA-4 blocking antibody242.
Since TGF-β is activated locally and unlikely to diffuse, CAFs respond locally to TGF-β provided by cancer cells, immune cells or other cell types in close proximity. The localized exposure of stromal fibroblasts to TGF-β produced by cancer cells may involve filopodial extensions over several cell diameters243,244. The response of stromal fibroblasts to TGF-β released by hyperplastic epithelial cells might be particularly relevant early in tumor formation when pre-neoplastic cells recruit fibroblasts to generate an ECM and TME. However, inactivation of TGF-β signaling in stromal fibroblasts potentiates neoplasia in adjacent epithelia245. The communication between CAFs and carcinoma cells through TGF-β is likely to contribute to tumor evolution throughout cancer progression.
Stromal fibroblasts are a major source of TGF-β in the TME40,227. Since TGF-β induces TGFB1 expression246, TGF-β1 released by cancer cells may activate TGF-β1 expression in CAFs. Consequently, CAFs show amplified autocrine TGF-β expression and activation, conceptually similar to autocrine TGF-β signaling in the cancer cell population. Whether stromal fibroblasts express primarily TGF-β1, similarly to carcinoma cells, and how latent TGF-β released by CAFs is activated are questions that remain to be addressed. However, TGF-β3 is clearly expressed by stromal CAFs and mesenchymal tumor cells41,70,247, and its role in relation to TGF-β1 remains to be better defined.
TGF-β signaling in stromal fibroblasts induces changes in their physiology and behavior that help define their roles in cancer progression. At very low levels, TGF-β act as chemo-attractant for fibroblasts248,249. Thus, TGF-β released and activated by CAFs may promote recruitment of fibroblasts, similarly to TGF-β secreted by cancer cells. Additionally, TGF-β promotes fibroblast proliferation110, and in this way may contribute to the expansion of the fibroblast population250 TGF-β signaling in fibroblasts also protects against cell death and promotes survival250. Conversely, however, pharmacological inhibition of the TβRI kinase enhances stromal fibroblast proliferation in a mouse melanoma model38.
TGF-β/Smad signaling, in cooperation with Erk MAPK and Akt-mTOR signaling, promotes activation and myofibroblast differentiation of CAFs, similar to fibroblast differentiation upon wounding and inflammation228,251,252. Persistent TGF-β signaling in the TME may drive fibroblast activation and myofibroblast differentiation in the tumor. It may also explain the expression and functions of EMT transcription factors in a fraction of stromal fibroblasts253,254. EMT transcription factor expression in response to TGF-β may be intrinsic to fibroblasts, but may also reflect an epithelial or endothelial origin of these CAFs. Indeed, epithelial and endothelial cells contribute, through EMT or endothelial-mesenchymal transition (EndMT), to the CAF population255,256, although the extent of their participation is a matter of debate. Concomitant with fibroblast activation, TGF-β induces ECM protein expression and ECM remodeling, thus making increased ECM deposition and remodeling a direct consequence of increased TGF-β signaling in CAFs. Additionally, with CAFs as a major source of TGF-β expression in the TME, CAF-mediated deposition of TGF-β in the ECM generates a reservoir of latent TGF-β for subsequent activation. Consequently, the architectural organization of TGF-β expression and signaling in stromal fibroblasts controls intratumoral immune cell functions and cytotoxic activities of NK and T cell populations (Figure 5), and thus cancer progression. Finally, TGF-β influences CAF expression of cytokines and chemokines that act on cancer, endothelial and/or immune cells, such as HGF245, VEGF, IL-6, IL-8, MCP1, PGE2 and CXCL12229,257–262. Release of such factors by CAFs influences the TME and may thus contribute to a pro-tumorigenic and immunotolerant state with e.g. inhibition of T cell trafficking and exclusion of T cells. TGF-β signaling in CAFs is increasingly seen as a determinant of resistance to immunotherapy21,23,77.
Figure 5:
Anti-TGF-β blockade stimulates efficient penetration of T cells into the tumor core, compared to anti-PD-1 monotherapy. Mice with established CCK168 SCC subcutaneous tumors were treated with two doses of antibody therapy on day 15 and 19 after tumor cell implantation, and examined four days later by immunohistochemistry to detect CD45+ total immune cells and CD8+ T cells. Note that anti-PD-1 stimulates CD45+ cell levels, but fails to provide efficient infiltration into the center of the tumor, whereas anti-TGF- monotherapy results in efficient penetration of T cells into the core. Combination therapy enhances CD8+ cytotoxic T cell activity and CD4+Th1:Treg ratio, resulting in tumor shrinkage (reproduced with modifications from Dodagatta-Marri et al.145.
For Figure 5, please refer to Figure 5 of Derynck, R., Turley, S.J., and Akhurst, R.J. (2021) TGFβ biology in cancer progression and immunotherapy. Nature Reviews in Clinical Oncology, 18, 9–34
Control of endothelial cell function and angiogenesis by TGF-β
The ability of tumor cells to induce new blood vessel formation is essential for tumor growth and metastatic cancer dissemination44,263. Various observations highlight activities of TGF-β on endothelial cells that may promote or repress angiogenesis, depending on the experimental setting264–266. Pro-angiogenic activities of TGF-β on endothelial cells are mediated by the ALK1 type I receptor and endoglin, which promote endothelial cell proliferation and migration265, whereas its anti-angiogenic activity signals through the canonical TβRI/ALK5 receptor266. TGF-β’s pro-angiogenic activities include promotion of capillary tube formation267, and expression of angiogenic factors, including VEGF-A262,268. However, TGF-β can also induce mesenchymal stem cells to differentiate into endothelial cells269. Overall, TGF-β has been convincingly shown ex vivo and in vivo to induce a pro-angiogenic environment and stimulate tumor angiogenesis270–272. Additionally, TGF-β secreted by endothelial cells promotes recruitment of perivascular pericytes and myofibroblasts that support functional vascular integrity273, and interaction between endothelial cells and pericytes confers localized and constitutive TGF-β activation, which is required for microvascular integrity274,275. It should be noted that increased TGF-β expression has been linked to increased microvessel density in certain tumor types271,272. TGF-β also indirectly acts on endothelial cells, through Treg cells, to establish an immune-protected endothelium with suppressed endothelial cell activation276. Conversely, endothelial cell-specific inactivation or repression of TGF-β signaling can suppress atherosclerosis-associated vascular inflammation by enhancing vascular integrity through reversal of EndMT277. These and other observations link TGF-β to tumor-induced angiogenesis and functional integrity of the tumor vasculature.
Lymphangiogenesis is essential for trafficking of immune cells and cellular debris, as well as drainage of interstitial fluid. Lymphatic vessels can also carry tumor cells and tumor-derived factors to draining lymph nodes, and thus contribute in multiple ways to tumor physiology, immunosuppression and cancer progression278,279. However, the roles of TGF-β in tumor-associated lymphangiogenesis and lymphatic function have been less studied280.
Therapeutic approaches to inhibit TGF-β signaling
As is apparent from the roles of TGF-β signaling in cancer cells and different cell types of the TME, increased TGF-β signaling promotes progression of carcinomas. Hence, inhibition of TGF-β signaling is expected to attenuate cancer progression through direct effects on cancer, immune and stromal cells. The notion of TGF-β signaling inhibition as a therapeutic anti-cancer approach was met with considerable hesitation for about two decades, and took many years to mature and be evaluated. A primary reason for this delay was that TGF-β was initially found to potently inhibit proliferation of epithelial cells, and therefore seen as a tumor suppressor of carcinomas110,111,281–284. The elucidation of underlying mechanisms of cell cycle inhibition281,285–287 provided high visibility to this concept, and justifiably raised concerns that TGF-β inhibition might give rise to tumors from pre-neoplastic epithelial cells287. Furthermore, the notions that TGF-β promotes EMT, that EMT contributes to cancer dissemination, and that EMT associates with an increase in cancer stem cell properties took a long time to become accepted127. Finally, although it was realized early on that immunosuppressive actions of TGF-β contribute to cancer progression288, the clinical successes with immune checkpoint blockade stimulated renewed interest in harnessing TGF-β signaling as an approach to enhance cancer therapies. Recent results in fact highlight the role of increased TGF-β signaling in intrinsic resistance to checkpoint blockade therapy21,23,38,71,145. The immunosuppressive role of TGF-β reminds us of earlier concerns, based primarily on knock-out mouse studies, that TGF-β1 depletion would lead to excessive and de-repressed inflammation and auto-immune responses197,199.
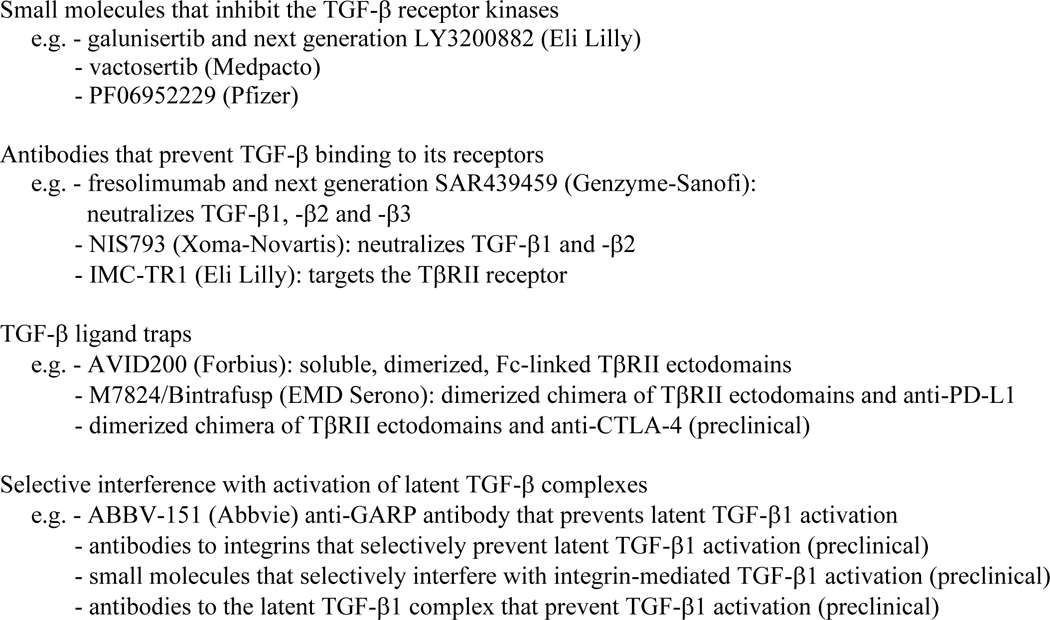
An array of therapeutic approaches that target individual steps of the canonical TGF-β/Smad signaling pathway and cell-directed approaches to inhibit TGF-β signaling have been developed and are under evaluation, with several of these in clinical trials. Besides inhibition of TGFB1 transcription289 or targeting TGFB1 or TGFBR2 mRNA for degradation290, most approaches to inhibit TGF-β signaling fall into four classes (Table 1). One class, exemplified by galunisertib (LY2157299; developed by Eli Lilly) as a well-studied therapeutic291,292 consists of small molecule inhibitors of TGF-β receptor kinases. These prevent ATP binding to the ATP binding pockets of the TGF-β receptors, primarily TβRI, thus blocking Smad2 and Smad3 activation in response to TGF-β binding. However, although simple to deliver orally, these inhibitors have poor pharmacokinetics and pharmacodynamics. Additionally, they do not target TGF-β receptors specifically, since they equally effectively inhibit related type I receptors for several other TGF-β-related proteins, such as activin, nodal and myostatin293, and may also inhibit other kinases, such as p38 MAPK292,294. Furthermore, while they prevent TGF-β-induced Smad activation, it is unclear whether they prevent TGF-β-induced PI3K-Akt-mTOR activation, since Akt activation through TβRI-TRAF6-TAK1 appears unaffected by this type of inhibitor in some cells but not others65,295,296. Consequently, lack of specificity and poor pharmacokinetics of current TGF-β receptor kinase inhibitors make dosing strategies difficult. Nevertheless, low production costs, ease of administration and promising results encourage the clinical evaluation of several such inhibitors, including Eli Lilly’s next generation LY3200882 (NCT02937272297) and vactosertib from Medpacto298,299(NCT03732274297).
Table 1.
