Abstract
During Trypanosoma brucei infections, the response against the variant surface glycoprotein (VSG) of the parasite represents a major interaction between the mammalian host immune system and the parasite surface. Since immune recognition of other parasite derived factors also occurs, we examined the humoral host response against trypanosome heat shock protein 60 (HSP60), a conserved antigen with an autoimmune character. During experimental T. brucei infection in BALB/c mice, the anti-HSP60 response was induced when parasites differentiated into stumpy forms. This response was characterized by a stage-specific immunoglobulin isotype switching as well as by the induction of an autoimmune response. Specific recognition of trypanosome HSP60 was found to occur during the entire course of infection. Immunoglobulin G2a (IgG2a) and IgG2b antibodies, induced mainly in a T-cell-independent manner, were observed during the first peak of parasitemia, whereas IgG1 and IgG3 antibodies were found at the end of the infection, due to a specific T-cell-mediated response. Comparative analysis of the kinetics of anti-HSP60, anti-invariant surface glycoprotein 70 (ISG70), and anti-VSG antibody responses indicated that the three trypanosome antigens give rise to specific and independent patterns of immunoglobulin isotype switching.
African trypanosomes are extracellular parasitic protozoa that can be transmitted by the bite of the tsetse fly. They are the causative agent of human sleeping sickness and the related cattle disease Nagana. To complete their life cycle in a mammalian host and to interact with the host immune system, they have developed a number of specific adaptations. The main parasitic mechanism involved in host immune system evasion is based on a continuous antigenic variation of the glycosylphosphatidylinositol-linked major surface protein variant surface glycoprotein (VSG). A dense layer of 107 copies of identical VSG molecules forms a protective coat for the trypanosome, and a regular switch in the expression of the VSG variants prevents efficient antibody-mediated parasite elimination (6, 23, 38, 40).
Despite the existence of the VSG, other trypanosome components of a conserved nature are part of a pronounced interaction between the host and the parasite. In the present study, we demonstrate that during the course of infection, the presence of trypanosome heat shock protein 60 (HSP60) is able to cause a significant host humoral immune response with an autoimmune character.
HSPs are highly conserved molecules produced by both prokaryotic and eukaryotic cells. Their main role is to preserve cellular functions under a variety of stress conditions. In particular, for a number of parasites it has been demonstrated that induction of HSP60 could be linked to the changing environmental conditions during passage from the mammalian host to the insect vector (20). Members of the HSP60 family function as molecular chaperones. They form a group of proteins that play a major role in folding, unfolding, and translocation of polypeptides as well as the assembly and disassembly of protein complexes (15, 16). During several infectious diseases such as with Mycobacterium tuberculosis, Chlamydia trachomatis, Toxoplasma gondii, Trypanosoma cruzi, and Leishmania major, HSP60 molecules are involved in the induction of host immune responses (13, 15, 16, 26). Due to the conserved nature of the amino acid sequence, infection-induced responses to pathogen HSPs may result in a final host response with an anti-self character. Although in mammalian cells HSP molecules are usually located within the intracellular compartment, an increasing body of evidence shows that during a progressing infection HSP60-derived epitopes can be recognized by the host immune system when expressed on the surface of mammalian cells (13, 39). In general, since both host cells and pathogens respond to stress by producing elevated HSP levels, an immune system recognition of the surface-expressed HSP molecules may be one part of a more complex interplay between the host and the pathogen.
In Trypanosoma brucei, HSP60 is a 63.7-kDa protein expressed by both bloodstream and procyclic forms. It has 51.3 and 94.5% amino acid identity to the mammalian homologue P1 protein and Trypanosoma cruzi HSP60, respectively (5).
Apart from the VSG and HSP molecules, another distinct group of antigens present on the trypanosome surface consists of several members of invariant surface glycoproteins (ISGs) (14, 44). Their invariant nature makes them an interesting tool for serological analyses of the samples from the infected host. ISG70 is much less abundant than the VSG (5.1 × 104 copies/cell) but is also distributed over the entire plasma membrane (14). In contrast to the VSG, ISG70 is not attached to the surface by a glycosylphosphatidylinositol anchor, so that the release of ISG70 is related to the elimination of trypanosomes during the infection (14).
In the present study, we analyzed a recognition of both trypanosome- and host-specific HSP60 peptides. This study showed that during the course of experimental T. brucei infections the induction of an anti-self humoral response takes place. Together with a recent report about the existence of autoreactive anti-VSG antibodies, these results pointed to the fact that autoimmune responses may play an important role in the interplay between the host and the parasite (21). Moreover, the profiles of immunoglobulin (Ig) isotype switching produced against HSP60, ISG70, and VSG were found to depend on both the antigen type and the stage of the infection.
MATERIALS AND METHODS
Mice and trypanosomes.
Both the pleomorphic AnTat 1.1E clone from the EATRO 1125 stock of T. brucei and a derived monomorphic AnTat 1.1 clone were kindly provided by N. Van Meirvenne (Institute of Tropical Medicine, Antwerp, Belgium). Parasite stabilates were stored in liquid nitrogen. To obtain parasites for infection studies, a mouse was infected intraperitoneally with a stabilate volume containing 50,000 living parasites. On day 3 of the infection, blood was taken, supplemented with heparin (15 U/ml), and used for infection of experimental groups of mice. To monitor the course of the parasitemia, 6- to 8-week old female BALB/c mice and athymic BALB/cnu/nu mice (Harlan) received an intraperitoneal injection of fresh blood, containing 5,000 parasites. At time intervals of 2 or 3 days, the number of parasites present in the blood was counted under a light microscope and an infectious serum sample was collected for the antibody titer analyses.
Preparation of trypanosome lysates and soluble VSG.
Trypanosomes were harvested from infected blood by DE52 chromatography with sterile phosphate-buffered saline (PBS) (pH 8.0) supplemented with 1% glucose for equilibration and elution. After separation, the parasites were washed and resuspended in sterile PBS. Crude parasite lysate was obtained by three freeze-thaw cycles in the presence of 1 mM Pefablock protease inhibitor (Boehringer, Mannheim, Germany) and 0.01 mM E64 (Sigma Chemical Co., St. Louis, Mo.). Soluble VSG was prepared from DE52-purified parasites by osmotic lysis for 5 min at 37°C at 109 cells/ml in 10 mM sodium phosphate (pH 8.0) containing 0.1 mM Nα-p-tosyl-l-lysine chloromethylketone (TLCK) and 0.1 mM phenylmethylsulfonyl fluoride (both from Boehringer). The supernatant was passed through a DE52 column equilibrated in 10 mM sodium phosphate (pH 8.0). Soluble VSG was further purified on a Sephacryl-S200 column (Pharmacia Biotech, Uppsala, Sweden), dialyzed against water overnight at 4°C, and freeze-dried. The protein concentration of the trypanosome lysates and VSG was estimated by using a detergent-compatible protein assay kit (Bio-Rad Laboratories, Hercules, Calif.) with bovine serum albumin (BSA) as a standard.
Trypanosome ISG70 (14) was kindly supplied by Paul Voorheis from Trinity College, Dublin, Ireland.
HSP60 synthesis.
HSP60 peptides were synthesized with an Applied Biosystems model 431 A peptide synthesizer by Merrifield's solid-phase Fmoc/TBTU-HOBT method. The reaction was performed on a 0.25 mmol scale. The initial Cys amino acid was esterified (0.5 mmol/g) on a 4-hydroxymethylphenoxy polystyrene–1% DVB resin (Wang resin). The following side chain protecting groups were used: Asp, Glu, and Thr, t-butyl; Asn and Gln, trityl; Arg, 2,2,5,7,8-pentamethyl-chroman-6-sulfonyl (Pmc); Lys, t-butyloxycarbonyl. After synthesis, dried resin-bound peptides were dissolved in a solution of 40:2:2:1:3 (by vol) TAE-water-thioanisole-ethanedithiol-phenol (10 ml) and incubated with continuous shaking for 2 h at room temperature. The peptides were filtered, washed, and precipitated from solutions by diethyl ether addition and were dissolved in 10% acetic acid and lyophilized. They were purified by reverse-phase chromatography on a Vydac C18 silica gel column (300-Å porosity,17-μm particle size; 25 by 25 mm) with a linear gradient solvent system. At the end of the procedure, peptides were additionally checked by both amino acid sequence analyses and by mass spectrometry. Their homogeneity was assessed by analytical high-pressure liquid chromatography (Pharmacia Biotech, Uppsala, Sweden). The peptides were found to be more than 98% pure.
Cloning and purification of T. brucei HSP60.
