Abstract
Background
We evaluated the potential role of birth characteristics in the etiology of early-onset meningioma.
Methods
Leveraging a population-based linkage of California birth records (from 1978 to 2015) and cancer registry data (from 1988 to 2015), we identified 362 nonmalignant meningioma cases aged 0–37 years and selected 18 100 controls matched on year of birth. Cases and controls were compared with regard to birth characteristics, with adjusted odds ratios (ORs) and 95% confidence intervals (CIs) estimated from unconditional multivariable logistic regression models. We also conducted stratified analyses by race/ethnicity and age.
Results
Female sex (compared to male: OR = 1.43, 95% CI: 1.16 to 1.79; P < .01) and Black race (compared to White: OR = 1.46, 95% CI: 1.02 to 2.07; P = .04) were associated with higher risk of meningioma. Higher birth order (OR = 0.90, 95% CI: 0.81 to 0.99 per additional birth position; P = .04) was associated with a lower risk. No significant associations were observed between birthweight, gestational age, delivery mode, maternal age, or maternal education and meningioma risk. In the non-Latino White subgroup, higher birthweight was associated with a higher risk of meningioma (OR = 1.20, 95% CI: 1.02 to 1.41 per 500 grams; P = .03), but this was not recapitulated in the Latino subgroup. In age-stratified analyses, female sex was a risk factor for those diagnosed at the age of 20–37 years but not among younger individuals.
Conclusions
In this large population-based study less prone to selection and recall bias, higher birth order was associated with a reduced risk of early-onset meningioma, while female sex and Black race were linked to an increased risk. There were also indications of differential associations by race/ethnicity and age of diagnosis.
Keywords: birth characteristics, birth order, birthweight, epidemiology, meningioma
Key Points.
The association between early-life exposures and risk of nonmalignant meningioma has not been explored.
Higher birth order was associated with a lower risk of meningioma.
Higher birth weight was associated with a higher risk of meningioma among non-Latino participants.
Importance of the Study.
Identification of risk factors for brain tumors has the potential to improve risk stratification and elucidate underlying mechanisms of pathogenesis. The association between early-life exposures and risk of meningioma has not been explored in prior studies. Here, we used a population-based data source less subject to selection and recall bias to examine associations between birth characteristics and risk of meningioma. We demonstrated inverse associations between birth order and meningioma risk. In stratified analyses, we also demonstrated positive associations between birthweight and meningioma risk among non-Latino White patients.
Meningioma is the most commonly diagnosed intracranial tumor, accounting for approximately one-third of all primary brain and central nervous system tumors.1–3 Although most often histologically benign, they can cause substantial morbidity due to their location adjacent to important neurovascular structures.4 In general, the incidence of meningioma increases with age, but diagnosis during young adulthood remains relatively common, particularly among women.2
The epidemiology of meningioma has been investigated in a variety of prospective and retrospective cohort and case-control studies, with convincing evidence for inherited susceptibility as well as for some environmental and behavioral risk factors, such as ionizing radiation.5 Women have been consistently shown to be approximately twice at risk developing of meningioma as men across the lifespan, suggesting the possibility that sex hormones may also play a role in pathogenesis.6
To date, few studies have examined early-life exposures, such as birth characteristics or parental sociodemographic features, and risk of meningioma. Early-life exposures are of interest, given that they have been indicated in the etiology of other tumors, such as pituitary tumors, sarcoma, and lymphoma.7–9 There has also been strong evidence for the developmental origin of breast cancer and other hormone-related cancers, suggesting that they begin in utero.10 Using the California Linkage Study of Early-onset Cancers (CALSEC), which links California statewide birth records with all cancer diagnoses reported to the statewide cancer registry, we examined potential associations between birth characteristics and risk of early-onset, nonmalignant meningioma.
