Abstract
Background
The development of safe and effective vaccines against SARS-CoV-2 and other viruses with high antigenic drift is of crucial importance to public health. Ferritin is a well characterized and ubiquitous iron storage protein that has emerged not only as a useful nanoreactor and nanocarrier, but more recently as an efficient platform for vaccine development.
Scope of review
This review discusses ferritin structure-function properties, self-assembly, and novel bioengineering strategies such as interior cavity and exterior surface modifications for cargo encapsulation and delivery. It also discusses the use of ferritin as a scaffold for biomedical applications, especially for vaccine development against influenza, Epstein-Barr, HIV, hepatitis-C, Lyme disease, and respiratory viruses such as SARS-CoV-2. The use of ferritin for the synthesis of mosaic vaccines to deliver a cocktail of antigens that elicit broad immune protection against different viral variants is also explored.
Major conclusions
The remarkable stability, biocompatibility, surface functionalization, and self-assembly properties of ferritin nanoparticles make them very attractive platforms for a wide range of biomedical applications, including the development of vaccines. Strong immune responses have been observed in pre-clinical studies against a wide range of pathogens and have led to the exploration of ferritin nanoparticles-based vaccines in multiple phase I clinical trials.
General significance
The broad protective antibody response of ferritin nanoparticles-based vaccines demonstrates the usefulness of ferritin as a highly promising and effective approaches for vaccine development.
Keywords: Ferritin, Vaccine, Antigen, Drug delivery, Nanoparticles, COVID-19, Bioengineering
Graphical abstract
1. Introduction
The COVID-19 pandemic has had devastating consequences on the population, economic, social, and health systems. According to the WHO statistics [1], there have been over 600 million confirmed cases worldwide including ∼6.6 million deaths, as of November 2022. Despite the fact that a large number of nations have employed non-pharmaceutical interventions such as personal protective equipment, social distancing, wide-spread testing, contact tracing, and shutdown measures to contain the spread of the virus, it became evident that long-term control of the virus would require effective vaccines. The causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a single-stranded positive-sense RNA (+ssRNA) virus containing four major structural proteins including spike (S) glycoprotein, small envelope (E) glycoprotein, membrane (M) glycoprotein, and nucleocapsid (N) protein, in addition to several accessory proteins. The spike (S) glycoprotein mediates entry into host cells and has been the major target of vaccine approaches [2,3]. A great effort of scientific research and global coordination resulted in an unprecedented rate of vaccine development and rollout leading to more than 40 vaccines being approved for general or emergency use [4] and over 12 billion doses given worldwide as of mid-2022 [5,6]. This success in vaccine development, however, has been impeded by the emergence of SARS-CoV-2 variants including alpha (B.1.17.7), beta (B.1351), epsilon (B1429), delta (B.1.617) and omicron (B.1.529) that evade vaccine-induced immunity [7,8], posing issues for viral transmission and global vaccination efforts. Consequently, there is a critical need for vaccines that offer high levels of immunogenicity, safety, and cross-protection between viral variants for SARS-CoV-2 and future pandemics.
A vast array of vaccine platforms of different generations such as virus-based (first generation), subunit-based (second generation) and RNA- or DNA- based (third generation) technologies have been employed in the development of a safe and more effective SARS-CoV-2 vaccine, with over 160 vaccines in clinical development and 200 in pre-clinical development as of December 2022 [6]. Despite the advantages of RNA/DNA-based vaccines, considerable hurdles are associated with their efficacy including premature degradation of molecules, and adjuvant and booster-shot requirements [9,10]. The last few decades have seen a considerable amount of research in the field of nanocarrier based vaccine delivery technologies including inorganic, lipid, polymeric, virus-like, micelle, and protein nanoparticles, which have been shown to improve antigen structure and stability [10] as well as provide broader [9,11,12] and more efficacious immune responses [13]. Among the multitude of nanocarriers, protein nanoparticles are particularly sophisticated and attractive targets for vaccine nanobiotechnology due to their biocompatibility, and flexibility of design by protein engineering [14,15]. Protein nanoparticles possess three distinct components that make them suitable for vaccine delivery: (i) a hollow interior cavity that can be loaded with vaccine antigens or nucleic acid cargos; (ii) an exterior surface that can be engineered to display vaccine antigens; and (iii) interfaces between subunits that may be engineered to allow controlled release [14,16,17]. One such protein nanoparticle that has emerged as a promising platform for the SARS-CoV-2 vaccine is ferritin, a ubiquitous iron storage and detoxification protein that protects cells from iron-induced oxidative damage [18]. Indeed, ferritin's remarkable chemical and thermal stability, reversible assembly and disassembly processes, and ability for engineering to display antigens has made the protein an attractive vaccine platform among other nanobiotechnology applications [19].
In this review, the structural and functional properties of ferritin is discussed, followed by an overview of ferritin nanoparticles production, purification, and functionalization and their applications to the field of nanomedicine, especially as a vaccine platform to augment immune response and enhance variant cross-protection.
2. Structural/functional, thermostability, and self-assembly properties of ferritin
2.1. Structural/functional
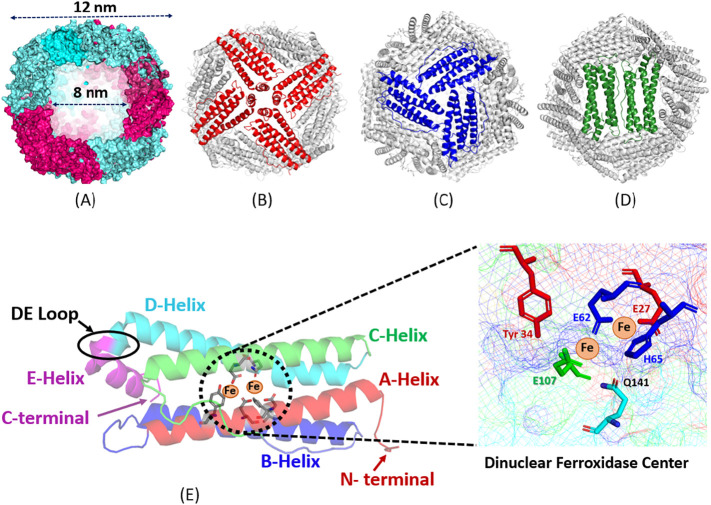
Ferritins are a family of highly conserved supramolecular nanostructures that play a critical role in iron homeostasis through sequestering thousands of Fe(III) atoms, protecting the cell from reactive oxygen species that may form from labile ferrous ions, and storing the oxidized iron in a mineralized core that is available for biological use [18,[20], [21], [22], [23], [24]]. The classical ferritin found in bacteria, plants, and animals is composed of 24 subunits, each of which is a 4-helical bundle, that assemble in octahedral (4/3/2) symmetry and feature a hollow spherical structure with an outer diameter of 12 nm and an internal cavity with a diameter of 8 nm [18,25] (Fig. 1 ). While these ubiquitous ferritins have highly stable 24-mer protein nanostructures, the hyperthermophilic obligate anaerobe ferritin from Thermotoga maritima exists naturally as a dimer that can reversibly associate into a 24-mer nanocage at high protein concentrations or in the presence of iron or dissociate back into dimers at low ionic strengths [26]. Mammalian ferritin exists largely as heteropolymers (apart from serum and mitochondrial ferritins) consisting of two distinct subunit types, H (heavy, ∼21 kDa) and L-type (light, ∼19 kDa), which co-assemble in various ratios (isoferritins) with a tissue-specific distribution [18,[20], [21], [22], [23], [24], [25]]. On the other hand, plant and bacterial ferritins are composed of one type of subunits (i.e. H- or H-like subunits) [18]. In addition, some bacteria and archaea produce other types of ferritins such as bacterioferritin where a heme moiety is found at the interface between subunit dimers, and/or a smaller 12-subunit mini-ferritin named DNA-binding proteins from starved cell [25].
Fig. 1.
(A) Computer model of a human heteropolymer ferritin with 70% H subunits (cyan) and 30% L subunits (red). (B, C, D) Ferritin view through the 4-fold (B), the 3-fold (C) and the 2-fold (D) channels. (E) Schematic view of an individual ferritin subunit showing the five-helices (A, B, C, D, and E) and the di‑iron ferroxidase center residues.
The H-subunit possesses a dinuclear ferroxidase center (Fig. 1E) that rapidly catalyzes the oxidation of Fe(II) to Fe(III), whereas the L-subunit lacks such center and consequently oxidizes iron at a much slower rate and is thought to play a role in iron mineral formation [18,[20], [21], [22], [23], [24], [25], [26], [27]]. Generally speaking, L-rich ferritins are characteristic of organs that store relatively large amounts of iron (≥ 1500 Fe atoms/molecule), while H-rich ferritins are found in organs with a low average iron content (≤ 1000 Fe atoms/molecule) [21]. While the mechanism through which ferritin H and L subunits co-assemble remains elusive, such assembly is likely a specific phenomenon since random interactions between H- and L-subunits would have led to the formation of isoferritin mixtures [28] in each tissue or organ, ranging from homopolymer H and H-rich ferritins, to L-rich and homopolymer L-ferritins. In vitro, heteropolymer ferritin reconstitution is a tedious process that yields very low amounts of functional proteins and may not represent naturally occurring ferritins; it occurs through denaturation and unfolding of recombinant homopolymers H- and L-subunits in high urea concentration and acidic or basic pH, followed by “renaturation” of the two subunits at neutral pH [21,[29], [30], [31], [32], [33], [34]]. In vitro ferritin reconstitution shows a clear preference for the formation of heteropolymers over homopolymers with a remarkably narrow distribution, suggesting the presence of preferential interactions between H and L chains [21,29,30,34]. This specific recognition is consistent with the fact that H and L homopolymers are poorly populated in mammalian tissues.
The 24-subunits of the highly conserved tertiary structure of classical ferritins are tightly packed together to create eight 3-fold, six 4-fold, and twelve 2-fold channels (Fig. 1), which are crucial to self-assembly and thought to provide permeation pathways for metal ions and a variety of small molecules [18,[21], [22], [23], [24],35,36]. Of particular interest are the 3-fold channels which are shown to be important for the transfer of iron ions through the protein shell to the di‑iron ferroxidase centers [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]]. In theory, fewer ferritin ferroxidase centers decrease the rate of iron oxidation, and therefore, the rate of iron deposition and core formation and should lead to more crystalline iron cores. Differences in the rates of biomineral formation, biomineral order, degree of crystallinity, and iron turnover have been observed in natural ferritins and found to be partially associated with their subunit composition [[43], [44], [45]]. Whereas the structure and core crystallinity of ferritin iron minerals across species can be quite variable with varying degrees of crystallinity, disordered mineral cores in animal ferritins are mostly observed in L-rich heteropolymers having a large number of catalytically inactive L-subunits, as those found in livers and spleens. In contrast, a more ordered ferritin core is typically reported in H-rich heteropolymers having more catalytically active H-subunits, as those found in hearts and brains [21,46]. These differences between natural ferritins and recombinant homopolymer ferritins suggest that the morphology of the ferritin iron core depends on the ferritin subunits' composition and is affected by the number of nucleation and ferroxidase sites present on the protein shell [47]. Additionally, the crystallinity of the mineral core has been shown to be related to the phosphate content of the iron core, varying from amorphous in plants and microbial ferritins having Fe:P ratio of ∼1:1 to nanocrystalline in animal ferritins with Fe:P of ∼8:1 [18,[48], [49], [50], [51], [52]]. A recent study from our laboratory employing spectroscopy and scanning transmission electron microscopy (STEM) revealed striking differences in the iron oxidation and mobilization kinetics and the resulting morphologies of the iron core; recombinant L-rich human ferritin exhibits spherical iron core morphologies whereas recombinant H-rich human ferritin showed more irregular core morphologies with rod and crescent like features [53], suggesting that the structure of the iron mineral may have a profound impact on the iron core reactivity with important implication on ferritin iron management in vivo.
2.2. Thermostability
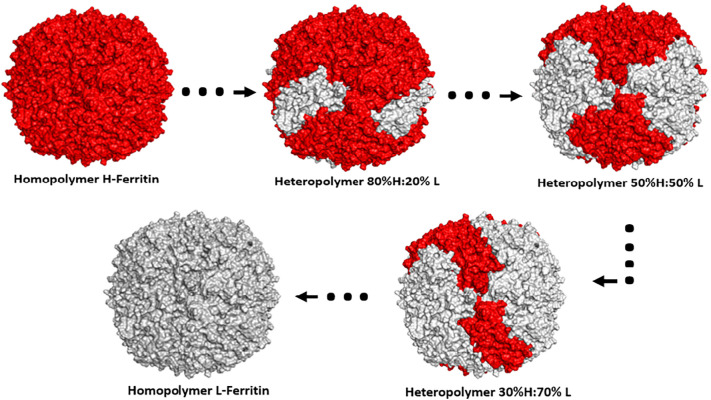
Owing to the unique architecture of the polymeric ferritin nanocage, ferritins exhibit remarkable resistance to physical and chemical denaturation. Native and recombinant mammalian ferritin have shown to withstand temperatures up to 100°C [[54], [55], [56]]. Moreover, hyper-thermostability has been demonstrated in plant ferritin (soybean seed ferritin exhibited a melting point (Tm) of 106°C) owing to an extra peptide (EP) domain at the N-terminal that is found in mature plant ferritin [55], in M. japonicus ferritin (Tm of 109°C) [57], as well as in ferritin from hyperthermophilic archaeal anaerobe P. furiosus which can withstand incubation at 100°C for 1 day or autoclaving at 120°C for half an hour without loss to activity [58,59]. Furthermore, resistance to chemical denaturation by agents such as urea and guanidium chloride [54,60], and pH stability (pH 3–10) [61] has been reported for ferritin, making it an ideal candidate for nanobiotechnology applications. Using an engineered plasmid design that enables the synthesis of complex ferritin nanostructures with specific H to L subunit ratios (Fig. 2 ), more recent work from our laboratory showed that homopolymer L- and heteropolymer L-rich ferritins exhibit a remarkable hyperthermostability (Tm = 115 ± 1°C) compared to H-rich ferritins [62]. The results revealed a significant linear correlation between protein thermal stability and the number of L subunits present on the ferritin shell. To our knowledge, this is the first report of recombinant human homo- and hetero-polymer ferritins that exhibit surprisingly high dissociation temperatures, the highest among all known ferritin species, including many known hyperthermophilic proteins and enzymes. This extraordinary thermostability may facilitate the use of human ferritin in a variety of applications, from a robust bio/nano template for the design of bio/nano materials, to the encapsulation and delivery of bioactive compounds and drugs.
Fig. 2.
(A) Computer generated models of human ferritin (homopolymers and heteropolymers) depicting an H24:L0 (homopolymer H-ferritin), H19L5, H12L12, and H7L17 heteropolymers, and an H0:L24 (homopolymer L-ferritin).
2.3. Self-assembly
Structural studies have revealed that the monomers of ferritins self-assemble into a 24-meric cage with octahedral symmetry through a series of assembly intermediates. The overall assembly mechanism of horse spleen apoferritin was first investigated by Gerl et al. [63,64] who monitored the protein's reconstitution using intrinsic fluorescence, far-UV circular dichroism, and glutaraldehyde cross-linking experiments and proposed that the complete self-assembled 24-mer forms from monomers and dimers via tetramers and hexamers.