The HSP60 cDNA was isolated from a λgt11 library screening by using serum of infected mice, and the entire HSP60 open reading frame was subcloned into expression vector p-CAL-nk-EK (Stratagene). T. brucei HSP60 was expressed as a fusion protein with a calmodulin binding peptide, in accordance with the specifications supplied by the Stratagene protocol. After an enzymatic cleavage, an additional purification step was performed with a Sephacryl-S200 column (Pharmacia Biotech). The purity of the recombinant trypanosome HSP60 was verified by Western blot analysis and Coomassie blue staining. The protein concentration was measured by using the protein assay kit (Bio-Rad Laboratories) with BSA as a standard.
Quantification of HSP60 in the trypanosome lysate.
A serial dilution (50 μl/well) of the purified standard HSP60 (30 down to 2.5 μg/ml) and 50 μl of a 1:10 dilution of trypanosome lysate (5 mg/ml) were blotted onto nitrocellulose filters by using a dot blot transfer apparatus. The filters were blocked for 1 h with a 1% skim milk–100 mM Tris-NaCl (pH 8.0) solution at room temperature. After multiple washes, the filters were incubated overnight at 4°C with rabbit anti-trypanosome HSP60 polyclonal antibodies (dilution, 1:500). The washed filters were incubated for 1 h at room temperature with a secondary anti-rabbit IgG antibody coupled to alkaline phosphatase (Promega) (dilution, 1:7,500). After several washes, the filters were stained for 15 min in a developing alkaline phosphatase solution (100 mM Tris-NaCl, 5 mM MgCl2 [pH 9.5]) containing two substrates: nitroblue tetrazolium (NBT) (Promega) and 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Promega). The trypanosome HSP60 was quantified with a GS 690 imaging densitometer apparatus (Bio-Rad Laboratories).
Detection of trypanosome HSP60 with infectious serum by using a dot blot technique.
Purified trypanosome HSP60 (1 μg) was spotted onto nitrocellulose filters, which were blocked overnight in 1% skim milk–100mM Tris-NaCl (pH8.0) solution at 4°C. After multiple washes, a 1:100 dilution of serum samples from different time points during the infection was added in triplicate to the nitrocellulose filters and incubated overnight at 4°C. The filters were washed and incubated for 1 h at room temperature with a mixture of the secondary goat anti-mouse IgG (Promega) (dilution, 1:7,500) and IgM (Sigma Chemical Co.) (dilution, 1:10,000) polyclonal antibodies, both coupled to alkaline phosphatase. Finally, the filters were washed and developed for 15 min by addition of NBT and BCIP substrates in alkaline phosphatase developing buffer (pH9.5). The images were analyzed with a Bio-Rad GS690 densitometer.
Immunodetection of anti-HSP60, anti-VSG, and anti-ISG70 responses in ELISA.
The titers and isotype pattern of the specific antibody response against total HSP60, HSP60-derived peptides, VSG, and ISG70 were determined by specific enzyme-linked immunosorbent assays (ELISAs). ELISA plates (Nunc) were coated overnight at 4°C with 5 μg of the trypanosome-derived antigens per ml. The plates were washed three times with PBS supplemented with 0.05% Tween 20 (Polylab Biochemicals) and further blocked for 1 h at room temperature with 1% skim milk solution in PBS. After three washes, serial dilutions of the individual infectious serum samples were added in triplicate to the plates and incubated for 1 h at room temperature. The washed plates were incubated for another 1 h at room temperature with a series of secondary antibodies: either anti-mouse IgG2a or IgG1 (Serotec) or anti-mouse IgM (Sigma Chemical Co.) coupled to alkaline phosphatase or anti-mouse IgG3 or IgG2b (Serotec) coupled to horseradish peroxidase. After 1 h of incubation, the plates were washed and incubated with the substrates, respectively 4-nitrophenylphosphate, disodium salt hexahydrate (NBT) (Acros Organics) for alkaline phosphatase and 3,3′,5,5-tetramethylbenzidine (TMB) (Sigma Chemical Co.) for horseradish peroxidase. The alkaline phosphatase-containing plates were left to develop for 30 min, and the optical density was measured at 405 and 690 nm to subtract nonspecific absorption. The horseradish peroxidase-containing plates were left to develop for 15 min, and the reaction was stopped by addition of 1 M H2SO4. The optical density was measured at 450 and 690 nm.
Serum titers were determined by comparing the OD values of serum samples from infected mice with those of control noninfected serum samples. End titer dilutions were determined as the highest serum dilution from infected mice that gave a similar OD value to the average control.
Immunodetection of trypanosome antigens by infectious serum in Western blotting.
The purified trypanosome HSP60 and VSG as well as total parasite lysates, were run under reducing conditions on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (10% polyacrylamide) and blotted onto the nitrocellulose filters by electrotransfer for 1 h at 100 V. The filters were blocked overnight in 1% skim milk–100 mM Tris-NaCl (pH 8) solution at 4°C. The washed filters were incubated overnight at 4°C with a 1:200 dilution of serum collected during the chronic phase of infection. After the washings, secondary anti-mouse IgG (Promega) and anti-mouse IgM (Sigma Chemical Co.) polyclonal antibodies, both coupled to alkaline phosphatase, were added for a 1-h incubation at room temperature. After the last wash, the filters were developed by the addition of NBT and BCIP substrates in alkaline phosphatase buffer.
Electron microscopy.
AnTat 1.1E bloodstream parasites were harvested from the blood of infected mice at the first peak of parasitemia. The parasites were fixed for 20 min in 3.5% paraformaldehyde–0.5% glutaraldehyde in PBS. After methanol dehydration, the parasites were embedded in Lowicryl polymerized under a UV light at −20°C. Ultrathin sections were collected on carbon-Formvar-coated copper grids and blocked in 10% normal goat serum (Sigma Chemical Co.) diluted in PBS solution containing 1% BSA. Rabbit anti-HSP60 polyclonal antibody (1:40 dilution in 1% BSA) was added for 1 h of incubation at room temperature. After three washes in PBS-BSA solution containing 0.05% Tween 20 (Polylab Biochemicals), secondary goat anti-rabbit IgG coupled to 15-nm gold particles (GAR15; British BioCell) (1:100 dilution in PBS–1% BSA) was added for another 1 h of incubation at room temperature. After three washes, sections were stained with uranyl acetate and lead citrate and images were observed in a AEI 6B electron microscope at 60 kV.
Immunofluorescence microscopy.
The trypanosomes were resuspended at a density of 106 cells/ml in PBS buffer and fixed by addition of 3.5% paraformaldehyde and 0.5% glutaraldehyde for 15 min at room temperature. They were permeabilized in 0.1% Triton X-100 (Sigma Chemical Co.) for another 15 min at room temperature. After two washes with PBS buffer, neutralizing 0.1 M glycine solution was added and the mixture was incubated for 15 min at room temperature. After three additional washes, 20 μl of parasite suspension was placed onto poly-l-lysine (1 mg/ml) (Sigma Chemical Co.)-coated coverslips and left to adhere for 30 min at room temperature. After several washes with PBS, the parasite-coated coverslips were blocked by the addition of 1% BSA–PBS solution for 1 h at room temperature. After repetitive washes, mouse anti-HSP60 antibody or rabbit anti-ATP synthase antibody, or both antibodies together, were added for an additional 1 h of incubation at room temperature. Anti-mitochondrial ATP synthase rabbit antibody from Crithidia fasciculata, which showed cross-reactivity with the ATP synthase β subunit of T. brucei, was a kind gift from R. Benne (Amsterdam) (24). After washes, the presence of ATP synthase was revealed with a secondary anti-rabbit Texas red-coupled antibody (Amersham) (1:100 dilution), while the HSP60 distribution was demonstrated by using an anti-mouse fluorescein isothiocyanate (FITC) (Amersham) (1:100 dilution)-coupled antibody for a 1-h incubation at room temperature. After a last wash, Vectashield mounting medium (Vector Laboratories) containing 100 ng of 4′,6-diamino-2-phenylindole (DAPI) (Sigma Chemical Co.) was added to the parasite-containing coverslips. The images were recorded with a charge-coupled device camera connected to a Zeiss Axioscope microscope and were processed with ISIS 3 software.
Intracellular flow cytometry analysis.
The trypanosomes were resuspended at a density of 106 cells/ml in PBS buffer and fixed by addition of 3.5% paraformaldehyde and 0.5% glutaraldehyde for 15 min at room temperature. They were permeabilized in 0.1% Triton X-100 for another 15 min at room temperature. After two washes with PBS buffer, neutralizing 0.1 M glycine solution was added and the mixture was incubated for 15 min at room temperature. After two washes with PBS, parasite aliquots were incubated for 30 min on ice with rabbit polyclonal anti-HSP60. The samples were washed twice in PBS and further incubated for 30 min on ice with a secondary sheep anti-rabbit FITC-coupled antibody (ICN). The samples were washed twice in PBS and analyzed on a Ventage SI apparatus (Beckton Dickinson).
RESULTS
Quantification and cellular distribution of the HSP60 in T. brucei.