Methods
Data Source
Detailed methods related to CALSEC data collection have been published elsewhere.8,11,12 In brief, statewide information on cancer diagnosis from 1988 to 2015 from the California Cancer Registry (CCR) were linked to California birth records maintained by the Vital Statistics Advisory Committee of the California Department of Public Health from 1978 to 2015. Reporting of birth and cancer diagnosis are both mandated by law, and are considered complete at the population level. Beginning in 2001, the CCR records incidence of nonmalignant tumors. From CALSEC, we identified cases who were born during 1978–2015 and diagnosed with first primary and incident meningioma during 1988–2015, at the age of 0–37 years, as well as 50 times as many controls who were frequency matched to cases on year of birth. For all participants, we extracted data on gestational age, birth weight, mode of delivery, birth plurality, and birth order. Parental sociodemographic information was also extracted, including parental race and ethnicity, maternal age at delivery, maternal education, and maternal nativity. The study protocol was approved by the Institutional Review Boards at the California Health and Human Services Agency, University of California, Berkeley (Berkeley, CA), and Yale University (New Haven, CT).
Outcomes
Diagnoses of nonmalignant brain tumors were recorded in CCR beginning in 2001. All patients diagnosed with nonmalignant meningioma in the CCR were included as cases according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), codes, using site “Any” and codes 9530/0,1, 9531/0, 9532/0, 9533/0, 9534/0, 9537/0, 9538/0, 9539/1. These ICD-O-3 codes specify nonmalignant meningioma of various types, including fibrous, angiomatous, psammomatous, and others. We excluded patients who had diagnoses of other benign or malignant tumors prior to the diagnosis of meningioma, out of concern that treatment for previous tumors might lead to a higher risk of second tumors. We identified a total of 362 cases who were born during 1978–2015 and diagnosed with meningioma during 2001–2015, to which 18 100 controls were matched on the birth year with a 50:1 ratio. The controls were randomly selected from statewide birth records, and none of the controls had been diagnosed with any cancer per CCR record at the time of linkage.
Statistical Analysis
Case and control characteristics were presented with frequencies and percentages, and Pearson’s χ 2 test were used to compare groups. We computed odds ratio (OR) estimates and 95% confidence intervals (CIs) using unconditional multivariable logistic regression models that adjusted for year of birth (ie, the matching variable). Such models provide valid and more efficient estimates of risk than conditional models in matched case-control studies.13 The multivariable models additionally included