Abstract
Lipid rafts, sterol- and sphingolipid-rich membrane microdomains, have been extensively studied in mammalian cells. Recently, lipid rafts have been shown to control virulence in a variety of parasites including Entamoeba histolytica, Giardia intestinalis, Leishmania spp., Plasmodium spp., Toxoplasma gondii, and Trypanosoma spp. Parasite rafts regulate adhesion to host and invasion, and parasite adhesion molecules often localize to rafts. Parasite rafts also control vesicle trafficking, motility, and cell signaling. Parasites disrupt host cell rafts; the dysregulation of host membrane function facilitates the establishment of infection and evasion of the host immune system. Discerning the mechanism by which lipid rafts regulate parasite pathogenesis is essential to our understanding of virulence. Such insight may guide the development of new drugs for disease management.
Keywords: lipid rafts, detergent-resistant membrane, protozoan parasite, cholesterol, host–parasite interaction
Lipid rafts: afloat in parasite membranes
Lipid rafts (see Glossary) are tightly packed, cholesterol- and sphingolipid-rich, membrane microdomains, which serve as a platform where protein–protein or protein–lipid interactions occur [1]. Lipid rafts can be extracted from the membrane through the use of cold non-ionic detergents; therefore, detergent-resistant membrane (DRM) is often considered representative of lipid raft populations. Although these terms are related, they are not necessarily interchangeable. Therefore, in this review, we have selected terminology that best represented the purification method utilized in the original studies. Rafts play roles in signaling pathways regulating several cellular processes including adhesion, motility, secretion, and invasion. The function of rafts often depends on the proteins found within these domains, and raft-association of these proteins is determined, in part, by the presence of post-translational modifications (Box 1). Rafts have been identified in several protozoan parasites including Entamoeba histolytica, Giardia intestinalis, Leishmania spp., Plasmodium spp., Toxoplasma gondii, and Trypanosoma spp. (Table 1).
Box 1. Protein modifications and raft lipids.
Lipid rafts contain a subset of proteins and lipids found in the plasma membrane. Certain post-translational modifications, such as GPI anchoring and acylation facilitate protein–raft interaction. The lipids found in rafts, such as sphingolipids, are also involved in maintaining raft formation and stability.
GPI-anchored proteins are commonly localized to rafts, but it is unclear why particular GPI-anchored proteins exhibit this localization pattern. In Leishmania spp., both GP63 and LPG are GPI-anchored in all life cycle stages; however, GP63 is localized to the DRM in both procyclic and metacyclic promastigotes, and LPG is localized only to DRM in metacyclic promastigotes [5]. Although their subcellular localization differs, the GPI anchor of LPG in both parasite life stages is identical [5]; it is currently unknown what factors contribute to raft versus non-raft localization of LPG.
In parasites, several studies have focused on the post-translational modifications of raft-associated proteins and the lipids found in rafts. The localization of T. cruzi PI-PLC to flagellar membrane lipid rafts depends on dual acylation [29]. The calcium sensors, FCaBP (T. cruzi) and calflagin Tb24 (T. brucei), depend on dual acylation for raft localization [31]. Palmitoyl acetyl transferases (PATs) are responsible for the addition of palmitoyl groups to proteins; tbPAT7 palmitolylates T. brucei calflagin [64].
Flagellar proteins in Leishmania major are targeted to lipid rafts through post-translational modifications. SMP-1, a small dually acylated protein, is targeted to flagellar rafts [27,28]. SMP-2 and SMP-4 are monoacylated (myristoylated) and localized to the flagellar pocket and cell body, respectively [27,34]. Similar to SMP-1, SMP-4 is associated with DRM; however, SMP-2 is solubilized in detergent [27]. Thus, dual acylation is not necessarily a raft-targeting signal. Reintroduced SMP-1 to double knockout SMP-1 and SMP-2 cells was protective against sphingolipid depletion [27]. This demonstrates that the role SMP-1 plays a role in stabilizing flagellar DRM [27].