HSPs are responsible for maintaining cellular functions under a variety of stress conditions related to a progressing infection. Besides their function as chaperones, they seem to be involved in induction of host immune response (15, 16). Here, HSP60 expression and anti-trypanosome HSP60 responses were analyzed during experimental trypanosome infections.
Pleomorphic bloodstream forms of T. brucei can cause a relative chronic infection in BALB/c mice. After intraperitoneal injection of 5,000 parasites, the first peak of parasitemia reaches a maximal density of 3 × 108 trypanosomes/ml within 5 days (Table 1). After this peak, the parasite load drops below the 106/ml detection limit within 3 days. Low parasite densities are maintained until day 11 of infection, after which parasite numbers begin to rise again, resulting in the further appearance of multiple parasitemia peaks until a final increase in parasite numbers results in the death of the host around day 35. Monomorphic bloodstream forms of T. brucei cause acute infection in BALB/c mice. After intraperitoneal injection of 5,000 parasites, exponential proliferation takes place and a lethal peak of parasitemia (>8 × 108 trypanosomes/ml blood) occurs within 5 days (data not shown).
TABLE 1.
Evolution of pleomorphic parasitemia in BALB/c and BALB/cnu/nu athymic mice infected intraperitoneally with 5,000 T. brucei AnTat 1.1E parasites
| Mouse strain | Parasitemia (106) in micea on day:
|
||||||
|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 10 | 20 | 30 | |
| BALB/c | 44 ± 12 | 288 ± 36 | 72 ± 15 | 6 ± 2 | NDb | 84 ± 23 | 122 ± 62 |
| BALB/cnu/nu | 68 ± 21 | 320 ± 57 | 32 ± 8 | 4 ± 2 | ND | 36 ± 18 | 80 ± 35 |
The experimental groups consisted of 10 mice each. Values are presented as means ± standard deviation.
ND, none detected.
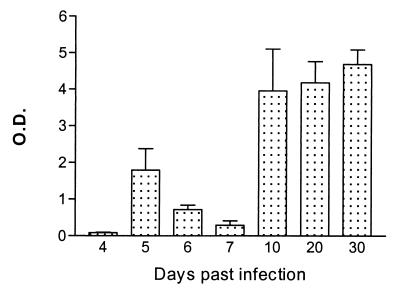
Dot blot densitometric analysis was used to analyze the kinetics of the total anti-HSP60 antibody response in serum collected at different time points during a pleomorphic AnTat 1.1E infection in BALB/c mice. The results presented in Fig. 1 indicated that total levels of anti-trypanosome HSP60 antibodies were most pronounced toward the end of the infection. Nitrocellulose-blotted trypanosome HSP60 was specifically recognized only by the serum of infected mice and not by the serum collected from noninfected control mice (data not shown).
FIG. 1.
Kinetics of anti-HSP60 antibody responses during experimental pleomorphic infection of BALB/c mice with T. brucei AnTat 1.1E. Serum samples were isolated from infected mice on the days indicated, and a densitometric dot blot analysis was performed with 1 μg of purified trypanosome HSP60 per datum point. Density quantification is expressed as absorbense units per square millimeter. Values are presented as mean optical density (O.D.) of three measurements and standard deviation.
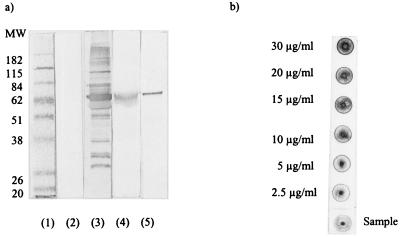
To study the role of HSP60 in trypanosome-host interaction, the amount of trypanosome HSP60, its cellular distribution, and its availability as a possible parasite antigen were analyzed. As shown in Fig. 2a (lane 3), the serum from BALB/c mice infected with the T. brucei AnTat 1.1E clone recognized a complex pattern of components in a total trypanosome lysate. These components included the major surface antigen, the VSG, and the trypanosome HSP60 (lanes 4 and 5, respectively). The amount of HSP60 present in the trypanosome lysate prepared from peak-stage pleomorphic DE52 purified parasites was estimated by a dot blot technique combined with an imaging densitometric analysis. The results presented in Fig. 2b indicate that 25 μg of total lysate contained between 0.125 and 0.25 μg of HSP60 (2.5 and 5 μg/ml). This result suggested that in the analyzed parasite lysate, HSP60 is at least 10 times less abundant that VSG, since it has been estimated before that VSG represents 10% of the total trypanosome protein content (6).
FIG. 2.
(a) Western blot analysis of T. brucei AnTat 1.1E soluble extracts immunostained with the infectious serum isolated from BALB/c mice during the chronic phase of pleomorphic infection. Lane 1 shows the molecular weight (MW) markers (in thousands), and lane 2 shows the background detection of a total trypanosome extract by the serum from non-infected mice. Lanes 3, 4, and 5 respectively contain total trypanosome extract, purified VSG, and purified trypanosome HSP60, stained with serum isolated from infected mice. (b) Quantification of the trypanosome HSP60 by the dot blot technique. A serial dilution of purified HSP60 (50 μl) was used to obtain a standard for densitometric analysis; 25 μg of total trypanosome extract was spotted per dot.
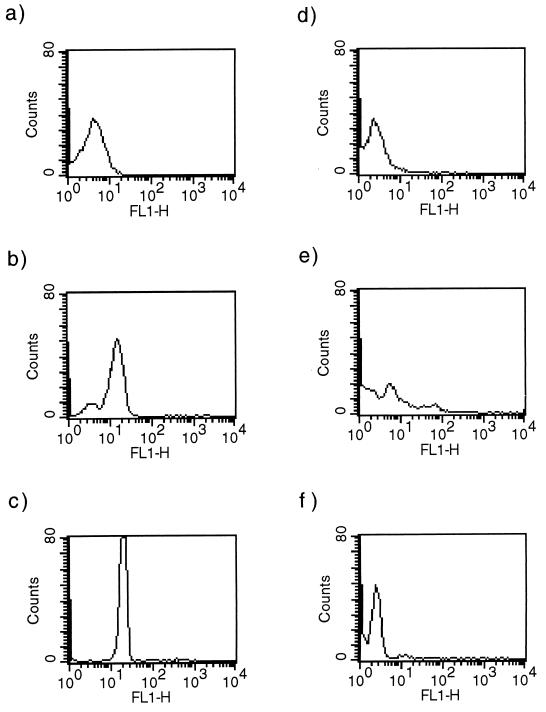
To evaluate the expression of HSP60 in trypanosomes throughout the whole course of an experimental infection, parasites were isolated at different time points and analyzed by intracellular flow cytometry. The results presented in Fig. 3 show that expression of HSP60 is upregulated in peak-stage parasites. This upregulation coincided with the occurrence of short stumpy forms of trypanosomes. On day 3 of infection (Fig. 3a), the vast majority of the parasites were long slender forms (24), expressing low levels of HSP60. On day 4 of infection (Fig. 3b), the long slender population was reduced to 25% and a short stumpy population, expressing high levels of HSP60, became dominant. On day 5 of infection (Fig. 3c), more than 90% of the parasites had differentiated into short stumpy forms expressing high HSP60 levels. On day 12 of infection (Fig. 3d), a first increase of parasitemia was measured after the clearance of the first infection peak. The parasite population occurred as homogeneous long slender trypanosomes, expressing low levels of HSP60. On day 35 (Fig. 3e), the final peak of parasitemia was reached. This population consisted of a mix of long slender and short stumpy forms, expressing low and high levels of HSP60, respectively. Blood isolated on day 5 of a monomorphic experimental infection contained only long slender parasites. Expression of HSP60 remained low in this trypanosome population (Fig. 3f).
FIG. 3.
Intracellular flow cytometry analyses of HSP60 expression in pleomorphic and monomorphic trypanosomes. (a to e) Expression of HSP60 in pleomorphic AnTat 1.1E trypanosomes was measured on days 3 (a), 4 (b), 5 (c), 12 (d), and 35 (e) of an experimental T. brucei infection in BALB/c mice. (f) HSP60 expression in monomorphic AnTat 1.1 trypanosomes was analyzed on day 5 of infection.