sex at birth, maternal race/ethnicity (Latino/Hispanic, non-Latino White, non-Latino Black, non-Latino Asian/Pacific Islander, and other), birthweight (grams), gestational age (weeks), birth plurality (singleton vs multiple), birth order (first, second, third or higher), mode of delivery (Cesarean vs vaginal), maternal age (years), maternal education (years), and maternal nativity (US vs other). Race/ethnicity stratification used in this study reflects the categorization allowed by the categories used by the federal Office of Management and Budget data collection available from the period of the study.14 Additional models were constructed stratified by ethnicity (non-Latino White vs Latino) and age (≤19, 20–37 years). All analyses were performed using SAS (version 9.4, SAS Institute). All tests were two-sided, and the threshold for statistical significance was 0.05.
Results
In our study population, the largest subgroup by race/ethnicity were non-Latino White individuals (43%), followed by Latinos (35%). Cases were more likely than controls to be female and less likely to have foreign-born mothers (Table 1).
Table 1.
Characteristics of Meningioma Cases and Controls in the California Linkage Study of Early-Onset Cancers
| Case (n = 362) | Control (n = 18 100) | P | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age at diagnosis (years) | 0–4 | 7 | 1.9 | |||
| 5–9 | 8 | 2.2 | ||||
| 10–14 | 19 | 5.2 | ||||
| 15–19 | 48 | 13.3 | ||||
| 20–24 | 87 | 24.0 | ||||
| 25–29 | 98 | 27.1 | ||||
| 30–34 | 79 | 21.8 | ||||
| 35–36 | 16 | 4.4 | ||||
| Sex | Female | 207 | 57.2 | 8870 | 49.0 | <.01 |
| Male | 155 | 42.8 | 9230 | 51.0 | ||
| Race/ethnicity | Non-Latino White | 157 | 43.4 | 7772 | 42.9 | .08 |
| Non-Latino Black | 44 | 12.2 | 1588 | 8.8 | ||
| Latino | 128 | 35.4 | 6834 | 37.8 | ||
| Non-Latino Asian | 24 | 6.6 | 1596 | 8.8 | ||
| Other | 9 | 2.5 | 310 | 1.7 | ||
| Birthweight (grams) | 250–2499 | 22 | 6.1 | 1063 | 5.9 | .65 |
| 2500–2999 | 49 | 13.5 | 2724 | 15.0 | ||
| 3000–3499 | 132 | 36.5 | 6940 | 38.3 | ||
| 3500–3999 | 110 | 30.4 | 5314 | 29.4 | ||
| 4000+ | 49 | 13.5 | 2059 | 11.4 | ||
| Gestational age (weeks) | 22–36 | 33 | 9.1 | 1564 | 8.6 | .91 |
| 37–41 | 253 | 69.9 | 12 825 | 70.9 | ||
| 42–44 | 41 | 11.3 | 1975 | 10.9 | ||
| Unknown | 35 | 9.7 | 1736 | 9.6 | ||
| Birth plurality | Singleton | 359 | 99.2 | 17 713 | 97.9 | .09 |
| Multiple | 3 | 0.8 | 387 | 2.1 | ||
| Birth order | 1st | 165 | 45.6 | 7605 | 42.0 | .39 |
| 2nd | 109 | 30.1 | 5729 | 31.7 | ||
| 3rd and higher | 88 | 24.3 | 4766 | 26.3 | ||
| Mode of delivery | Vaginal | 279 | 77.1 | 14 472 | 80.0 | .18 |
| C-section | 83 | 22.9 | 3628 | 20.0 | ||
| Year of birth | 1978–1982 | 138 | 38.1 | 6900 | 38.1 | 1.00 |
| 1983–1987 | 103 | 28.5 | 5150 | 28.5 | ||
| 1988–1992 | 60 | 16.6 | 3000 | 16.6 | ||
| 1993–2013 | 61 | 16.9 | 3050 | 16.9 | ||