Sphingolipids are a major component of lipid rafts. Therefore, researchers have investigated whether sphingolipids are necessary for raft formation in parasites. RNAi-mediated inhibition of serine palmitoyltransferase (SPT2) prevents T. brucei sphingolipid biosynthesis. Knockdown of SPT2 in procyclic stage parasites did not affect calflagin localization to flagellar rafts; however, exposure of bloodstream T. brucei to myriocin, which also inhibits serine palmitoyltransferases, causes loss of association of calflagins with DRMs [65]. Differences in membrane composition between the parasite stages, including the inclusion of ergosterol in procyclic forms, may account for these differences. Ergosterol may allow procyclic rafts to be more resistant to the removal of sphingolipids from their membranes [65]. Lipid rafts in Leishmania spp. are also able to form in sphingolipid-deficient parasites, possibly due to the presence of ergosterol in Leishmania [5]. Disruption of sphingolipid biosynthesis in L. major by myriocin has no effect on SMP-1 localization in rafts [28]. Knockout of LmLCB2, a subunit of one serine palmitoyltransferase in Leishmania, yields parasites that cannot synthesize sphingolipids or ceramide. This deletion delays the association of GP63 with DRMs and changes the localization of LPG from non-raft to raft-associated [5]. These changes affect the ability of the parasite to form infective metacyclic promastigotes [5].
Table 1.
Overview of cholesterol-rich membrane microdomain functions
| Parasite | Functionsa |
|---|---|
| Entamoeba histolytica | • Facilitate attachment to host collagen and host cells • Regulate fluid phase endocytosis • Adhesion molecules: Gal/GalNAc lectin localized to rafts in a PIP2- and calcium-dependent manner |
| Giardia intestinalis | • Facilitate attachment to host intestinal cells |
| Leishmania spp. | • Facilitate attachment to, entry into, and replication within host macrophages • Adhesion molecules: GP63 family of parasite adhesion molecules is localized to rafts • Regulate motility (flagellar proteins localize to lipid rafts) • Host rafts: parasite protein, GP63, enters host rafts and cleaves host phosphatases which are important in IFN-γ signaling. Infection results in mislocalization of host raft proteins, CD1d and CD40, which leads to alterations in IL12- and IL10-based signaling |
| Plasmodium spp. | • Facilitate attachment to and invasion of erythrocytes • May control protein sorting in rhoptries (rhoptry proteins, such as Pf34 and RAMA, localize to DRM) • Regulate motility (glideosome protein complexes localize to DRM) • Host rafts: composition of host rafts is altered during invasion |
| Toxoplasma gondii | • Regulate motility (glideosome protein complexes localize to DRM) |
| Trypanosoma brucei | • May regulate calcium signaling (proteins involved in calcium signaling, such as calflagin Tb24, localize to lipid rafts) |
| Trypanosoma cruzi | • Facilitate invasion of host cells by trypomastigotes, but not amastigotes • Regulate receptor-mediated endocytosis of transferrin • Control flagellar signaling • May regulate calcium signaling (proteins involved in calcium signaling, such as PI-PLC and FCaBP, localize to lipid rafts) • Host rafts: host rafts are required for stage-specific adhesion, internalization, and intracellular survival of the parasite |
Details about these functions and relevant references are found throughout the text.
Protozoan parasites with an intracellular life cycle stage, such as Leishmania spp., Plasmodium spp., and Trypanosoma cruzi, have also developed several mechanisms to manipulate host cell lipid rafts for invasion, colonization, and immune system evasion. In addition to traditional lipid rafts, host cell caveolae, which are a specialized type of lipid raft containing caveolin proteins, are also manipulated by intracellular pathogens.
Parasites utilize lipid rafts during multiple life cycle stages. In E. histolytica and G. intestinalis, for example, rafts mediate initial attachment to the host epithelial layer. DRM-associated proteins in Plasmodium spp. are required for invasion of red blood cells (RBCs). Leishmania spp. manipulate signaling pathways emanating from host lipid rafts to evade the immune system. The reliance on lipid rafts for survival truly makes these membrane domains a ‘life raft’ for parasites; the use of these life rafts determines the success of parasitic infections, and whether the parasites will ‘sink or swim’.