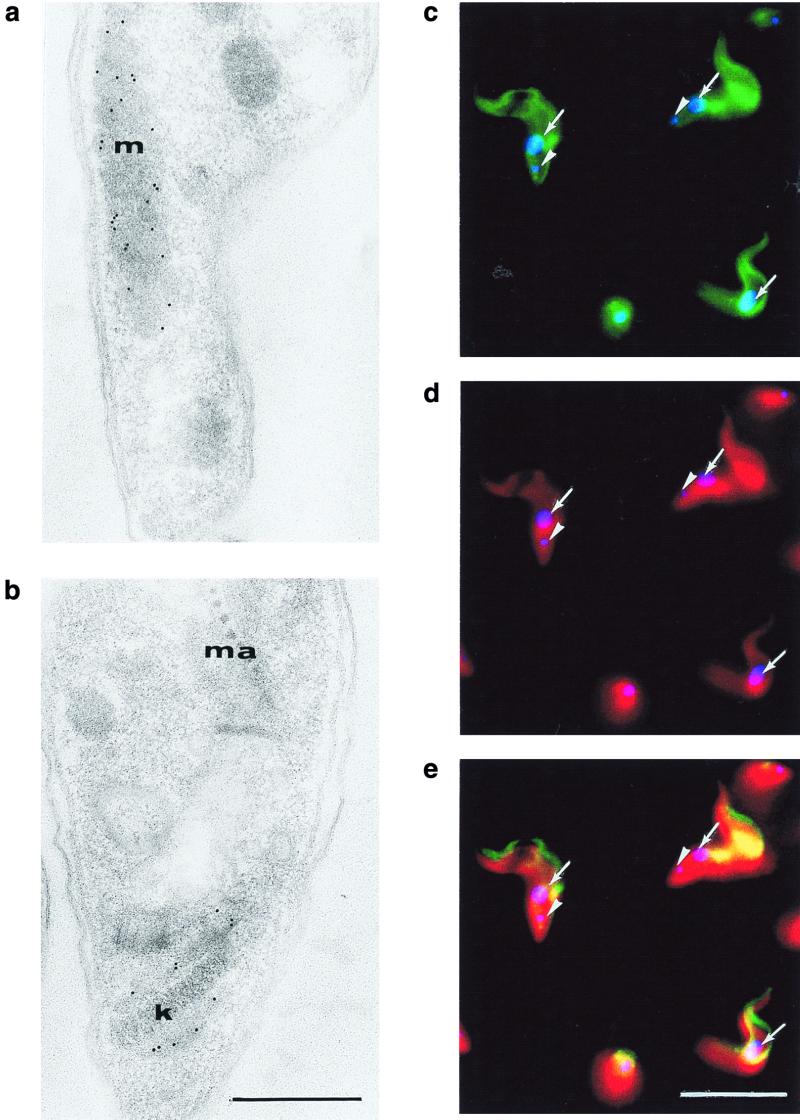
To localize cellular distribution of trypanosome HSP60, ultrathin sections of pleomorphic AnTat1.1E trypanosomes isolated on the first peak of parasitemia were analyzed by transmission electron microscopy. Rabbit anti-HSP60 polyclonal antibodies clearly labeled the mitochondrion (Fig. 4a). This localization was in agreement with the coincident staining of the mitochondrion with both anti-HSP60 (FITC staining) and anti-mitochondrial ATP synthase (Texas red) antibodies observed in a double-immunofluorescence experiment (Fig. 4c to e). Detailed analysis of electron microscopy images revealed that HSP60 was also present in the region of the kinetoplast (Fig. 4b).
FIG. 4.
Transmission electron microscopy and immunofluorescence microscopy analysis of the cellular localization of trypanosome HSP60. (a and b) Indirect immunogold labeling reveals the presence of HSP60 in the mitochondrion (m) and around the kinetoplast (k) (ma, maculae adherentes of the junction complex between the body and the flagellum). Bar, 0.5 μm. (c to e) Immunofluorescence shows the colocalization of HSP60 (FITC staining) (c and e) with the mitochondrial marker ATP synthase (Texas red staining) (d and e) in the mitochondrion. The DAPI blue fluorescence was used to indicate the nucleus (solid arrows) and the kinetoplast (arrowheads). Bar, 10 μm.
Apart from the analysis of peak-stage pleomorphic trypanosomes, which consisted of a majority population of short stumpy parasites, the localization of HSP60 was also performed on long slender parasites isolated during early-stage pleomorphic infections or peak-stage monomorphic infections. The obtained data did not show any alteration in cellular distribution (results not shown) but indicated a reduced abundance of expressed molecules, confirming the previously described flow cytometry measurements. No evidence was observed for a surface localization, indicating that an induction of host humoral response to HSP60 must be linked to the occurrence of spontaneously degrading short stumpy forms arising at the beginning of the first peak of parasitemia.
Humoral responses to the trypanosome HSP60.
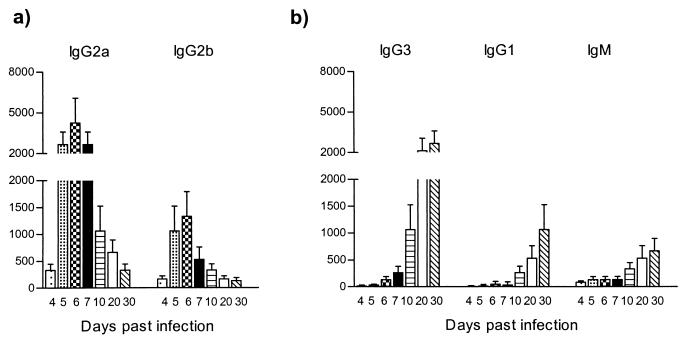
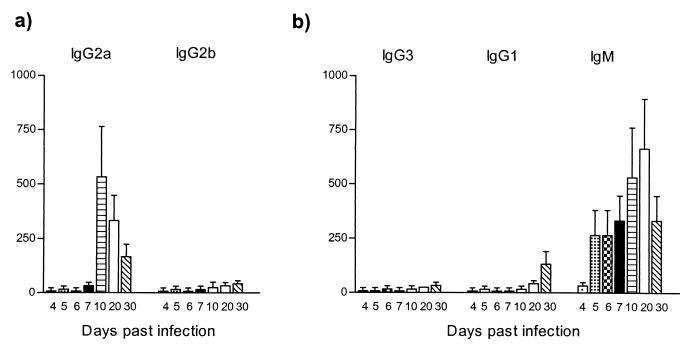
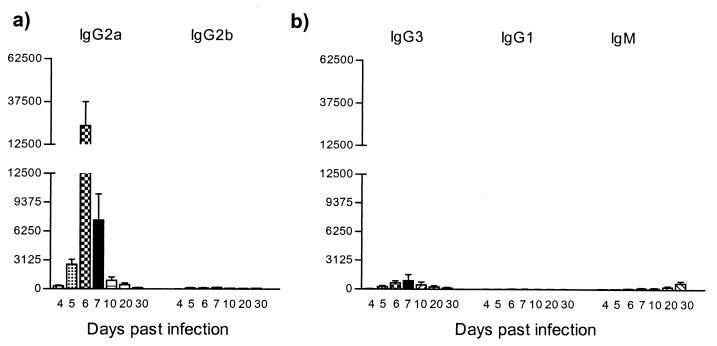
To determine specific titers and patterns of antibody isotypes recognizing the trypanosomal HSP60, serum was collected on days 4, 5, 6, 7, 10, 20, and 30 of pleomorphic infection and analyzed in an trypanosome HSP60-specific ELISA. As shown in Fig. 5a, trypanosome HSP60 was recognized as early as 4 days after infection. IgG2a antibody titers were predominant and reached peak values on days 5, 6, and 7, encompassing the whole first peak of parasitemia. The induction of IgG2a was accompanied by an IgG2b antibody response, however at lower magnitude. Therefore, both IgG2a and IgG2b antibodies induction was restricted to the first line of host immune response.
FIG. 5.
ELISA detection of anti-HSP60 antibody isotype responses during experimental pleomorphic infection of BALB/c mice with T. brucei AnTat 1.1E parasites. Serial dilutions of serum collected at various time points of infection were added to trypanosome HSP60-coated ELISA plates (5 μg/ml), with the starting dilution being 1/25. (a) Detection of IgG2a and IgG2b anti-HSP60 antibodies. (b) Detection of IgG3, IgG1, and IgM anti-HSP60 antibodies. The measurements were made with sera from five individual mice, and the values represent the means of specific end-point serum titers and standard deviation, with noninfected serum as a background detection limit.
A contrasting picture was obtained for the IgG3 and IgG1 antibody response (Fig. 5b). Both IgG3 and IgG1 titers became detectable just after the first peak of parasitemia and progressively increased toward the end of infection. Similarly, a maximal IgM response was recorded only toward the end of infection. Taken together, these results showed that HSP60 is recognized throughout the whole course of parasitemia, with a clear pattern of immunoglobulin isotype switching from early induction of IgG2a and IgG2b to late induction of IgG3, IgG1, and IgM. There was no significant induction of IgA and IgE antibody levels (data not shown).