Major classes of TGF-β signaling inhibitors that are pursued therapeutically
|
Another class of therapeutics comprises monoclonal antibodies that prevent TGF-β receptors from binding ligand145,300. While neutralizing TGF-β antibodies have exquisite ligand specificity, they need to effectively interfere with the very high binding affinity (10−11 M range) of TGF-β to cell surface receptor complexes65. Antibodies are expensive to manufacture and may be less efficiently distributed within the tumor than small molecule inhibitors, but provide superior pharmacokinetic properties. The TGF-β antibody fresolimumab, a human IgG4k monoclonal antibody based on the 1D11 antibody that neutralizes all three TGF-β isoforms, was developed by Cambridge Antibody Technology/Genzyme-Sanofi, and showed promising results in a phase 1 clinical trial300. A next generation pan-TGF-β antibody, SAR439459 (NCT03192345297), was developed by Sanofi, and is seen as a lead candidate in anti-cancer mono- and combination therapies. Xoma Corporation developed TGF-β blocking antibodies with distinct specificities for the three TGF-βs301, and selected one that neutralizes TGF-β1 and -β2 but not TGF-β3 for clinical testing, with the rationale that TGF-β3 may oppose effects of TGF-β1 and -β2, and may be less commonly expressed in tumors302. However, CAFs do express TGF-β3, as do some mesenchymal tumors21,23,41,69. TGF-β3’s role in cancer progression needs to be better defined. Novartis is pursuing a clinical phase 1/1b dose escalation study to evaluate anti-TGF-β1/β2 antibody, NIS793, in combination with anti-PD-1 antibody in patients with advanced cancers (NCT02947165297). An antibody, developed by Eli Lilly, that blocks activated TGF-β1 but not TGF-β2 or TGF-β3, completed phase 1 clinical evaluation as monotherapy303. Finally, a TβRII receptor antibody (IMC-TRI, LY3022859) under development by Eli Lilly showed efficacy in preclinical evaluation against mammary cancer304, but was not further pursued due to onset of uncontrolled cytokine release syndrome at higher doses before therapeutic benefit was observed305 (Table 1).
Other inhibitors are designed as soluble, high-affinity ligand traps to prevent TGF-β binding to its receptors306,307. These comprise Fc-stabilized dimers of TβRII extracellular domains that are aimed to sequester TGF-β1 and -β3, but not TGF-β2, and thus prevent their binding to transmembrane TβRII receptors307–309. The utility of a TGF-β ligand trap in blocking tumor dissemination was first shown in mice that express such a trap and were challenged by intravenous or orthotopic implantation of breast carcinoma cells306. Distinct TGF-β traps have now been developed with differences in ligand binding specificities, and incorporation of the betaglycan ectodomain was shown to enable TGF-β2 binding310. Further engineering led to the development of a smaller ligand trap with a 100–1000-fold increased ligand-binding efficacy311. Based on this design, AVID200, developed by Forbius, entered the clinic for therapeutic evaluation against advanced malignancies (NCT03834662297). The Fc-TβRII trap was also the basis for the development of a bi-specific trap that combines TGF-β binding by the TβRII ectodomains with a human PD-L1-blocking IgG1308,309,312.
Finally, some antibodies and small molecules target the TGF-β activation process, and thus provide selective cell- or tissue-type inhibition of TGF-β signaling. Latent TGF-β1 activation involves interaction at the cell surface of the prosegments of TGF-β1 with selected β integrins in complexes with αv integrin. Since αvβ1, αvβ6 and αvβ8 mediate TGF-β1 activation81, targeted interference with these interactions prevents TGF-β1 activation in a cell type-selective manner without systemic TGF-β inhibition. Antibodies against integrins β1, β6 and β8 were shown to selectively impair TGF-β1 activation85,313–315, and small molecules have been developed that prevent integrin β1- and integrin β6-mediated latent TGF-β1 activation316–318. Such antibodies and small molecules are now under preclinical and clinical evaluation. Notably, antibodies against integrin β8, which is expressed on tumor cells, Treg cells and DCs, have anti-tumor activity as monotherapy in some syngeneic cancer models82,85,319. This activity, thought to result from interference with TGF-β activation, is the basis of a clinical evaluation of PF-06940434, which targets αvβ8 (NCT04152018297). Since GARP, in cooperation with integrin αvβ8, is required for TGF-β1 activation at the surface of Treg cells82,320, antibodies that block GARP-mediated TGF-β1 activation have also been evaluated for their efficacy in enhancing the cytotoxic immune response in cancers64. Since GARP is also expressed by endothelial cells, fibroblasts, megakaryocytes and platelets73,74, the selectivity of such anti-GARP antibodies for the cytotoxic immune response remains to be further validated. Finally, an antibody was developed that binds the TGF-β1 prosegment and prevents dissociation of mature TGF-β1 from LAP, thus keeping TGF-β1 latent without affecting TGF-β2 or TGF-β3 activation71. This antibody is expected to systemically prevent TGF-β1 activation and is under preclinical evaluation71.
Efficacy of anti-TGF-β monotherapy
Success of classical cancer therapies, i.e. chemotherapy, radiation and molecular targeted therapies, is based on early regression of the primary tumor, using Response Evaluation Criteria In Solid Tumors, or RECIST321, which commonly results from direct cytotoxic effects on cancer cells. However, while some anticipated direct antitumoral activities of TGF-β inhibition, TGF-β signaling inhibitors in general do not incite direct cytotoxic effects on malignant cells. Inhibition of cancer cell dissemination or cancer stem cell properties is expected from anti-TGF-β therapy; however, these are not primary RECIST criteria and not easily scored. Success of TGF-β inhibition in anticancer therapy is likely to arise from combined effects on cells of the TME, e.g. inhibition of immunosuppressive activities, effects on stromal fibroblasts that repress ECM production and allow for immune cell infiltration (Figure 5), and impaired angiogenesis. These effects are not easily assayed in immuno-compromised human xenograft models, but require syngeneic immune-competent mouse tumor models, and clinical outcomes should be assessed using guidelines developed for immune checkpoint blockade therapies322,323. Notably, therapeutic effects of anti-TGF-β monotherapy or combination therapies on immune or other cells in the TME do not depend on TGF-β responsiveness of the cancer cells23,324.
TGF-β inhibition as monotherapy, using neutralizing antibodies, ligand traps or receptor kinase inhibitors, has seen some success in mouse models of carcinomas, primarily in reducing metastatic spread rather than reducing growth of the primary tumor325 (Table 1). Early examples showed reduction of breast cancer metastasis in response to anti-TGF-β antibodies or soluble TβRII-based ligand trap306,326,327. Indeed, transgenic expression of a ligand trap suppressed metastatic outgrowth of mouse mammary tumors, with no apparent adverse effects306. Other studies using TβRI small molecule inhibitors confirmed attenuation of metastasis in various carcinoma types325.
In clinical trials that used conventional RECIST criteria, monotherapy with the TβRI kinase inhibitor galunisertib provided unimpressive clinical responses, although success may have been pre-empted by restricted dosing regimens imposed to avoid possible non-tolerated adverse effects328–330 (Table 1). The anti-pan-TGF-β antibody fresolimumab showed promising results in a phase 1 trial of 29 melanoma and renal cell carcinoma patients, without dose-limiting toxicity up to 15 mg/kg dose. One melanoma patient achieved a partial response, and six had stable disease with a median progression-free survival of 24 weeks300. In contrast, Eli Lilly’s TGF-β1-specific neutralizing antibody, developed for renal fibrosis, did not show clinical benefit in a small biomarker study of patients with metastatic cancer303. Additional, ongoing studies in monotherapy are designed to assess potential toxicities of novel TGF-β inhibitors. However, based on increasing usage of combination therapies, and restrictions due to compliance with standard of care practices, TGF-β inhibition as monotherapy is unlikely to be a clinical path forward.
Potential Adverse Effects of TGF-β signaling blockade
Although anti-TGF-β treatment is well documented to attenuate cancer cell dissemination and metastasis in mouse models, some pre-clinical data nevertheless raise concerns about possible adverse outcomes. Increased mammary cancer metastasis was seen in response to the TβRI kinase inhibitor, LY364947 (Eli Lilly)331. Additionally, treatment with a pan-TGF-β antibody reduced metastasis in only three of twelve syngeneic mouse mammary carcinoma models, and increased cancer dissemination in another three332. The suppression of cancer dissemination by anti-TGF-β was not seen in immune-deficient mice, whereas its prometastatic activity was immune-independent, associated with a gene expression signature similar to ER+ human breast cancers. This effect may result from enhanced cancer cell proliferation since ER+-like carcinomas retain growth inhibition by TGF-β332. Additionally, TGF-β2 was shown in a mouse model to support metastatic breast cancer dormancy within bone, thus raising concerns about tumor promoting effects of TGF-β2 inhibition333,334,335. These cautionary observations illustrate the importance of identifying biomarkers that predict responses to TGF-β inhibition of distinct tumor types and distinguish effects of TGF-β blockade on cancer cell proliferation and dissemination.
Like many small molecule inhibitors, resistance to TGF-β receptor kinase inhibitors may develop. Long term LY2109761 treatment of mice harboring chemically induced SCCs resulted in outgrowth of tumors with increased, drug-refractile Smad2 activation and phenotypes suggestive of elevated TGF-β signaling336. Treatment of colon carcinoma cell lines with galunisertib resulted in activation of AXL and p38 MAPK, suggesting an adaptive mechanism of cancer cell resistance337. Short-term treatment schedules and/or “drug holidays”, routinely used with targeted therapies, may avoid acquisition of such resistance. Consequently, most clinical trials utilizing galunisertib or next generation relatives follow a regimen of two weeks on and two weeks off drug treatment.
Clinical studies of TGF-β antibodies or TGF-β signaling inhibitors allow an evaluation of undesirable clinical adverse effects, including those that had been major causes for concern and contributed to extensive delays in clinical evaluation. Such fears were that inhibition of TGF-β signaling might induce metaplasia and tumor outgrowth, consistent with the tumor suppressor role of TGF-β signaling during carcinoma development, or that de-repressed immune responses might lead to inflammation and auto-immune manifestations. Our current knowledge, primarily derived from clinical trials with galunisertib, fresolimumab, and a bi-specific anti-PDL-1/anti-TGF-β chimeric trap, M7824, indicate that some patients on high drug doses develop sporadic keratoacanthomas338,339. Since such low-grade pre-malignant cutaneous squamous lesions are common manifestations with several other oncology drugs, and easily monitored and surgically managed, their occurrence is less of a barrier than originally perceived, especially with the hope for complete and durable remission of the treated malignancy. Additionally, grade 1 or 2 skin rashes have been reported with fresolimumab and M7824 inhibition338,339. No dose-limiting adverse effects on the immune system have been reported in clinical trials using small molecule TβRI kinase inhibitors or TGF-β antibodies. However, escalating doses of a TβRII antibody (LY3022859, Eli Lilly) in patients with advanced carcinomas resulted in effects indicative of a cytokine release syndrome, prior to reaching a dose that was considered efficacious for cancer treatment305. The anti-TGF-β1 antibody LY2382770 was found to be safe, with fatigue, nausea and diarrhea as common side effects, in a phase 1 study in metastatic cancer patients, but without efficacy303.
Initial dose escalation studies of galunisertib or other TβRI kinase inhibitors mandated monitoring for cardiotoxicity due to heart valve dysplasia that was observed in rats and dogs receiving high doses of drug340,341. However, valve malformations were seen only at doses beyond those required for anti-cancer efficacy. Cardiovascular toxicity with histopathological changes in the heart was also seen in mice and Cynomolgus monkeys treated with an antibody that potently neutralizes all three TGF-βs342. However, in contrast to treatment with the TβRI kinase inhibitor LY2109761 or a pan-TGF-β antibody, antibody-mediated repression of TGF-β1 activation did not result in cardiac valvulopathies in a rat toxicity model71. In human clinical trials using galunisertib, cardiac monitoring revealed treatment-related cardiovascular effects, but these did not reach grade 3 toxicity even at the highest doses341.
Additionally, concerns about effects on vascular integrity leading to hemorrhagic lesions need to be considered when designing anti-TGF-β therapy regimens, especially since TGF-β1 is required for endothelial integrity through effects on pericytes273,275. Treatment of mice and monkey in toxicology models with a potent, neutralizing pan-TGF-β antibody resulted in persistent hemorrhagic bleeding and associated pathologies342. Clinical trials of M7824 reveal an association with manageable mucosal bleeding attributed to TGF-β blockade339. Overall, these findings strongly suggest that potent and systemic inhibition of all three TGF-βs may confer substantial toxicity that, however, is attenuated with less efficient inhibition or targeting TGF-β1 only. Nevertheless, small molecule inhibitors, anti-TGF-β-antibodies and M7824, using appropriate dosage and treatment regimens, have manageable safety profiles, even though the therapeutic window is narrow. Ongoing studies will expand our knowledge on adverse effects and their impact on therapeutic modalities.