| Maternal age (years) | <20 | 46 | 12.7 | 2240 | 12.4 | .57 |
| 20–24 | 104 | 28.7 | 5322 | 29.4 | ||
| 25–29 | 115 | 31.8 | 5359 | 29.6 | ||
| 30–34 | 60 | 16.6 | 3552 | 19.6 | ||
| >=35 | 37 | 10.2 | 1627 | 9.0 | ||
| Maternal education | Up to 8 years | 14 | 3.9 | 954 | 5.3 | .68 |
| 9–11 years | 22 | 6.1 | 1178 | 6.5 | ||
| 12 years | 44 | 12.2 | 2035 | 11.2 | ||
| 13–15 years | 27 | 7.5 | 1315 | 7.3 | ||
| 16 or more years | 25 | 6.9 | 1067 | 5.9 | ||
| Unknown | 230 | 63.5 | 11 551 | 63.8 | ||
| Mother’s place of birth | US | 259 | 71.5 | 11 764 | 65.0 | <.01 |
| Foreign | 103 | 28.5 | 6336 | 35.0 |
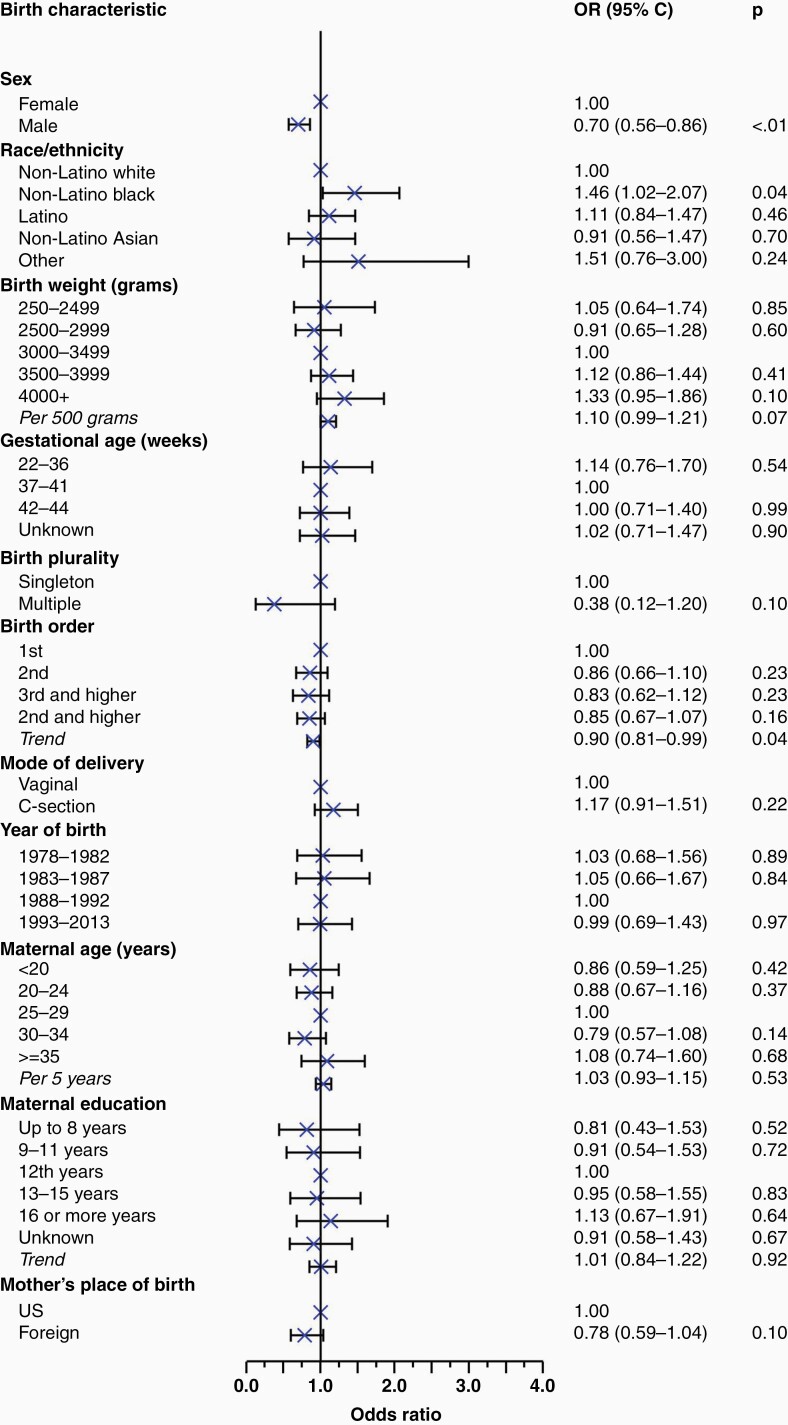
Based on results from a multivariable logistic regression model, female sex (compared to male: OR = 1.43, 95% CI: 1.16 to 1.79; P < .01) and Black race (compared to White: OR = 1.46, 95% CI: 1.02 to 2.07; P = .04) were associated with a higher risk of meningioma. Higher birth order (OR = 0.90, 95% CI: 0.81 to 0.99 per additional birth position; P = .04) was associated with lower meningioma risk. No significant associations were observed between birthweight, gestational age, mode of delivery, maternal age, or maternal education and risk of meningioma (Figure 1). A nonsignificant inverse association was observed between multiplicity and meningioma risk (OR = 0.38, 95% CI: 0.12 to 1.20, P = .10 comparing multiple births to singletons).
Figure 1.
Forest plot demonstrating the odds ratios resulting from a multivariable logistic regression model examining the association between various birth characteristics and incidence of meningioma. All variables were included as categorical variables in the model for mutual adjustment, with P-trends calculated to assess semicontinuous associations between characteristics and meningioma incidence.
When stratified by race/ethnicity (Supplementary Tables 1 and 2), power was significantly reduced. Among non-Latino White participants, there were 157 cases. Higher birthweight was associated with a higher risk of meningioma (OR = 1.20, 95% CI: 1.02 to 1.41 per 500 grams; P = .03), as was low maternal education (OR = 5.15, 95% CI: 1.29 to 20.45; P = .02). These observations were not recapitulated in the subgroup of 128 Latino cases and 6400 Latino controls, but the statistical comparison was limited by small numbers in the Latino subgroup.
Stratified analyses by age at meningioma diagnosis (≤19 vs ≥20 years, Supplementary Tables 3 and 4) demonstrated that female sex was not a risk factor for pediatric meningioma (OR = 1.05, 95% CI: 0.68 to 1.66; P = .81). The observed disparity comparing Black to non-Latino White race was smaller and not statistically significant among those who are aged ≤ 19 years (OR = 0.97, 95% CI: 0.41 to 2.27, P = .94), but remained statistically significant among those who are ≥ 20 years (OR = 1.62, 95% CI: 1.10 to 2.39, P = .04).