Adhesion: anchoring parasites to hosts
Adhesion to host cells by parasites is an essential first step in the invasion process and may be mediated by parasite lipid rafts. During infection, intestinal pathogens such as G. intestinalis and E. histolytica attach to host epithelial cells of the intestinal tract. Exposure of G. intestinalis to methyl-β-cyclodextrin (MβCD), a cyclic compound that chelates cholesterol and disrupts lipid raft domains, abolishes its ability to adhere to Caco-2/TC7 cells [2]. Parasite adhesion to host is unaffected when Caco-2/TC7 cells are exposed to MβCD, demonstrating that the lipid rafts of G. intestinalis, and not those of host cells, regulate adhesion [2]. Exposure of E. histolytica to MβCD reduces adhesion to host cell targets, such as collagen [3] and Chinese hamster ovary (CHO) cells [4]. Thus, parasite lipid rafts appear to regulate binding of intestinal parasites to host. The role of host cell rafts in E. histolytica infection has not been investigated to date.
The role of rafts in attachment to host is further illustrated by the existence of parasite adhesion proteins within raft domains. One class of adhesion molecules is the GP63 family of zinc-dependent metalloproteases in Leishmania spp [5]. GP63, which is glycophosphatidylinositol (GPI)-anchored, is localized to lipid rafts of Leishmania amazonensis [5]. In E. histolytica, the best characterized of the cell surface adhesion molecules is the galactose/N-acetylgalactosamine (Gal/GalNAc) lectin, which is composed of heavy (Hgl), light (Lgl), and intermediate (Igl) subunits. This protein complex binds to galactose and N-acetylgalactosamine residues on host components. Igl, which is GPI-anchored, is constitutively localized to rafts. Hgl, a transmembrane protein, and Lgl, a GPI-anchored protein, form a covalent dimer that is only transiently-associated with rafts. For example, physical interaction between E. histolytica and Gal/GalNAc ligands on RBCs or collagen [6 leads to the enrichment of Hgl–Lgl dimers in rafts and, thus, colocalization of all three subunits. Cholesterol loading of the membrane similarly enhances the enrichment of the Hgl–Lgl dimer in rafts [4]. Interestingly, colocalization of these subunits in lipid rafts during cholesterol loading correlates with increased adhesion to CHO cells [4]. Therefore, in E. histolytica, rafts may regulate the assembly and function of adhesion molecules.
In addition to adhesion, lipid rafts mediate the invasion process of several pathogens with intracellular life cycle stages. For example, invasion is inhibited in MβCD-treated trypomastigotes, but not MβCD-treated amastigotes of T. cruzi [7]. This suggests the involvement of rafts in infection is stage specific in T. cruzi [7]. Infection by Leishmania viannia braziliensis involves attachment to and then subsequent phagocytosis by macrophages. MβCD exposure of the parasite reduces the infection rate of this pathogen in macrophages [8].
Parasite armament: DRM-associated rhoptry and surface proteins open the gangway for Plasmodium spp.
Plasmodium falciparum proteins that function in invasion of RBCs are localized to the DRM of P. falciparum. Many of these DRM-associated proteins were identified in proteomic analyses, an overview of which can be found in Table 2. Proteins that comprise the rhoptry, an organelle which is responsible for secreting proteins into host cytoplasm, were identified among the DRM-associated proteins (Figure 1). Detergent-resistant proteins were found in both the rhoptry bulb and rhoptry neck. For example, Pf34, a rhoptry neck protein, and rhoptry-associated membrane antigen (RAMA), a rhoptry bulb marker, are present in the DRM proteome of P. falciparum [9,10]. The localization of RAMA to DRM domains is necessary for proper trafficking of other proteins, such as the rhoptry-associated protein (RAP) family, to the correct rhoptry compartment for secretion [11]. Pf34 is proposed to be an adhesin that functions during invasion of erythrocytes [12]. Pv34, the Plasmodium vivax homolog of Pf34, is also DRM-associated, although its exact localization, rhoptry bulb or neck, is currently unknown [13]. Rhoptry neck protein 1 (RON1), which is conserved across Apicomplexa, is a DRM-associated protein expressed in the schizont stage of P. vivax [14]. Understanding the trafficking and the subcellular localization of rhoptry proteins is important as they are predicted to be involved in RBC invasion.