Combining TGF-β blockade with established anti-cancer therapies
Since inhibition of TGF-β signaling is expected to repress immunosuppressive activities, angiogenic responses and activation of the CAF population in the TME, TGF-β inhibition lends itself to enhance the efficacies of other therapeutic approaches. Furthermore, EMT of cancer cells, driven by TGF-β signaling, also promotes cancer drug resistance and cancer stem cell properties. Consequently, targeted inhibition of TGF-β signaling and/or EMT of cancer cells is expected to inhibit cancer progression when combined with cytotoxic or radiation therapies (Table 1).
Radiation induces both a rapid release of bioactive TGF-β1 from latent complexes and increased TGF-β1 expression343,344. Consequently, tumor irradiation enhances TGF-β1 activation within the TME that promotes ECM deposition and fibroblast proliferation, and contributes to fibrosis345. Enhanced TGF-β may also promote radiation-induced increase in drug resistance, and p53-ATM-mediated DNA mismatch repair as a protective mechanism against radiation-induced damage156. Radiation also increases tumor antigenicity by increasing antigen presentation on cancer cells and release of tumor neoantigens from necrotic cells346. This anti-tumor immune activation in response to irradiation accounts for the abscopal effect of radiation therapy, whereby irradiation of a single metastatic lesion can induce regression of dispersed non-irradiated lesions within the same animal or patient346.
Several preclinical and clinical trials combine irradiation with TGF-β signaling inhibition (Table 1), with the expectation of maximizing DNA damage, reducing fibrosis, and elevating immune-mediated tumor clearance347. A small clinical trial with fresolimumab in combination with radiotherapy for metastatic breast cancer failed to meet the endpoint of activation of an abscopal effect following radiation therapy, but did show enhanced overall survival in patients receiving a high drug dose347. In this study, elevated memory CD8+ T cells led the authors to postulate that a combination of PD-1/PD-L1 blockade with fresolimumab and radiation would be required for durable anti-tumor responses347.
Combinations of TβRI kinase inhibitors with chemotherapy are also pursued348 (Table 1). Most completed studies used galunisertib in combination with the methylating agent lomustine for recurrent glioblastoma291,349, sorafenib for unresectable hepatocellular carcinoma350,351, or gemcitabine for unresectable pancreatic cancer352. Eli Lilly’s TβRI small molecule inhibitor, LY3200882, is also under evaluation in combination with capecitabine, a fluoropyrimidine, for advanced colorectal cancer, and other chemotherapeutic agents for a variety of solid tumor types (NCT02937272297). Other TβRI kinase inhibitors, including vactosertib298, are being evaluated in combination with paclitaxel in metastatic gastric cancer patients (NCT03698825297), pomalidomide for multiple myeloma (NCT03143985297), and FOLFOLI for pancreatic cancer (NCT03666832297).
Combining TGF-β blockade with immune checkpoint blockade
In light of the current emphasis on immunotherapies for cancer treatment, and appreciation of TGF-β’s immunosuppressive activities on innate and adaptive immune cells, avenues towards combining anti-TGF-β with checkpoint inhibitors are under intense investigation. An underlying and predominant rationale focuses on the ability of TGF-β released and activated by cancer cells, stromal fibroblasts and/or immune cells to dampen anti-tumor immunity and responses to immunotherapeutics through direct and indirect mechanisms21,23,71,145,353,354. Thus, blocking TGF-β signaling should represses these immunosuppressive activities and enhance the success of checkpoint blockade inhibition or other immunotherapeutic approaches in cancer patients.
Complementary to immunosuppression by TGF-β, the transmembrane PD-L1 ligand, expressed by carcinoma cells, tumor infiltrating DCs and macrophages, binds the PD-1 receptor on CD8 T lymphocytes and represses their anti-tumor functions201. Notably, EMT of carcinoma cells in response to increased TGF-β signaling is accompanied, in some tumors, by increased PD-L1 expression37,355,356. CTLA-4, another inhibitory receptor that is expressed on CD4+ Treg and activated CD8+ T cells, restrains costimulation of T cells and thus also serves as immune checkpoint357,358. Blockade of the CTLA-4 receptor or PD-1-PD-L1 interaction enables treatment of cancers in which either checkpoint restricts immune rejection of the tumor. Anti-CTLA-4 therapy has been somewhat effective in melanoma and several other cancers, and blockade of the PD-1/PD-L1 axis is now first-line therapy for various tumor types, particularly melanoma and non-small cell lung carcinoma, with their combination being superior at the expense of enhanced adverse effects359,360. Unfortunately, the therapeutic response to anti-CTLA-4, anti-PD-1 or anti-PD-L1 antibodies is limited, not only by tumor type, but also by other parameters, such as mutation load and immune cell penetration into the tumor parenchyma. For most tumor types, response rates to single agent therapies in clinical trials ranges between 10 and 25 %, depending on tumor type. Melanomas and urothelial carcinomas that are intrinsically resistant to anti-PD-1/PD-L1 therapy show elevated TGF-β signaling, particularly in the stromal compartment of immune excluded tumors21,77,228,361. Moreover, anti-PD-1 therapy by itself promotes intratumoral TGF-β signaling in mouse models145. The contributions of TGF-β expression, activation and responsiveness in the different cell populations of different tumor types and grades, and the mechanisms by which T cell infiltration into the tumor are impaired remain to be defined; they involve activated CAFs, immunosuppressive myeloid cells, Treg cells and the ECM architecture of the TME21,145,361.
Considering that TGF-β, CTLA-4 and PD-L1/PD-1 signaling act as parallel immunosuppressive pathways that repress cytotoxic T cell, NK cell and macrophage activities through distinct mechanisms, treatments that combine TGF-β inhibition with immune checkpoint inhibition are likely to increase therapeutic efficacy21,23,71,145,346,362. The rationale for such combinations is strengthened by observations that TGF-β-induced EMT enhances PD-L1 expression by tumor cells363, TGF-β/Smad3 signaling enhances antigen-induced PD-1 expression on tumor infiltrating T lymphocytes364, and anti-PD-1 treatment increases Smad3 activation and Treg cell differentiation in carcinomas145, conferring increased TGF-β-driven immunosuppression by cancer cells.
Combination therapies have been pre-clinically evaluated in murine cancer models. Depending on the model and experimental design, anti-PD-1 or anti-PD-L1 enhance the anti-tumor responses of anti-TGF-β, and vice versa, and result in potent and durable cytotoxic T cell responses that can clear even large and aggressive primary tumors, and suppress cancer metastasis21,23,145,346,353,362. Galunisertib23,353, neutralizing anti-TGF-β antibodies21,145, an antibody that prevents TGF-β1 activation71 and anti-integrin β8 antibodies that prevent TGF-β activation85,319 were shown to enhance the anti-tumor efficacy of PD-1/PD-L1 blockade. Increased efficacy of anti-PD-1/PD-L1 with these blocking antibodies was accompanied by enhanced T cell penetration into the tumor parenchyma and activation of CD8+ cytotoxic T cell and CD4+ Th cell populations145,319 (Figure 5). With rapidly expanding preclinical evaluation of combinations of immune checkpoint blockade, with TGF-β signaling inhibition, clinical trials have commenced (Table 1). A Novartis trial of anti-PD-1 and anti-TGFβ1/2 (NCT02947165297) initiated following preclinical studies on chemically induced carcinoma and other syngeneic models. A major question in this context relates to the extent to which TGF-β2 and/or TGF-β3 inhibition contribute to the therapeutic efficacy of TGF-β blockade. TGF-β2 was reported to contribute to metastatic tumor dormancy333–335, and TGF-β3 was seen, in some cases, to antagonize TGF-β1 activity302. It is also unknown to what extent anti-integrin β8 antibody affects integrin β8 functions beyond TGF-β1 activation. Other questions relate to possible adverse effects of TGF-β inhibition on previously established therapeutic windows for anti-PD-1, anti-PD-L1 and anti-CTLA-4 therapies. Combined on- and off-target toxicities will determine clinical design, therapeutic regimens and efficacies.
Bifunctional fusion proteins that bind and block both TGF-β and CTLA-4 or PD-L1 have been generated, thus biochemically linking immune checkpoint inhibition with blocking TGF-β signaling308,309,365. By design, these chimeras, based on the TβRII ligand trap, should sequester TGF-β1 and -β3 only, similarly to dimerized TβRII receptor65. However, M7824, the chimeric fusion protein that combines dimerized TβRII ectodomains with a monoclonal antibody to PD-L1 depletes both TGF-β1 and TGF-β2 in patients339, likely due to altered TGF-β binding properties of the TβRII trap when fused to anti-PD-L1. This bi-specific drug may enable targeting of TGF-β blockade to sites of high PD-L1 expression, i.e. within tumor or its draining lymph nodes. Whether a single molecule can simultaneously neutralize TGF-β and either PD-L1 or CTLA-4 in vivo may depend on steric constraints related to localized TGF-β activation and PD-L1 or CTLA-4 expression in the tumor.
Preclinical evaluation of an anti-CTLA-4-TβRII chimera in comparison with anti-CTLA-4 showed increased repression of Treg cell specification and a switch from IL17-expressing CD4+ Th17 cells to IFN-γ expressing Th1 cells309. Anti-CTLA-4-TβRII had superior anti-tumor activity in a breast cancer mouse model, compared with anti-TGF-β or a combination of anti-CTLA-4 and anti-TGF-β309. The anti-PD-L1-TβRII chimera, M7824, also demonstrated increased efficacy of tumor regression in some mouse models, when compared to TβRII trap alone or anti-PD-L1 monotherapy366, consistent with results from combinations of anti-TGF-β or anti-PD-1/PD-L1145. M7824 and an independently developed anti-PDL-1-TβRII chimera also outperformed a combination of TβRII-Fc with anti-PD-1 antibody in tumor growth inhibition, and were found to repress mesenchymal properties of carcinoma cells in culture and in vivo309,367, consistent with TGF-β’s role as driver of EMT in cancer progression. Furthermore, M7824 reduced Treg-mediated repression of CD8+ cytotoxic T cell activation in culture, and tumor infiltration of T cells and NK cells in vivo312,366. Clinical studies suggest that M7824 exerts more durable responses than historic responses for anti-PDL-1 monotherapy339. With M7824 under clinical evaluation365, its safety profile is seen as manageable, with a narrow therapeutic window. Keratoacanthomas, skin rashes, and impaired vascular integrity that results in mucosal bleeding are expected side effects of TGF-β inhibition, whereas other adverse effects, including immune-related changes, resemble those seen with anti-PD-1 or anti-PD-L1 treatment.
With these successes, the key role of CD4+ T cells in driving anti-TGF-β tumor immune responses is increasingly recognized. CD4+ T cells are more prevalent than CD8+ cytotoxic T cells and, as mentioned, CD4+ T cells can acquire cytotoxic activity when TGF-β responses are inactivated216. Importantly, CD4+ T cells show considerable plasticity within tumors, dependent on the cytokine milieu, and particularly TGF-β levels. The balance of CD4+ Th1, Th2, Th17 and Treg cells can shift dramatically following therapy, with reprogramming from one CD4+ T cell subset towards another368,369 (Figure 4). In a preclinical study of six SCC lines, the CD4+ T cell composition distinguished responsive lines from those refractile to anti-TGF-β/anti-PD-1 combination therapy in vivo145. Checkpoint blockade enhanced their Th1/ Treg ratio, and this was reversed and further diminished by TGF-β blockade, accounting for anti-tumor synergy between both drugs145. In a mouse model of anti-CTLA4-resistant metastatic prostate cancer, high CD4+ Th17 cell levels in bone metastases provided vulnerability towards TGF-β blockade by reprogramming these cells towards an anti-tumorigenic CD4+ Th1 phenotype354. Thus, high CD4+ T cell content, whether Treg or Th17 cells, may be prognostic for favorable response to TGF-β blockade.
TGF-β blockade promotes therapeutic activity of cancer vaccines and CAR-T cells
Before the synergy of TGF-β signaling inhibition with checkpoint blockade was discovered, the potentiating activity of TGF-β blockade towards cancer vaccines was recognized. Indeed, the earliest clinical trials targeting the TGF-β pathway for cancer treatment were designed to potentiate anti-tumor immunity using a tumor vaccine for non-small lung carcinoma, genetically modified by including an anti-sense TGF-β2 RNA370. The concept was ahead of its time; the technology and choice of TGFB2 as target now seem outdated, and a phase 3 clinical trial did not meet its endpoint of increased overall survival371. TGF-β blockade using a TβRI kinase inhibitor, SM16, enhanced the antitumor effects of an immune-activating adenoviral vector encoding IFN-β, and an adenoviral HPV E7 vaccine therapy in models of mesothelioma and lung cancer372.