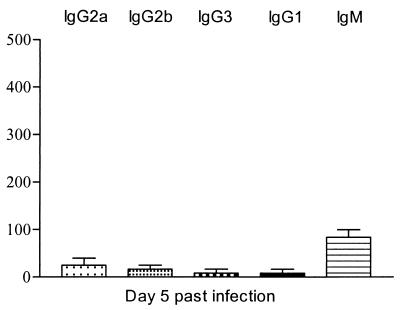
In contrast to the results obtained during the pleomorphic T. brucei AnTat 1.1E infection, experimental infections with monomorphic T. brucei AnTat 1.1 parasites did not result in substantial anti-HSP60 antibody induction, as shown in Fig. 6. These results suggest that short stumpy form parasites play a key role a in the induction of anti-HSP60 responses during pleomorphic trypanosome infections.
FIG. 6.
ELISA detection of anti-HSP60 antibody isotype responses during experimental monomorphic infection of BALB/c mice with T. brucei AnTat 1.1E parasites. Serial dilutions of serum collected on day 5 postinfection were added to trypanosome HSP60-coated ELISA plates (5 μg/ml), with the starting dilution being 1/25. Detection of IgG2a, IgG2b, IgG3, IgG1, and IgM anti-HSP60 antibodies was performed. The measurements were made with sera from five individual mice, and the values represent the means of specific end-point serum titers and standard deviation, with noninfected serum as a background detection limit.
Anti-HSP60 responses in an athymic BALB/cnu/nu mice.
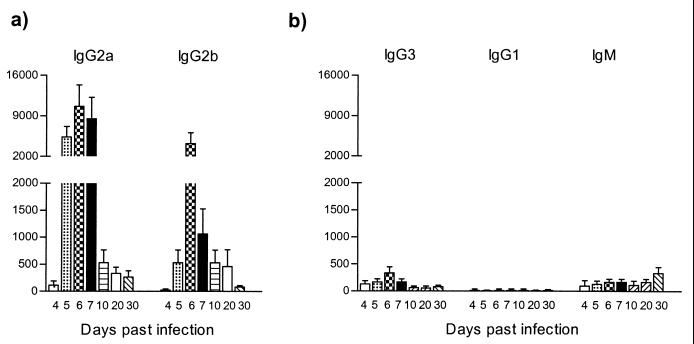
To be able to estimate the relative importance of the T-cell mediated response during experimental trypanosome infection, the T. brucei AnTat 1.1E parasitemia pattern in athymic BALB/cnu/nu mice was analyzed. First, the results in Table 1 showed no major differences between the parasitemia in these mice and that in wild-type BALB/c mice. Both the levels of parasitemia and survival time were similar. As in the wild-type BALB/c-infected mice, HSP60 recognition was characterized by an early appearance of high IgG2a and IgG2b antibody titers (Fig. 7a). Although IgG2a antibody induction was predominant, both isotypes were induced equally fast and reached their maximum level at the first peak of the parasitemia. Thus, the early induction of IgG2a and IgG2b anti-HSP60 response does not require T-cell help and probably results from a short stumpy form-induced polyclonal B-cell activation. On the other hand, the results also suggest that the late-stage anti-HSP60 responses (IgG3, IgG1, and IgM) require T-cell help, since they were strongly reduced in athymic BALB/cnu/nu mice (Fig. 7b).
FIG. 7.
ELISA detection of anti-HSP60 antibody isotype responses during experimental pleomorphic infection of athymic BALB/cnu/nu mice with T. brucei AnTat 1.1E parasites. The conditions are identical to those used in the experiment in Fig. 5.
Analyses of the autoimmune character of anti-HSP60 humoral responses.
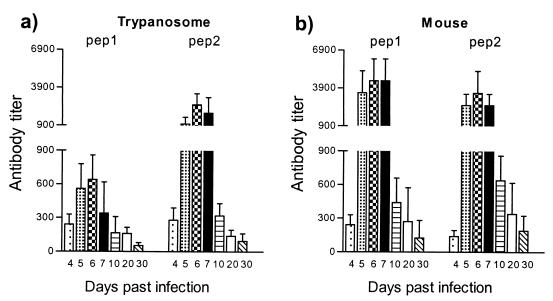
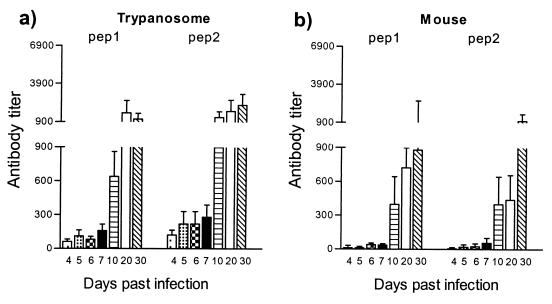
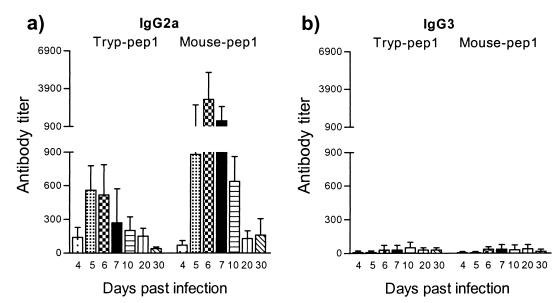
Trypanosome and host HSP60 have high sequence identity, suggesting the possible induction of self-recognition and autoantibody formation. This hypothesis was evaluated by monitoring the antibody response to trypanosome-derived and mouse-derived HSP60 peptides. By sequence alignment, two distinct regions with minimal homology (amino acids 152 to 171 and 368 to 387) were identified (Table 2). These trypanosome-specific peptides were used for the detection of an anti-HSP60 antibody response during the course of a T. brucei infection. The infection-related recognition of the trypanosome-specific peptides was compared to the recognition of host-specific HSP60 peptides from the same region of minimal homology. End-point antibody titers against two trypanosome-derived peptides and two host-derived peptides were determined at the level of both early-induced IgG2a response and late-induced IgG3 antibody response. IgG2a antibodies recognizing both trypanosome- and host-derived HSP60 peptides were induced with similar kinetics (Fig. 8). Moreover, the kinetics of peptide-specific IgG2a antibody induction were similar to the antibody induction pattern measured for the whole trypanosome HSP60 molecule (Fig. 5a). Further, elevated IgG3 isotype responses were also recorded by using both trypanosome- and host-specific HSP60 peptides. However, antibody titers against host-specific peptides were slightly reduced compared to titers detected against the specific trypanosome peptides (Fig. 9). Again, the kinetics of peptide-specific IgG3 antibody induction were similar to those of the antibody induction pattern recorded for the whole-trypanosome HSP60 molecule (Fig. 5b). In both cases, a significant increase in antibody titers in serum started to occur around day 10.
TABLE 2.
Amino acid sequence alignment of trypanosome and mouse HSP60 within the selected region of minimal sequence homology
| Region | Origin | Sequencea |
|---|---|---|
| P1 | Trypanosome | 152-EVILKNIESQSRTVTNTENV-171 |
| P1a | Mouse | DAVIAELKK**KP**TP*EI |
| P2 | Trypanosome | 368-DVAMMKERVDLVRGLIERET-387 |
| P2a | Mouse | *K*HIEK*IQEITEQLDIT* |
Amino acid identity is indicated as *. Alignment was done with the Pileup program of the Genetics Computer Group package.
FIG. 8.
ELISA detection of IgG2a anti-HSP60 antibody isotype responses during experimental pleomorphic infection of BALB/c mice with T. brucei AnTat 1.1E parasites. The screening was performed on synthetic trypanosome HSP60 peptides (pep 1, pep 2) (a) and synthetic mouse HSP60 peptides (pep 1, pep 2) (5 μg/ml) (b) with serial dilutions of serum collected at various time points of infection; the starting serum dilution was 1/25. Measurements were made with the sera collected from 5 individual mice, and the values represents the means of specific end-point serum titers and standard deviation, calculated with noninfected serum as a background detection limit.
FIG. 9.
ELISA detection of IgG3 anti-HSP60 antibody isotype responses during experimental pleomorphic infection of BALB/c mice with T. brucei AnTat 1.1E parasites. The conditions are identical to those used in the experiment in Fig. 8.
To reevaluate the T-cell dependency of the observed anti-HSP60-peptide responses, antibody titers were determined in the serum of T. brucei-infected BALB/cnu/nu mice. The results in Fig. 10a indicate that these mice mounted an early IgG2a responses that was not significantly different from the response observed in wild-type infected BALB/c mice (Fig. 8). This confirms the T-cell-independent nature of the early-stage anti-HSP60 response. Furthermore, the results in Fig. 10b show that T. brucei-infected BALB/cnu/nu lack the late-stage IgG3 anti-HSP60 peptide response that was observed in wild-type infected BALB/c mice. This confirms the T-cell-dependent nature of the late-stage anti-HSP60 response.
FIG. 10.