Discussion
This study examines potential associations between birth characteristics, parental sociodemographic features, and risk of early-onset meningioma, the most common primary intracranial tumor. Using population-based data that are less prone to selection or recall bias, we identified an inverse association between birth order and risk of meningioma, and a positive association between Black race and meningioma. Among non-Latino White individuals, higher birthweight was also associated with a higher risk of meningioma. Stratification by age in this relatively young cohort of patients with meningioma further demonstrated that disparities in incidence by sex and race were not present in those who are aged ≤ 19 years.
Prior work examining risk factors for meningioma has similarly identified disparities in incidence by race/ethnicity, with higher incidence among Black individuals compared to those of other racial and ethnic backgrounds.3 High-dose radiation and radiation from dental X-rays have both been established as risk factors, as well as higher body mass index and a variety of familial cancer syndromes, such as neurofibromatosis type 2.5,6,15 Additionally, atopic diseases such as allergies, asthma, and eczema may play a protective role, perhaps through heightened immune surveillance.16–18 Although investigated extensively, head injury, alcohol use, and cell phone exposure are likely not risk factors for meningioma based on the current literature.6 It is important to note that most previous studies included predominantly older patients. Our study is the first that focused on early-onset meningioma.
Despite restriction to early-onset meningioma (ie, those diagnosed at 0–37 years), we nevertheless demonstrated similar disparities in incidence by race as those reported elsewhere in the literature. Specifically, risk of early-onset meningioma was about 50% higher in Black compared to non-Latino White individuals, driven solely by differential risk in those older than 20 years, though tests for statistical interaction were limited by small subgroups. Therefore, if driven primarily by differential access to care or neuroimaging, our data suggest that these differences are not present during childhood, given the lack of differences by race among those who are younger than 20 years. If driven by exposure to an as-yet unidentified risk factor, these data suggest that such a risk factor would have an effect solely among young adults and older, which may inform future studies. Similarly, our study is in line with others that describe the disparity in meningioma incidence by sex as a post-pubertal phenomenon, with no significant difference in incidence by sex for those younger than 20 years, but 30% lower risk among men compared to women in those aged 20 years or older.
Novel findings of the current study include a 10% reduced risk of meningioma for each additional increase in birth order, meaning that compared to firstborns, later-born children were at a lower risk of early-onset meningioma. Although not specified as an a priori hypothesis given the exploratory nature of this analysis, similar findings have been identified for early-onset synovial sarcoma,8 early-onset non-Hodgkin lymphoma,9 and pediatric thyroid cancer.11 In general, birth order is not thought to be a causal risk factor for tumor development, but rather a proxy for other factors that may influence tumorigenesis. Compared to first-borns, later-born children have a higher degree of nutrition while in utero due to more efficient placentation.19 As a result of this overall in utero nutritional restriction, first-born children tend to have reduced insulin sensitivity at birth and greater concentrations of IGF-1, which may play a role in tumorigenesis.20 Firstborns are also taller on average, which itself may be a risk marker for meningioma, possibly through a similar association with circulating growth factors.21
In addition, later birth order is associated with a higher amount of exposure to infectious agents on average (through the earlier-born children, ie, the older siblings), which may affect the development of the immune system and conditions that depend on it, such as allergy and atopic disease.17 Given that these conditions themselves may have a protective association with meningioma and are likely more common in first-born than in later-born children, it is less likely that this underlying mechanism explains the observed finding for meningioma.6,16