In a study employing time-resolved small-angle X-ray scattering (SAXS), Sato et al. demonstrated the time-dependent changes in the SAXS profiles of E. coli ferritin during its pH induced reassembly process and found that monomers and trimers are unlikely intermediates in the self-assembly process due to their unstable nature [65]. These differences may be attributed to differences in self-assembly between ferritins of varying subunit composition (i.e., heteropolymer of H and L in horse spleen ferritin vs. homopolymer H ferritin in E. coli ferritin), or limitations in the ability to identify all possible oligomer intermediates using SAXS data. Mohanty et al. tracked the kinetics of bullfrog M ferritin (structural and functional analogue of human H ferritin) self-assembly by laser light scattering and observed a biphasic profile during assembly kinetics consisting of a rapid folding phase of partially unstructured monomers/dimers into unidentified oligomers, followed by a slower reassembly/reorganization process to form the 24-mer within 10 min, the rates of which were accelerated by increasing protein concentration and ionic strength [66]. An interesting area of research particularly useful to the field of nanobiotechnology involves understanding ferritin's propensity to form heteropolymers made of different ratios of H-and L-chain subunits (in mammals), the driving forces that influence subunits assembly and their distribution/arrangement around the 24-mer ferritin molecule. Carmona et al. monitored the self-assembly process of human ferritin heteropolymers by fluorescence resonance energy transfer (FRET) technology using conjugate fluorophores bound to exposed residues on the subunits and found that the presence of L-chains displaced the formation of H—H dimers, thereby demonstrating preferential formation of H/L heterodimers over the homopolymers [34]. Additionally, it was found that the H-chain distribution on the heteropolymeric ferritin shell is not random, but instead occupies preferential sites at distant positions up until the number of H-subunits on the heteropolymer reaches 8, at which point they begin to co-localize [34]. This observation coincided with the ferroxidase activity increasing with the H-chain content until a plateau of 8 H-chains per shell was reached, suggesting that the steric distribution of ferroxidase activity present on the H-chains is not random, and plays a role in the functionality of the protein [34]. To study ferritin self-assembly, mutational studies have been performed to assess the role of various residues and regions on the assembly and function of the ferritin nanocage and are elegantly reviewed by Zhang and Orner [67], as well as by Jin et al. [68]. Mutational studies showed that the C-terminus region has a major role in ferritin stability and assembly capacity but may be protein specific. For instance, deletion of the E helix from E. coli bacterioferritin resulted in a protein that existed solely as a dimer [69], demonstrating that the E helix is fundamental to the formation of higher order oligomers in bacteria, however, the E helix was demonstrated to be non-essential for human ferritin H-homopolymer assembly [70,71]. Nonetheless, mutations around the 4-fold channel or in the DE loop (Fig. 1E) can elicit conformational changes. For instance, alteration of the hydrophobic profile of the 4-fold channel results in an incorrect “flop” conformation where the E helix points outside the ferritin cavity [70]. On the other hand, mutations to the N-terminus region, which points outside of the ferritin cage, has been demonstrated to be less deleterious to proper self-assembly. For instance, deletion of the first thirteen residues at the N-terminus of human H-chain ferritin resulted in a fully assembled and functional protein [72]. Although the overall mechanism of ferritin self-assembly remains to be solved, numerous research groups have proposed and executed methods to control ferritin's self-assembly and structure-function relations to synthesize nanomaterials which will be discussed in the interface modification section of this review.
3. Preparation of ferritin nanoparticles
3.1. Production and purification of ferritin
Because the ferritin family of proteins is found in all kingdoms of life, there exists numerous methods, albeit of varying complexities, to isolate and purify the protein. Simple ferritin nanoparticle constructs are successfully produced in E. coli and then purified, but bacteria lack the ability to express glycosylated proteins and/or complex antigens. Thus, modified ferritin nanoparticles that require mammalian post-translational modifications must be produced in eukaryotic cells [73]. The simple recombinant ferritins derived from bacteria are typically synthesized by transforming the cells with vectors that express H and/or L subunits under a strong and inducible promotor [74]. Despite the discovery of recombinant human heteropolymer ferritins more than 4 decades ago, difficulties in cloning, expressing, and reconstitution of heteropolymer H/L ferritin have led to studies of ferritins being performed almost exclusively with homopolymer ferritins [74]. Our lab has engineered a novel plasmid design that enables the synthesis of ferritins of various H to L ratios (Fig. 2) depending on the concentrations of two inducers to mimic natural human heteropolymers [74]. Regardless of the H to L subunit composition, ferritin from bacterial cultures is purified through a series of steps in which the cells are disrupted through sonication, clarified by centrifugation to remove debris, then heat treated (70–75°C) and centrifuged to denature and precipitate up to 80% of native E. coli proteins and leave only the thermostable ferritin in solution [74,75]. Alternatively, or in tandem with heat treatment, the supernatant can be subjected to protein precipitation through incubation with ammonium sulfate where ferritin precipitates and native E. coli proteins remain soluble [74,75]. Following precipitation and clarification steps, bacterial derived ferritin may be further purified through size-exclusion chromatography (SEC), differential centrifugation and/or ion-exchange chromatography (IEC) [75]. The precipitation steps may be skipped entirely to preserve the integrity of antigen/epitope whereby ferritin nanoparticles may be separated by (i) affinity chromatography using an inserted His-tag on the protein [76] or (ii) IEC followed by SEC [75]. On the other hand, purification of ferritin from animal cell cultures is rather straightforward, requiring simple centrifugation or filtration followed by further purification through chromatographic approaches [75]. Protein quantification may be achieved using established protocols including the BCA assay at A562nm, whereas overall structure and composition can be characterized through native and SDS PAGE, or methods such as mass spectrometry, capillary electrophoresis, dynamic light scattering (DLS), transmission electron microscopy (TEM), circular dichroism (CD), among others. Recombinant ferritin often contains a significant amount of iron within its mineral core upon purification and depending on the final application the iron core may be removed by reduction followed by chelation [[77], [78], [79]].
The formulation of ferritin-based vaccines, their design, production, and purification have been discussed elsewhere [75]. Several studies have used ferritin from different sources and different expression systems for ferritin-based vaccine production, including A. fulgidus, T. ni, and P. furiosus ferritins [75]. It was suggested that the un-glycosylated forms of ferritins, such as those produced in E. coli or in H. pylori have the advantage of preventing undesired interactions between the glycan and certain chemical groups on the proteins of interest that are fused onto ferritin [75], thus facilitating their native tertiary structures and potential immunogenicity. These mutations allow the antigen to acquire a native tertiary structure and potential immunogenicity.
4. Interface modification and encapsulation
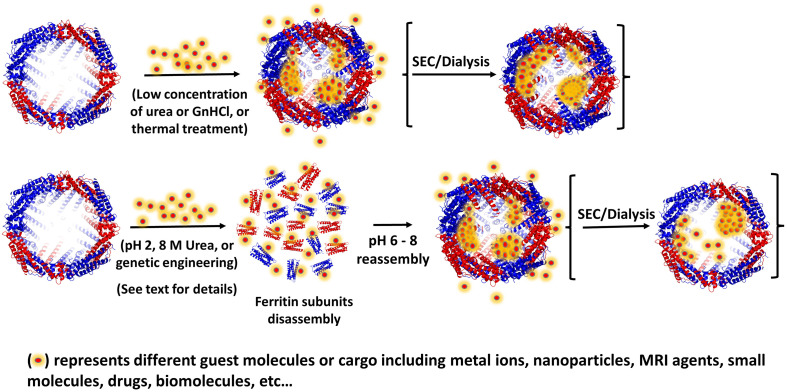
Ferritin's cage architecture as well as its self-assembly and disassembly properties has been exploited for its ability to incorporate active molecules within the protein structure such as peptides, drugs, and imaging agents. While the ferritin protein has remarkable stability, it has been shown to disassemble into subunits under strongly acidic (pH < 2–3) or basic (pH 11–12) conditions and reassemble at neutral pH conditions [80]. Li et al. demonstrated that ferritin dimers maintain their secondary and tertiary structures at acidic pH, thereby substantiating that the dimer is the essential unit to ferritin's self-assembly [81]. This phenomenon has been taken advantage of through reassembling the protein by increasing the pH in the presence of highly concentrated cargo materials, in such a way that many molecules, statistically, become encapsulated within the ferritin cavity [[82], [83], [84]] (Fig. 3 ). However, this technique typically suffers from low protein yields (only 60% recovery), loss of the protein structure (hole defects), low encapsulation efficiency, and loss of cargo bioactivity associated with the harsh pH treatment required for loading the ferritin cage [80,[85], [86], [87]].
Fig. 3.
Schematic of direct cargo loading into ferritin (top row) or acid-denaturation/disassembly process in the presence of guest molecules (bottom row) followed by reassembly and refolding at neutral pH.
Similarly to pH-dependent disassembly, high concentrations of chaotropic agents such as urea (8 M) could accomplish disassembly of the 24-mer through destroying its non-covalent forces [88]. Subsequent dialysis of the disassembled protein in stepwise gradients of urea from 8.0 M to 0 M in buffer reassembles the ferritin shell [88]. For this method, however, the formation of soluble aggregates and insoluble precipitates is common, leading to low ferritin recovery yields [89]. In recent years, researchers have sought to solve the issue of cargo and ferritin-shell integrity through developing milder approaches with higher efficiency to encapsulate guest molecules within ferritin nanocages. Chen et al. employed genetic engineering to delete the last 23 amino acids involved in the formation of the DE-turn and E helix on HuHF, resulting in a fully intact protein shell which can be dissociated into subunits at pH 4.0 and reassembled at pH 7.5 [89]. On the other hand, while the deletion of these amino acids had very little effect on the assembly of ferritin, Chen et al. found that the thermal stability of HuHF decreased to 72.7°C [90]. Similarly, Yang et al. engineered phytoferritin (plant-ferritin) capable of disassembly at pH 4.0 and reassembly at pH 6.7 upon deletion of its EP domain, though the α-helix content decreased by 5.5% and the melting temperature decreased by ∼4°C [90]. Recently, Gu et al. introduced His6 motifs at the 4-fold channel interfaces of two different recombinant ferritins, rHuHF and recombinant shrimp (Marsupenaeus japonicus) ferritin (rSF) which enabled the ferritin nanocages to disassemble into tetramers at pH 7.5, and reversibly reassemble back into intact protein nanocages upon increasing the pH to 10.0 or incubation with transition metal ions, owing to histidine motifs bearing a pKa of 6.6 and their tendency to form a coordination bond with transition metal ions [91]. His-mediated ferritin nanocage self-assembly allowed for encapsulation of curcumin and doxorubicin under relatively mild conditions meanwhile keeping the protein shell completely intact, as confirmed by X-ray crystal structure, and demonstrating markedly higher loading efficiency compared to the reported values for the pH dependent disassembly/reassembly encapsulation method [91]. Other physical methods including atmospheric cold plasma (ACP), pulsed electric field (PEF), and manothermosonication (MTS), have been employed to destabilize treated ferritin, allowing for disassembly at milder pH values (pH 3–4) and reassembly into intact ferritin cages at neutral pH, thereby avoiding the harmful effects associated with harsh acidic treatment [[92], [93], [94]]. Apart from the property of reversible self-assembly, ferritin channels, which allow small molecules to enter and exit the protein, have been exploited as a method for encapsulation. As mentioned earlier, high concentrations of chaotropic agents are able to dissociate ferritin into its subunits, however, low concentrations appear to expand the four-fold channels of phytoferritin and allow encapsulation of cargo. For instance, Yang et al. was successful in using 20 mM urea and 2.0 mM guanidine hydrochloride (GnHCl) to encapsulate molecules into phytoferritin at comparable efficiency to complete chaotropic disassembly without protein damage, although it is important to note that these methods may trap urea/GnHCl molecules within the ferritin cavity [[95], [96], [97]].
While ferritin is able to withstand temperatures over 80°C, the channels are reported to be more sensitive to temperature [60,98,99] with initial iron release rates reaching a maximum value at 60°C in phytoferritin [99], suggesting that cargo may be loaded into the ferritin cavity through the expansion of the ferritin channels caused by temperature changes. Through crystal structure and protein mutation analyses, Jiang et al. showed that ferritin's four-fold channels expand from 0.9 nm to 1.0 nm upon thermal treatment (60°C) and recovered completely to the original upon decreasing the temperature to 20°C [100]. Notably, the thermal expansion channel-based drug loading strategy achieves higher drug loading efficiency, better protein yield, and better stability compared to pH or chaotropic agent dependent disassembly, however, it is worth noting that many bioactive molecules are sensitive to temperature and may degrade during such thermal treatment. By modifying interactions at the subunit-subunit interface in ferritin we may not only control self-assembly to encapsulate cargoes of interest but also potentially regulate the release of the contents at acidic pH in a target cell environment. In addition to the pH-dependent disassembly/reassembly strategy in acidic compartments, other cargo delivery strategies include genetic or chemical conjugations and surface functionalization of ferritin using peptides, antibodies, and functional ligands, have been employed [101]. In addition to ferritin's natural function to oxidize and store iron, ferritin can bind several other metal ions, especially divalent cations [102]. Taking advantage of this phenomena, several metal-based nanoparticles and metal-containing compounds can be encapsulated within the cavity of ferritin through incubation of a metal-salt solution with apoferritin and a reducing agent, followed by dialysis to remove unincorporated molecules [102]. Another metal-based assembly strategy designed by Huard et al. involves modifying the C2 interface of ferritin via reverse metal-templated interface redesign (rMeTIR) to form a ferritin cage whose assembly is dependent on Cu(II) binding, with cage-assembly being irreversible after removal of copper ions [103]. Ferritin from the hyperthermophilic archaeon Archaeoglobus fulgidus (AfFtn) was demonstrated to disassemble in solutions of low ionic strength and reassemble in high ionic strength [104] seemingly due to ionic charges alleviating electrostatic repulsion between the negatively charged residues at subunit interfaces [105]. Additionally, AfFtn was demonstrated to be able to self-assemble around highly charged cargo such as gold nanoparticles without adjusting the ionic strength of the medium [[106], [107]] raising the prospect of encapsulating larger cargo without complete disassembly of the nanocage.
5. Interior modifications
The interior cavity of ferritin is commonly modified to encapsulate, and better accommodate various cargos including diagnostic or therapeutic agents. While the negatively charged interior of ferritin can readily coordinate metal ions, it has been demonstrated that mutating interior-facing surface residues to hold a net positive charge can facilitate electrostatic-mediated encapsulation such as supercharged fluorescent proteins and nucleic acids [[108], [109], [110]]. Similarly, the inner surface of the ferritin nanocage may be engineered by genetically fusing hydrophobic peptides to the C-terminal end of ferritin subunits to increase absorption of insoluble, hydrophobic cargo [111,112]. Introducing cysteine residues to the interior of the nanocage has been a widely used approach to facilitate covalent attachment of dye molecules, drugs, or other active compounds through disulfide bonds [113] or to enable specific chemical reactions such as copper-free click chemistry [114].
6. Exterior modifications
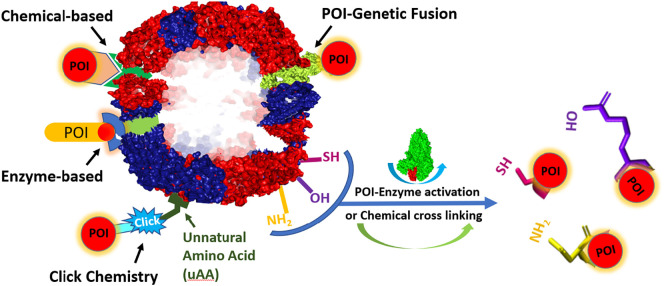
Perhaps the most exploited component of ferritin in the field of nanomedicine, and the most pertinent to the scope of this review is the external surface of the protein nanocage, which can be bioengineered to display specific molecules. The goal of such modifications is to present antigens to immune cells, deliver encapsulated molecules to specific targets, increase functionality, or even increase their circulatory half-life and decrease their immunogenicity. Similarly to interior modification methods, chemical conjugations, and genetic fusions are commonly used to modify the exterior surface of ferritin to attach antigens and trigger specific cellular responses. Below, we provide a brief overview of the most commonly employed surface modifications methods (Fig. 4 ).
Fig. 4.
An illustration of different strategies for the functionalization of ferritin nanocages. For greater details, please refer to the section “Exterior Modifications” of the text.