ELISA detection of IgG2a (a) and IgG3 (b) anti-HSP60 antibody isotype responses during experimental pleomorphic infection of athymic BALB/cnu/nu mice with T. brucei AnTat 1.1E parasites. The screening was performed with synthetic trypanosome HSP60 peptide (Tryp-pep1) and synthetic mouse HSP60 peptide (Mouse-pep1). Serial dilutions of serum collected at various time points of infection were tested; the starting serum dilution was 1/25. Measurements were made with the sera collected from five individual mice, and the values represent the means of specific end-point serum titers and standard deviation, calculated with noninfected serum as a background detection limit.
Together, the results indicate that during experimental T. brucei infection, an immune response is generated against trypanosome-derived HSP60 epitopes and that an autoreactive recognition of host-derived HSP60 epitopes occurs simultaneously. The observation that the early-stage IgG2a response has a T-cell-independent nature explains the fact that in T. brucei-infected wild-type BALB/c mice, responses generated to both trypanosome- and mouse-specifice HSP60-derived peptides can occur with similar kinetics and magnitude. The late-stage T-cell-dependent nature of the anti-HSP60 responses could explain why trypanosome-specific anti-HSP60-peptide IgG3 responses are higher in magnitude than are autoreactive mouse-specific IgG3 responses.
Comparison between the anti-HSP60 response and the responses to ISG70 and VSG.
To compare the antibody isotype pattern observed against HSP60 with the isotype pattern of antibodies directed against an ISG, the anti-ISG70 response was analyzed during the T. brucei infection. Only IgG2a and IgM antibodies were induced, and both were maximal during the late phase of the infection. IgG2b, IgG3, and IgG1 anti-ISG70 antibody titers were detectable but remained low during the whole course of infection (Fig. 11). In athymic BALB/cnu/nu mice, anti-ISG70 antibody titers remained minimal during the whole course of infection (results not shown). Therefore, in contrast to the anti-HSP60 humoral response, anti-ISG70 antibody induction requires the involvement of a specific T-cell compartment.
FIG. 11.
ELISA detection of anti-ISG70 antibody isotype responses during experimental pleomorphic infection of BALB/c mice with T. brucei AnTat 1.1E parasites. The screening was performed on trypanosome ISG70 (5 μg/ml). The conditions are identical to those used in the experiment in Fig. 5.
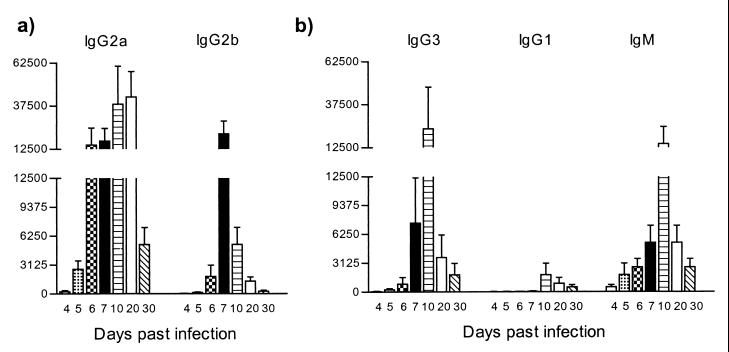
To link the results obtained above with previous reports on antibody isotype switching during trypanosome infections, the pattern of anti-VSG antibody isotype induction in both wild-type and athymic BALB/cnu/nu mice was determined. Infection of wild-type mice with T. brucei AnTat 1.1E led to a rapid anti-VSG response. High IgG2a and intermediate IgG2b levels (Fig. 12a), as well as high IgG3 and IgM responses combined with low IgG1 antibody titers (Fig. 12b), were recorded with a maximum around day 10 of infection. As shown in Fig. 13 athymic BALB/cnu/nu mice responded to the T. brucei infection by the rapid predominant induction of specific IgG2a antibodies and low levels of specific IgG3 antibodies. Only low levels of IgG2b, IgG1, and IgM anti-VSG antibodies could be detected in the serum of these mice, showing that the majority of the anti-VSG response in BALB/c mice was T-cell dependent.
FIG. 12.
ELISA detection of anti-VSG antibody isotype responses during experimental pleomorphic infection of BALB/c mice with T. brucei AnTat 1.1E parasites. The screening was performed on purified trypanosome VSG (5 μg/ml). The conditions are identical to those used in the experiment in Fig. 5.
FIG. 13.
ELISA detection of anti-VSG antibody isotype responses during experimental pleomorphic infection of athymic BALB/cnu/nu mice with T. brucei AnTat 1.1E parasites. The screening was performed on purified trypanosome VSG (5 μg/ml). The conditions are identical to those used in the experiment in Fig. 5.
Taken together, these results indicated that experimental pleomorphic infection with T. brucei leads to complex antibody isotype switching which is antigen specific.
DISCUSSION
Experimental T. brucei infections in BALB/c mice are characterized by the appearance of several peaks of parasitemia followed by repeated elimination of parasites in the liver and lymphoid tissue of the host (1, 41). It has been proposed that antibody-mediated killing of parasites by macrophages (3, 9, 17), spontaneous short stumpy form trypanosome degradation (4, 31), cytokine-induced toxicity (18), and direct NO action (12) are the main effector mechanisms that finally lead to the typical repeating pattern of infection. Continuous antigenic variation of the VSG is obviously a major mechanism involved in this process, since it allows an efficient evasion from host immune responses. However, the VSG cannot be considered the unique parasite component crucial for the interplay with the host, since other trypanosome molecules appear to be involved in the induction of host cytokines and the development of immunosuppression (8, 37). Despite a general downregulation of both interleukin-2 (IL-2) and its receptor expression, it would nevertheless appear that the host maintains a specific T-cell-dependent antibody response throughout the whole course of infection (8, 29, 30, 36). In this study we characterized the induction of a humoral response against the trypanosome HSP60, a molecule with a high degree of sequence identity to that of the host, and compared this response with those induced against two other parasite antigens, ISG70 and variant VSG. We concluded that the three antigens lead to specific and independent profiles of immunoglobulin isotype switching including both T-cell-dependent and T-cell-independent mechanisms.
During the course of a T. brucei infection, the host immune system is confronted with the presence of parasite-derived HSP60, due to the regular trypanosome elimination during the progressing disease. The results presented here have shown that HSP60 expression is significantly upregulated in the spontaneously degenerating short stumpy form trypanosomes. This event might be an evolutionary parasite adaptation to be able to cope with sudden changes in environmental stress during the passage from the mammalian host to the insect vector.
During pleomorphic trypanosome infections in BALB/c mice, a high induction of antibodies recognizing trypanosome HSP60 occurred as rapidly as the induction of antibodies against the VSG surface coat. In contrast, anti-HSP60 antibody responses were minimal in monomorphic infections, where no parasite differentiation from long slender forms to short stumpy forms takes place. In wild-type BALB/c mice, the antibody response against HSP60 occurred in two distinct phases. During the first days of infection, HSP60 recognition was marked by the appearance of a high IgG2a antibody titer, accompanied by moderate IgG2b levels. In contrast, during the late phase of infection, it was limited to a clear induction of IgG3 and IgG1 isotypes. IgM response occurred throughout the whole infection, although its maximal level was recorded just before the end of the parasitemia. It is important to mention here that, as demonstrated before (22), serum from uninfected mice contains significant levels of IgM antibodies that recognize trypanosome components. In the context of trypanosome HSP60 recognition, our results indicate that at the beginning of a trypanosome infection, the difference between the levels of these IgM antibodies and infection-induced IgM antibodies is very low. At the end of the infection, however, a clear increase in IgM antibody titers was observed. In athymic BALB/cnu/nu mice, the humoral response to HSP60 during the first peak of infection consisted mainly of IgG2a and IgG2b. Only low levels of IgM, IgG1, and IgG3 antibodies were observed in the late phase. Therefore, a T-cell-independent recognition of HSP60 was dominant during the early phase of parasitemia and a T-cell-dependent response was mounted afterward.
Since trypanosome HSP60 shows homology to its host counterpart, HSP60 recognition was analyzed in the context of a possible anti-host HSP60 autoimmune recognition. The use of both trypanosome- and host-specific HSP60 peptides allowed us to confirm this hypothesis, since a clear infection-induced production of both IgG2a and IgG3 antibodies against these molecules could be demonstrated. Similar to the responses to the complete trypanosome HSP60, IgG2a anti-trypanosome and anti-host peptide recognition was associated with the early stage of the infection and was generally T-cell independent. The presence of the spontaneously degenerating short stumpy population is crucial for this response, since monomorphic parasite infections failed to induce it. Moreover, the response was shown to be antigen specific, since the kinetics of anti-HSP60 antibody induction was clearly distinct from the kinetics of induction of antibodies against the other parasite molecules tested. In contrast to the observed IgG2a response, the IgG3 anti-HSP60 response was associated with the late stage of infection and was T-cell dependent. This was observed at the level of the responses generated to the whole trypanosome molecule, as well as at the level of responses to both trypanosome-derived and host-derived HSP60 peptides. As such, the results indicate a mixed nature of both anti-trypanosome and anti-host antibody responses.