Finally, levels of circulating estrogen are higher in first-borns than in later borns.22 This is particularly of interest for meningioma, where a consistently higher risk in women compared to men during adulthood has long suggested the possibility that circulating estrogens/progesterones may play a causal role. Some reports have even identified regression of meningioma after cessation of exogenous hormones, but in population studies, the association remains less clear.23 Some case-control studies have identified relatively strong positive associations between the use of exogenous hormones and incidence of meningioma,24–26 while others have not.27 Overall, there is a suggestive positive association with menopausal hormone therapy and more limited data for oral contraceptives.2 In our data, some observations may be related to estrogen exposure including the 5-fold risk for meningioma in less educated non-Latino White individuals (<8 years compared to 12 years of education), given known associations between high body mass index and low education levels in Californians.28 Estrogens and early hormonal risk factors are associated with body mass index among adolescents and young adults,29 which may accelerate development in girls with delays in boys, mirroring the sex-specific meningioma incidence trends that appear after puberty and extend through the reproductive years.2
Interestingly, despite relatively strong findings demonstrating an association between higher adult body mass index and later incidence of meningioma, we found a statistically significant positive association between birthweight, which is itself associated with adult body mass index, and meningioma only among non-Latino White individuals, with the noted limitation that the Latino subgroup was small enough to prohibit adequate statistical comparison.15,24,27
This study is distinguished by its population-based design, and conceptually it is a nested case-control study within the California birth cohort. The linkage covered all births and cancer diagnoses in California, the most populous and racially/ethnically diverse state in the United States, over a long period of time. No cases or controls had to be traced or consented to for participation in this study, minimizing selection bias. All data on birth characteristics are retrieved from existing birth records that were collected before meningioma diagnosis, eliminating recall bias. In addition, data on race/ethnicity and age at diagnosis allowed for stratified analyses to delineate more clearly various associations of interest. Despite this, limitations of our study include the younger age of participants, with the oldest being only 37 years. Given that meningioma is more common in older age, it is possible that the findings reported here may not be generalizable. In addition, because this was a hypothesis-generating study, we conducted statistical tests on several different strata, making it possible that some of the statistically significant results could be false positives. For validation, future studies should aim to examine these same factors, particularly if data on incidence and risk factors are available across the entire lifespan.
Conclusion
In this large population-based study not prone to selection and recall bias, higher birth order was associated with a reduced risk of early-onset meningioma, while female sex and Black race were linked to an increased risk. There were also indications of differential associations by race/ethnicity and age of diagnosis. Future studies with a large number of early-onset cases may help shed light on the possible underlying mechanisms.
Supplementary Material
Contributor Information
David J Cote, Department of Neurosurgery, Keck School of Medicine of the University of Southern California, Los Angeles, California, USA; Center for Genetic Epidemiology, Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, California, USA.
Rong Wang, Department of Chronic Disease Epidemiology, Yale School of Public Health, New Haven, Connecticut, USA.
Libby M Morimoto, Department of Epidemiology, School of Public Health, University of California, Berkeley, California, USA.
Catherine Metayer, Department of Epidemiology, School of Public Health, University of California, Berkeley, California, USA.
Jessica Stempel, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Gabriel Zada, Department of Neurosurgery, Keck School of Medicine of the University of Southern California, Los Angeles, California, USA.
Xiaomei Ma, Department of Chronic Disease Epidemiology, Yale School of Public Health, New Haven, Connecticut, USA.
Joseph L Wiemels, Center for Genetic Epidemiology, Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, California, USA.
Funding
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Instituteʼs Surveillance, Epidemiology, and End Results Program (contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute); and the Centers for Disease Control and Preventionʼs National Program of Cancer Registries (agreement U58DP003862-01 awarded to the California Department of Public Health).
Conflict of Interest
The authors have nothing to disclose.
Author Contributions
Conceptualization (All authors), Methodology (R.W., L.M.M., C.M., X.M., J.L.W.), Formal Analysis (D.J.C., R.W.), Writing—Original Draft (D.J.C.), Writing—Review and Editing (All authors), Supervision (X.M., J.L.W.). The work reported in the paper has been performed by the authors, unless clearly specified in the text.