6.1. Genetic fusion
A protein of interest (POI) may be conjugated to ferritin's surface through genetic fusion of the gene encoding the POI to the N-terminal end of ferritin subunits, which are exposed to the protein's outer surface, using established recombinant DNA technologies for subsequent production in a chosen expression system [115]. From the expression of simple peptides to complex trimers, genetic fusion of POI on ferritin's external surface has had wide applications in the field of vaccine development, including the search for vaccines against influenza [[116], [117], [118]] Epstein-Barr virus [119], hepatitis C [73,120,121], HIV-1 [73,122], Lyme [123], RSV [124], and SARS-CoV-2 [[125], [126], [127], [128], [129], [130], [131], [132], [133]] which will be discussed in a later section. A pitfall to expressing a protein of interest on ferritin's exterior through genetic fusion, however, is that care must be taken to recombinantly express antigens in a way that does not impair their stability or conformation, nor impair ferritin's inter-subunit interactions during the self-assembly process [75].
6.2. Chemical crosslinking
The separate production, or modular assembly, of the nanoparticle and POI followed by their conjugation, is often used to bypass the steric issues that may arise from surface functionalization by genetic fusion [75]. One approach to chemical crosslinking involves the chemical treatment of a reactive residue on either the nanoparticle or the POI to conjugate with a reactive amino acid on the other respective protein, often exploiting conjugation of surface exposed cysteine (-SH group) residues with primary amines (−NH2) found in lysine residues, or less often hydroxyl groups found in serine and threonine residues [75,134]. The main drawbacks to this technique, however, are the lack of control over the number of cross-linkers attached or their orientation which can lead to the formation of aggregates or uneven decoration of the antigen on the surface of the protein nanocage which may hinder efficient presentation to target cells [135].
6.3. Intermediate based conjugation
To increase specificity of POI attachment to ferritin, intermediate based conjugation strategies may be appropriate. Chemically inducible dimerization (CID), or the use of a small molecule as an intermediate to induce the binding of two proteins, has been shown to successfully conjugate AOIs (antigen of interest) or POIs, to ferritin [75,136]. One example is the FKBP/FRB/rapamycin system, in which the FKBP and FRB peptides are genetically fused to the scaffold protein and POI and upon addition rapamycin form a ternary FKBP-FRB-rapamycin complex, which brings the POI to the target site on the scaffold protein [137]. Unlike classical crosslinking, CID allows for greater specificity, however conjugation via this method is restricted to the N- or C-terminals of the conjugated proteins as in the case of genetic fusion. Similarly to CID, enzyme-mediated conjugation involves using an enzyme intermediate to bind two peptides through activating certain residues to enable binding or conjugation between genetically encoded motifs [138]. However, unlike CID, enzyme-mediated conjugation does not restrict conjugation to the N- or C-terminals [75].
6.4. Click chemistry
Click chemistry involves the incorporation of unnatural amino acids (uaas) to the protein nanoparticle and AOI or POI, thereby introducing functionalized side chains such as those bearing biorthogonal reactive groups such as azides, alkynes, alkenes, and tetrazines, followed by their conjugation often through azide-alkyne coupling [139]. A commonly used system for the introduction of uaas relies on the generation of orthogonal aminoacyl-tRNA synthetases and tRNAs that function independently from those endogenous to the chosen biological expression system (such as E. coli) [140]. Using this approach, Khoshnejad et al. inserted the 4-azidophenylalanine (4-AzF) uaa at residue 5 of L-chain ferritin which allowed for conjugation of Dibenzylcyclooctyne (DBCO)-functionalized IgG, enabling specific pulmonary targeting in mice [141]. While click chemistry enjoys the benefits of high specificity and yield, the main drawback is the associated costs which would confer a great disadvantage in large scale productions such as vaccine manufacturing [75].
6.5. Genetically encodable linkers
The most common modular assembly strategy for protein-protein conjugation involves incorporation of genetically encodable peptide or protein tags. Most notable is the SpyTag (peptide tag of 13 residues) and SpyCatcher (protein of 138 residues) based conjugation mediated by the SpyLigase enzyme leading to irreversible isopeptide bond formation under a wide range of pH and temperature conditions [75,142]. Using this system, the Tag and the Catcher are genetically fused to the N- or C-terminal of both proteins, and upon mixing spontaneously form a stable covalent bond, thereby attaching the protein nanoparticle to the POI [75,142]. Other notable linkers include His tags (interact with Ni-NTA columns), Halotags (interact with chloroalkane-linked moieties), SNAP-tags (interact with alkyl-guanine ligands), Clip-tag (interact with benzyl cytosine derivatives), and biotin acceptor peptides (conjugation of biotin-linked moieties) [138]. As with genetic fusion, care must be taken when optimizing linkers for ferritin conjugation purposes in order to enable the correct assembly of the scaffold and displayed protein and thus enhance biological response [75].
6.6. “Stealth” coatings
In addition to displaying molecules on the ferritin nanocage for the purpose of eliciting an immune response, surface modifications could be engineered to increase half-life or improve delivery. For instance, glycosylated ferritin has been shown to be cleared at a slower rate by hepatocytes, therefore introducing glycans to ferritin's exterior surface would prolong half-life [75]. Similarly, “stealth” coatings such as polyethylene glycol (PEG) can be conjugated to the protein's surface which would mask the nanoparticle from cell receptors that may sense it as foreign agents, thereby increasing target specificity and improving gene delivery [143,144]. However, although ferritin biocompatibility and safety for human medicine development have been demonstrated, additional challenges remain including immunogenicity caused by cargo loading or ferritin surface functionalization, homogeneity of the final assembled product, and/or the immunogenic response of multi-antigen designs. Whereas a single antigen has limited immunogenicity, a highly organized supramolecular particle like ferritin can yield an improved immune response by forming a highly organized nano structure that mimics the original pathogen. Furthermore, because of its uniform structure, good plasticity, and desirable thermal and chemical stabilities, ferritin can be engineered to carry different antigens and expose immunogens in a repetitive and well-organized manner. Nonetheless, several studies have demonstrated great promises for the development of ferritin-based vaccines and the potential of these constructs to elicit immune responses against a wide range of pathogens, and some ferritin-based vaccines have already moved to Phase 1 clinical trials, as discussed below.
7. Biomedical applications of ferritin
7.1. Nanoreactor
The unique size, optical, magnetic, and chemical properties of inorganic nanoparticles (NPs) have shown to be highly useful in nanomedicine. However, several obstacles exist to their implementation as diagnostic and therapeutic agent including toxicity, biodistribution, and bioaccumulation [145]. When inorganic nanoparticles are introduced to a biological medium, they inevitably face significant physiochemical changes due to protein corona (PC) formation at the nanoparticle surface [105]. The adsorption of proteins on the surface of the nanoparticle can influence its body distribution, bioactivity, and cytotoxicity [146], which may or may not be beneficial to the diagnostic or therapeutic purpose of these nanoparticles. On one hand, the presence of a PC may increase biocompatibility of NPs, but on the other hand they can mask functionalized molecules on the NP surface thereby inhibiting their interaction with specific receptors on target cells, causing aggregation of the NPs, inducing conformational changes in the proteins, or increasing NP size and therefore affecting their biodistribution [147]. Furthermore, the size of nanoparticles can greatly affect their circulation, biodistribution, and clearance. For instance, smaller nanoparticles (< 20 nm) are more easily transported to the lymph nodes [148] and are most capable of perturbing membranes [149,150], whereas intermediate-sized nanoparticles (20–100 nm) are most capable of circulating in the blood for long periods of time and avoiding clearance [148]. Superparamagnetic iron oxide, platinum, gold, silver, quantum dots (composed of group IIB-VIA or group IIIA-VA elements on the periodic table), and other inorganic nanoparticles have been demonstrated to be useful to a wide range of biomedical applications including targeted delivery of drugs, biosensing, imaging, antimicrobial therapy, and photothermal therapy [105,[150], [151], [152], [153], [154], [155]]. Drawbacks to their clinical use, however, include their toxicity, induced by harmful metal ions or uncontrolled protein corona formation, poor solubility, and propensity to aggregate into structures larger than their optimal nano-size [152,154,156,157]. Ferritin's ability to restrict the size and shape of nanoparticles synthesized within the nanocage enhance their solubility, mitigate thier toxicity, and avoid nonspecific interactions due to PC formation. These highly desirable characteristics have allowed for the application of ferritin as an “inorganic material” in medicine [[152], [153], [154]]. For instance, apoferritin has been successfully used as a biotemplate for synthesizing CdSe, CdS, ZnSe, ZnS, PbS, and other quantum dots within its protein shell, mimicking ferritin's natural iron biomineralization process, and allowing for enhanced biocompatibility of these materials [151].
7.2. Nanocarrier
While significant progress has been made in the diagnosis of cancer over the past few decades, drug therapy still suffers from serious drawbacks including nonspecific distribution, uncontrolled drug release, and toxicity. Protein nanoparticles have the potential to overcome these obstacles by improving biocompatibility and tumor targeting ability. Seaman et al. demonstrated that human heavy chain ferritin can be recognized by the transferrin receptor-1 (TfR1) that is overexpressed on tumor cells [158] from various types of cancer, including lung and breast cancer [159]. In addition, ferritin has been shown to bind to a few other receptors including Tim-1, Tim-2, Scara5, and CXCR4 [160]. Ferritin's intrinsic tumor-selective properties, as well as the ability to engineer additional tumor-targeting moieties on its surface has been the subject of many studies on ferritin-coated chemotherapeutic drugs, ferritin in photothermal therapy (PTT) and ferritin in photodynamic therapy (PDT), and in bioimaging and other biomedical uses [[86], [87], [88],105,151,[158], [159], [160], [161], [162]] (Fig. 5 ). Since many chemotherapy drugs are hydrophobic, encapsulation within ferritin has been shown to dramatically increase their solubility and thus their biocompatibility, as well as achieve significant anti-cancer effects in cell and animal models [147]. Using encapsulation processes discussed earlier, many anti-cancer drugs have been loaded inside ferritin nanocages including doxorubicin, carboplatin, and cisplatin, exhibiting improved circulation time, decreased toxicity, and greater accumulation at the tumor sites [87,147,161,162]. Ferritin has also been used to encapsulate gadolinium and superparamagnetic iron oxide nanoparticles for application in magnetic resonance imaging (MRI), resulting in reduced toxicity, higher relaxivity of the MRI contrast agent, and significant signal increase with greater specificity for cancer cells [87,115,151,161,162]. The use of ferritin as a delivery platform for photothermal agents and photosensitizers, which generate heat and reactive oxygen species, respectively, has been shown to reduce the side effects of these therapies in normal tissues [115,148], improve cellular uptake [115], and inhibit tumor growth [87,115]. For thorough reviews on the encapsulation of inorganic nanoparticles, the use of ferritin nanoparticles in cancer therapies and diagnoses, and their application to nanomedicine and biotechnology, we refer the reader to several excellent reviews by Sun et al. [87], Jutz et al. [115] Mohanty et al. [151], Truffi et al. [161], and He et al. [162].
Fig. 5.
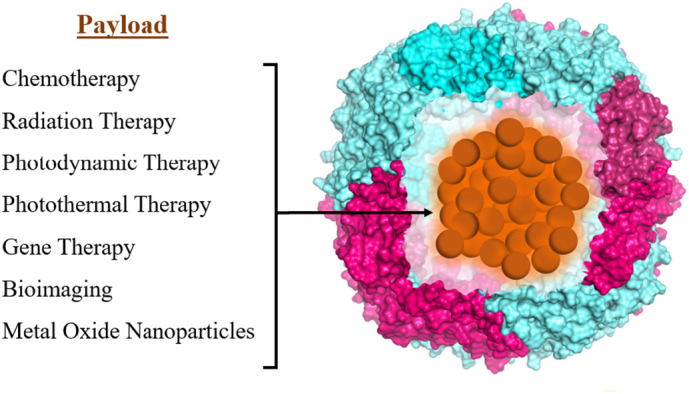
Schematic of various cargo loaded-ferritin represented by ( ).
).
8. Vaccine platform
A vast array of vaccination platforms has been employed to circumvent diseases caused by pathogens throughout history. To date, vaccines are developed from attenuated or killed whole organisms, and RNA/DNA/protein subunits of viral pathogens. Despite the advantages of each method, there exists several drawbacks to their safety and efficacy. For instance, while inactivated, live-attenuated, or recombinant viral vaccines generally produce strong and long term immune responses, they are associated with more frequent adverse effects such as existing levels of immunity, reducing effectivity, and risk of residual illness [163]. RNA/DNA vaccines that code for a virulent antigen, while very safe, well tolerated, and fast to produce, often suffer from lower immunogenicity, and extensive logistical challenges for distribution and administration (i.e. very low temperature storage for mRNA-based vaccines) and the need for a special delivery system [163]. Subunit vaccines, in which a protein antigen from the pathogen is utilized to elicit an immune response, are an attractive option for reasons of safety and stability. However, they are typically less immunogenic and often require an adjuvant and/or multiple booster shots to elicit long term immune responses [163]. One strategy to overcome the lower levels of protective immunity of modern vaccines is the design of multiple antigen presenting nanoparticles that integrate various antigen components to improve antibody response [102,164]. Ferritin's architecture, biocompatibility, capacity for self-assembly into symmetrical, monodisperse architectures, ease of production with relatively large yields, and potential for engineering at multiple interfaces makes the protein an attractive target for the development of vaccines. This is corroborated by a recent study demonstrating that a minimum of 20–25 epitopes spaced by 5–10 nm are required to elicit sufficient B-cell activation [164]. Other studies have demonstrated that epitope-protein nanoparticles elicit stronger immune responses compared to soluble antigens [73]. Furthermore, intramuscular injections of spherical protein nanoparticles have been shown to have the ideal shape and size (∼ 20–150 nm in diameter) to be taken up by peripheral or lymph node dendritic cells [165]. Altogether, the highly symmetrical architectures of the ferritin nanocages offer promising platforms for vaccine development, particularly in light of our ability to engineer their external surfaces with multi-copy antigen displays.
8.1. Influenza virus
The first ferritin vaccine, developed against an H1N1 influenza virus by Kanekiyo et al., featured the influenza virus haemagglutinin (HA) fused to the surface of Helicobacter pylori ferritin at the interface of adjacent subunits, generating eight trimeric viral spikes on its surface [116]. Immunization of mice with this influenza ferritin vaccine elicited antibody titers over 10-fold higher compared to the trivalent inactivated influenza vaccine (TIV) (current commercial vaccine), with a single immunization inducing an immune response comparable to two immunizations of the TIV vaccine [116]. Remarkably, the influenza ferritin vaccine demonstrated cross-reactivity against two independent highly conserved epitopes of H1N1 [116], suggesting a powerful potential use for a ferritin nanoparticle-based antigen display platform for a universal influenza vaccine. In a more recent work, Yassine et al. demonstrated that a ferritin-based nanoparticle immunogen displaying only the highly conserved stem region of the H1 HA glycoprotein was capable of eliciting broadly cross-reactive antibodies in mice and ferrets and was successful in protecting the immunized animals from lethal doses of the H5N1 influenza virus [117]. These powerful results in non-human animal trials prompted three vaccines against influenza to be examined in human Phase 1 clinical trials. In the first vaccine, Houser et al. developed a H. pylori ferritin nanoparticle influenza vaccine (NCT03186781) against an H2N2 strain and demonstrated a broad neutralizing antibody response after a single dose against not only H2-naïve and H2-exposed populations, but also against seasonal H1 and avian H5 subtypes, due to their targeting of the highly conserved HA stem domain [118]. The results of the other two vaccine trials, NCT03814720 and NCT04579250, are still underway.