Adoptive transfer of ex vivo-expanded tumor-homing T lymphocytes for immunotherapy was clinically introduced over thirty years ago373. In preclinical studies, TGF-β blockade using a TβRI kinase inhibitor, SM16, was shown to augment the persistence of adoptively transferred T cells in spleen, lymph nodes, and tumors of thymoma-bearing mice, showed increased activation of tumor infiltrating T cells, enhanced tumor necrosis factor-α and decreased arginase expression in the TME374. Clinically, adoptive T cell therapy was rapidly followed by genetic manipulations to enhance targeting of T cells to tumor cells and T cell activation using chimeric antigen receptors (CARs)375, and CAR-T cell therapy has been successful for hematological malignancies. Expansion and adoptive transfer of autologous cytotoxic T lymphocytes from patients, selected for targeting one or two key Epstein-Barr Virus Latent Membrane Proteins expressed on B-lymphoma cells, resulted in sustained complete responses with little toxicity376. Since TGF-β represses cytotoxic T lymphocyte activation, TGF-β inhibition has been pursued to enhance the efficacy of CAR-T cell therapies. The TβRI inhibitor SM16A enhanced the adoptive T cell transfer and anti-tumor efficacy in mesothelioma-bearing mice374. With refinements in T cell engineering, incorporation of a dominant-negative, cytoplasmically truncated TβRII receptor within T cells attenuated immunosuppression by TGF-β377. In a small number of patients with relapsed and refractory EBV-positive Hodgkin’s lymphoma, the engineered lymphocytes showed considerable expansion in vivo, persisting in blood, and induced complete regression without a lymphoproliferative phenotype or cytokine release syndrome211. Further modifications of CAR-T-mediated approaches resulted in engineering of T cells expressing a chimeric T cell antigen receptor with an extracellular, single chain variable fragment derived from a neutralizing TGF-β antibody. These CAR-T cells respond to TGF-β by dimerizing the TGF-β binding CAR that results in activation of T cells. Thus, rather than exerting its normal immunosuppressive role, TGF-β acts as a potent immunostimulant of these CAR-T cells378,379. Such design may enable targeting of CAR-T cells to areas in the TME with high levels of TGF-β.
Summarizing these clinical developments, repression of TGF-β signaling appears to enhance the efficacy of some cancer treatments, independent of the cancer cells’ TGF-β responsiveness. Preclinical studies, supported by early clinical observations, show enhanced efficacies of immunotherapies when TGF-β signaling is repressed and its immunosuppressive activities are consequently attenuated. Ongoing and future clinical trials may fully validate these findings in cancer treatment.
Concluding remarks and future directions
TGF-β signaling plays key roles in cancer progression, through direct effects on cancer cells, immune cells and other cell types in the TME. Autocrine and paracrine TGF-β signaling in cancer cells directs profound changes in gene expression that repress the epithelial phenotype and facilitate invasion and dissemination, promote stem cell properties and cancer drug resistance, and release of immunosuppressive cytokines. TGF-β released by cancer cells, stromal fibroblasts and other cell types in the TME shapes the architecture of the TME through ECM protein expression, and suppresses cytotoxic activities of immune cells, thus making the TME an immune tolerant environment that prevents or attenuates the efficacies of cancer immunotherapies. Considering this plethora of TGF-β activities, therapeutic avenues to inhibit TGF-β signaling should target both the cancer cells and immune and stromal components of the TME to ensure durable regression.
High TGF-β signaling has been implicated in resistance to various anti-cancer treatments including chemotherapies and molecularly-targeted therapies149,380,381. Increased TGF-β signaling within tumors also revealed itself as a substantial impediment of responsiveness to immunotherapies, to the extent that only a fraction of patients respond to anti-PD-1 or -PD-L1, anti-CTLA-4 and CAR-T cell therapies, and these patients show low TGF-β signaling signatures. As new immunotherapies are evaluated, TGF-β signaling in the TME will again reveal itself as impeding response and extension of survival time. These will undoubtedly benefit from the considerable effort that is directed towards therapeutically repressing TGF-β signaling to enhance anti-PD-1/-PD-L1 responsiveness. In contrast to chemotherapy or oncogene-targeted drugs, immunotherapy, including TGF-β blockade, does not routinely result in short-term endpoints that are assessed by RECIST321. Rather, responses can be considerably delayed and unpredictable, in part due to the cascade of immune events that are required for a productive anti-tumor cytotoxic response382. Since cytotoxic responses to anti-TGF-β monotherapies, when they occur, are immune-mediated and followed by sustained immunity to tumor challenge23,145,319,353, responses to TGF-β blockade should be scored using Immune-Related Response Criteria322,323. Delayed anti-tumor responses and long-term survival may take years of patient follow-up to meet statistical significance.
Considering the normal roles of TGF-β signaling in normal physiological processes, it is not surprising that systemic suppression of TGF-β signaling is accompanied by adverse effects that limit the therapeutic window and design of treatment regimens. Concerns for potential adverse effects resulting from normal physiological activities of TGF-β signaling impeded the pursuit of anti-TGF-β therapies. Together with only marginal clinical efficacy of anti-TGF-β monotherapy, these fears stalled drug development for many years, even though severe adverse effects are common when exploring novel cancer treatment modalities that interfere with normal physiology yet extend patient life for months. While therapeutic approaches and regimens clearly need further exploration, current, early clinical trials have shown adverse effects to be generally of low grade and manageable, with substantial and durable responses to TGF-β blockade, when combined with checkpoint inhibition. Going forward, selectively targeting TGF-β inhibition to the tumor should greatly decrease the severity of adverse effects and enhance therapeutic opportunities. TGF-β inhibition using bispecific drugs or through interference with TGF-β signaling components with restricted tissue expression, e.g. blocking integrin- or GARP-mediated TGF-β activation, is likely to enable novel therapies. More precise targeting may also be provided by the use of pro-bodies383. Incorporation of TGF-β inhibitors into tumor-targeted CAR-T cells, viral vectors, nanoparticles, or oncolytic viruses384 may provide additional opportunities. Some may require smaller TGF-β inhibitors that are packaged into viral vectors, such as TGF-β1 mini-monomers that act as potent dominant negative inhibitors385, or the use of nanobodies386.
As we learn more about TGF-β’s activities on individual TME compartments, it becomes clear that key cellular and molecular features that define responsiveness to TGF-β blockade may differ between tumor types. For example, TGF-β blockade responses of PDAC, with its extensive stromal elaboration, frequent SMAD4 inactivation and low tumor mutation load387, may differ from those of head and neck SCCs that have high tumor mutation loads and CD4+ T cell content114,388. Distinct tumor mutation loads, oncogenic drivers, and other genetic and epigenetic differences in cancer cells dictate immune infiltration and responses to checkpoint blockade, and will help define responses to TGF-β blockade.
Additionally, germline genetic variations389–392 and microbiome differences between individuals359,393–395 are likely to influence therapeutic responses and immune-related adverse effects. GWAS studies revealed genetic associations between germline variants in genes encoding TGF-β signaling molecules and susceptibilities to cancer and autoimmune disorders396,397, and preclinical studies highlight extensive influences of germline genetic modifiers of TGF-β activity on tumorigenesis, vascular integrity and allergy392,398–400. Also the gut microbiome may help define responses to TGF-β blockade. For example, Tgfb1+/− mice are healthy in a germ-free environment, but develop colon inflammation, colitis and colon adenocarcinoma when housed under less clean conditions401. Furthermore, the colon microbiome can also influence systemic responses to immune checkpoint blockade393,394,402. It will therefore be important to also take this variable into consideration when studying responses to TGF-β blockade.
Substantial research on cancers of different organ sites is still needed to evaluate the extent to which tumor architecture, genetic and phenotypic heterogeneity of malignant and TME cells and their TGF-β responsiveness define the efficacy of diverse immunotherapies, without or in combination with TGF-β inhibition. Only in this way can we move toward a reliable personalized prediction of responsiveness to therapy and effects of anti-TGF-β immunotherapy.
Footnotes
Competing Interests:
Rik Derynck is a co-Founder of Pliant Therapeutics. Shannon Turley is an employee of Genentech Inc., and Rosemary Akhurst has sponsored research agreements with Pfizer Corp. and BMS and is a co-inventor of a patent on the use of anti-TGF-β antibodies that is co-owned by UCSF and Xoma.
References
- 1.Moses HL, Roberts AB & Derynck R. The discovery and early days of TGF-β: a historical perspective. Cold Spring Harb Perspect Biol 8, a021865 (2016).. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moses HL, Branum EL, Proper JA & Robinson RA Transforming growth factor production by chemically transformed cells. Cancer Res 41, 2842–2848 (1981). [PubMed] [Google Scholar]
- 3.Roberts AB, Anzano MA, Lamb LC, Smith JM & Sporn MB New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Nat Acad Sci USA 78, 5339–5343 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derynck R. et al. Human transforming growth factor-β complementary DNA sequence and expression in normal and transformed cells. Nature 316, 701–705 (1985). [DOI] [PubMed] [Google Scholar]
- 5.Derynck R. et al. Synthesis of messenger RNAs for transforming growth factors α and β and the epidermal growth factor receptor by human tumors. Cancer Res 47, 707–712 (1987). [PubMed] [Google Scholar]
- 6.Tucker RF, Shipley GD, Moses HL & Holley RW Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science 226, 705–707 (1984). [DOI] [PubMed] [Google Scholar]
- 7.Siegel PM & Massagué J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer 3, 807–821 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Miettinen PJ, Ebner R, Lopez AR & Derynck R. TGF-β-induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol 127, 2021–2036 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsuno Y, Lamouille S. & Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 25, 76–84 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Fridman WH, Pages F, Sautes-Fridman C. & Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12, 298–306 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Fridman WH, Zitvogel L, Sautes-Fridman C. & Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14, 717–734 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Turley SJ, Cremasco V. & Astarita JL Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol 15, 669–682 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Peske JD, Woods AB & Engelhard VH Control of CD8 T-Cell Infiltration into Tumors by Vasculature and Microenvironment. Adv Cancer Res 128, 263–307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolas-Boluda A. & Donnadieu E. Obstacles to T cell migration in the tumor microenvironment. Comp Immunol Microbiol Infect Dis 63, 22–30 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Krummel MF, Bartumeus F. & Gerard A. T cell migration, search strategies and mechanisms. Nat Rev Immunol 16, 193–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce JA & Fearon DT T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Chen DS & Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Swartz MA & Lund AW Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer 12, 210–219 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Aspelund A. & Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 124, 878–887 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengyel E, Makowski L, DiGiovanni J. & Kolonin MG Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer 4, 374–384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariathasan S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarthy A, Khan L, Bensler NP, Bose P. & De Carvalho DD TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun 9, 4692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauriello DVF et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Thorsson V. et al. The immune landscape of cancer. Immunity 48, 812–830 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahmani A. & Delisle JS TGF-β in T cell biology: implications for cancer immunotherapy. Cancers 10, 194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ungefroren H. Blockade of TGF-β signaling: a potential target for cancer immunotherapy? Expert Opin Ther Targets 23, 679–693 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Batlle E. & Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieweke MH, Thompson NL, Sporn MB & Bissell MJ Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-β. Science 248, 1656–1660 (1990). [DOI] [PubMed] [Google Scholar]
- 29.Akhurst RJ, Fee F. & Balmain A. Localized production of TGF-β mRNA in tumour promoter-stimulated mouse epidermis. Nature 331, 363–365 (1988). [DOI] [PubMed] [Google Scholar]
- 30.DiPaolo JA, DeMarinis AJ, Evans CH & Doniger J. Expression of initiated and promoted stages of irradiation carcinogenesis in vitro. Cancer Lett 14, 243–249 (1981). [DOI] [PubMed] [Google Scholar]
- 31.Harjunpaa H, Llort Asens M, Guenther C. & Fagerholm SC Cell Adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front Immunol 10, 1078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onnis A. & Baldari CT Orchestration of immunological synapse assembly by vesicular trafficking. Front Cell Dev 7, 110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro-Sanchez P, Aguilar-Sopena O, Alegre-Gomez S, Ramirez-Munoz R. & Roda-Navarro P. Regulation of CD4+ T cell signaling and immunological synapse by protein tyrosine phosphatases: molecular mechanisms in autoimmunity. Front Immunol 10, 1447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibue T. & Weinberg RA EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 14, 611–629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brabletz T, Kalluri R, Nieto MA & Weinberg RA EMT in cancer. Nat Rev Cancer 18, 128–134 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Nieto MA, Huang RY, Jackson RA & Thiery JP EMT: 2016. Cell 166, 21–45 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Dongre A. & Weinberg RA New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20, 69–84 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Zhao F. et al. Stromal fibroblasts mediate anti-PD-1 resistance via MMP-9 and dictate TGFβ inhibitor sequencing in melanoma. Cancer Immunol Res 6, 1459–1471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohlund D, Elyada E. & Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med 211, 1503–1523 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 16, 582–598 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Cui W. et al. TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell 86, 531–542 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Portella G. et al. Transforming growth factor β is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ 9, 393–404 (1998). [PubMed] [Google Scholar]