Table 2.
Detergent-resistant membrane proteome of Plasmodium spp. and vector
| Protein categories | Plasmodium falciparum a,b | Anopheles gambiae midgutc,d | Plasmodium berghei e,f | Plasmodium berghei hoste |
|---|---|---|---|---|
| Chaperones | • | |||
| Cytoskeletal proteins | • | • | ||
| Formation of parasitophorous vacuole | • | |||
| Glycosyl hydrolases | • | |||
| GPI-anchored proteins | • | • | ||
| GPI-binding proteins | • | |||
| Immunoglobulin-like proteins | • | |||
| Inner membrane complex | • | |||
| Known ookinete-interacting proteinsg | • | |||
| Lectins/receptors | • | |||
| Membrane fusion events | • | |||
| Merozoite surface proteins | • | |||
| Multimembrane spanning proteins | • | |||
| Multidrug resistance | • | |||
| Peptidases | • | |||
| Protease inhibitors | • | |||
| Protein complex assembly | • | |||
| Protein folding | • | • | ||
| Protein sorting | • | • | ||
| Proteins involved in adhesion/invasion | • | • | • | |
| Rhoptry-associated proteins | • | |||
| Trafficking | • | • | ||
| Transporters | • | |||
| Variant antigen superfamily | • |
From [9].
From [24].
From [68].
A. gambiae is the insect vector of Plasmodium spp.
From [69].
P. berghei, which infect rodents, is a closely related species to P. falciparum.
Six known ookinete-interacting proteins (aminopeptidase N, three annexin-like proteins, carboxypeptidase B, and scavenger receptor, croquemort homolog) were identified in the DRM proteome of A. gambiae and are candidates for use in transmission blocking vaccines.
Figure 1.
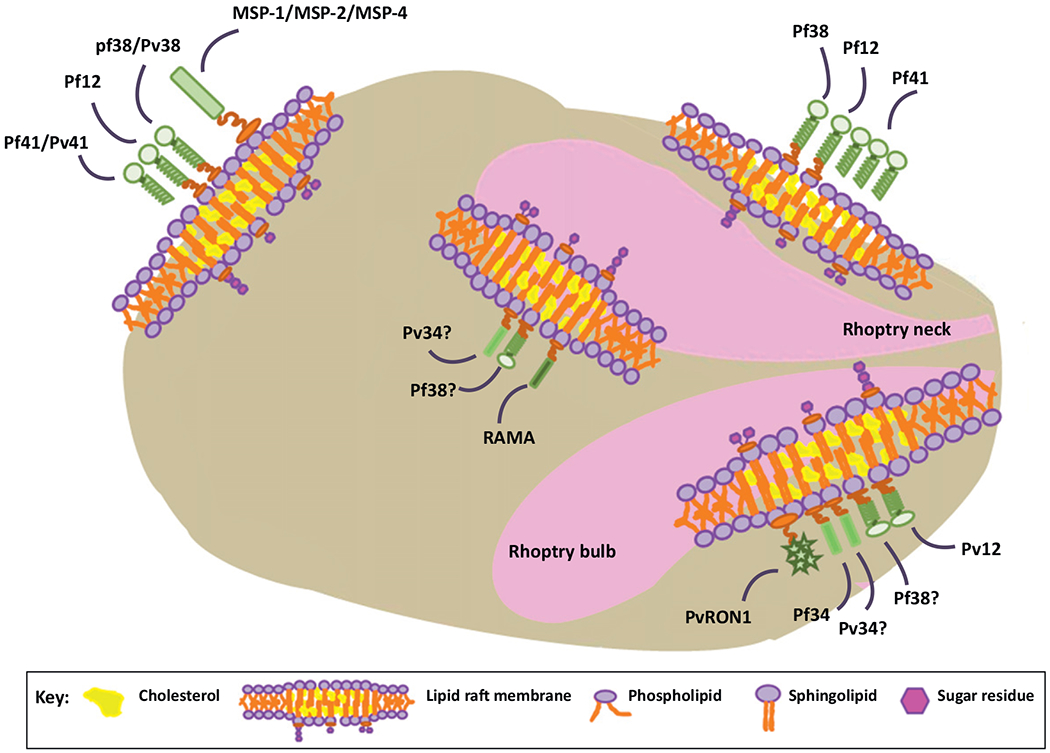
Proteins associated with detergent-resistant membrane (DRM) in Plasmodium. Several studies have identified DRM-associated proteins in Plasmodium. DRM proteins are commonly found on the merozoite surface and within rhoptries. Merozoite surface protein 1 (MSP-1), MSP-2, and MSP-4 are glycophosphatidylinositol (GPI)-anchored surface proteins identified in studies using Plasmodium falciparum [9]. Although illustrated at the basal end, these proteins are probably distributed in DRMs throughout the parasite surface [9]. Additionally, a group of three proteins containing Cys6 domains, Pf41, Pf12, and Pf38, were identified in the DRM; Pf12 and Pf38 are probably GPI-anchored, whereas