8.2. Epstein-Barr virus
The receptor binding domains (RBDs) are viral fragments that allow attachment host cell membranes. They are the target of major neutralizing antibodies and have been investigated as vaccine candidates against numerous viruses. Despite being sites of vulnerability in viruses, isolated RBDs are often weakly immunogenic due to steric hinderance from surrounding glycans, and protein misfolding/destabilization/conformational changes in a soluble form [119]. To improve immunogenicity and circumvent these issues, nanoparticle technology has been employed to design immunogens with modified configuration that would otherwise be impossible to apply to nonrecombinant vaccine platforms. For instance, Kanekiyo et al. designed a ferritin-based nanoparticle immunogen displaying the conserved receptor-binding domain of the gp350 antigen from Epstein-Barr virus, a major envelope glycoprotein which is the target of neutralizing antibodies in naturally infected individuals [119]. In mice and non-human primates, the nanoparticles conjugated to the gp350 RBD generated neutralizing antibody responses that significantly exceeded those obtained with the soluble gp350 protein, suggesting that gp350 is conformationally stabilized when conjugated to the ferritin nanoparticles, thereby focusing the immune response more efficiently [119]. In recent developments, the gp350-ferritin nanoparticle vaccine with a saponin-based Matrix-M adjuvant is being investigated in a phase 1 clinical trial (NCT04645147), although the trial is expected to last for a few years, and developments have yet to be published.
8.3. Hepatitis C
Conventional approaches such as attenuated or live viruses and purified or recombinant viral proteins, though successful against a great range of pathogens, falter against highly variable viruses such as HIV-1 and hepatitis C virus (HCV), which have evolved mechanisms to evade host immunity. As a result, a great deal of effort has been made to develop vaccines directed towards conserved regions which produce broadly neutralizing antibodies (bnAbs) against a wide spectrum of genotypes. Recently, two cross-neutralizing antigenic sites of HCV glycoproteins E1 (residues 314–324) and E2 (residues 412–423) have been identified as major targets for the antibody response [120]. He et al. presented in silico studies of multivalent E1 and E2 epitopes on ferritin nanoparticles and found that conjugation of the epitopes to the ferritin nanoparticles significantly increased antibody binding [120]. A more recent in vivo study demonstrated that a ferritin nanoparticle vaccine displaying the E2 antigen markedly enhanced the neutralizing antibody response against several clinically adaptive HCV strains compared to the unfused antigen in murine models [121].
8.4. HIV
Ferritin has also been used as an antigen display platform in the search for a safe and effective vaccine against HIV, particularly focusing on the viral gp120 glycoprotein, the major component of the viral envelope [73]. Zhou et al. demonstrated that grafting of a glycopeptide from the variable region 3 on gp120 onto ferritin nanoparticles resulted in successful recognition by neutralizing antibodies from three different donors whereas the free antigen failed to be recognized. These results suggest that the structural integrity provided by the ferritin scaffold was critical to elicit immunogenicity [122].
8.5. Lyme disease
Lyme disease, the most common tick-borne disease in the United States and Europe caused by Borrelia burgdorferi, poses extreme challenges to public health given the challenges in its diagnosis and treatment, and lack of a vaccine. In a recent study, OspA, a lipoprotein expressed on the outer membrane surface of B. burgdorferi and the main target of antibody response, was genetically fused to the N-terminus of H. pylori ferritin to produce a vaccine displaying 24 OspA proteins on the nanoparticle surface [123]. The OspA-ferritin nanoparticle vaccine elicited high antibody titers against seven major serotypes in mice and non-human primates, higher than those achieved by a previously licensed vaccine [123]. In addition, the high antibody titer was demonstrated to be maintained in rhesus macaques for at least 6 months following vaccination [123], suggesting this multivalent ferritin vaccine has the potential to offer broad, and long-lasting immune protection against Borrelia infection.
8.6. Respiratory syncytial virus (RSV)
Respiratory syncytial virus (RSV), a leading cause of respiratory illness in immunocompromised individuals, has also been a target of ferritin vaccine development. Swanson et al. engineered H. pylori ferritin to express eight prefusion RSV moieties on its surface, and upon immunization elicited strong pre-F specific neutralizing antibodies in mice, nonhuman primates, and in vitro human cells when combined with the squalene-based adjuvant AF03, especially compared to the antigen alone [124].
8.7. SARS-CoV-2
The unprecedented development and roll-out of hundreds of vaccines to mitigate the SARS-CoV-2 pandemic also involved the use of ferritin and other nanoparticles as platforms. In an early approach, 72 amino acids of the receptor binding motif of the Sars-cov2 spike were fused to the N-terminus of human H ferritin. The construct was expressed in E. coli as an insoluble protein that could be renatured and was able to bind the receptor ACE2 [166]. Most of the following studies were based on Helicobacter pylori ferritin to avoid the induction of antibodies of the human protein [167]. For example, Guo et al. modified the receptor binding domain (RBD) of the spike protein to include four additional glycosylation sites, and upon covalent attachment to H. pylori ferritin the complex was shown to elicit more potent neutralizing antibody responses than the wild-type RBD in mice [167]. A similar approach of fusing the RBD directly to the N-terminus of H. pylori ferritin and expressing the protein in mammalian cells was used in other works [125,168,169]. These vaccines were tested in mice, ferrets or monkeys and always produced neutralizing antibodies at levels much higher than the RBD alone.
The SpyTag-SpyCatcher system was then used in various studies to produce RBD exposed to H. pylori ferritin. Zhang et al. [170] added at the N-terminus a signal peptide followed by a spy-tag, and then a glycosylation site was included to facilitate expression and secretion by mammalian cells. The product purified by the supernatant of the transfected cells was then bound to SARS-CoV-2 spike glycoprotein containing a spy-catcher. The complex was stable and considered for vaccine development. Other studies inserted SpyCatcher sequence at the N-terminus of H. pylori ferritin that was expressed in E. coli and bound it to RBD containing SpyTag and the products were shown to induce strong immune response in mice [[162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173]]. In one study the RBD was added to a modified heptad repeat 2 (HR2) that increased stability and antibody titer [174]. The RBD was also coupled to the 12-mer ferritin-like Dps from hyperthermophilic Sulfolobus islandicus and it elicited neutralizing antibodies [175].
A more recent challenge to SARS-COV2 vaccines is the identification of variants of concern (VOCs), five of which have been defined by The World Health Organization since the spread of the original Wuhan Hu-1 strain of SARS-CoV-2, four of which have previously circulated; (i) Alpha (B.1.1.7), (ii) Beta (B.1.351), (iii) Gamma (P.1), and Delta (B.1.617.2), as well as one currently circulating; (iv) Omicron (B.1.1.529) [176,177]. Multiple studies have observed a substantial reduction in vaccine-elicited neutralization capacity across all variants of concern, especially the recent Omicron variant [155,[176], [177], [178], [179], [180], [181]]. Given the number of infections and re-infections, as well as the cost and supply-chain issues of booster programs with currently available vaccines requiring ultra-cold storage, there exists a great rationale for the development of a safe, cost effective, and thermostable SARS-CoV-2 vaccine that provides broad protection against future emerging variants, and possibly other sarbecoviruses. Given the success of the multimerization platform approach of presenting epitopes on ferritin nanoparticles in preclinical trials against viruses, there has been a great interest in the use of a ferritin platform as a second-generation vaccine against SARS-CoV-2. Powell et al. designed H. pylori ferritin nanoparticles fusing one of two SARS-CoV-2 spikes: (i) full length ectodomain (residues 1–1143) or (ii) a C-terminal 70 amino-acid deletion ectodomain, resulting in protein nanoparticles displaying eight copies of a trimeric antigen on the surface of the 3-fold axis [125]. Following a single immunization with either immunogen, mice exhibited neutralizing antibody titers 2-fold greater than those in convalescent plasma from COVID-19 patients, whereas immunization with unconjugated trimers elicited little to no neutralizing titers with the same dose [122]. In a similar study, researchers at the Walter Reed Army Institute of Research and their collaborators designed a spike-domain ferritin nanoparticle (SpFN) vaccine by genetically linking an H. pylori ferritin molecule to the C-terminal region of the spike protein ectodomain (residues 12–1158), generating a ferritin nanoparticle displaying eight trimeric spikes [126]. Following a single immunization in mice, SpFN elicited potent neutralizing antibody titers against both SARS-CoV-2 (infective dose ID50 > 10,000) and SARS-CoV-1(ID50 > 2,000) which has 26% sequence divergence in the spike protein, thereby demonstrating broad cross protection against different coronaviruses [126,127]. Furthermore, the immune response elicited by these ferritin nanoparticle vaccines were tested in combination with the adjuvants ALFQ (Army Liposome Formulation containing QS-210) and Alhydrogel (AH) and were found to be consistently superior with the ALFQ adjuvant in mice [126]. In a companion study in non-human primates, Joyce et al. demonstrated that the SpFN vaccine combined with the ALFQ adjuvant elicited high titers of antibodies that neutralized SARS-CoV-2 and rapidly eliminated the virus in the upper and lower airways and lung parenchyma [129]. Remarkably, the adjuvanted SpFN vaccine elicited neutralization activity that was either equivalent to, or higher than the wild-type virus against four variants of concern [B.1.1.7 (alpha variant), B.1.351 (beta variant), P.1 (gamma variant), and B.1.617.2 (delta variant)], as well as against SARS-CoV-1 [129]. Similarly, Wuertz et al. demonstrated that SpFN-ALFQ generates a robust immune response against the RBD and spike proteins of the alpha and beta variants with a 2-dose regimen in hamsters [130]. In a comprehensive study of the cellular immune responses induced by the adjuvanted SpFN vaccine, Carmen et al. found that after immunization, mice showed a potent multifactorial immune response, particularly with increased frequency of polyfunctional spike-specific memory CD4+ T cells, and long-lived memory CD8+ T cells with effective cytolytic function and distribution to the lungs [131]. The effectivity and broad protection of the SpFN-ALFQ vaccine against multiple VOCs of SARS-CoV-2 in rodent and non-human primate models has led to its evaluation in human phase I clinical trial (NCT04784767), although no results have been posted as of November 2022.
In addition to the SpFN vaccine, Joyce and collaborators have developed a secondary vaccine candidate in which bullfrog-H. pylori chimeric ferritin was genetically linked to the C-terminal region of the receptor binding domain (RBD) on the S1 subunit (residues 331–527) [126]. In mice, two doses of the RBD-ferritin vaccine elicited comparable neutralizing antibody titers to just one dose of the SpFN vaccine, however the RBD-ferritin vaccine elicited higher SARS-CoV-1 neutralizing responses compared to SpFN, which is 36% aa sequence divergent from SARS-CoV-2 in the RBD [126,127]. In a companion study evaluating the RBD-ferritin vaccine in combination with the ALFQ adjuvant in macaques, King et al. found that a two-dose regimen resulted in a robust humoral and cellular immune response, as well as protection against viral replication and lung pathology following respiratory exposure to a high dose of SARS-CoV-2 virus [128]. Additionally, RBD-ferritin vaccination in macaques demonstrated cross reactivity to the alpha (B.1.1.7) and beta (B.1.351) VOCs as well as to SARS-CoV-1 [128]. While the SpFN-ALFQ vaccine had been chosen to continue into clinical testing due to the level of immune response observed after a single immunization, further investigation of the RBD-ferritin vaccine is warranted given its potent immunogenicity. Given that expression of a large and complex antigen takes a considerable amount of energy, one advantage to the RBD-ferritin vaccine is its smaller size compared to the SpFN vaccine, manifesting as greater production yields (> 20 mg/L compared to 5 mg/L, respectively) [126], which may be beneficial to large-scale manufacturing efforts.
8.8. Mosaic vaccines
As described above, protein nanocages engineered with multiple copies of a single antigen have been very successful in eliciting a robust immune response and broad protection against viral pathogens. However, the prospect of furbishing nanocages with multiple different proteins from different viral strains remains mostly unexplored, but has great potential in stimulating broader immune protection, especially against viruses with high mutation rates. Successful examples of vaccination with multiple antigenic types include four FDA licensed pneumococcal vaccines; Prevar13 ®, Vaxneuvance®, Prevnar20®, and Pneumovax23®, which contain purified preparations of pneumococcal bacteria capsular polysaccharides from 13, 15, 20, and 23 different serotypes, respectively [182], as well as the FDA licensed Gardasil-9 ® vaccine which contains purified proteins from 9 human papillomavirus (HPV) types [183]. While these multivalent vaccines have shown to be highly effective in humans, similar approaches may not be as successful against pathogens with high rates of mutation which results in substantial antigenic variation. A solution to this issue may be to engineer multivalent vaccines to present more conserved domains or subdomains of viral proteins to elicit response by cross-reactive immune cells. Kanekiyo et al. demonstrated that co-display of RBDs from hemagglutinin antigens from different influenza strains spanning over 90-years on bullfrog-H. pylori chimeric ferritin nanoparticles elicited superior antibody responses in mice than those induced by the antigenically homotypic immunogens, even when the separate homotypic RBD-np vaccines are mixed [12]. Similarly, a trivalent mosaic HIV-1 vaccine displaying envelope (Env) sequences provided 66% protection against heterologous challenges with simian immunodeficiency virus (SIV) in Rhesus monkeys [184] and is currently being evaluated in human clinical trials. This immunofocusing approach has also been used to construct mosaic nanoparticle vaccine co-displaying the RBD of SARS-CoV-2 along with RBDs from seven animal sarbecoviruses on a 60-mer protein nanoparticle [133]. This approach has been demonstrated to elicit broad binding and neutralizing responses against both human and bat viruses in mice and macaques [132,133]. Immunization of animals with the homotypic and the mosaic vaccine resulted in protection against SARS-CoV-2 challenge, despite the mosaic vaccine containing just 1/8th of the SARS-CoV-2 RBDs compared to the homotypic vaccine [132]. Moreover, only animals immunized with the mosaic vaccine demonstrated significant protection against SARS-CoV-1 and animal sarbecoviruses whose RBDs were not displayed on the nanoparticles [132]. Additionally, it has been demonstrated that a conserved internal nucleoprotein antigen peptide from influenza virus can be encapsulated within apoferritin, which when coupled with HA-surface conjugation mimicked the structure of the natural influenza virus. Such arrangement provided 100% protection against lethal doses of H1N1 strains in mice compared to just 60% protection in the vaccine containing only the HA protein on the outer surface [185]. Given the potential to display RBDs on the outer surface as well as encapsulate antigenic peptides within the protein cavity, ferritin can be used as a platform for development of a mosaic vaccine (Fig. 6 ) against SARS-CoV-2 to confer broad protection against viral variants [186].
Fig. 6.
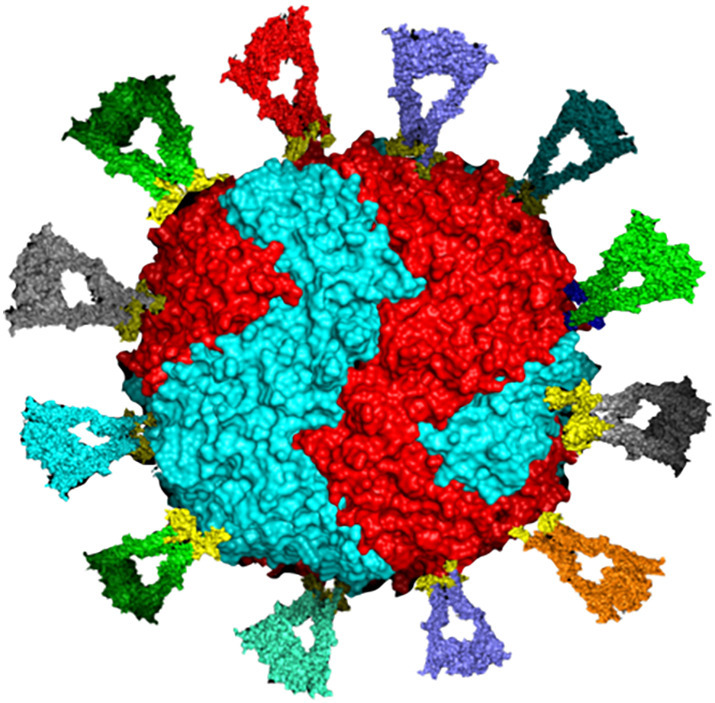
Schematic illustration of surface engineering of human heteropolymer ferritin with 12 different and randomly placed RBDs to represent the prospect of a universal mosaic ferritin nanocage vaccine candidate that could offer broader neutralizing antibody capability. This design could carry up to 24 RBDs and an antigenic peptide inside the protein cavity to improve immune response.