- 43.Robertson IB & Rifkin DB Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb Perspect Biol 8, a021907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 285, 1182–1186 (1971). [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N. VEGF as a therapeutic target in cancer. Oncology 69 Suppl 3, 11–16 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Goel S, Wong AH & Jain RK Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med 2, a006486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson-Percival A, He B. & Ganss R. Immunomodulation of tumor vessels: it takes two to tango. Trends Immunol 39, 801–814 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto M. et al. CD8 T Cell Exhaustion in chronic nfection and cancer: opportunities for interventions. Annu Rev Med 69, 301–318 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Chiossone L, Dumas PY, Vienne M. & Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 18, 671–688 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Fu C. & Jiang A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol 9, 3059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hildner K. et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wculek SK et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20, 7–24 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Engelhardt JJ et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell 21, 402–417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salmon H. et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engblom C, Pfirschke C. & Pittet MJ The role of myeloid cells in cancer therapies. Nat Rev Cancer 16, 447–462 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Shalapour S. et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z. et al. Gr-1+CD11b+ cells are responsible for tumor promoting effect of TGF-β in breast cancer progression. Int J Cancer 131, 2584–2595 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen ML et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proc Natl Acad Sci U S A 102, 419–424 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marie JC, Letterio JJ, Gavin M. & Rudensky AY TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 201, 1061–1067 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tone Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature immunology 9, 194–202 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Sanjabi S, Oh SA & Li MO Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol 9, a022236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu M, Li S. & Li MO TGF-β control of adaptive immune tolerance: a break from Treg cells. Bioessays 40, e1800063 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donkor MK, Sarkar A. & Li MO TGF-β1 produced by activated CD4+ T cells antagonizes T cell surveillance of tumor development. Oncoimmunology 1, 162–171, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuende J. et al. Monoclonal antibodies against GARP/TGF-β1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Sci Transl Med 7, 284ra256 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Derynck R. & Budi EH Specificity, versatility, and control of TGF-β family signaling. Sci Signal 12, aav5183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Assoian RK, Komoriya A, Meyers CA, Miller DM & Sporn MB Transforming growth factor-β in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 258, 7155–7160 (1983). [PubMed] [Google Scholar]
- 67.Cheifetz S. et al. Heterodimeric transforming growth factor β. Biological properties and interaction with three types of cell surface receptors. J Biol Chem 263, 10783–10789 (1988). [PubMed] [Google Scholar]
- 68.Cui W, Kemp CJ, Duffie E, Balmain A. & Akhurst RJ Lack of transforming growth factor-β1 expression in benign skin tumors of p53null mice is prognostic for a high risk of malignant conversion. Cancer Res 54, 5831–5836 (1994). [PubMed] [Google Scholar]
- 69.Qin X. et al. TGFβ3-mediated induction of periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep 6, 20587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hachim MY, Hachim IY, Dai M, Ali S. & Lebrun JJ Differential expression of TGFβ isoforms in breast cancer highlights different roles during breast cancer progression. Tumour Biol 40, 1010428317748254 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Martin CJ et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med 12, eaay8456 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Stockis J, Dedobbeleer O. & Lucas S. Role of GARP in the activation of latent TGF-β1. Mol Biosyst 13, 1925–1935 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Metelli A. et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-β. Sci Transl Med 12, eaay4860 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roubin R. et al. Structure and developmental expression of mouse Garp, a gene encoding a new leucine-rich repeat-containing protein. Int J Dev Biol 40, 545–555 (1996). [PubMed] [Google Scholar]
- 75.Qin Y. et al. A milieu molecule for TGF-β required for microglia function in the nervous system. Cell 174, 156–171 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma W, Qin Y, Chapuy B. & Lu C. LRRC33 is a novel binding and potential regulating protein of TGF-β1 function in human acute myeloid leukemia cells. PLoS One 14, e0213482 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dominguez CX et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov 10, 232–253 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Purcell JW et al. LRRC15 is a novel mesenchymal protein and stromal target for antibody-drug conjugates. Cancer Res 78, 4059–4072 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Shi M. et al. Latent TGF-β structure and activation. Nature 474, 343–349 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henderson NC & Sheppard D. Integrin-mediated regulation of TGFβ in fibrosis. Biochim Biophys Acta 1832, 891–896 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown NF & Marshall JF Integrin-mediated TGFβ activation modulates the tumour microenvironment. Cancers 11, 1221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stockis J. et al. Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin αvβ8. Proc Natl Acad Sci U S A 114, E10161–E10168 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arnold TD et al. Defective retinal vascular endothelial cell development as a consequence of impaired integrin αvβ8-mediated activation of transforming growth factor-β. J Neurosci 32, 1197–1206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arnold TD et al. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Development 141, 4489–4499 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takasaka N. et al. Integrin αvβ8-expressing tumor cells evade host immunity by regulating TGF-β activation in immune cells. JCI Insight 3, e122591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hata A. & Chen YG TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol 8, a022061 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hill CS Transcriptional control by the SMADs. Cold Spring Harb Perspect Biol 8, a022079 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heldin CH & Moustakas A. Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol 8, a022053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang YE Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol 9, a022129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lemmon MA & Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Budi EH, Muthusamy BP & Derynck R. The insulin response integrates increased TGF-β signaling through Akt-induced enhancement of cell surface delivery of TGF-β receptors. Sci Signal 8, ra96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu L. & Derynck R. Essential role of TGF-β signaling in glucose-induced cell hypertrophy. Dev Cell 17, 35–48 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J. et al. The regulation of TGF-β/SMAD signaling by protein deubiquitination. Protein Cell 5, 503–517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu C, Xu P, Lamouille S, Xu J. & Derynck R. TACE-mediated ectodomain shedding of the type I TGF-β receptor downregulates TGF-β signaling. Mol Cell 35, 26–36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oft M, Akhurst RJ & Balmain A. Metastasis is driven by sequential elevation of H-Ras and Smad2 levels. Nat Cell Biol 4, 487–494 (2002). [DOI] [PubMed] [Google Scholar]
- 96.Birchenall-Roberts MC et al. Transcriptional regulation of the transforming growth factor β1 promoter by v-src gene products is mediated through the AP-1 complex. Mol Cell Biol 10, 4978–4983 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thatikunta P, Raj GV, Kundu M, Khalili K. & Amini S. The transcription factor E2F-1 modulates TGF-β1 RNA expression in glial cells. Oncogene 14, 2959–2969 (1997). [DOI] [PubMed] [Google Scholar]
- 98.Cui W. et al. Concerted action of TGF-β1 and its type II receptor in control of epidermal homeostasis in transgenic mice. Genes Dev 9, 945–955 (1995). [DOI] [PubMed] [Google Scholar]
- 99.Mayer IA & Arteaga CL The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med 67, 11–28 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Manning BD & Toker A. AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo L. et al. MicroRNAs, TGF-β signaling, and the inflammatory microenvironment in cancer. Tumour Biol 37, 115–125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lamouille S, Subramanyam D, Blelloch R. & Derynck R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol 25, 200–207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blahna MT & Hata A. Regulation of miRNA biogenesis as an integrated component of growth factor signaling. Curr Opin Cell Biol 25, 233–240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horiguchi K. et al. TGF-β drives epithelial-mesenchymal transition through δEF1-mediated downregulation of ESRP. Oncogene 31, 3190–3201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Howley BV & Howe PH TGF-β signaling in cancer: post-transcriptional regulation of EMT via hnRNP E1. Cytokine 118, 19–26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hynes RO The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hubmacher D. & Apte SS The biology of the extracellular matrix: novel insights. Curr Opin Rheumatol 25, 65–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heldin CH, Rubin K, Pietras K. & Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer 4, 806–813 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Massagué J, Blain SW & Lo RS TGFβ signaling in growth control, cancer, and heritable disorders. Cell 103, 295–309 (2000). [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Alexander PB & Wang XF TGF-β family signaling in the control of cell proliferation and survival. Cold Spring Harb Perspect Biol 9, a022145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seoane J. & Gomis RR TGF-β family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol 9, a022277 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ikushima H. & Miyazono K. TGFβ signalling: a complex web in cancer progression. Nat Rev Cancer 10, 415–424 (2010). [DOI] [PubMed] [Google Scholar]
- 113.Pino MS et al. Epithelial to mesenchymal transition is impaired in colon cancer cells with microsatellite instability. Gastroenterology 138, 1406–1417 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517, 576–582, doi: 10.1038/nature14129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cho SY et al. Sporadic early-onset diffuse gastric cancers have high frequency of somatic CDH1 alterations, but low frequency of somatic RHOA mutations compared with late-onset cancers. Gastroenterology 153, 536–549 e526 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Korkut A. et al. A pan-cancer analysis reveals high-frequency genetic alterations in mediators of signaling by the TGF-β superfamily. Cell Syst 7, 422–437 e427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blackford A. et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 15, 4674–4679 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Descargues P. et al. IKKα is a critical coregulator of a Smad4-independent TGFβ-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc Natl Acad Sci USA 105, 2487–2492 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levy L. & Hill CS Smad4 dependency defines two classes of transforming growth factor β target genes and distinguishes TGF-β-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol 25, 8108–8125 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang L. & Karin M. Roles of tumor suppressors in regulating tumor-associated inflammation. Cell Death Differ 21, 1677–1686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Inman GJ et al. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine-associated mutational signature. Nat Commun 9, 3667 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Busch S. et al. TGF-β receptor type-2 expression in cancer-associated fibroblasts regulates breast cancer cell growth and survival and is a prognostic marker in pre-menopausal breast cancer. Oncogene 34, 27–38 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Suriyamurthy S, Baker D, ten Dijke P. & Iyengar PV Epigenetic reprogramming of TGF-β signaling in breast cancer. Cancers 11, 726 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miyazawa K. & Miyazono K. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol 9, a022095 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang J, Gifford CC, Samarakoon R. & Higgins PJ Deregulation of negative controls on TGF-β1 signaling in tumor progression. Cancers 10, 159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119, 1417–1419 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Derynck R. & Weinberg RA EMT and cancer: more than meets the eye. Dev Cell 49, 313–316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lamouille S, Xu J. & Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15, 178–196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ramachandran A. et al. TGF-β uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. Elife 7, e31756 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tam WL & Weinberg RA The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 19, 1438–1449 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeisberg M. & Neilson EG Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119, 1429–1437 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Derynck R, Muthusamy BP & Saeteurn KY Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol 31, 56–66 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chaffer CL, San Juan BP, Lim E. & Weinberg RA EMT, cell plasticity and metastasis. Cancer Metastasis Rev 35, 645–654 (2016). [DOI] [PubMed] [Google Scholar]