References
- 1. Ostrom QT, Adel Fahmideh M, Cote DJ, et al. . Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019;21(11):1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021;23(Suppl 3):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brastianos PK, Galanis E, Butowski N, et al. . Advances in multidisciplinary therapy for meningiomas. Neuro Oncol. 2019;21(Suppl 1):i18–i31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braganza MZ, Kitahara CM, Berrington de González A, et al. . Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14(11):1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh KM. Epidemiology of meningiomas. Handb Clin Neurol. 2020;169:3–15. [DOI] [PubMed] [Google Scholar]
- 7. Cote DJ, Smith TR, Kaiser UB, Laws ER, Stampfer MJ. Body habitus across the lifespan and risk of pituitary adenoma. J Clin Endocrinol Metab. 2021;106(4):e1591–e1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiemels JL, Wang R, Feng Q, et al. . Birth characteristics and risk of early-onset synovial sarcoma. Cancer Epidemiol Biomarkers Prev. 2020;29(6):1162–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dwyer KE, Wang R, Cozen W, et al. . Mode of delivery, birth characteristics, and early-onset non-hodgkin lymphoma in a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2021;30(12):2286–2293. [DOI] [PubMed] [Google Scholar]
- 10. Chiam K, Tilley WD, Butler LM, Bianco-Miotto T. The dynamic and static modification of the epigenome by hormones: a role in the developmental origin of hormone related cancers. Biochim Biophys Acta. 2009;1795(2):104–109. [DOI] [PubMed] [Google Scholar]
- 11. Deziel NC, Zhang Y, Wang R, et al. . Birth characteristics and risk of pediatric thyroid cancer: a population-based record-linkage study in California. Thyroid. 2021;31(4):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris DH, Kwong SL, Schlag R.. Research Utilizing the California Cancer Registry. California: California Department of Health Services, Cancer Surveillance Section; 2000. [Google Scholar]
- 13. Wan F, Colditz GA, Sutcliffe S. Matched versus unmatched analysis of matched case-control studies. Am J Epidemiol. 2021;190(9):1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aragones A, Hayes SL, Chen MH, González J, Gany FM. Characterization of the Hispanic or latino population in health research: a systematic review. J Immigr Minor Health. 2014;16(3):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sergentanis TN, Tsivgoulis G, Perlepe C, et al. . Obesity and risk for brain/CNS tumors, gliomas and meningiomas: a meta-analysis. PLoS One. 2015;10(9):e0136974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wiemels JL, Wrensch M, Sison JD, et al. . Reduced allergy and immunoglobulin E among adults with intracranial meningioma compared to controls. Int J Cancer. 2011;129(8):1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wigertz A, Lönn S, Schwartzbaum J, et al. . Allergic conditions and brain tumor risk. Am J Epidemiol. 2007;166(8):941–950. [DOI] [PubMed] [Google Scholar]
- 18. Turner MC, Krewski D, Armstrong BK, et al. . Allergy and brain tumors in the INTERPHONE study: pooled results from Australia, Canada, France, Israel, and New Zealand. Cancer Causes Control. 2013;24(5):949–960. [DOI] [PubMed] [Google Scholar]
- 19. Gluckman PD, Hanson MA, Hanson MA. Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med. 2004;9(5):419–425. [DOI] [PubMed] [Google Scholar]
- 20. Ayyavoo A, Savage T, Derraik JG, Hofman PL, Cutfield WS. First-born children have reduced insulin sensitivity and higher daytime blood pressure compared to later-born children. J Clin Endocrinol Metab. 2013;98(3):1248–1253. [DOI] [PubMed] [Google Scholar]
- 21. Ben-Zion Berliner M, Katz LH, Derazne E, et al. . Height as a risk factor in meningioma: a study of 2 million Israeli adolescents. BMC Cancer. 2020;20(1):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernstein L, Depue RH, Ross RK, et al. . Higher maternal levels of free estradiol in first compared to second pregnancy: early gestational differences. J Natl Cancer Inst. 1986;76(6):1035–1039. [PubMed] [Google Scholar]
- 23. Vadivelu S, Sharer L, Schulder M. Regression of multiple intracranial meningiomas after cessation of long-term progesterone agonist therapy. J Neurosurg. 2010;112(5):920–924. [DOI] [PubMed] [Google Scholar]
- 24. Jhawar BS, Fuchs CS, Colditz GA, Stampfer MJ. Sex steroid hormone exposures and risk for meningioma. J Neurosurg. 2003;99(5):848–853. [DOI] [PubMed] [Google Scholar]
- 25. Wigertz A, Lönn S, Hall P, et al. . Reproductive factors and risk of meningioma and glioma. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2663–2670. [DOI] [PubMed] [Google Scholar]
- 26. Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26(2):279–282. [DOI] [PubMed] [Google Scholar]
- 27. Benson VS, Pirie K, Green J, Casabonne D, Beral V; Million Women Study Collaborators. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008;99(1):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ettner SL, Grzywacz Joseph G, Grzywacz JG. Socioeconomic status and health among Californians: an examination of multiple pathways. Am J Public Health. 2003;93(3):441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burt Solorzano CM, McCartney Christopher R, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140(3):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.