9. Concluding remarks and future prospects
The intracellular trafficking and in vivo behavior and fate of vaccines administered via various routes and delivery systems have been recently reviewed [187]. In general, because of limited accessibility and/or inefficient cell permeation, most vaccine molecules are not efficiently recognized by the immune system and thus are susceptible to degradation. However, effective vaccine delivery systems should encompass protection from enzymatic degradation, improvement of pharmacokinetic properties through surface engineering strategies such as PEGylation, and sophisticated vaccine targeting and delivery strategies, as discussed in detail elsewhere [187]. Ferritin nanoparticles are remarkably stable, biocompatible, and amenable to genetic fusion and chemical conjugation, making the protein nanocage a very attractive platform for a wide range of biomedical applications, especially as an antigen display platform in the development of vaccines. The ability to express and purify ferritin in bacterial and animal cultures using industrial processes already in place allows for cost-effective large-scale manufacturing. More importantly, given the ability to selectively engineer antigen display and fine-tune immune focusing, ferritin vaccines are one effective and practical approach that have shown good safety profiles in comparison to more traditional approaches such as whole pathogen vaccines. Indeed, the pre-clinical successes of ferritin vaccines eliciting immune responses against a wide range of pathogens has led to its investigation in three phase I clinical trials, including a SpFN-ALFQ vaccine against SARS-CoV-2 [126]. However, there remain challenges in the design and manufacturing of antigen conjugated nanoparticles vaccines due to steric and conformational constraints. In silico studies and computational models to optimize conjugation of antibodies, targeting efficiency, and improve immunogenicity, will be crucial to vaccine development and preclinical evaluation. A different approach to developing a multivalent vaccine is an mRNA vaccine encoding conserved antigens from multiple viral strains. For instance, an experimental mRNA vaccine encoding 20 HA influenza antigens has been shown to elicit high levels of cross reactive and subtype-specific antibodies in mice and is moving toward clinical trials in humans [188]. While a multi-valent mRNA vaccine avoids the steric challenges posed by antigen conjugation to a protein nanoparticle, the requirement for ultra-cold storage provides rationale for further exploration of thermostable ferritin vaccines. While ferritin vaccines hold a great promise in terms of eliciting broad protection and circulation of neutralizing antibodies in the bloodstream against SARS-CoV-2 variants of concerns, mucosal vaccines are another potential avenue to block mild covid-related illnesses and prevent transmission. A nasal vaccine for instance could spur a broader and more lasting immune response, by targeting parts of the body (i.e. nose and throat) where SARS-CoV-2 prefers to lodge before spreading to the lungs and other organs. Thus, instead of focusing on a circulating, whole body immune response, a mucosal vaccine would activate immune cells in the mucosal tissue of the upper respiratory tract. Currently, there are several nasal vaccines with two leading efforts, one at the Coalition for Epidemic Preparedness Innovations in Norway, and the other at the U.S. National Institute of Allergy and Infectious Diseases. It is worth noting that two SARS-CoV-2 vaccines administered through the nose or mouth, developed by the Chinese companies CanSino Biologics and Beijing Wantai Biological Pharmacy Enterprise Co Ltd, have been approved for use as boosters in China [[189], [190]]. If proven safe and effective, mucosal vaccines in tandem with a potentially universal ferritin nanoparticle vaccine may offer reliable solutions to cease viral transmission and boost global immunity against the current SARS-CoV-2 pandemic, and future zoonotic viruses. In addition to mosaic nanoparticles approach, there is about a dozen major universal or pan-coronavirus vaccines that are currently in development designed to induce immunity against SARS-CoV-2 and other related coronaviruses. The broad protective antibody response of these different vaccines, compared to a homotypic nanoparticle approach, demonstrate the usefulness of the 24-mer ferritin (12 nm diameter), and perhaps other ferritin-like architectures such as the 48-mer assembled ferritin (17 nm) [191] or the 60, 180 or 240-mer encapsulin nanoparticles (20 to 42 nm diameter) [192], as highly promising and effective approaches for vaccine development.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by the US National Science Foundation, Division of Molecular and Cellular Biosciences (MCB) award 1934666 (F.B.-A.), the US National Institute of Health grant R15GM104879 (F.B.-A.), and a Cottrell Instrumentation Supplements Award from the Research Corporation for Science Advancement award 27452 (F.B.-A.)
Data availability
No data was used for the research described in the article.
References
- 1.The World Health Organization https://covid19.who.int
- 2.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2021;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Zu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Vaccine Development and Approvals Tracker https://covid19.trackvaccines.org
- 5.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Charlie G., Ródes-Guirao L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 6.COVID-19 vaccine tracker and landscape World Health Organization. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 7.Greaney A.J., Loes A.N., Crawford K.H.D., Starr T.N., Malone K.D., Chu H.Y., Bloom J.D. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021;29:463–476. doi: 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., Iketani S., Guo Y., Chan J.F.W., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., Yu J., Chik K.K.H., Yuen T.T.T., Yoon C., To, K. K. W, Chen H., Yin M.T., Sobieszczyk M.E., Huang Y., Wang H.H., Sheng Z., Yuen K.Y., Ho D.D. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 9.Kanekiyo M., Wei C.J., Yassine H.M., McTamney P.M., Boyington J.C., Whittle J.R.R., Rao S.S., Kong W.P., Wang L., Nabel G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013;499:102–106. doi: 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pati R., Shevtsov M., Sonawane A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018;9:2224. doi: 10.3389/fimmu.2018.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darricarrère N., Qiu Y., Kanekiyo M., Creanga A., Gillespie R.A., Moin S.M., Saleh J., Sancho J., Chou T.H., Zhou Y., Zhang R., Dai S., Moody A., Saunders K.O., Crank M.C., Mascola J.R., Graham B.S., Wei C.J., Nabel G.J. Broad neutralization of H1 and H3 viruses by adjuvanted influenza HA stem vaccines in nonhuman primates. Sci. Transl. Med. 2021;13(583) doi: 10.1126/scitranslmed.abe5449. [DOI] [PubMed] [Google Scholar]
- 12.Kanekiyo M., Joyce M.G., Gillespie R.A., Gallagher J.R., Andrews S.F., Yassine H.M., Wheatley A.K., Fisher B.E., Ambrozak D.R., Creanga A., Leung K., Yang E.S., Boyoglu-Barnum S., Georgiev I.S., Tsybovsky Y., Prabhakaran M.S., Anderson H., Kong W.P., Baxa U., Zephir K.L., Ledgerwood J.E., Koup R.A., Kwong P.D., Harris A.K., McDermott A.B., Mascola J.R., Graham B.S. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 2019;20:362–372. doi: 10.1038/s41590-018-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanekiyo M., Bu W., Joyce M.G., Meng G., Whittle J.R.R., Baxa U., Yamamoto T., Narpala S., Todd J.P., Rao S.S., McDermott A.B., Cohen J.I., Nabel G.J. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell. 2015;162(5):1090–1100. doi: 10.1016/j.cell.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhaskar S., Lim S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017;9 doi: 10.1038/am.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curley S.M., Putnam D. Biological nanoparticles in vaccine development. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.867119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vu M.N., Kelly H.G., Kent S.J., Wheatley A.K. Current and future nanoparticle vaccines for COVID-19. Lancet. 2021;74 doi: 10.1016/j.ebiom.2021.103699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habibi N., Mauser A., Ko Y., Lahann J. Protein nanoparticles: uniting the power of proteins with engineering design approaches. Adv. Sci. (Weinh). 2022;9(8):2104012. doi: 10.1002/advs.202104012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bou-Abdallah F. The iron redox and hydrolysis chemistry of the ferritins. Biochim. Biophys. Acta. 2010;719-731 doi: 10.1016/j.bbagen.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Khoshnejad M., Parhiz H., Shuvaev V.V., Dmochowski I.J., Muzykantov V.R. Ferritin-based drug delivery systems: hybrid nanocarriers for vascular immunotargeting. J. Control. Release. 2018;282:13–24. doi: 10.1016/j.jconrel.2018.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews S.C. Iron storage in bacteria. Adv. Microb. Physiol. 1988;40:281–351. doi: 10.1016/s0065-2911(08)60134-4. [DOI] [PubMed] [Google Scholar]
- 21.Harrison P.M., Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta. 1996;1275(22):161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 22.Chasteen N.D., Ferritin. Uptake, storage, and release of iron. Met. Ions Biol. Syst. 1998;35:479–514. [PubMed] [Google Scholar]
- 23.Arosio P., Ingrassia R., Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta. 2009;1790(7):589–599. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Theil E.C. The ferritin family of iron storage proteins. Adv. Enzymol. Relat. Areas Mol. Biol. 1990;63:421–496. doi: 10.1002/9780470123096.ch7. [DOI] [PubMed] [Google Scholar]
- 25.Arosio P., Elia L., Poli M. Ferritin, cellular iron storage and regulation. IUBMB Life. 2017;69:414–422. doi: 10.1002/iub.1621. [DOI] [PubMed] [Google Scholar]
- 26.Ceci P., Forte E., Di Cecca G., Fornara M., Chiancone E. The Characterization of Thermotoga Maritima ferritin reveals an unusual subunit dissociation behavior and efficient DNA protection from iron-mediated oxidative stress. Extremophiles. 2011;15:431–439. doi: 10.1007/s00792-011-0374-3. [DOI] [PubMed] [Google Scholar]
- 27.Bou-Abdallah F., Biasiotto G., Arosio P., Chasteen N.D. The putative “nucleation site” in human H-chain ferritin is not required for mineralization of the iron core. Biochemistry. 2004;43(14):4332–4337. doi: 10.1021/bi0498813. [DOI] [PubMed] [Google Scholar]
- 28.Drysdale J.W., Adelman T.G., Arosio P., Casareale D., Fitzpatrick P., Harzard J.T., Yokota M. Human isoferritins in normal and disease states. Semin. Hematol. 1977;14:71–88. [PubMed] [Google Scholar]
- 29.Santambrogio P., Levi S., Cozzi A., Rovida E., Albertini A., Arosio P. Production and characterization of recombinant heteropolymers of human Ferritin H and L Chains. J. Biol. Chem. 1993;268:12744–12748. [PubMed] [Google Scholar]
- 30.Levi S., Santambrogio P., Cozzi A., Rovida E., Corsi B., Tamborini E., Spada A., Albertini A., Arosio P. The role of the L- chain in Ferritin iron incorporation. J. Mol. Biol. 1994;238:649–654. doi: 10.1006/jmbi.1994.1325. [DOI] [PubMed] [Google Scholar]
- 31.Lee J., Seo H.Y., Jeon E.S., Park O.S., Lee K.M., Park C.U., Kim K.S. Cooperative activity of subunits of human Ferritin heteropolymers in Escherichia coli. Biochem. Mol. Biol. 2001;34:365–370. [Google Scholar]
- 32.Kim H.J., Kim H.M., Kim J.H., Ryu K.S., Park S.M., Jahng K.Y., Yang M.S., Kim D.H. Expression of heteropolymeric ferritin improves iron storage in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2003;69:1999–2005. doi: 10.1128/AEM.69.4.1999-2005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rucker P., Torti F.M., Torti S.V. Recombinant ferritin: 755 modulation of subunit stoichiometry in bacterial expression systems. Protein Eng. 1997;10:967–973. doi: 10.1093/protein/10.8.967. [DOI] [PubMed] [Google Scholar]
- 34.Carmona F., Poli M., Bertuzzi M., Gianoncelli A., Gangemi F., Arosio P. Study of ferritin self-assembly and heteropolymer formation by the use of Fluorescence Resonance Energy Transfer (FRET) technology. Biochim. Biophys. Acta. 2017;1861(3):522–532. doi: 10.1016/j.bbagen.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Theil E.C. Ferritins: dynamic management of biological iron and oxygen chemistry. Acc. Chem. Res. 2005;38(3):167–175. doi: 10.1021/ar0302336. [DOI] [PubMed] [Google Scholar]
- 36.Douglas T., Ripoli D.R. Calculated electrostatic gradients in recombinant human H-chain ferritin. Protein Sci. 1998;7(5):1083–1091. doi: 10.1002/pro.5560070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahaski T., Kuyucak S. Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophys. J. 2003;84(4):2256–2263. doi: 10.1016/S0006-3495(03)75031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bou-Abdallah F., Arosio P., Levi S., Janus-Chandler C., Chasteen N.D. Defining metal ion inhibitor interactions with recombinant human H- and L-chain ferritins and site-directed variants: an isothermal titration calorimetry study. J. Biol. Inorg. Chem. 2003;8:489–497. doi: 10.1007/s00775-003-0455-6. [DOI] [PubMed] [Google Scholar]
- 39.Bou-Abdallah F., Arosio P., Santambrogio P., Yang X., Janus-Chandler C., Chasteen N.D. Ferrous ion binding to recombinant human H-chain ferritin. An isothermal titration calorimetry study. Biochemistry. 2002;41:11184–11191. doi: 10.1021/bi020215g. [DOI] [PubMed] [Google Scholar]
- 40.Theil E.C., Takagi H., Small G.W., He L., Tipton A.R., Danger D. The ferritin iron entry and exit problem. Inorg. Chim. Acta. 2000;297(297):242–251. [Google Scholar]
- 41.Levi S., Santambrogio P., Corsi B., Cozzi A., Arosio P. Evidence that residues exposed on the three-fold channels have active roles in the mechanism of ferritin iron incorporation. Biochem. J. 1996;317:467–473. doi: 10.1042/bj3170467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bou-Abdallah F., Zhao G., Biasiotto G., Poli M., Arosio P., Chasteen N.D. Facilitated diffusion of iron(II) and dioxygen substrates into human H-chain ferritin, a fluorescence and absorbance study employing the ferroxidase center substitution. J. Am. Chem. Soc. 2008;130(52):17801–17811. doi: 10.1021/ja8054035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theil E.C. Ferritin: the protein nanocage and iron biomineral in health and in disease. Inorg. Chem. 2013;52(21):12223–12233. doi: 10.1021/ic400484n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel F.M., Ehm L., Antao S.M., Lee P.L., Chupas P.J., Liu G., Strongin D.R., Schoonen M.A.A., Phillips B.L., Parise J.B. The structure of ferrihydrite, a nanocrystalline material. Science. 2007;316:1726–1729. doi: 10.1126/science.1142525. [DOI] [PubMed] [Google Scholar]