- 134.Pastushenko I. & Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol 29, 212–226 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Jolly MK et al. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol Ther 194, 161–184 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Mishra AK, Campanale JP, Mondo JA & Montell DJ Cell interactions in collective cell migration. Development 146, dev172056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Celia-Terrassa T. & Jolly MK Cancer stem cells and epithelial-to-mesenchymal transition in cancer metastasis. Cold Spring Harb Perspect Med a036905 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aponte PM & Caicedo A. Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int 2017, 5619472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scheel C. et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145, 926–940 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scheel C. & Weinberg RA Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol 22, 396–403 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dongre A. et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res 77, 3982–3989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Katsuno Y. et al. Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci Signal 12, aau8544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Soundararajan R. et al. Targeting the interplay between epithelial-to-mesenchymal-transition and the immune system for effective immunotherapy. Cancers 11, 714 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Terry S. et al. New insights into the role of EMT in tumor immune escape. Mol Oncol 11, 824–846 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dodagatta-Marri E. et al. α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J Immunother Cancer 7, 62 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang Y. & Zhan H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett 468, 72–81 (2020). [DOI] [PubMed] [Google Scholar]
- 147.Reddy SM, Carroll E. & Nanda R. Atezolizumab for the treatment of breast cancer. Expert Rev Anticancer Ther 20, 151–158 (2020). [DOI] [PubMed] [Google Scholar]
- 148.Schmid P. et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21, 44–59 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Singh A. & Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Staalduinen J, Baker D, ten Dijke P. & van Dam H. Epithelial-mesenchymal-transition-inducing transcription factors: new targets for tackling chemoresistance in cancer? Oncogene 37, 6195–6211 (2018). [DOI] [PubMed] [Google Scholar]
- 151.Fischer KR et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oshimori N, Oristian D. & Fuchs E. TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 160, 963–976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nunes T. et al. Targeting cancer stem cells to overcome chemoresistance. Int J Mol Sci 19, 4036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.De Angelis ML, Francescangeli F. & Zeuner A. Breast cancer stem cells as drivers of tumor chemoresistance, dormancy and relapse: new challenges and therapeutic opportunities. Cancers 11, 1569 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Steinbichler TB et al. Therapy resistance mediated by cancer stem cells. Semin Cancer Biol 53, 156–167 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Ewan KB et al. Transforming growth factor-β1 mediates cellular response to DNA damage in situ. Cancer Res 62, 5627–5631 (2002). [PubMed] [Google Scholar]
- 157.Glick AB, Weinberg WC, Wu IH, Quan W. & Yuspa SH Transforming growth factor β1 suppresses genomic instability independent of a G1 arrest, p53, and Rb. Cancer Res 56, 3645–3650 (1996). [PubMed] [Google Scholar]
- 158.Comaills V. et al. Genomic instability is induced by persistent proliferation of cells undergoing epithelial-to-mesenchymal transition. Cell Rep 17, 2632–2647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pal D. et al. TGF-β reduces DNA ds-break repair mechanisms to heighten genetic diversity and adaptability of CD44+/CD24− cancer cells. Elife 6, e21615 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martincorena I. et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Matsushita H. et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morvan MG & Lanier LL NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16, 7–19 (2016). [DOI] [PubMed] [Google Scholar]
- 163.Trinh TL et al. Immune evasion by TGFβ-induced miR-183 repression of MICA/B expression in human lung tumor cells. Oncoimmunology 8, e1557372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nam JS et al. An anti-transforming growth factor β antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res 68, 3835–3843 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Friese MA et al. RNA interference targeting transforming growth factor-β enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res 64, 7596–7603 (2004). [DOI] [PubMed] [Google Scholar]
- 166.Lazarova M. & Steinle A. Impairment of NKG2D-mediated tumor immunity by TGF-β. Front Immunol 10, 2689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sadallah S. et al. Platelet-derived ectosomes reduce NK cell function. J Immunol 197, 1663–1671 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Naganuma H. et al. Transforming growth factor-β inhibits interferon-γ secretion by lymphokine-activated killer cells stimulated with tumor cells. Neurol Med Chir 36, 789–795 (1996). [DOI] [PubMed] [Google Scholar]
- 169.Mantovani A, Sozzani S, Locati M, Allavena P. & Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23, 549–555 (2002). [DOI] [PubMed] [Google Scholar]
- 170.Hildenbrand R. et al. Transforming growth factor-β stimulates urokinase expression in tumor-associated macrophages of the breast. Lab Invest 78, 59–71 (1998). [PubMed] [Google Scholar]
- 171.Laoui D. et al. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol 55, 861–867 (2011). [DOI] [PubMed] [Google Scholar]
- 172.Standiford TJ et al. TGF-beta-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth. Oncogene 30, 2475–2484 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fridlender ZG et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li H, Han Y, Guo Q, Zhang M. & Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β1. J Immunol 182, 240–249 (2009). [DOI] [PubMed] [Google Scholar]
- 175.Takeuchi M, Kosiewicz MM, Alard P. & Streilein JW On the mechanisms by which transforming growth factor-β2 alters antigen-presenting abilities of macrophages on T cell activation. Eur J Immunol 27, 1648–1656 (1997). [DOI] [PubMed] [Google Scholar]
- 176.Demidem A, Taylor JR, Grammer SF & Streilein JW Comparison of effects of transforming growth factor-β and cyclosporin A on antigen-presenting cells of blood and epidermis. J Invest Dermatol 96, 401–407 (1991). [DOI] [PubMed] [Google Scholar]
- 177.Seeger P, Musso T. & Sozzani S. The TGF-β superfamily in dendritic cell biology. Cytokine Growth Factor Rev 26, 647–657 (2015). [DOI] [PubMed] [Google Scholar]
- 178.Siegert A, Denkert C, Leclere A. & Hauptmann S. Suppression of the reactive oxygen intermediates production of human macrophages by colorectal adenocarcinoma cell lines. Immunology 98, 551–556 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nelson BJ, Ralph P, Green SJ & Nacy CA Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-β1. J Immunol 146, 1849–1857 (1991). [PubMed] [Google Scholar]
- 180.Yang L. et al. Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13, 23–35 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mohammed J. et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat Immunol 17, 414–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Roberts EW et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kobie JJ et al. Transforming growth factor β inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res 63, 1860–1864 (2003). [PubMed] [Google Scholar]
- 184.Sato K. et al. TGF-β1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J Immunol 164, 2285–2295 (2000). [DOI] [PubMed] [Google Scholar]
- 185.Gujar R. & Sen P. Transforming growth factor-β1 impairs lymph node homing of dendritic cells by downregulating C-type lectin receptor-2 expression. Cytokine 110, 39–43 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Fenton TM et al. Inflammatory cues enhance TGFβ activation by distinct subsets of human intestinal dendritic cells via integrin αvβ8. Mucosal Immunol 10, 624–634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Coombes JL et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med 204, 1757–1764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu J. & Lanier LL Natural killer cells and cancer. Adv Cancer Res 90, 127–156 (2003). [DOI] [PubMed] [Google Scholar]
- 189.Lugli E, Galletti G, Boi SK & Youngblood BA Stem, effector, and hybrid states of memory CD8+ T Cells. Trends Immunol 41, 17–28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou J, Dudley ME, Rosenberg SA & Robbins PF Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother 28, 53–62 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gill S. & June CH Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev 263, 68–89 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Ahmed N. et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res 67, 5957–5964 (2007). [DOI] [PubMed] [Google Scholar]
- 193.June CH & Levine BL T cell engineering as therapy for cancer and HIV: our synthetic future. Philos Trans R Soc Lond B Biol Sci 370, 20140374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Grosser R, Cherkassky L, Chintala N. & Adusumilli PS Combination immunotherapy with CAR-T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 36, 471–482 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Amaria RN et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 24, 1649–1654 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ribas A. & Wolchok JD Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kulkarni AB et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90, 770–774 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Diebold RJ et al. Early-onset multifocal inflammation in the transforming growth factor β1-null mouse is lymphocyte mediated. Proc Natl Acad Sci U S A 92, 12215–12219 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shull MM et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 359, 693–699 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gorelik L. & Flavell RA Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nat Med 7, 1118–1122 (2001). [DOI] [PubMed] [Google Scholar]
- 201.Pardoll DM The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Novitskiy SV et al. Deletion of TGF-β signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol 92, 641–651 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Park IK, Shultz LD, Letterio JJ & Gorham JD TGF-β1 inhibits T-bet induction by IFN-γ in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J Immunol 175, 5666–5674 (2005). [DOI] [PubMed] [Google Scholar]
- 204.Choudhry MA, Sir O. & Sayeed MM TGF-β abrogates TCR-mediated signaling by upregulating tyrosine phosphatases in T cells. Shock 15, 193–199 (2001). [DOI] [PubMed] [Google Scholar]
- 205.Das L. & Levine AD TGF-β inhibits IL-2 production and promotes cell cycle arrest in TCR-activated effector/memory T cells in the presence of sustained TCR signal transduction. J Immunol 180, 1490–1498 (2008). [DOI] [PubMed] [Google Scholar]
- 206.Thomas DA & Massagué J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005). [DOI] [PubMed] [Google Scholar]
- 207.Mackay LK et al. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43, 1101–1111 (2015). [DOI] [PubMed] [Google Scholar]
- 208.Adusumilli PS et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 6, 261ra151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang D. et al. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight 3, 99048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang Y. et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med 9, eaag1209 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bollard CM et al. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed Hodgkin lymphoma. J Clin Oncol 36, 1128–1139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hung K. et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 188, 2357–2368 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Corthay A. et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity 22, 371–383 (2005). [DOI] [PubMed] [Google Scholar]
- 214.Perez-Diez A. et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 109, 5346–5354 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Murphy KA & Griffith TS CD8 T cell-independent antitumor response and its potential for treatment of malignant gliomas. Cancers 8, 71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Marie JC, Liggitt D. & Rudensky AY Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity 25, 441–454 (2006). [DOI] [PubMed] [Google Scholar]
- 217.Mangan PR et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006). [DOI] [PubMed] [Google Scholar]
- 218.Donkor MK et al. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-β1 cytokine. Immunity 35, 123–134 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Moreno Ayala MA, Li Z. & DuPage M. Treg programming and therapeutic reprogramming in cancer. Immunology 157, 198–209 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ralainirina N. et al. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol 81, 144–153 (2007). [DOI] [PubMed] [Google Scholar]
- 221.Zemmour D. et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat Immunol 19, 291–301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cremasco V. et al. FAP delineates heterogeneous and functionally divergent stromal cells in immune-excluded breast tumors. Cancer Immunol Res 6, 1472–1485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Boesch M. et al. Interleukin 7-expressing fibroblasts promote breast cancer growth through sustenance of tumor cell stemness. Oncoimmunology 7, e1414129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Costa A. et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33, 463–479 (2018).. [DOI] [PubMed] [Google Scholar]
- 225.Elyada E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov 9, 1102–1123, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ohlund D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214, 579–596 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Calon A. et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Calon A. et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet 47, 320–329 (2015). [DOI] [PubMed] [Google Scholar]
- 229.Feig C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 110, 20212–20217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fearon DT The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res 2, 187–193 (2014). [DOI] [PubMed] [Google Scholar]
- 231.Lakins MA, Ghorani E, Munir H, Martins CP & Shields JD Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T cells to protect tumour cells. Nat Commun 9, 948 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hinz B. Myofibroblasts. Exp Eye Res 142, 56–70 (2016). [DOI] [PubMed] [Google Scholar]
- 233.Calon A, Tauriello DV & Batlle E. TGF-β in CAF-mediated tumor growth and metastasis. Semin Cancer Biol 25, 15–22 (2014). [DOI] [PubMed] [Google Scholar]
- 234.LeBleu VS & Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech 11, dmm.029447 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Park JE et al. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem 274, 36505–36512 (1999). [DOI] [PubMed] [Google Scholar]
- 236.Yin M. et al. TGF-β signaling, activated stromal fibroblasts, and cysteine cathepsins B and L drive the invasive growth of human melanoma cells. Am J Pathol 181, 2202–2216 (2012). [DOI] [PubMed] [Google Scholar]