Although HSP60 molecules are usually not present on the cellular surface, an increasing body of evidence shows that during parasitic infection, host macrophages are able to express HSP60-derived epitopes on their surface when stressed by high gamma interferon (IFN-γ) concentrations (13, 35). In this case, host cells expressing HSP60-derived epitopes can become a target for CD8+ subsets of T cells, leading to autolysis (28, 35). Since a rapid induction of IFN-γ production is one of the features accompanying T. brucei infection (8), the appearance of self-reactive anti-HSP60 antibodies could possibly lead to the recognition and lysis of HSP60-expressing macrophages during the progressing infection.
Given that in other parasitic infections members of the HSP70 and HSP90 families are also able to induce a significant autoimmune response, we do not exclude the possibility that T. brucei-related anti-HSP60 antibody induction is part of a larger array of an anti-HSP recognition. In other parasitic infections, the induction of anti-HSP antibodies has been associated with the occurrence of autoimmune disorders accompanied by HSP-related pathology (15, 16, 43). In this context, it has even been shown that the induction of anti-VSG antibody responses originates partly from a self-reactive compartment (21, 23). Moreover, trypanosomiasis-related induction of autoantibodies was noted for other host-derived components such as DNA (7), cytoskeleton (22), neurofilament-associated proteins (2), calmodulin (27), and thyroglobulin (7).
In contrast to the high anti-HSP60 antibody levels, humoral responses to the specific ISG70 were weaker. Only low levels of IgG2a and IgM were detected, and the levels reached a maximum toward the end of pleomorphic infection. The low anti-ISG70 antibody titers could be explained by the marked predominance of antibody response against the VSG and HSP60 rather than by the limited immunogenicity of the ISG70 protein itself, since mice or rabbits immunized with ISG70 alone were able to generate specific antibodies (14). The minimal anti-ISG70 antibody induction obtained in athymic BALB/cnu/nu mice suggests that the immune response to this antigen requires the involvement of T cells. It is possible that the mechanisms involved in the differential induction of high polyclonal antibody induction against homologous determinants and the low, T-cell-restricted antibody response to trypanosome-specific determinants evolved as a part of the trypanosome immune escape mechanism.
To gain a more general insight into the pattern of trypanosome-induced antibody isotypes, the anti-VSG immunoglobulin isotypes were analyzed as well. The experimental pleomorphic infection of BALB/c mice with T. brucei AnTat 1.1E gave rise to the simultaneous appearance of IgG2a, IgG2b, IgG3, IgG1, and IgM antibodies, which reached maximal titers when the parasite load fell below the detection limit just after the first peak of infection. IgG2a, IgG3, and IgG2b antibody production was predominant, while IgG1 and IgM responses were induced at lower levels. These patterns were similar in general to the previously described anti-VSG responses in T. rhodesiense-infected C57BL/6 mice (30). However, a marked difference occurred in the magnitudes of anti-VSG responses, which appeared to be significantly higher in the T. brucei BALB/c infection model (30). This difference may be due to an enhanced capability of BALB/c mice to maintain a polyclonal B-cell antibody response. Another difference was observed in athymic mice. While infection of BALB/cnu/nu mice gave rise to a pronounced IgG2a response and a low IgG3 response, infection in C57BL/6nu/nu mice led to elevated IgG3 and IgM antibody titers (30). Thus, VSG triggers both T-cell-dependent and T-cell-independent antibody responses, with isotype patterns that may depend upon the mouse strain used and the parasite model studied (25).
In in vitro B-cell activation assays, as well as during a number of parasitic infections, the local cytokine environment appears to determine the nature of immunoglobulin isotypes (10, 11, 33, 42). While IFN-γ seems to be responsible for a combined induction of the IgG2a and IgG3 antibodies, IL-4 may trigger the switching IgE and IgG1, whereas transforming growth factor β may be involved in the induction of IgA and IgG2b (33). Since T cells are considered to play a crucial role in isotype switching, a link is often made between Th1/Th2 cytokine patterns and the appearance of isotype profiles. However, cytokines themselves are insufficient to trigger an Ig switch in resting B cells. An additional stimulation is required by a B-cell activator, an antigen receptor cross-linker, or an activated T cell. In the absence of T cells, immunoglobulin isotype switching events can occur as well. For instance in athymic mice, NK cells can be the source of IFN-γ, while IL-4 can be secreted by the activated mast cells and transforming growth factor β production can be linked to macrophage and B-cell stimulation (32–34). During experimental T. brucei infections in BALB/c mice, the observed antibody isotype pattern was complex and could not be easily related to a distinct Th1 or Th2 T-cell response, especially when circulating antibody titers against different trypanosomal antigens were determined. For example, when trypanosome-induced anti-VSG responses were analyzed, both IgG2a and IgG3 isotype antibodies were detected throughout the course of the infection. However, when anti-HSP60 responses were studied, IgG2a isotype induction was observed in the absence of an IgG3 response, although both isotypes are considered to occur due to a IFN-γ-driven response. Moreover, the anti-ISG70 responses consisted mainly of IgG2a isotypes, and IgG3 was not detectable. Along these lines, it is relevant to add that although an early IgG2b isotype response to HSP60 was found to be mainly T-cell independent, the early IgG2b anti-VSG response was induced in a T-cell-dependent manner. Finally, although most isotype patterns observed would suggest an overall Th1-like antibody response, the induction of early-stage anti-VSG IgG1 antibodies as well as late-stage anti-HSP60 IgG1 antibodies should be considered to be IL-4 mediated, confirming the complexity of the humoral anti-trypanosome response. Therefore, it would appear that during the course of a T. brucei infection, several switch factors and B-cell activation pathways can determine the humoral response. One of the possible explanations for this observation is that these responses result from mixed anti-parasite and anti-host immune reactions.
ACKNOWLEDGMENTS
Work presented here was financed by the Belgian FRSM and FRC-IM; a research contact with the Communaute Francaise de Belgique; the Interuniversity Poles of Attraction Program—Belgian State, Prime Minister's Office—Federal Office for Scientific, Technical and Cultural Affairs; and the Agreement for Collaborative Research between ILRI (Nairobi) and Belgian Research Centers. M.R. was supported by a WHO TDR contract. S.M. is a Postdoctoral Fellow of the Foundation for Scientific Research-Flanders (FWO). A.M. and M.G. are senior research associates of the National Foundation for Scientific Research (FNRS).