- 45.Theil E.C. Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry. Curr. Opin. Chem. Biol. 2011;15:304–311. doi: 10.1016/j.cbpa.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St. Pierre T., Tran K.C., Webb J., Macey D.J., Heywood B.R., Sparks N.H., Wade V.J., Manna S., Pootrakul P. Organ-specific crystalline structures of ferritin cores inthalassemia/hemoglobin E. Biol. Met. 1991;4:162–165. doi: 10.1007/BF01141308. [DOI] [PubMed] [Google Scholar]
- 47.López-Castro J.D., Delgado J.J., Perez-Omil J.A., Gálvez N., Cuesta R., Watt R.K., Domínguez-Vera M. A new approach to the ferritin iron core growth: influence of the H/L ratio on the core shape. Dalton Trans. 2012;41:1320–1324. doi: 10.1039/c1dt11205h. [DOI] [PubMed] [Google Scholar]
- 48.Mann S., Williams J.M., Treffry A., Harrison P.M. Reconstituted and native iron-cores of bacterioferritin and ferritin. J. Mol. Biol. 1987;198:405–416. doi: 10.1016/0022-2836(87)90290-7. [DOI] [PubMed] [Google Scholar]
- 49.Wade V.J., Treffry A., Laulher̀e J.P., Bauminger E.R., Cleton M.I., Mann S., Briat J.-F., Harrison P.M. Structure and composition of ferritin cores from pea seed (Pisum sativum) Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1993;1161:91–96. doi: 10.1016/0167-4838(93)90201-2. [DOI] [PubMed] [Google Scholar]
- 50.Michel F.M., Barrón V., Torrent J., Morales M.P., Serna J., Boily J.F., Liu Q., Ambrosini A., Cismasu A.C., Brown G.E. Ordered ferrimagnetic form of ferrihydrite reveals links among structure, composition, and magnetism. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2787–2792. doi: 10.1073/pnas.0910170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao G. Phytoferritin and its implications for human health and nutrition. Biochim. Biophys. Acta. 2010;1800:815–823. doi: 10.1016/j.bbagen.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Rohrer J.S., Islam Q.T., Watt G.D., Sayers D.E., Theil E.C. Iron environment in ferritin with large amounts of phosphate, from Azotobacter vinelandii and horse spleen, analyzed using extended X-ray absorption fine structure (EXAFS) Biochemistry. 1990;29:259–264. doi: 10.1021/bi00453a035. [DOI] [PubMed] [Google Scholar]
- 53.Reutovich A.A., Srivastava A.K., Smith G.L., Foucher A., Yates D.M., Stach E.A., Papaefthymiou G.C., Arosio P., Bou-Abdallah F. Effect of phosphate and ferritin subunit composition on the kinetics, structure, and reactivity of the iron core in human homo- and heteropolymer ferritins. Biochemistry. 2022;61(19):2106–2117. doi: 10.1021/acs.biochem.2c00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stefanini S., Cavallo S., Wang C.Q., Tataseo P., Vecchini P., Giartosio A., Chiancone E. Thermal stability of horse spleen apoferritin and human recombinant H apoferritin. Arch. Biochem. Biophys. 1996;325:58–64. doi: 10.1006/abbi.1996.0007. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X., Zang J., Chen H., Zhou K., Zhang T., Lv C., Zhao G. Thermostability of protein nanocages: the effect of natural extra peptide on the exterior surface. RSC Adv. 2019;9(43):24777–24782. doi: 10.1039/c9ra04785a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNally J.R., Mehlenbacher M.R., Luscieti S., Smith G.L., Reutovich A.A., Maura P., Arosio P., Bou-Abdallah F. Mutant L-chain ferritins that cause neuroferritinopathy alter ferritin functionality and iron permeability. Metallomics. 2019;11(10):1635–1647. doi: 10.1039/c9mt00154a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan X., Liu Y., Zang J., Zhang T., Zhao G. Hyperthermostability of prawn ferritin nanocage facilitates its application as a robust nanovehicle for nutraceuticals. Int. J. Biol. Macromol. 2021;191:152–160. doi: 10.1016/j.ijbiomac.2021.09.067. [DOI] [PubMed] [Google Scholar]
- 58.Tatur J., Hagedoorn P.L., Overeijnder M.L., Hagen W.R. A highly thermostable ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus. Extremophiles. 2006;10(2):139–148. doi: 10.1007/s00792-005-0484-x. [DOI] [PubMed] [Google Scholar]
- 59.Tatur J., Hagen W.R., Matias P.M. Crystal structure of the ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus. J. Biol. Inorg. Chem. 2007;12(5):615–630. doi: 10.1007/s00775-007-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Jin W., Theil E.C. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3653–3658. doi: 10.1073/pnas.0636928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khoshnejad M., Parhiz H., Shuvaev V.V., Dmochowski I.J., Muzykantov V.R. Ferritin-based drug delivery systems: hybrid nanocarriers for vascular immunotargeting. J. Control. Release. 2018;282:13–24. doi: 10.1016/j.jconrel.2018.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srivastava A.K., Scalcione L.J., Arosio P., Bou-Abdallah F. Hyperthermostable recombinant human heteropolymer ferritin derived from a novel plasmid design. Protein Sci. 2022 doi: 10.1002/pro.4543. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerl M., Jaenicke R. Mechanism of the self-assembly of apoferritin from horse spleen. Eur. Biophys. J. 1987;15:103–109. doi: 10.1007/BF00257503. [DOI] [PubMed] [Google Scholar]
- 64.Gerl M., Jaenicke R., Smith J.M.A., Harrison P.M. Self-assembly of apoferritin from horse spleen after reversible chemical modification with 2,3-Dimethylmaleic anhydride. Biochemistry. 1988;27:4089–4096. doi: 10.1021/bi00411a027. [DOI] [PubMed] [Google Scholar]
- 65.Sato D., Ohtomo H., Yamada Y., Hikima T., Kurobe A., Fujiwara K., Ikeguchi M. Ferritin assembly revisited: a time-resolved small-angle X-ray scattering study. Biochemistry. 2016;55:287–293. doi: 10.1021/acs.biochem.5b01152. [DOI] [PubMed] [Google Scholar]
- 66.Mohanty A., Mithra K., Jena S.S., Behera R.K. Kinetics of ferritin self-assembly by laser light scattering: impact of subunit concentration, pH and ionic strength. Biomacromolecules. 2021;22:1389–1398. doi: 10.1021/acs.biomac.0c01562. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y., Orner B.P. Self-assembly in the Ferritin Nano-cage protein superfamily. Int. J. Mol. Sci. 2011;12(8):5406–5421. doi: 10.3390/ijms12085406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin Y., He J., Fan K., Yan X. Ferritin variants: inspirations for rationally designing protein nanocarriers. Nanoscale. 2019;11:12449. doi: 10.1039/c9nr03823j. [DOI] [PubMed] [Google Scholar]
- 69.Fan R., Boyle A.L., Cheong V.V., Ng S.L., Orner B.P. A helix swapping study of two protein cages. Biochemistry. 2009;48:5623–5630. doi: 10.1021/bi900387t. [DOI] [PubMed] [Google Scholar]
- 70.Luzzago A., Cesareni G. Isolation of point mutations that affect the folding of the H-chain of human ferritin in E. coli. EMBO J. 1989;8:569–576. doi: 10.1002/j.1460-2075.1989.tb03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levi S., Luzzago A., Franceschinelli F., Santambrogio P., Cesareni G., Arosio P. Mutational analysis of the channel and loop sequences of human ferritin H-chain. Biochem. J. 1989;264:381–388. doi: 10.1042/bj2640381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levi S., Luzzago A., Cesareni G., Cozzi A., Franceschinelli F., Albertini A., Arosio P. Mechanism of ferritin iron uptake: activity of the H-Chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human-liver, recombinant H-Chain ferritins, and of 2 H-Chain deletion mutants. J. Biol. Chem. 1988;263:18086–18092. [PubMed] [Google Scholar]
- 73.Olshefsky A., Richardson C., Pun S.H., King N.P. Engineering self-assembling protein nanoparticles for therapeutic delivery. Bio. Conj. Chem. 2022;33(11):2018–2034. doi: 10.1021/acs.bioconjchem.2c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srivastava A.K., Arosio P., Poli M., Bou-Abdallah F. A novel approach for the synthesis of human heteropolymer ferritins of different H to L subunit ratios. J. Mol. Biol. 2021;433(19) doi: 10.1016/j.jmb.2021.167198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodrigues M.Q., Alves P.M., Roldão A. Functionalizing ferritin nanoparticles for vaccine development. Pharmaceuticals. 2021;13(10):1621. doi: 10.3390/pharmaceutics13101621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weidenbacher P., Musunuri S., Powell A.E., Tang S., Do J., Sanyal M., Kim P.S. Simplified purification of glycoprotein-modified ferritin nanoparticles for vaccine development. Biochemistry. 2022 doi: 10.1021/acs.biochem.2c00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson L.E., Wilkinson T., Arosio P., Melman A., Bou-Abdallah F. Effect of chaotropes on the kinetics of iron release from ferritin by flavin nucleotides. Biochim. Biophys. Acta. 2017;1861:3257–3262. doi: 10.1016/j.bbagen.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bou-Abdallah F., Paliakkara J.J., Melman G., Melman A. Reductive mobilization of iron from intact ferritin: mechanisms and physiological implication. Pharmaceuticals. 2018;11(4) doi: 10.3390/ph11040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Melman A., Bou-Abdallah F. Iron mineralization and core dissociation in mammalian homopolymeric H-Ferritin: current understanding and future perspectives. Biochim. Biophys. Acta. 2020;1864(11) doi: 10.1016/j.bbagen.2020.129700. [DOI] [PubMed] [Google Scholar]
- 80.Kim M., Rho Y., Jin K.S., Ahn B., Jung S., Kim H., Ree M. pH dependent structures of ferritin and apoferritin in solution: disassembly and reassembly. Biomacromolecules. 2011;12(5):1629–1640. doi: 10.1021/bm200026v. [DOI] [PubMed] [Google Scholar]
- 81.Li Z., Maity B., Hishikawa Y., Ueno T., Lu D. Importance of the subunit-subunit interface in ferritin disassembly: a molecular dynamics study. Langmuir. 2022;38(3):1106–1113. doi: 10.1021/acs.langmuir.1c02753. [DOI] [PubMed] [Google Scholar]
- 82.Aime S., Frullano L., Crich S.G. Compartmentalization of a gadolinium complex in the apoferritin cavity: a route to obtain high relaxivity contrast agents for magnetic resonance imaging. Angew. Chem. Int. Ed. Eng. 2002;41(6):1017–1019. doi: 10.1002/1521-3773(20020315)41:6<1017::aid-anie1017>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 83.Simsek E., Akif Kilic M. Magic ferritin: a novel chemotherapeutic encapsulation bullet. J. Magn. Magn. Mater. 2005;293:509–513. [Google Scholar]
- 84.Domínguez-Vera J., Colacio E. Nanoparicles of Prussian blue ferritin: a new route for obtaining nanomaterials. Inorg. Chem. 2003;42(22):6983–6985. doi: 10.1021/ic034783b. [DOI] [PubMed] [Google Scholar]
- 85.Sana B., Johnson E., Lim S. The unique self-assembly/disassembly property of Archaeoglobus fulgidus ferritin and its implications on molecular release from the protein cage. Biochim. Biophys. Acta. 2015;1850(12):2544–2551. doi: 10.1016/j.bbagen.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Kilic M.A., Ozlu E., Calis S. A novel protein-based anticancer drug encapsulating nanosphere: Apoferritindoxorubicin complex. J. Biomed. Nanotechnol. 2012;8:508–514. doi: 10.1166/jbn.2012.1406. [DOI] [PubMed] [Google Scholar]
- 87.Sun X., Hong Y., Gong Y., Zheng S., Xie D. Bioengineered ferritin nanocages for cancer therapy. Int. J. Mol. Sci. 2021;22(13):7023. doi: 10.3390/ijms22137023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang M., Fan K., Zhou M., Duan D., Zheng J., Yang D., Feng J., Yan X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. U. S. A. 2014;111:14900–14905. doi: 10.1073/pnas.1407808111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H., Zhang S., Xu C., Zhao G. Engineering protein interfaces yields ferritin disassembly and reassembly under benign experimental conditions. Chem. Commun. 2016;52:7402–7405. doi: 10.1039/c6cc03108k. [DOI] [PubMed] [Google Scholar]
- 90.Yang R., Liu Y., Meng D., Blanchard C.L., Zhou Z. Alcalase enzymolysis of red bean (adzuki) ferritin achieves nanoencapsulation of food nutrients in a mild condition. J. Agric. Food Chem. 2018;66:1999–2007. doi: 10.1021/acs.jafc.7b05656. [DOI] [PubMed] [Google Scholar]
- 91.Gu C., Zhang T., Lv C., Wang Y., Zhao G. His-meditated reversible self-assembly of ferritin nanocages through two different switches for Encapsulation of Cargo molecules. ACS Nano. 2020;14:17080–17090. doi: 10.1021/acsnano.0c06670. [DOI] [PubMed] [Google Scholar]
- 92.Yang R., Liu Y., Meng D., Wang D., Blanchard C.L., Zhou Z. Effect of atmospheric cold plasma on structure, activity, and reversible assembly of the phytoferritin. Food Chem. 2018;264:41–48. doi: 10.1016/j.foodchem.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 93.Meng D., Wang B., Zhen T., Zhang M., Yang R. Pulsed electric fields-modified ferritin realizes loading of rutin by a moderate pH transition. J. Agric. Food Chem. 2018;66:12404–12411. doi: 10.1021/acs.jafc.8b03021. [DOI] [PubMed] [Google Scholar]
- 94.Yildiz G., Andrade J., Engeseth N.E., Feng H. Functionalizing soy protein nano-aggregates with pH-shifting and mano-thermo-sonication. J. Colloid Interface Sci. 2017;505:836–846. doi: 10.1016/j.jcis.2017.06.088. [DOI] [PubMed] [Google Scholar]
- 95.Yang R., Liu Y., Meng D., Chen Z., Blanchard C.L., Zhou Z. Urea-driven epigallocatechin gallate (EGCG) permeation into the ferritin cage, an innovative method for fabrication of protein-polyphenol co-assemblies. J. Agric. Food Chem. 2017;65:1410–1419. doi: 10.1021/acs.jafc.6b04671. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y., Yang R., Liu J., Meng D., Zhou Z., Zhang Y., Blanchard C. Fabrication, structure, and function evaluation of the ferritin based nano-carrier for food bioactive compounds. Food Chem. 2019;299 doi: 10.1016/j.foodchem.2019.125097. [DOI] [PubMed] [Google Scholar]
- 97.Yang R., Liu Y., Blanchard C., Zhou Z. Channel directed rutin nano-encapsulation in phytoferritin induced by guanidine hydrochloride. Food Chem. 2018;240:935–939. doi: 10.1016/j.foodchem.2017.07.088. [DOI] [PubMed] [Google Scholar]
- 98.Yang R., Tian J., Liu Y., Yang Z., Wu D., Zhou Z. Thermally induced encapsulation of food nutrients into phytoferritin through the flexible channels without additives. J. Agric. Food Chem. 2017;65:9950–9955. doi: 10.1021/acs.jafc.7b03949. [DOI] [PubMed] [Google Scholar]
- 99.Chattopadhyay S., Chen J.Y., Chen H.W., Hu C.M.J. Nanoparticle vaccines adopting virus-like features for enhanced immune potentiation. Nanotheranostic. 2017;1(3):244–260. doi: 10.7150/ntno.19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang B., Chen X., Sun G., Chen X., Yin Y., Jin Y., Mi Q., Ma L., Yang Y., Yan X., Fan K. A natural drug entry channel in the ferritin nanocage. Nanotoday. 2020;35 [Google Scholar]
- 101.Chen H., Tan X., Han X., Ma L., Dai H., Fu Y., Zhang Y. Ferritin nanocage based delivery vehicles: from single-, co- to compartmentalized- encapsulation of bioactive or nutraceutical compounds. Biotechnol. Adv. 2022;61 doi: 10.1016/j.biotechadv.2022.108037. [DOI] [PubMed] [Google Scholar]