- 237.Barker HE, Bird D, Lang G. & Erler JT Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res 11, 1425–1436 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wei Y. et al. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J Clin Invest 127, 3675–3688 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- 240.Karagiannis GS et al. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res 10, 1403–1418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Monteran L. & Erez N. The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol 10, 1835 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ozdemir BC et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fereres S, Hatori R, Hatori M. & Kornberg TB Cytoneme-mediated signaling essential for tumorigenesis. PLoS Genet 15, e1008415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kornberg TB Distributing signaling proteins in space and time: the province of cytonemes. Curr Opin Genet Dev 45, 22–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bhowmick NA et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851 (2004). [DOI] [PubMed] [Google Scholar]
- 246.Kim SJ et al. Autoinduction of transforming growth factor β1 is mediated by the AP-1 complex. Mol Cell Biol 10, 1492–1497 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Flanders KC et al. Quantitation of TGF-β proteins in mouse tissues shows reciprocal changes in TGF-β1 and TGF-β3 in normal vs neoplastic mammary epithelium. Oncotarget 7, 38164–38179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Acharya PS et al. Fibroblast migration is mediated by CD44-dependent TGF β activation. J Cell Sci 121, 1393–1402 (2008). [DOI] [PubMed] [Google Scholar]
- 249.Postlethwaite AE, Keski-Oja J, Moses HL & Kang AH Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor β. J Exp Med 165, 251–256 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Huang H. et al. Targeting TGFβR2-mutant tumors exposes vulnerabilities to stromal TGFβ blockade in pancreatic cancer. EMBO Mol Med 11, e10515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Biffi G. et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 9, 282–301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Foster DS, Jones RE, Ransom RC, Longaker MT & Norton JA The evolving relationship of wound healing and tumor stroma. JCI Insight 3, 99911 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Alba-Castellon L. et al. Snail1-dependent activation of cancer-associated fibroblast controls epithelial tumor cell invasion and metastasis. Cancer Res 76, 6205–6217 (2016). [DOI] [PubMed] [Google Scholar]
- 254.Baulida J. Epithelial-to-mesenchymal transition transcription factors in cancer-associated fibroblasts. Mol Oncol 11, 847–859 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kalluri R. & Weinberg RA The basics of epithelial-mesenchymal transition. J Clin Invest 119, 1420–1428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yu W. et al. The endothelial-mesenchymal transition (EndMT) and tissue regeneration. Curr Stem Cell Res Ther 9, 196–204 (2014). [DOI] [PubMed] [Google Scholar]
- 257.Qi W. et al. TGF-β1 induces IL-8 and MCP-1 through a connective tissue growth factor-independent pathway. Am J Physiol Renal Physiol 290, F703–709 (2006). [DOI] [PubMed] [Google Scholar]
- 258.Shi J. et al. Targeted blockade of TGF-β and IL-6/JAK2/STAT3 pathways inhibits lung cancer growth promoted by bone marrow-derived myofibroblasts. Sci Rep 7, 8660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Matsumura T. et al. Regulation of transforming growth factor-β-dependent cyclooxygenase-2 expression in fibroblasts. J Biol Chem 284, 35861–35871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yu PF et al. Downregulation of CXCL12 in mesenchymal stromal cells by TGFβ promotes breast cancer metastasis. Oncogene 36, 840–849 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ahirwar DK et al. Fibroblast-derived CXCL12 promotes breast cancer metastasis by facilitating tumor cell intravasation. Oncogene 37, 4428–4442 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pertovaara L. et al. Vascular endothelial growth factor is induced in response to transforming growth factor-β in fibroblastic and epithelial cells. J Biol Chem 269, 6271–6274 (1994). [PubMed] [Google Scholar]
- 263.Folkman J, Watson K, Ingber D. & Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339, 58–61(1989). [DOI] [PubMed] [Google Scholar]
- 264.Larrivee B. et al. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell 22, 489–500 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Goumans MJ et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol Cell 12, 817–828 (2003). [DOI] [PubMed] [Google Scholar]
- 266.Goumans MJ, Lebrin F. & Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-β receptor signaling pathways. Trends Cardiovasc Med 13, 301–307 (2003). [DOI] [PubMed] [Google Scholar]
- 267.Madri JA, Pratt BM & Tucker AM Phenotypic modulation of endothelial cells by transforming growth factor-β depends upon the composition and organization of the extracellular matrix. J Cell Biol 106, 1375–1384 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hayashi T. et al. Transforming growth factor β receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin Cancer Res 10, 7540–7546 (2004). [DOI] [PubMed] [Google Scholar]
- 269.Batlle R. et al. Regulation of tumor angiogenesis and mesenchymal-endothelial transition by p38α through TGF-β and JNK signaling. Nat Commun 10, 3071 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yang EY & Moses HL Transforming growth factor β1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol 111, 731–741 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lin H. et al. High immunohistochemical expression of TGF-β1 predicts a poor prognosis in cervical cancer patients who harbor enriched endoglin microvessel density. Int J Gynecol Pathol 31, 482–489 (2012). [DOI] [PubMed] [Google Scholar]
- 272.Li GC et al. Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor β1. Oncol Lett 11, 1089–1094 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Goumans MJ & ten Dijke P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb Perspect Biol 10, a022210 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sato Y, Tsuboi R, Lyons R, Moses H. & Rifkin DB Characterization of the activation of latent TGF-β by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol 111, 757–763 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Walshe TE et al. TGF-β is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One 4, e5149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.He S, Li M, Ma X, Lin J. & Li D. CD4+CD25+Foxp3+ regulatory T cells protect the proinflammatory activation of human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol 30, 2621–2630 (2010). [DOI] [PubMed] [Google Scholar]
- 277.Chen PY et al. Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nat Metab 1, 912–926 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Scavelli C, Vacca A, Di Pietro G, Dammacco F. & Ribatti D. Crosstalk between angiogenesis and lymphangiogenesis in tumor progression. Leukemia 18, 1054–1058 (2004). [DOI] [PubMed] [Google Scholar]
- 279.Stacker SA, Achen MG, Jussila L, Baldwin ME & Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2, 573–583 (2002). [DOI] [PubMed] [Google Scholar]
- 280.Niessen K, Zhang G, Ridgway JB, Chen H. & Yan M. ALK1 signaling regulates early postnatal lymphatic vessel development. Blood 115, 1654–1661 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Seoane J. et al. TGFβ influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol 3, 400–408 (2001). [DOI] [PubMed] [Google Scholar]
- 282.Coffey RJ Jr. et al. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res 48, 1596–1602 (1988). [PubMed] [Google Scholar]
- 283.Pierce DF Jr. et al. Mammary tumor suppression by transforming growth factor β1 transgene expression. Proc Natl Acad Sci U S A 92, 4254–4258 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Arteaga CL et al. Growth stimulation of human breast cancer cells with anti-transforming growth factor β antibodies: evidence for negative autocrine regulation by transforming growth factor β. Cell Growth Differ 1, 367–374 (1990). [PubMed] [Google Scholar]
- 285.Laiho M, DeCaprio JA, Ludlow JW, Livingston DM & Massagué J. Growth inhibition by TGF-β linked to suppression of retinoblastoma protein phosphorylation. Cell 62, 175–185 (1990). [DOI] [PubMed] [Google Scholar]
- 286.Koff A, Ohtsuki M, Polyak K, Roberts JM & Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-β. Science 260, 536–539 (1993). [DOI] [PubMed] [Google Scholar]
- 287.Akhurst RJ TGF-β antagonists: why suppress a tumor suppressor? J Clin Invest 109, 1533–1536 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Arteaga CL et al. Anti-transforming growth factor (TGF)-β antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-β interactions in human breast cancer progression. J Clin Invest 92, 2569–2576 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Igarashi J. et al. Preclinical study of novel gene silencer pyrrole-imidazole polyamide targeting human TGF-β1 promoter for hypertrophic scars in a common marmoset primate model. PLoS One 10, e0125295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jachimczak P. et al. The effect of transforming growth factor-β2-specific phosphorothioate-anti-sense oligodeoxynucleotides in reversing cellular immunosuppression in malignant glioma. J Neurosurg 78, 944–951 (1993). [DOI] [PubMed] [Google Scholar]
- 291.Brandes AA et al. A phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol 18, 1146–1156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yingling JM et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-β receptor type I inhibitor. Oncotarget 9, 6659–6677 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Inman GJ et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62, 65–74 (2002). [DOI] [PubMed] [Google Scholar]
- 294.Fu K. et al. SM16, an orally active TGF-β type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler Thromb Vasc Biol 28, 665–671 (2008). [DOI] [PubMed] [Google Scholar]
- 295.Budi EH, Mamai O, Hoffman S, Akhurst RJ & Derynck R. Enhanced TGF-β signaling contributes to the insulin-induced angiogenic responses of endothelial cells. iScience 11, 474–491 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hamidi A. et al. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci Signal 10, eaal4186 (2017). [DOI] [PubMed] [Google Scholar]
- 297. https://clinicaltrials.gov. [Google Scholar]
- 298.Jung SY et al. Pharmacokinetic characteristics of vactosertib, a new activin receptor-like kinase 5 inhibitor, in patients with advanced solid tumors in a first-in-human phase 1 study. Invest New Drugs doi: 10.1007/s10637-019-00835-y (2019). [DOI] [PubMed] [Google Scholar]
- 299.Jung SY et al. Population pharmacokinetics of vactosertib, a new TGF-beta receptor type Iota inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol 85, 173–183 (2019). [DOI] [PubMed] [Google Scholar]
- 300.Morris JC et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-β (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One 9, e90353 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bedinger D. et al. Development and characterization of human monoclonal antibodies that neutralize multiple TGFβ isoforms. MAbs 8, 389–404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Laverty HG, Wakefield LM, Occleston NL, O’Kane S. & Ferguson MW TGF-β3 and cancer: a review. Cytokine Growth Factor Rev 20, 305–317 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Cohn A. et al. A phase I dose-escalation study to a predefined dose of a transforming growth factor-β1 monoclonal antibody (TβM1) in patients with metastatic cancer. Int J Oncol 45, 2221–2231 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhong Z. et al. Anti-transforming growth factor β receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin Cancer Res 16, 1191–1205 (2010). [DOI] [PubMed] [Google Scholar]
- 305.Tolcher AW et al. A phase 1 study of anti-TGFβ receptor type-II monoclonal antibody LY3022859 in patients with advanced solid tumors. Cancer Chemother Pharmacol 79, 673–680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yang YA et al. Lifetime exposure to a soluble TGF-β antagonist protects mice against metastasis without adverse side effects. J Clin Invest 109, 1607–1615 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hinck AP & O’Connor-McCourt MD Structures of TGF-β receptor complexes: implications for function and therapeutic intervention using ligand traps. Curr Pharm Biotechnol 12, 2081–2098 (2011). [DOI] [PubMed] [Google Scholar]
- 308.Lan Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med 10, eaan5488 (2018). [DOI] [PubMed] [Google Scholar]
- 309.Ravi R. et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun 9, 741 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Qin T. et al. A novel highly potent trivalent TGF-β receptor trap inhibits early-stage tumorigenesis and tumor cell invasion in murine Pten-deficient prostate glands. Oncotarget 7, 86087–86102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zwaagstra JC et al. Engineering and therapeutic application of single-chain bivalent TGF-β family traps. Mol Cancer Ther 11, 1477–1487 (2012). [DOI] [PubMed] [Google Scholar]
- 312.Knudson KM et al. M7824, a novel bifunctional anti-PD-L1/TGFβ trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology 7, e1426519 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Park CC, Zhang HJ, Yao ES, Park CJ & Bissell MJ β1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res 68, 4398–4405 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Park CC et al. β1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res 66, 1526–1535 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Van Aarsen LA et al. Antibody-mediated blockade of integrin αvβ6 inhibits tumor progression in vivo by a transforming growth factor-β-regulated mechanism. Cancer Res 68, 561–570 (2008). [DOI] [PubMed] [Google Scholar]
- 316.Miller LM, Pritchard JM, MacDonald SJF, Jamieson C. & Watson AJB Emergence of small-molecule non-RGD-mimetic inhibitors for RGD Integrins. J Med Chem 60, 3241–3251 (2017). [DOI] [PubMed] [Google Scholar]
- 317.Reed NI et al. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med 7, 288ra279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Schaub J. et al. Targeted disruption of TGF-B activation by an αvβ1 integrin inhibitor significantly reduces liver fibrosis in CCl4 mice and human NASH liver slices. J. Hepatol. 70, Suppl April 2019, e57–e58 (2019). [Google Scholar]
- 319.Dodagatta-Marri E. et al. Integrin avβ8 on T cells is responsible for suppression of anti-tumor immunity in syngeneic models and is a promising target for tumor immunotherapy. CANCER-CELL-D-20-00307 under review, Available at SSRN: https://SSRN.com/ (2020). [Google Scholar]
- 320.Liénart S. et al. Structural basis of latent TGF-β1 presentation and activation by GARP on human regulatory T cells. Science 362, 952–956 (2018). [DOI] [PubMed] [Google Scholar]
- 321.Eisenhauer EA et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 322.Hodi FS et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): Refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 36, 850–858 (2018). [DOI] [PubMed] [Google Scholar]
- 323.Seymour L. et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18, e143–e152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Subramanian G. et al. Targeting endogenous transforming growth factor β receptor signaling in SMAD4-deficient human pancreatic carcinoma cells inhibits their invasive phenotype1. Cancer Res 64, 5200–5211 (2004). [DOI] [PubMed] [Google Scholar]