Pf41 has no membrane anchor and may be complexed with other GPI-anchored proteins, such as Pf12 and Pf38 [9,15,16]. Although Pf12 and Pf38 were classified as ‘surface proteins’, the location of Pf41 was more precisely determined. The majority of Pf41 was concentrated at the apical end of merozoite stage parasites [9,15]. Localization studies suggest that Pf38 is also localized to rhoptries, although the precise location within the rhoptry was not determined [9]. Some DRM-associated proteins were localized to rhoptries, including rhoptry-associated membrane antigen (RAMA), a known rhoptry bulb protein, and Pf34, Pv12, and Plasmodium vivax rhoptry neck protein 1 (PvRON1), known rhoptry neck proteins [13,14,17]. Interestingly, Pv12 and Pf12 are found in different DRM locations in their respective Plasmodium species. Pf38 is localized to the merozoite surface in addition to rhoptries, although its precise localization within rhoptries has not been determined [15]. Pv34, the P. vivax homolog of Pf34, is also localized to rhoptries, although it is uncertain whether it is associated with the bulb or neck region [13].
Surface proteins that are raft-associated are also important in invasion by Plasmodium spp. (Figure 1) and several are potential vaccine targets. GPI-anchored merozoite surface proteins, including MSP-1, MSP-2, and MSP-4, were identified in the DRM proteome [9]. In addition to its rhoptry localization, detergent-resistant Pf34 is also found on the surface of the parasite [9,15,16]. Three detergent-resistant proteins containing Cys6 domains, Pf38/Pv38, Pf41/Pv41, and Pf12/Pv12, are present in P. falciparum and P. vivax, respectively. Pf38 is a GPI-anchored protein in P. falciparum and is localized to the merozoite surface as well as to the rhoptries [9]. P. falciparum Pf12 is localized to the merozoite surface, but its homolog, Pv12, is localized to the rhoptry neck in P. vivax [17]. These Cys6 proteins are strongly recognized by antibodies of malaria-infected individuals [9,15]. Also, exogenous addition of these proteins moderately inhibited merozoite invasion [18], supporting their role in virulence. Together, these data support the potential of detergent-resistant surface proteins as vaccine targets.