- 102.Zhang C., Zhang X., Zhao G. Ferritin nanocage: a versatile nanocarrier utilized in the field of food, nutrition, and medicine. Nanomaterials. 1894;2020:10. doi: 10.3390/nano10091894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huard D.J.E., Kane K.M., Tezcan F.A. Re-engineering protein interfaces yields copper-inducible ferritin cage assembly. Nat. Chem. Biol. 2013;9(3):169–176. doi: 10.1038/nchembio.1163. [DOI] [PubMed] [Google Scholar]
- 104.Johnson E., Cascio D., Sawaya M., Gingery R.M., Schröder I. Crystal structures of a tetrahedral open pore ferritin from the hyperthermophilic archaeon Archaeoglobus fulgidus. Structure. 2005;13(4):637–648. doi: 10.1016/j.str.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 105.Pulsipher K.W., Dmochowski I.J. Ferritin: versatile host, nanoreactor, and delivery agent. Isr. J. Chem. 2016;56:660–670. [Google Scholar]
- 106.Swift J., Butts C.A., Cheung-Lau J., Yerubandi V., Dmochowski I.J. Efficient self-assembly of Archaeoglobus fulgidus ferritin around metallic cores. Langmuir. 2009;25(9):5219–5225. doi: 10.1021/la8040743. [DOI] [PubMed] [Google Scholar]
- 107.Cheung-Lau J.C., Liu D., Pulsipher K.W., Liu W., Dmochowski I.J. Engineering a well-ordered, functional protein-gold nanoparticle assembly. J. Inorg. Biochem. 2014;130:59–68. doi: 10.1016/j.jinorgbio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 108.Bale J.B., Gonen S., Liu Y., Sheffler W., Ellis D., Thomas C., Cascio D., Yeates T.O., Gonen T., King N.P. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science. 2016;353(6297):389–394. doi: 10.1126/science.aaf8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Butterfield G.L., Lajoie M.J., Gustafson H.H., Sellers D.L., Nattermann U., Ellis D., Bale J.B., Ke S., Lenz G.H., Yehdego A., Ravichandran R., Pun S.H., King N.P. Evolution of a designed protein assembly encapsulating its own RNA genome. Nature. 2017;552(7685):415–420. doi: 10.1038/nature25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim S., Kim G.S., Seo J., Rangaswamy G.G., So I.S., Park R.W., Lee B.H., Kim I.S. Double-chambered ferritin platform: dual-function payloads of cytotoxic peptides and fluorescent protein. Biomacromolecules. .2016;17(1):12–19. doi: 10.1021/acs.biomac.5b01134. [DOI] [PubMed] [Google Scholar]
- 111.Wang Z., Zhao Y., Zhang S., Chen Z., Sun G., Zhang B., Jiang B., Yang Y., Yan X., Fan K. Re-engineering the inner surface of ferritin nanocages enables dual drug payloads for synergistic tumor therapy. Theranostics. 2022;12(4):1800–1815. doi: 10.7150/thno.68459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Swift J., Wehbi W.A., Kelly B.D., Stowell X.F., Saven J.G., Dmochowski I.J. Design of functional ferritin-like proteins with hydrophobic cavities. J. Am. Chem. Soc. 2006;128(20):6611–6619. doi: 10.1021/ja057069x. [DOI] [PubMed] [Google Scholar]
- 113.Uchida M., Klem M.T., Allen M., Suci P., Flenniken M., Gillitzer E., Carpness Z., Liepold L.O., Young M., Douglas T. Biological containers: protein cages as multifunctional nanoplatforms. Adv. Mater. 2007;19:1025–1042. [Google Scholar]
- 114.Wang Y.H., Jian M.L., Chen P.J., Tsou J.C., Truong L.P., Wang Y.S. Ferritin conjugates with multiple clickable amino acids encoded by C-terminal engineered pyrrolysyl-tRNA synthetase. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.779976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jutz G., van Rijn P., Miranda B.S., Böker A. Ferritin: a versatile building block for bionanotechnology. Chem. Rev. 2015;115:1653–1701. doi: 10.1021/cr400011b. [DOI] [PubMed] [Google Scholar]
- 116.Kanekiyo M., Wei C.J., Yassine H.M., McTamney P.M., Boyington J.C., Whittle J.R.R., Rao S.S., Kong W.P., Wang L., Nabel G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013;499:102–106. doi: 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yassine H.M., Boyington J.C., McTamney P.M., Wei C.J., Kanekiyo M., Kong W.P., Gallagher J.R., Wang L., Zhang Y., Joyce M.G., Lingwood D., Moin S.M., Andersen H., Okuno Y., Rao S.S., Harris A.K., Kwong P.D., Mascola J.R., Nabel G.J., Graham B.S. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015;21(9):1065–1070. doi: 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- 118.Houser K.V., Chen G.L., Carter C., Crank M.C., Nguyen T.A., Florez M.C.B., Berkowitz N.M., Mendoza F., Hendel C.S., Gordon I.J., Coates E.E., Vazquez S., Steim J., Case C.L., Lawlor H., Carlton K., Gaudinski M.R., Strom L., Hofstetter A.R., Liang C.J., Narpala S., Hatcher C., Gillespie R.A., Creanga A., Kanekiyo M., Raab J.E., Andrews S.F., Zhang Y., Yang E.S., Wang L., Leung K., Kong W.P., Freyn A.W., Nachbagauer R., Palese P., Bailer R.T., McDermott A.B., Koup R.A., Gall J.G., Arnold F., Mascola J.R., Graham B.S., Ledgerwood J.E. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat. Med. 2022;28:383–391. doi: 10.1038/s41591-021-01660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanekiyo M., Bu W., Joyce M.G., Meng G., Whittle J.R.R., Baxa U., Yamamoto T., Narpala S., Todd J.P., Rao S.S., McDermott A.B., Cohen J.I., Nabel G.J. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell. 2015;162(5):1090–1100. doi: 10.1016/j.cell.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He L., Cheng Y., Kong L., Azadnia P., Giang E., Kim J., Wood M.R., Wilson I.A., Law M., Zhu J. Approaching rational epitope design for hepatitis C virus with meta-server and multivalent scaffolding. Sci. Rep. 2015;5:12501. doi: 10.1038/srep12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yan Y., Wang X., Lou P., Hu Z., Qu P., Li D., Li Q., Xu Y., Niu J., He Y., Zhong J., Zhong H. A nanoparticle-based hepatitis C virus vaccine with enhanced potency. J. Infect. Dis. 2020;221(8):1304–1314. doi: 10.1093/infdis/jiz228. [DOI] [PubMed] [Google Scholar]
- 122.Zhou T., Zhu J., Yang Y., Gorman J., Ofek G., Srivatsan S., Druz A., Lees R.C., Lum G., Soto C., Stuckey J., Burton D.R., Koff W.C., Connors M., Kwong P.D. Transplanting supersites of HIV-1 vulnerability. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0099881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kamp H.D., Swanson K.A., Wei R.R., Dhal P.K., Dharanipragada R., Kern A., Sharma B., Sima R., Hajdusek O., Hu L.T., Wei C.J., Nabel G.J. Design of a broadly reactive Lyme disease vaccine. NPJ Vaccines. 2020;5:33. doi: 10.1038/s41541-020-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Swanson K.A., Rainho-Tomko J.N., Williams Z.P., Lanza L., Peredelchuk M., Kishko M., Pavot V., Alamares-Sapuay J., Adhikarla H., Gupta S., Chivukula S., Gallichan S., Zhang L., Jackson N., Yoon H., Wei C.J., Nabel G.J. A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci. Immunol. 2020;5(47):eaba6466. doi: 10.1126/sciimmunol.aba6466. [DOI] [PubMed] [Google Scholar]
- 125.Powell A.E., Zhang K., Sanyal M., Tang S., Weidenbacher P.A., Li S., Pham T.D., Pak J.E., Chiu W., Kim P.S. A single immunization with spike-functionalized ferritin vaccines elicits neutralizing antibody responses against SARS-CoV-2 in mice. ACS Cen. Sci. 2021;7(1):183–199. doi: 10.1021/acscentsci.0c01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Joyce M.G., Chen W.H., Sankhala R.S., Hajduczki A., Thomas P.V., Choe M., Martinez E.J., Chang W.C., Peterson C.E., Morrison E.B., Smith C., Chen R.E., Ahmed A., Wieczorek L., Anderson A., Case J.B., Li Y., Oertel T., Rosado L., Ganesh A., Whalen C., Carmen J.M., Mendez-Rivera L., Karch C.P., Gohain N., Villar Z., McCurdy D., Beck Z., Kim J., Shrivastava S., Jobe O., Dussupt V., Molnar S., Tran U., Kannadka C.B., Soman S., Kuklis C., Zemil M., Khanh H., Wu W., Cole M.A., Duso D.K., Kummer L.W., Lang T.J., Muncil S.E., Currier J.R., Krebs S.J., Polonis V.R., Rajan S., McTamney P.M., Esser M.T., Reiley W.W., Rolland M., de Val N., Diamond M.S., Gromowski G.D., Matyas G.R., Rao M., Michael N.L., Modjarrad K. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. Cell Rep. 2021;37(12) doi: 10.1016/j.celrep.2021.110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Verma J., Subbarao N. A comparative study of human betacoronavirus spike proteins: structure, function and therapeutics. Arch. Virol. 2021;166:697–714. doi: 10.1007/s00705-021-04961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.King H.A.D., Joyce M.G., Lakhal-Naour I., Ahmed A., Cincotta C.M., Subra C., Peachman K.K., Hack H.R., Chen R.E., Thomas P.V., Chen W.H., Sankhala R.S., Hajduczki A., Martinez E.J., Peterson C.E., Chang W.C., Choe M., Smith C., Headley J.A., Elyard H.A., Cook A., Anderson A., McGuckin Wuertz K., Dong M., Swafford I., Case J.B., Currier J.R., Lal K.G., Amare M.F., Dussupt V., Molnar S., Daye S.P., Zheng X., Barkei E.K., Alfson K., Staples H.M., Carrion R., Krebs S.J., Paquin-Proulx D., Karasavvas N., Polonis V.R., Jagodzinski L.L., Vasan S., Scott P.T., Huang Y., Nair M.S., Ho D.D., de Val N., Diamond M.S., Lewis M.G., Rao M., Matyas G.R., Gromowski G.D., Peel S.A., Michael N.L., Modjarrad K., Bolton D.L. Efficacy and breadth of adjuvanted SARS-CoV-2 receptor-binding domain nanoparticle vaccine in macaques. Proc. Natl. Acad. Sci. U. S. A. 2021;11(38) doi: 10.1073/pnas.2106433118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Joyce M.G., King H.A.D., Elakhal-Naouar I., Ahmed A., Peachman K.K., Macedo Cincotta C., Subra C., Chen R.E., Thomas P.V., Chen W.H., Sankhala R.S., Hajduczki A., Martinez E.J., Peterson C.E., Chang W.C., Choe M., Smith C., Lee P.J., Headley J.A., Taddese M.G., Elyard H.A., Cook A., Anderson A., McGuckin Wuertz K., Dong M., Swafford I., Case J.B., Currier J.R., Lal K.G., Molnar S., Nair M.S., Dussupt V., Daye S.P., Zeng X., Barkei E.K., Staples H.M., Alfson K., Carrion R., Krebs S.J., Paquin-Proulx D., Karasavva N., Polonis V.R., Jagodzinski L.L., Amare M.F., Vasan S., Scott P.T., Huang Y., Ho D.D., de Val N., Diamond M.S., Lewis M.G., Rao M., Matyas G.R., Gromowski G.D., Peel S.A., Michael N.L., Bolton D.L., Modjarrad K. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Sc. Transl. Med. 2021;14:623. doi: 10.1126/scitranslmed.abi5735. [DOI] [PubMed] [Google Scholar]
- 130.Wuertz K.M., Barkei E.K., Chen W.H., Martinez E.J., Lakhal-Naouar I., Jagodzinski L.L., Paquin-Proulx D., Gromowski G.D., Swafford I., Ganesh A., Dong M., Zeng X., Thomas P.V., Sankhala R.S., Hajduczki A., Peterson C.E., Kuklis C., Soman S., Wieczorek L., Zemil M., Anderson A., Darden J., Hernandez H., Grove H., Dussupt V., Hack H., de la Barrera R., Zarling S., Wood J.F., Froude J.W., Gagne M., Henry A.R., Mokhtari E.B., Mudvari P., Krebs S.J., Pekosz A.S., Currier J.R., Kar S., Porto M., Winn A., Radzyminski K., Lewis M.G., Vasan S., Suthar M., Polonis V.R., Matyas G.R., Boritz E.A., Douek D.C., Seder R.A., Daye S.P., Rao M., Peel S.A., Joyce M.G., Bolton D.L., Michael N.L., Modjarrad K. A SARS-CoV-2 spike ferritin nanoparticle vaccine protects hamsters against alpha and Beta virus variant challenge. NPJ Vaccines. 2021;6:129. doi: 10.1038/s41541-021-00392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carmen J.M., Shrivastava S., Lu Z., Anderson A., Morrison E.B., Sankhala R.S., Chen W.H., Chang W.C., Bolton J.J., Matyas G.R., Michael N.L., Joyce M.G., Modjarrad K., Currier J.R., Bergmann-Leitner E., Malloy A.M.W., Rao M. SARS-CoV-2 ferritin nanoparticle vaccine induces robust innate immune activity driving polyfunctional spike-specific T cell responses. NPJ Vaccines. 2021;6:151. doi: 10.1038/s41541-021-00414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohen A.A., Doremalen N.V., Greaney A., Anderson H., Sharma A., Starr T.N., Keeffe J.R., Fan C., Schulz J.E., Gnanapragasam P.N.P., Kakutani L.M., West A.P.J., Saturday G., Lee Y.E., Gao H., Jette C.A., Lewis M.G., Tan T.K., Townsend A.R., Bloom J.D., Munster V.J., Bjorkman P.J. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science. 2022;377:618. doi: 10.1126/science.abq0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cohen A.A., Gnanapragasam P.N.P., Lee Y.E., Hoffman P.R., Ou S., Kakutani L.M., Keeffe J.R., Wu H.J., Howarth M., West A.P., Barnes C.O., Nussenzweig M.C., Bjorkman P.J. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science. 2021;371(6530):735–741. doi: 10.1126/science.abf6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brune K.D., Howarth M. New routes and opportunities for modular construction of particulate vaccines: stick, click, and glue. Front. Immunol. 2018;9:1432. doi: 10.3389/fimmu.2018.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leneghan D.B., Miura K., Taylor I.J., Li Y., Jin J., Brune K.D., Bachmann M.F., Howarth M., Long C.A., Biswas S. Nanoassembly routes stimulate conflicting antibody quantity and quality for transmission-blocking malaria vaccines. Sci. Rep. 2017;7:3811. doi: 10.1038/s41598-017-03798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ducasse R., Wang W.A., Navarro M.G.J., DeBons N., Colin A., Gautier J., Guigner J.M., Guyot F., Gueroui Z. Programmed self-assembly of a biochemical and magnetic scaffold to trigger and manipulate microtubule structures. Sci. Rep. 2017;7:11344. doi: 10.1038/s41598-017-10297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DeRose R., Miyamoto T., Inoue T. Manipulating signaling at wil:chemically-inducible dimerization (CID) techniques resolve problems in cell biology. Pflugers Arch. 2013;465(3):409–417. doi: 10.1007/s00424-012-1208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khoshnejad M., Parhiz H., Shuvaev V.V., Dmochowski I.J., Muzykantov V.R. Ferritin-based drug delivery systems: hybrid nanocarriers for vascular immunotargeting. J. Control. Release. 2018;282:13–24. doi: 10.1016/j.jconrel.2018.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patel K.G., Swartz J.R. Surface functionalization of virus-like particles by direct conjugation using azide-alkyne click chemistry. Bioconjug. Chem. 2011;22:376–387. doi: 10.1021/bc100367u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Santoro S.W., Anderson J.C., Lakshman V., Schultz P.G. An archaeobacteria-derived glutamyl-tRNA synthetase and tRNA pair for unnatural amino acid mutagenesis of proteins in Escherichia coli. Nucleic Acids Res. 2003;31(23):6700–6709. doi: 10.1093/nar/gkg903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khoshnejad M., Greineder C.F., Pulsipher K.W., Villa C.H., Altun B., Pan D.C., Tsourkas A., Dmochowski I.J., Muzykantov V.R. Ferritin nanocages with biologically orthogonal conjugation for vascular targeting and imaging. Bioconjug. Chem. 2018;29(4):1209–1218. doi: 10.1021/acs.bioconjchem.8b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fierer J.O., Veggiani G., Howarth M. SpyLigase peptide-peptide ligation polymerizes affibodies to enhance magnetic cancer cell capture. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E1176–E1181. doi: 10.1073/pnas.1315776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vannucci L., Falvo E., Fornara M., Di Micco P., Benada O., Krizan J., Svoboda J., Hulikova-Capkova K., Morea V., Boffi A., Ceci P. Selective targeting of melanoma by PEG-masked protein-based multifunctional nanoparticles. Int. J. Nanomedicine. 2012;7:1489–1509. doi: 10.2147/IJN.S28242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Giner-Casares J.J., Henriksen-Lacey M., Coronado-Puchau M., Liz-Marzán L.M. Inorganic nanoparticles for biomedicine: where materials scientists meet medical research. Mattod. 2015;19(1):19–28. [Google Scholar]