- 325.Akhurst RJ & Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 11, 790–811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Arteaga CL et al. Anti-transforming growth factor (TGF)-β antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-β interactions in human breast cancer progression. J Clin Invest 92, 2569–2576 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Muraoka RS et al. Blockade of TGF-β inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest 109, 1551–1559 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Rodon J. et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs 33, 357–370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Fujiwara Y. et al. Phase 1 study of galunisertib, a TGF-β receptor I kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 76, 1143–1152 (2015). [DOI] [PubMed] [Google Scholar]
- 330.Herbertz S. et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-β signaling pathway. Drug Des Devel Ther 9, 4479–4499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Biswas T, Gu X, Yang J, Ellies LG & Sun LZ Attenuation of TGF-β signaling supports tumor progression of a mesenchymal-like mammary tumor cell line in a syngeneic murine model. Cancer Lett 346, 129–138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yang Y. et al. The outcome of TGFβ antagonism in metastatic breast cancer models in vivo reflects a complex balance between tumor-suppressive and proprogression activities of TGFβ. Clin Cancer Res 26, 643–656 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Bragado P. et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat Cell Biol 15, 1351–1361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yumoto K. et al. Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow. Sci Rep 6, 36520 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Jiang J. et al. PRRX1 regulates cellular phenotype plasticity and dormancy of head and neck squamous cell carcinoma through miR-642b-3p. Neoplasia 21, 216–229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.C Connolly EC et al. Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long term TβRI/II kinase inhibition with LY2109761. Cancer Res 71, 1–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ciardiello D. et al. Inhibition of TGFb in colorectal cancer cells is associated with compensatory activation of AXL and p38 MAPK signaling pathways. in Proceedings of the American Association for Cancer Research Annual Meeting Abstract nr 2627 (AACR; Philadelphia (PA): ). doi: 10.1158/1538-7445.AM2019-2627 (2019). [DOI] [Google Scholar]
- 338.Lacouture ME et al. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor β by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol Immunother 64, 437–446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Strauss J. et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res 24, 1287–1295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Anderton MJ et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol 39, 916–924 (2011). [DOI] [PubMed] [Google Scholar]
- 341.Kovacs RJ et al. Cardiac safety of TGF-β receptor I kinase inhibitor LY2157299 monohydrate in cancer patients in a first-in-human dose study. Cardiovasc Toxicol 15, 309–323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Mitra MS et al. A potent pan-TGFβ neutralizing monoclonal antibody elicits cardiovascular toxicity in mice and Cynomolgus monkeys. Toxicol Sci doi: 10.1093/toxsci/kfaa024 (2020). [DOI] [PubMed] [Google Scholar]
- 343.Barcellos-Hoff MH & Dix TA Redox-mediated activation of latent transforming growth factor-β1. Mol Endocrinol 10, 1077–1083 (1996). [DOI] [PubMed] [Google Scholar]
- 344.Martin M. et al. Coactivation of AP-1 activity and TGF-β1 gene expression in the stress response of normal skin cells to ionizing radiation. Oncogene 15, 981–989 (1997). [DOI] [PubMed] [Google Scholar]
- 345.Barcellos-Hoff MH Radiation-induced transforming growth factor β and subsequent extracellular matrix reorganization in murine mammary gland. Cancer Res 53, 3880–3886 (1993). [PubMed] [Google Scholar]
- 346.Vanpouille-Box C. et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res 75, 2232–2242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Formenti SC et al. Focal irradiation and systemic TGFβ blockade in metastatic breast Cancer. Clin Cancer Res 24, 2493–2504 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Bhola NE et al. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest 123, 1348–1358 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Capper D. et al. Biomarker and histopathology evaluation of patients with recurrent glioblastoma treated with Galunisertib, Lomustine, or the combination of Galunisertib and Lomustine. Int J Mol Sci 18, 995 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ungerleider N, Han C, Zhang J, Yao L. & Wu T. TGFβ signaling confers sorafenib resistance via induction of multiple RTKs in hepatocellular carcinoma cells. Mol Carcinog 56, 1302–1311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kelley RK et al. A phase 2 study of Galunisertib (TGF-β1 receptor type I inhibitor) and Sorafenib in patients with advanced hepatocellular carcinoma. Clin Transl Gastroenterol 10, e00056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Melisi D. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 119, 1208–1214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Holmgaard RB et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer 6, 47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Jiao S. et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177–1190 (2019). [DOI] [PubMed] [Google Scholar]
- 355.Noman MZ et al. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology 6, e1263412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lequeux A. et al. Impact of hypoxic tumor microenvironment and tumor cell plasticity on the expression of immune checkpoints. Cancer Lett 458, 13–20 (2019). [DOI] [PubMed] [Google Scholar]
- 357.Leach DR, Krummel MF & Allison JP Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996). [DOI] [PubMed] [Google Scholar]
- 358.Wolchok JD et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 1291, 1–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wei SC et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A 116, 22699–22709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Larkin J. et al. Five-year survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med 381, 1535–1546 (2019). [DOI] [PubMed] [Google Scholar]
- 361.Hugo W. et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 165, 35–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Akhurst RJ & Padgett RW Matters of context guide future research in TGFβ superfamily signaling. Sci Signal 8, re10 (2015). [DOI] [PubMed] [Google Scholar]
- 363.Funaki S. et al. Chemotherapy enhances programmed cell death 1/ligand 1 expression via TGF-β induced epithelial mesenchymal transition in non-small cell lung cancer. Oncol Rep 38, 2277–2284 (2017). [DOI] [PubMed] [Google Scholar]
- 364.Park BV et al. TGFβ1-mediated SMAD3 enhances PD-1 expression on antigen-specific T cells in cancer. Cancer Discov 6, 1366–1381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Lind H. et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J Immunother Cancer 8, e000433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Jochems C. et al. Analyses of functions of an anti-PD-L1/TGFβR2 bispecific fusion protein (M7824). Oncotarget 8, 75217–75231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.David JM et al. A novel bifunctional anti-PD-L1/TGF-β trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology 6, e1349589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Wang D. et al. Targeting EZH2 reprograms intratumoral regulatory T cells to enhance cancer immunity. Cell Rep 23, 3262–3274 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.McClymont SA et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 186, 3918–3926 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Nemunaitis J. et al. Phase II trial of Belagenpumatucel-L, a TGF-β2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther 16, 620–624 (2009). [DOI] [PubMed] [Google Scholar]
- 371.Giaccone G. et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer 51, 2321–2329 (2015). [DOI] [PubMed] [Google Scholar]
- 372.Kim S. et al. Systemic blockade of transforming growth factor-β signaling augments the efficacy of immunogene therapy. Cancer Res 68, 10247–10256 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Muul LM, Director EP, Hyatt CL & Rosenberg SA Large scale production of human lymphokine activated killer cells for use in adoptive immunotherapy. J Immunol Methods 88, 265–275 (1986). [DOI] [PubMed] [Google Scholar]
- 374.Wallace A. et al. Transforming growth factor-β receptor blockade augments the effectiveness of adoptive T-cell therapy of established solid cancers. Clin Cancer Res 14, 3966–3974 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Hwu P. & Rosenberg SA The genetic modification of T cells for cancer therapy: an overview of laboratory and clinical trials. Cancer Detect Prev 18, 43–50 (1994). [PubMed] [Google Scholar]
- 376.Grupp SA et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New Engl J Med 368, 1509–1518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Foster AE et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-β receptor. J Immunother 31, 500–505 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Chang ZL et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol 14, 317–324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Hou AJ, Chang ZL, Lorenzini MH, Zah E. & Chen YY TGF-β-responsive CAR-T cells promote anti-tumor immune function. Bioeng Transl Med 3, 75–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Huang S. et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell 151, 937–950(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Panda M. & Biswal BK Cell signaling and cancer: a mechanistic insight into drug resistance. Mol Biol Rep 46, 5645–5659 (2019). [DOI] [PubMed] [Google Scholar]
- 382.Gulley JL et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst 109 djw261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Autio KA, Boni V, Humphrey RW & Naing A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin Cancer Res 26, 1–6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Hamid O, Hoffner B, Gasal E, Hong J. & Carvajal RD Oncolytic immunotherapy: unlocking the potential of viruses to help target cancer. Cancer Immunol Immunother 66, 1249–1264 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kim SK et al. An engineered transforming growth factor β (TGF-β) monomer that functions as a dominant negative to block TGF-β signaling. J Biol Chem 292, 7173–7188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Chanier T. & Chames P. Nanobody engineering: toward next generation immunotherapies and immunoimaging of cancer. Antibodies 8, 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 32, 185–203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Lechner A. et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 8, 44418–44433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Van Allen EM et al. Long-term benefit of PD-L1 blockade in lung cancer associated with JAK3 activation. Cancer Immunol Res 3, 855–863 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Trefny MP et al. A variant of a killer cell immunoglobulin-like receptor is associated with resistance to PD-1 blockade in lung cancer. Clin Cancer Res 25, 3026–3034 (2019). [DOI] [PubMed] [Google Scholar]
- 391.Valle L. et al. Germline allele-specific expression of TGFBR1 confers an increased risk of colorectal cancer. Science 321, 1361–1365 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kawasaki K. et al. Genetic variants of Adam17 differentially regulate TGFβ signaling to modify vascular pathology in mice and humans. Proc Natl Acad Sci USA 111, 7723–7728 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Routy B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 394.Jin Y. et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol 14, 1378–1389 (2019). [DOI] [PubMed] [Google Scholar]
- 395.Dubin K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 7, 10391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Pasche B. et al. TGFBR1*6A and cancer: a meta-analysis of 12 case-control studies. J Clin Oncol 22, 756–758 (2004). [DOI] [PubMed] [Google Scholar]
- 397.Izad M. et al. Cytokines genes polymorphisms and risk of multiple sclerosis. Am J Med Sci 339, 327–331 (2010). [DOI] [PubMed] [Google Scholar]
- 398.Bonyadi M. et al. Mapping of a major genetic modifier of embryonic lethality in TGF β1 knockout mice. Nat Genet 15, 207–211 (1997). [DOI] [PubMed] [Google Scholar]
- 399.Benzinou M. et al. Mouse and human strategies identify PTPN14 as a modifier of angiogenesis and hereditary haemorrhagic telangiectasia. Nat Commun 3, 616 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Freimuth J. et al. Epistatic interactions between Tgfb1 and genetic loci, Tgfbm2 and Tgfbm3, determine susceptibility to an asthmatic stimulus. Proc Natl Acad Sci U S A 109, 18042–18047 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Engle SJ et al. Elimination of colon cancer in germ-free transforming growth factor β1-deficient mice. Cancer Res 62, 6362–6366 (2002). [PubMed] [Google Scholar]
- 402.Gopalakrishnan V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Santini V. et al. Phase II study of the ALK5 inhibitor Galunisertib in very low-, low-, and intermediate-risk myelodysplastic syndromes. Clin Cancer Res 25, 6976–6985 (2019). [DOI] [PubMed] [Google Scholar]
- 404.Stevenson JP et al. Immunological effects of the TGFβ-blocking antibody GC1008 in malignant pleural mesothelioma patients. Oncoimmunology 2, e26218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Formenti SC et al. Baseline T cell dysfunction by single cell network profiling in metastatic breast cancer patients. J Immunother Cancer 7, 177 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Melisi D. et al. TGFβ receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol 83, 975–991 (2019). [DOI] [PubMed] [Google Scholar]
- 407.Gueorguieva I. et al. Population pharmacokinetics and exposure-overall survival analysis of the transforming growth factor-β inhibitor galunisertib in patients with pancreatic cancer. Cancer Chemother Pharmacol 84, 1003–1015 (2019). [DOI] [PubMed] [Google Scholar]
- 408.Rodon J. et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res 21, 553–560 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Ikeda M. et al. A phase 1b study of transforming growth factor-β receptor I inhibitor galunisertib in combination with sorafenib in Japanese patients with unresectable hepatocellular carcinoma. Invest New Drugs 37, 118–126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ikeda M. et al. Phase 1b study of galunisertib in combination with gemcitabine in Japanese patients with metastatic or locally advanced pancreatic cancer. Cancer Chemother Pharmacol 79, 1169–1177 (2017). [DOI] [PubMed] [Google Scholar]
- 411.Pei H. et al. LY3200882, a novel, highly selective TGFβRI small molecule inhibitor. Proceedings of the American Association for Cancer Research Annual Meeting Abstract 955 Cancer Res 77, doi: 10.1158/1538-7445.AM2017-955 (2017). [DOI] [Google Scholar]
- 412.Keedy VL et al. Association of TGF-β responsive signature with anti-tumor effect of vactosertib, a potent, oral TGF-β receptor type I (TGFBRI) inhibitor in patients with advanced solid tumors. J Clin Oncol 36, 3031–3031 (2018).30199311 [Google Scholar]
- 413.Yap T. et al. AVID200, first-in-class TGF-b1 and -b3 selective inhibitor: results of a phase 1 monotherapy dose escalation study in solid tumors and evidence of targeted engagement in patients. in SITC 34th Annual Meeting Abstract P856 (2019). [Google Scholar]