Endocytosis: taking on nutrients
Endocytic processes are important for parasite nutrient uptake and, thus, parasite growth and survival. Both nonspecific and receptor-mediated endocytosis are mediated by vesicle trafficking, and studies suggest that vesicle trafficking in parasites relies on lipid rafts. For example, in E. histolytica, raft disruption by MβCD inhibits fluid phase endocytosis, a nonspecific vesicle trafficking event [19]. Additionally, regions of the plasma membrane where receptor-mediated uptake of transferrin occurs in T. cruzi colocalize with a lipid raft stain, cholera B toxin, and a raft marker, flotillin-1, suggesting that transferrin uptake occurs in lipid raft regions [20]. In the presence of MβCD or filipin, two raft-disrupting agents, transferrin uptake was inhibited in T. cruzi [21]. Together, these data highlight the importance of parasite lipid rafts for survival. Interestingly, host cell endocytic pathways are manipulated by intracellular parasites during the invasion process. Several examples of host cell ‘hijacking’ can be found in the following sections: ‘Host cell lipid rafts: more than just docks’ and ‘Maintaining stowaway status: avoiding phagolysosomal acidification’.
Parasite motility: full steam ahead
The role of lipid rafts in motility has been established for several protozoan parasites. Apicomplexan parasites employ a glideosome, which contains the molecular machinery needed for motility [22]. The glideosome of Toxoplasma gondii contains two myosin proteins, myosin A heavy chain and myosin light chain. It also contains two glideosome-associated proteins (GAPs), GAP45 and GAP50, both of which anchor the glideosome to the inner membrane complex (IMC) [22]. In T. gondii, the glideosome is first assembled as a soluble complex containing myosin A heavy chain, myosin light chain 1, and GAP45 [22]. This complex then becomes associated with cholesterol-rich DRM domains of the IMC, and this association can be disrupted by MβCD [22,23]. Glideosomes also regulate motility in P. falciparum. The P. falciparum homologs of T. gondii IMC proteins PfGAP50, PfGAP45, and myosin A, and two additional glideosome-associated proteins, PfGAPM1 and PfGAPM2, are also detergent-resistant [9,24]. Therefore, it is clear that lipid rafts are involved in apicomplexan glideosome-mediated motility.
Lipid rafts, and their associated proteins, are enriched in the flagellar membrane of kinetoplasts and also participate in cellular motility [22,25,26]. In Leishmania major, small myristoylated protein 1 (SMP-1), a small dually acylated protein, is targeted to lipid rafts in the flagellum [27,28]. Another L. major SMP protein, SMP-2, has been identified, which is non-raft-associated [26]. Double knockout parasites, with loss of SMP-1 and SMP-2, exhibited shortened flagella and motility defects [27]. Although the phenotype of SMP-1 single knockouts has not been discussed in the literature, the phenotype of the double knockout is rescued by reintroduction of DRM-associated SMP-1, but not SMP-2 [27]. This demonstrates that DRM-associated proteins, such as SMP-1, are critical for flagellar function.
Cell signaling: aye, aye, captain
The connection between lipid rafts and temporal and spatial regulation of cell signaling is well established. Cell signaling in the parasite may result in changes within the parasite or within the host. For example, in E. histolytica, sufficient levels of phosphatidylinositol (4,5) bisphosphate (PIP2) and intracellular calcium, which are important signaling molecules, are required for Gal/GalNAc lectin localization in lipid rafts [6]. In T. cruzi, phosphatidylinositol-specific phospholipase C (PI-PLC) resides in flagellar lipid rafts [29]. PI-PLC hydrolyzes PIP2 into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), which regulate downstream calcium signaling. Surface expression of PI-PLC in T. cruzi occurs simultaneously with depletion of PIP2 from host cells, host cytoskeletal changes, and calcium signaling [29]. Therefore, the localization of PI-PLC in outer membrane lipid rafts of the flagellum may facilitate changes in the host during invasion.
Cytosolic calcium in Trypanosoma spp. regulates several important cellular processes such as host invasion by T. cruzi [30]. Therefore, calcium-binding proteins are important for virulence. Flagellar calcium binding protein (FCaBP), a calcium binding protein in T. cruzi, is localized to lipid rafts, and its flagellar localization depends on binding of calcium ions [31]. Calflagin Tb24, a calcium sensor in Trypanosoma brucei, is also localized to lipid rafts [25]. Mice infected with calflagin-deficient parasites survived for longer periods of time than mice infected with wild type parasites, indicating a role for calflagin in virulence [32]. However, calflagin-deficient parasites were not altered in growth, morphology, motility, or ability to clear antibodies from their surface [32]. Thus, the role of calflagin in virulence is not precisely understood.