REFERENCES
- 1.Albright J W, Long G W, Albright J F. The liver as a major site of immunological elimination of murine trypanosome infection, demonstrated with the liver perfusion model. Infect Immun. 1990;58:1965–1970. doi: 10.1128/iai.58.6.1965-1970.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayed Z, Bouteille B, Van Miervenne N, Douna F, Houinto D, Dumas M, Jauberteau M O. Detection and characterization of autoantibodies directed against neurofilament proteins in human African trypanosomiasis. Am J Trop Med Hyg. 1997;57:1–6. [PubMed] [Google Scholar]
- 3.Black S J, Sendashonga C N, Webster P, Koch G L, Shapiro S Z. Regulation of parasite-specific antibody responses in resistant (C57BL/6) and susceptible (C3H/HE) mice infected with Trypanosoma (trypanozoon) brucei brucei. Parasite Immunol. 1986;8:425–442. doi: 10.1111/j.1365-3024.1986.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 4.Black S J, Hewett R S, Sendashonga C N. Trypanosoma brucei surface antigen is released by degrading parasites but not by actively divided parasites. Parasite Immunol. 1982;4:233–244. doi: 10.1111/j.1365-3024.1982.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 5.Bringaud F, Peyruchaud S, Baltz D, Giroud C H, Simpson L, Baltz T. Molecular characterization of the mitochondrial heat shock protein 60 gene from Trypanosoma brucei. Mol Biochem Parasitol. 1995;74:119–123. doi: 10.1016/0166-6851(95)02486-7. [DOI] [PubMed] [Google Scholar]
- 6.Cross G A M. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- 7.Daniel-Ribeiro C, Tirard S, Monjour L, Homberg J C, Gentilini M. Relevance of autoantigens to autoimmunity in African trypanosomiasis: study of DNA and thyroglobulin antibodies. Acta Trop. 1983;40:321–329. [PubMed] [Google Scholar]
- 8.Darji A, Sileghem M, Heremans H, Brys L, de Baetselier P. Inhibition of T-cell responsiveness during experimental infections with Trypanosoma brucei: active involvement of endogenous gamma-interferon. Infect Immun. 1993;61:3098–3102. doi: 10.1128/iai.61.7.3098-3102.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey W L, Mansfield J M. Lymphocyte function in experimental trypanosomiasis. Role of antibody and mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983;130:405–411. [PubMed] [Google Scholar]
- 10.Finkelman F D, Urban J., Jr Cytokines: making the right choice. Parasitol Today. 1992;8:311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- 11.Finkelman F D, Katona I M, Urban J F J. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci USA. 1986;83:9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobert A P, Semballa S, Daulouede S, Lesthelle S, Taxile M, Veyret B, Vincendeau P. Murine macrophages use oxygen-and nitric oxide-dependent mechanisms to synthesize S-nitroso-albumin and to kill extracellular trypanosomes. Infect Immun. 1998;66:4068–4072. doi: 10.1128/iai.66.9.4068-4072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisaeda H, Himeno K. The role of host-derived heat-shock protein in immunity against Toxoplasma gondii infection. Parasitol Today. 1997;13:465–468. doi: 10.1016/s0169-4758(97)01128-9. [DOI] [PubMed] [Google Scholar]
- 14.Jackson D G, Windle H J, Voorheis P. The identification, purification, and characterization of two invariant surface glycoproteins located beneath the surface coat barrier of bloodstream forms of Trypanosoma brucei. J Biol Chem. 1993;268:8085–8095. [PubMed] [Google Scholar]
- 15.Kaufmann S H E. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann S H E, Schoel B, van Embden J D, Koga T, Wand-Wurttenberger A, Munk M E, Steinhoff U. A heat-shock protein 60: implications for pathogenesis of and protection against bacterial infections. Immunol Rev. 1991;121:67–90. doi: 10.1111/j.1600-065x.1991.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 17.MacAskill J A, Holmes P H, Whitelaw D D, Jennings F W, Urquhart G M. Immune response in C57BL mice genetically resistant to Trypanosoma congolense infection. II. Aspects of the humoral response. Parasite Immunol. 1983;5:577–586. doi: 10.1111/j.1365-3024.1983.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 18.Magez S, Geuskens M, Beschin A, del Favero H, Verschuren H, Lucas R, Pays E, de Baetselier P. Specific uptake of tumor necrosis factor-α is involved in growth control of Trypanosoma brucei. J Cell Biol. 1997;137:715–727. doi: 10.1083/jcb.137.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansfield J M, Kreier J P. Autoimmunity in experimental Trypanosoma congolense infections of rabbits. Infect Immun. 1972;5:648–656. doi: 10.1128/iai.5.5.648-656.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maresca B, Carrat L. The biology of the heat shock response in parasites. Parasitol Today. 1992;8:260–266. doi: 10.1016/0169-4758(92)90137-q. [DOI] [PubMed] [Google Scholar]
- 21.Muller N, Mansfield J M, Seebeck T. Trypanosome variant glycoproteins are recognized by self-reactive antibodies in uninfected hosts. Infect Immun. 1996;64:4593–4597. doi: 10.1128/iai.64.11.4593-4597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller N, Imbodem M, Detmer E, Mansfield J M, Seebeck T. Cytoskeleton-associated antigens from African trypanosomes are recognized by self-reactive antibodies of uninfected mice. Parasitology. 1993;107:411–417. doi: 10.1017/s0031182000067767. [DOI] [PubMed] [Google Scholar]
- 23.Pays E, Vanhamme L, Berberof M. Genetic control for expression of surface antigens in African trypanosomes. Annu Rev Microbiol. 1994;48:25–52. doi: 10.1146/annurev.mi.48.100194.000325. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Morga D, Pays E. A protein linked to mitochondrion development in Trypanosoma brucei. Mol Biochem Parasitol. 1999;101:161–172. doi: 10.1016/s0166-6851(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 25.Reintiz D M, Mansfield J. T-cell-independent and T-cell-dependent variant surface glycoprotein epitopes in Trypanosoma-infected mice. Infect Immun. 1990;58:2337–2342. doi: 10.1128/iai.58.7.2337-2342.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey-Ladino J A, Josi P B, Singh B, Gupta R, Reiner N E. Leishmania major: molecular cloning, sequencing, and expression of the heat shock protein 60 reveals unique carboxy terminal peptide sequence. Exp Parasitol. 1997;85:249–263. doi: 10.1006/expr.1996.4137. [DOI] [PubMed] [Google Scholar]
- 27.Ruben L, Patton C L. Antibodies to calmodulin during experimental Trypanosoma brucei rhodesiense infections in rabbits. Immunology. 1985;56:227–233. [PMC free article] [PubMed] [Google Scholar]
- 28.Schett G, Metzler B, Mayr M, Amberger A, Niederwieser D, Gupta R S, Mizzen L, Xu Q, Wick G. Macrophage-lysis mediated by autoantibodies to heat shock protein 65/60. Atherosclerosis. 1997;128:27–38. doi: 10.1016/s0021-9150(96)05975-8. [DOI] [PubMed] [Google Scholar]
- 29.Schleifer K W, Mansfield J M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993;150:5492–5503. [PubMed] [Google Scholar]
- 30.Schopf L R, Filutowicz H, Bi X J, Mansfield J M. Interleukin-4-dependent immunoglobulin G1 isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect Immun. 1998;66:451–461. doi: 10.1128/iai.66.2.451-461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sendashonga C N, Black S J. Humoral responses against Trypanosoma brucei variable surface antigen are induced by degenerating parasites. Parasite Immunol. 1982;4:245–257. doi: 10.1111/j.1365-3024.1982.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 32.Snapper C M, Mond J J. A model for induction of T cell-independent humoral immunity in response to polysacharide antigens. J Immunol. 1996;157:2229–2233. [PubMed] [Google Scholar]
- 33.Snapper C M, Mond J J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 34.Snapper C M, Tamaguchi H, Moorman M A, Sneed R, Smoot D, Mond J J. Natural killer cells induce activated murine B cells to secrete Ig. J Immunol. 1993;151:5251–5260. [PubMed] [Google Scholar]
- 35.Steinhof U, Zugel U, Wand-Wurttenberger A, Hengel H, Rosch R, Munk M E, Kaufmann S H E. Prevention of autoimmune lysis by T cells with specificity for a heat shock protein by antisense oligonucleotide treatment. Proc Natl Acad Sci USA. 1994;91:5085–5088. doi: 10.1073/pnas.91.11.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sternberg J M, Mabbot N A. Nitric oxide-mediated suppression of T cell responses during trypanosoma brucei infection-soluble trypanosome products and interferon-gamma are synergistic inducers of nitric oxide synthase. Eur J Immunol. 1996;26:539–543. doi: 10.1002/eji.1830260306. [DOI] [PubMed] [Google Scholar]
- 37.Vaidyla T, Bakhiet M, Hill K L, Olson T, Kristensson K, Donelson J E. The gene for T lymphocyte triggering factor from African trypanosomes. J Exp Med. 1997;186:433–438. doi: 10.1084/jem.186.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vickermann K, Tetely L, Hendry K A K, Turner C M R. Biology of African trypanosomes in the tsetse fly. Biol Cell. 1988;64:109–119. doi: 10.1016/0248-4900(88)90070-6. [DOI] [PubMed] [Google Scholar]
- 39.Wand-Wurttenberger A, Schoel B, Ivanyi J, Kaufmann S H E. Surface expression by mononuclear phagocytes of an epitope shared with mycobacterial heat shock protein 60. Eur J Immunol. 1991;21:1089–1092. doi: 10.1002/eji.1830210437. [DOI] [PubMed] [Google Scholar]
- 40.Webb H, Carnall N, Vanhamme L, Rolin S, Van Den Abbeele J, Welburn S, Pays E, Carrington M. The GPI-phospholipase C of Trypanosoma brucei is nonessential but influences parasitaemia in mice. J Cell Biol. 1997;139:103–114. doi: 10.1083/jcb.139.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitelaw D D, Bell I R, Holmes P H. Immunological clearance of 75SE-labelled Trypanosoma brucei in goats. Acta Trop. 1989;46:101–106. doi: 10.1016/0001-706x(89)90003-x. [DOI] [PubMed] [Google Scholar]
- 42.Widhe M, Ekerfelt C, Forsberg P, Bergstrom S, Ernerudh J. IgG subclasses in Lyme borreliosis: a study of specific IgG subclasses distribution in an interferon-gamma-predominated disease. Scand J Immunol. 1998;47:575–581. [PubMed] [Google Scholar]
- 43.Ye Y L, Suen J L, Chen Y Y, Chiang B L. Phenotypic and functional analysis of activated B cell of autoimmune NZBxNZW F1 mice. Scand J Immunol. 1998;47:122–126. doi: 10.1046/j.1365-3083.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 44.Ziegelbauer K, Overath P. Organization of two invariant surface glycoproteins in the surface coat of Trypanosoma brucei. Infect Immun. 1993;61:4540–4545. doi: 10.1128/iai.61.11.4540-4545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]