- 146.Kopac T. Protein corona, understanding the nanoparticle-protein interactions and future perspectives: a critical review. Int. J. Biol. Macromol. 2021;169:290–301. doi: 10.1016/j.ijbiomac.2020.12.108. [DOI] [PubMed] [Google Scholar]
- 147.Corbo C., Molinaro R., Parodi A., Furman N.E.T., Salvatore F., Tasciotti E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine (London) 2016;1191:81–100. doi: 10.2217/nnm.15.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Díaz-Torres R., López-Arellano R., Escobar-Chávez J.J., García-García E., Domínguez-Delgado C.L., Ramírez-Noguera P. Handbook of Nanoparticles. 2015. Effect of size and functionalization of pharmaceutical nanoparticles and their interaction with biological systems; pp. 1–17. [Google Scholar]
- 149.Contini C., Hindley J.W., Macdonald T.J., Barritt J.D., Ces O., Quirke N. Size dependency of gold nanoparticles interacting with model membranes. Comm. Chem. 2020;3(130) doi: 10.1038/s42004-020-00377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Laurent S., Mahmoudi M. Superparamagnetic iron oxide nanoparticles: promises for diagnosis and treatment of cancer. Int. J. Mol. Epidemiol. Genet. 2011;2(4):367–390. [PMC free article] [PubMed] [Google Scholar]
- 151.Mohanty A., Parida A., Raut R.K., Behera R.K. Ferritin: a promising nanoreactor and nanocarrier for bionanotechnology. ACS Bio. Med. Chem. Au. 2022;2(3):258–281. doi: 10.1021/acsbiomedchemau.2c00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Theil E.C., Tosha T., Behera R.K. Solving Biology’s iron chemistry problem with Ferritin protein Nanocages. Acc. Chem. Res. 2016;49(5):784–791. doi: 10.1021/ar500469e. [DOI] [PubMed] [Google Scholar]
- 153.Theil E.C., Behera R.K., Tosha T. Ferritins for chemistry and for life. Coord. Chem. Rev. 2013;257:579–586. doi: 10.1016/j.ccr.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mohanty A., Mithra K., Jena S.S., Behera R.K. Kinetics of ferritin self-assembly by laser light scattering: impact of subunit concentration, pH, and ionic strength. Biomacromolecules. 2021;22(4):1389–1398. doi: 10.1021/acs.biomac.0c01562. [DOI] [PubMed] [Google Scholar]
- 155.Singh S., Dhawan A., Karhana S., Bhat M., Dinda A.K. Quantum dots: an emerging tool for point-of-care testing. Micromachines. 2020;11(12):1058. doi: 10.3390/mi11121058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Y., Xu C., Chang Y., Zhao L., Zhang K., Zhao Y., Gao F., Gao X. Ultrasmall superparamagnetic iron oxide nano- particle for T2-weighted magnetic resonance imaging. ACS Appl. Mater. Interfaces. 2017;9(34):28959–28966. doi: 10.1021/acsami.7b10030. [DOI] [PubMed] [Google Scholar]
- 157.Chen C., Ge J., Gao Y., Chen L., Cui J., Zeng J., Gao M. Ultrasmall superparamagnetic iron oxide nanoparticles: a next generation contrast agent for magnetic resonance imaging. WIREs Nanomed. Nanobiotechnol. 2022;14(e1740) doi: 10.1002/wnan.1740. [DOI] [PubMed] [Google Scholar]
- 158.Li L., Fang C.J., Ryan J.C., Niemi E.C., Lebrón J.A., Björkman P.J., Arase H., Torti S.V., Nakamura M.C., Seaman W.E. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3505–3510. doi: 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fan K., Cao C., Pan Y., Lu D., Yang D., Feng J., Song L., Liang M.L., Yan X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012;7(7):459–464. doi: 10.1038/nnano.2012.90. [DOI] [PubMed] [Google Scholar]
- 160.Chiou B., Connor J.R. Emerging and dynamic biomedical uses of ferritin. Pharmaceuticals. 2018;11:124. doi: 10.3390/ph11040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Truffi M., Fiandra L., Sorrentino L., Monieri M., Corsi F., Mazzucchelli S. Ferritin nanocages: a biological platform for drug delivery, imaging, and theranostics in cancer. Pharmacol. Res. 2016;107:57–65. doi: 10.1016/j.phrs.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 162.He J., Fan K., Yan X. Ferritin drug carrier (FDC) for tumor targeting therapy. J. Control. Release. 2019;311-312:288–300. doi: 10.1016/j.jconrel.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 163.Ahire E.D., Kshirsagar S.J. Immune responses induces by different vaccine platforms against coronavirus disease-19. Explor. Immunol. 2021;1:243–257. [Google Scholar]
- 164.Dintzis R.Z., Vogelstein B., Dintzis H.M. Specific cellular stimulation in the primary immune response: experimental test of a quantized model. Proc. Natl. Acad. Sci. U. S. A. 1982;79:884–888. doi: 10.1073/pnas.79.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reddy S.T., Swartz M.A., Hubbell J.A. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27(12):573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 166.Yao D., Lao F., Zhang Z., Liu Y., Cheng J., Ding F., Wang X., Xi L., Wang C., Yan X., Zhang R., Ouyang F., Ding H., Ke T. Human H-ferritin presenting RBM of spike glycoprotein as potential vaccine of SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.05.25.115618. 05.25.115618. [DOI] [Google Scholar]
- 167.Guo Y., He W., Mou H., Zhang L., Chang J., Peng S., Ojha A., Tavora R., Parcells M.S., Luo G., Li W., Zhong G., Choe H., Farzan M., Quinlan B.D. An engineered receptor-binding domain improves the immunogenicity of multivalent SARS-CoV-2 vaccines. ASM J./mBio. 2021;12(3) doi: 10.1128/mBio.00930-21. e00930–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim Y.I., Kim D., Yu K.M., Seo H.D., Lee S.A., Casel M.A.B., Jang S.G., Kim S., Jung W., Lai C.J., Choi Y.K., Jung J.U. Development of spike receptor-binding domain nanoparticles as a vaccine candidate against SARS-CoV-2 infection in ferrets. ASM J./mBio. 2021;12(2) doi: 10.1128/mBio.00230-21. e00230–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li H., Guo L., Zheng H., Li J., Zhao X., Li J., Liang Y., Yang F., Zhao Y., Yang J., Xue M., Zuo Y., Zhou J., Chen Y., Yang Z., Li Y., Jin W., Shi H., He Z., Li Q., Liu L. Self-assembling nanoparticle vaccines displaying the receptor binding domain of SARS-CoV-2 elicit robust protective immune responses in rhesus monkeys. Bioconjug. Chem. 2021;32(5):1034–1046. doi: 10.1021/acs.bioconjchem.1c00208. [DOI] [PubMed] [Google Scholar]
- 170.Zhang B., Chao C.W., Tsybovsky Y., Abiona O.M., Hutchinson G.B., Moliva J.I., Olia A.S., Pegu A., Phung E., Stewart-Jones G.B.E., Verardi R., Wang L., Wang S., Werner A., Yang E.S., Yap C., Zhou T., Mascola J.R., Sullivan N.J., Graham B.S., Corbett K.S., Kwong P.D. A platform incorporating trimeric antigens into self-assembling nanoparticles reveals SARS-CoV-2-spike nanoparticles to elicit substantially higher neutralizing responses than spike alone. Sci. Rep. 2020;10(1):18149. doi: 10.1038/s41598-020-74949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ma X., Zou F., Yu F., Li R., Yuan Y., Zhang Y., Zhang X., Deng J., Chen T., Song Z., Qiao Y., Zhan Y., Liu J., Zhang J., Zhang X., Peng Z., Li Y., Lin Y., Liang L., Wang G., Chen Y., Chen Q., Pan T., He X., Zhang H. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity. 2020;53(6):1315–1330.e9. doi: 10.1016/j.immuni.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kang Y.F., Sun C., Zhuang Z., Yuan R.Y., Zheng Q., Li J.P., Zhou P.P., Chen X.C., Liu Z., Zhang X., Yu X.H., Kong X.W., Zhu Q.Y., Zhong Q., Xu M., Zhong N.S., Zeng Y.X., Feng G.K., Ke C., Zhao J.C., Zeng M.S. Rapid development of SARS-CoV-2 spike protein receptor-binding domain self-assembled nanoparticle vaccine candidates. ACS Nano. 2021;15(2):2738–2752. doi: 10.1021/acsnano.0c08379. [DOI] [PubMed] [Google Scholar]
- 173.Wang W., Huang B., Zhu Y., Tan W., Zhu M. Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice. Cell. Mol. Immunol. 2021;18(3):749–751. doi: 10.1038/s41423-021-00643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.He L., Lin X., Wang Y., Abraham C., Sou C., Ngo T., Zhang Y., Wilson I.A., Zhu J. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021;7(12) doi: 10.1126/sciadv.abf1591. 19. eabf1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Salzer R., Clark J.J., Vaysburd M., Chang V.T., Albecka A., Kiss L., Sharma P., Gonzalez Llamazares A., Kipar A., Hiscox J.A., Owen A., Aricescu A.R., Stewart J.P., James L.C., Löwe J. Single-dose immunisation with a multimerised SARS-CoV-2 receptor binding domain (RBD) induces an enhanced and protective response in mice. FEBS Lett. 2021;595(18):2323–2340. doi: 10.1002/1873-3468.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Caniels T.G., Bontjer I., Van der Straten K., Poniman M., Burger J.A., Appelman B., Lavell A.H.A., Oomen M., Godeke G.J., Valle C., Mögling R., Van Willigen H.D.G., Wynberg E., Schinkel M., Van Vught L., Guera D., Snitselaar J.L., Chaturbhuj D.N., Martin I.C., Moore J.P., De Jong M.D., Reusken C., Sikkens J.J., Bomers M.K., De Bree G.J., Eggink D., Sanders R.W. Emerging SARS-CoV-2 variants of concern evade humoral immune responses from infection and vaccination. Sc. Adv. 2021;7(36) doi: 10.1126/sciadv.abj5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.The World Health Organization (WHO) Naming SARS-CoV-2 variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants
- 178.Rössler A., Riepler L., Bante D., von Laer D., Kimpel J. SARS-CoV-2 omicron variant neutralization in serum from vaccinated and convalescent persons. N. Engl. J. Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., San J.E., Cromer D., Scheepers C., Amoako D.G., Karim F., Bernstein M., Lustig G., Archary D., Smith M., Ganga Y., Jule Z., Reedoy K., Hwa S.H., Giandhari J., Blackburn J.M., Gosnell B.I., Abdool Karim S.S., Hanekom W., von Gottberg A., Bhiman J.N., Lessells R.J., Moosa M.S., Davenport M.P., de Oliveira T., Moore P.L., Sigal A. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cameroni E., Bowen J.E., Rosen L.E., Saliba C., Zepeda S.K., Culap K., Pinto D., VanBlargan L.A., De Marco A., di Iulio J., Zatta F., Kaiser H., Noack J., Farhat N., Czudnochowski N., Havenar-Daughton C., Sprouse K.R., Dillen J.R., Powell A.E., Chen A., Maher C., Yin L., Sun D., Soriaga L., Bassi J., Silacci-Fregni C., Gustafsson C., Franko N.M., Logue J., Iqbal N.T., Mazzitelli I., Geffner J., Grifantini R., Chu H., Gori A., Riva A., Giannini O., Ceschi A., Ferrari P., Cippà P.E., Franzetti-Pellanda A., Garzoni C., Halfmann P.J., Kawaoka Y., Hebner C., Purcell L.A., Piccoli L., Pizzuto M.S., Walls A.C., Diamond M.S., Telenti A., Virgin H.W., Lanzavecchia A., Snell G., Veesler D., Corti D. Broadly neutralizing antibodies overcome SARS-CoV-2 omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Evans J.P., Zeng C., Qu P., Faraone J., Zheng Y.M., Carlin C., Bednash J.S., Zhou T., Lozanski G., Mallampalli R., Saif L.J., Oltz E.M., Mohler P.J., Xu K., Gumina R.J., Liu S.L. Neutralization of SARS-CoV-2 omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe. 2022;30(8):1093–1102. doi: 10.1016/j.chom.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Center for Disease Control and Prevention. Vaccines and Preventable Diseases https://www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html
- 183.Yang D.Y., Bracken K. Update on the new 9-valent vaccine for human papillomavirus prevention. Can. Fam. Physician. 2016;62(5):399–402. [PMC free article] [PubMed] [Google Scholar]
- 184.Barouch D.H., Tomaka F.L., Wegmann F., Stieh D.J., Alter G., Robb M.L., Michael N.L., Peter L., Nkolola J.P., Borducchi E.N., Chandrashekar A., Jetton D., Stephenson K.E., Li W., Korber B., Tomaras G.D., Montefiori D.C., Gray G., Frahm N., McElrath M.J., Baden L., Johnson J., Hutter J., Swann E., Karita E., Kibuuka H., Mpendo J., Garrett N., Mngadi K., Chinyenze K., Priddy F., Lazarus E., Laher F., Nitayapan S., Pitisuttithum P., Bart S., Campbell T., Feldman R., Lucksinger G., Borremans C., Callewaert K., Roten R., Sadoff J., Scheppler L., Weijtens M., Feddes-de Boer K., van Manen D., Vreugdenhil J., Zahn R., Lavreys L., Nijs S., Tolboom J., Hendriks J., Euler Z., Pau M.G., Schuitemaker H. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomized, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19) Lancet. 2018;392(10143):P232–P243. doi: 10.1016/S0140-6736(18)31364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wei J., Li Z., Yang Y., Ma G., Su Z., Zhang S. An apoferritin-hemagglutinin conjugate vaccine with encapsulated nucleoprotein antigen peptide from influenza confers enhanced cross protection. Bioconjug. Chem. 2020;31:1948–1959. doi: 10.1021/acs.bioconjchem.0c00308. [DOI] [PubMed] [Google Scholar]
- 186.Ebrahimi K.H. Ferritin as a platform for creating antiviral mosaic nanocages: prospects for treating COVID-19. ChemBioChem. 2021;22:1371–1378. doi: 10.1002/cbic.202000728. [DOI] [PubMed] [Google Scholar]
- 187.Lee J., Kim D., Byun J., Wu Y., Park J., Oh Y.-K. In vivo fate and intracellular trafficking of vaccine delivery systems. Adv. Drug Deliv. Rev. 2022;186 doi: 10.1016/j.addr.2022.114325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Arevalo C.P., Bolton M.J., Le Sage V., Ye N., Furey C., Muramatsu H., Alameh M.G., Pardi N., Drapeau E.M., Parkhouse K., Garretson T., Morris J.S., Moncla L.H., Tam Y.K., Fan S.H.Y., Lakdawala S.S., Weissman D., Hensley S.E. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science. 2022;378(6622):899–904. doi: 10.1126/science.abm0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.How nasal-spray vaccines could change the pandemicNature (News Features) 2022;(06 September) https://www.nature.com/articles/d41586-022-02824-3 [Google Scholar]
- 190.Nasal spray Covid-19 vaccine co-developed by Hong Kong scientists approved for emergency use in mainland China in ‘historic breakthrough’. South China Morning Post. 06 December 2022. https://www.scmp.com/news/hong-kong/health-environment/article/3202179/nasal-spray-covid-19-vaccine-co-developed-hong-kong-scientists-approved-emergency-use-mainland-china.
- 191.Zang J., Chen H., Zhang X., Zhang C., Guo J., Du M., Zhao G. Disulfide-mediated conversion of 8-mer bowl-like protein architecture into three different nanocages. Nat. Commun. 2019;10:778. doi: 10.1038/s41467-019-08788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eren E., Wang B., Winkler D.C., Watts N.R., Steven A.C., Wingfield P.T. Structural characterization of the Myxococcus xanthus encapsuling and ferritin-like cargo system gives insight into its iron storage mechanism. Structure. 2022;30(4):551–563.e4. doi: 10.1016/j.str.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.