The existence of distinct lipid raft domains within biological membranes has been previously proposed [33] and may also occur in protozoan parasites. The earliest study in parasites to support the existence of multiple raft domains was performed using membrane ‘raft patching’ in Leishmania spp. [5]. This technique results in aggregated lipid raft domains. Both metacyclic lipophosphoglycan (LPG) and hydrophilic acylated surface protein B (HASPB) are DRM-associated in Leishmania, but do not colocalize to the same DRM ‘patches’ [5].
Similarly, other raft isolation protocols have been used to support the existence of multiple lipid raft domains. Sucrose gradient fractionation is a widely accepted method for separating buoyant ‘raft’ from denser ‘non-raft’ fractions. Raft domains may span several fractions. In P. falciparum, the fractionation pattern for the detergent-resistant rhoptry protein, Pf34, differs from that of another detergent-resistant rhoptry protein, RAMA, suggesting that multiple DRM populations exist within rhoptries [10]. This is consistent with the observation that Pf34 is localized to the rhoptry neck, whereas RAMA is localized to the rhoptry body (Figure 1) [10]. Multiple lipid raft domains are also likely to be present in E. histolytica. The sucrose gradient flotation properties of the Gal/GalNAc lectin differs in parasites bound to RBCs compared with those bound to collagen [6]. In L. major, SMP-1, which is dually acylated, and another SMP protein, SMP-4, which is monoacylated, are also localized to distinct DRM fractions [34]. Although raft domains probably regulate cell signaling in these parasites by segregating specific proteins, the existence of subpopulations of different types of rafts adds an additional level of control that may be important to virulence.
Host cell lipid rafts: more than just docks
Parasites manipulate host rafts to facilitate invasion. Although endocytosis is not a naturally occurring phenomenon in RBCs, P. falciparum induces the formation of the parasitophorous vacuole during invasion [35]. As a means to study parasite modulation of host rafts, primaquine was used to induce endovesicular formation in RBCs. Primaquine-induced endovesicles are buoyant in sucrose gradients, cholesterol-rich, and contain proteins normally found in rafts, such as flotillin and stromatin, and may be isolated with non-detergent methods. Thus, they represent parasite-free, detergent-free, control endomembranes that may be compared to P. falciparum parasitophorous vacuoles. Primaquine-induced endovesicles contained a specific lipid profile, including phosphatidylserine and PIP2; however, P. falciparum-induced vesicles did not harbor PIP2. This evidence suggests that P. falciparum remodels RBC rafts during the invasion process [35].
Several studies have demonstrated the importance of host rafts during T. cruzi invasion. Chelation of cholesterol by MβCD or blocking of cholesterol synthesis in mammalian cells (macrophage, HEp2, HeLa, or Vero cells) interferes with adhesion and internalization of T. cruzi [7,36,37], implicating host lipid rafts in the attachment and invasion of T. cruzi. Host placental alkaline phosphatase (PLAP) can regulate internalization of T. cruzi [38]. PLAP is a GPI-anchored protein that resides in DRM microdomains [38,39] and can be liberated by cholesterol chelation [37]. Therefore, the loss of PLAP after cholesterol chelation may explain the inability of T. cruzi to invade MβCD-treated host cells [38]. Exposure of HeLa or Vero cells to cholera toxin B subunit, which binds to ganglioside GM1, a marker of lipid rafts, or to cholesterol chelating agents, MβCD or filipin, also inhibited invasion of both T. cruzi trypomastigotes and amastigotes, supporting the importance of rafts in host cell internalization of the parasite [36].
Similar to other host lipid rafts, host caveolae also serve as ports of entry for parasites. Caveolae are characterized by the presence of a family of proteins known as caveolins. Caveolin-1 knockout mice were employed to determine the role of caveolin-1 in the pathogenesis of T. cruzi [40]. There was no difference in parasite load in the heart cells or macrophages of wild type or caveolin-1 null mice, suggesting that caveolin-1 is not essential for parasite entry or survival [40]. By contrast, a separate study showed that caveolin-1 colocalizes with the point of contact between macrophages and trypomastigotes [36]. In addition, during phagocytosis of metacyclic promastigotes by macrophages, parasites localize to areas of the macrophage membrane containing caveolin-1 [41]. Thus, the role of caveolin-1 in parasite invasion is still being contended.
Despite the similarity in parasite load, T. cruzi-infected caveolin-1 knockout mice did not survive as long as T. cruzi-infected wild type mice [40]. Because caveolin-1 also regulates the release of chemokines, cytokines, and nitric oxide from immune cells, it is possible that an immune defect was responsible for increased virulence of the parasite in caveolin-1 knockout mice [40]. Also, caveolin-3 levels were decreased in cardiac cells after infection with T. cruzi [42]. Caveolin-1/caveolin-3 double knockout mice exhibit symptoms of cardiomyopathy similar to that which is characteristic of Chagas disease [43]. This suggests that cardiac symptoms of Chagas disease may be attributed to the effect of T. cruzi on host caveolae.
Maintaining stowaway status: avoiding phagolysosomal acidification
Parasites may also manipulate host cell lipid rafts or caveolae in a manner that allows for evasion of the host cell lysosomal pathway [44] and, interestingly, this may occur in a parasite stage-specific manner. For example, Leishmania infantum chagasi promastigotes require intact host caveolae for entry into macrophages and postinvasion replication [45]. When promastigotes enter the host through caveolae, fusion of the vesicles containing promastigotes with lysosomes is delayed by 24–48 h [46,47]. By contrast, the entry and survival of the amastigote form of L. i. chagasi is not affected by the loss of host caveolae, nor do amastigotes depend on this alternative route to avoid fusion with the lysosome, because they are better adapted to deal with phagolysosomal conditions [46]. Likewise, in T. cruzi, phagocytosis of metacyclic, but not avirulent promastigotes is associated with delayed parasitophorous vacuole-lysosomal fusion, and intracellular survival is enhanced [36]. This suggests that particular life cycle stages of Leishmania and T. cruzi require intact lipid rafts or caveolae to evade lysosomal processing for survival.
Leishmania spp. also use other mechanisms to evade the host lysosomal pathway. Leishmania donovani promastigotes transfer LPG from their membranes to the membrane of macrophages, where it disrupts lipid rafts and prevents F-actin assembly. This, in turn, disrupts phagosomal maturation and results in reduced phagocytosis of additional parasites [48,49]. Specifically, LPG insertion into macrophages inhibits the recruitment of the exocytosis regulator synaptin V to the nascent phagosome [45,50]. Synaptin V is required for the recruitment of vacuolar ATPase, which is responsible for phagolysosomal acidification [49]. Exclusion of synaptin V is beneficial for the parasite because cytotoxic acidification is prevented.
Safe harbor: evading the host immune system
Parasites must also evade the host immune system to survive. One way parasites accomplish this is through direct manipulation of the host immune response. Leishmania spp. secrete the metalloprotease GP63, which is then taken up by host macrophages through their lipid raft domains [51,52]. Internalization of GP63 in host macrophages is associated with cleavage of the subunits of the early activator protein 1 (AP-1) signalosome such as C-Jun [52]. This disrupts antimicrobial activity of macrophages (Figure 2) [52]. In addition to the AP-1 transcription factor, GP63 cleaves host cell protein tyrosine phosphatases (PTPs), which regulate interferon (IFN)-γ signaling in macrophages [51,53]. Cleavage of PTPs is lower in macrophages infected by GP63-null L. major [51]. Additionally, disruption of macrophage lipid rafts by MβCD inhibits cleavage of PTPs by GP63 [51]. Together, these data demonstrate the importance of GP63 in modulating immune cell activities through host lipid rafts.
Figure 2.
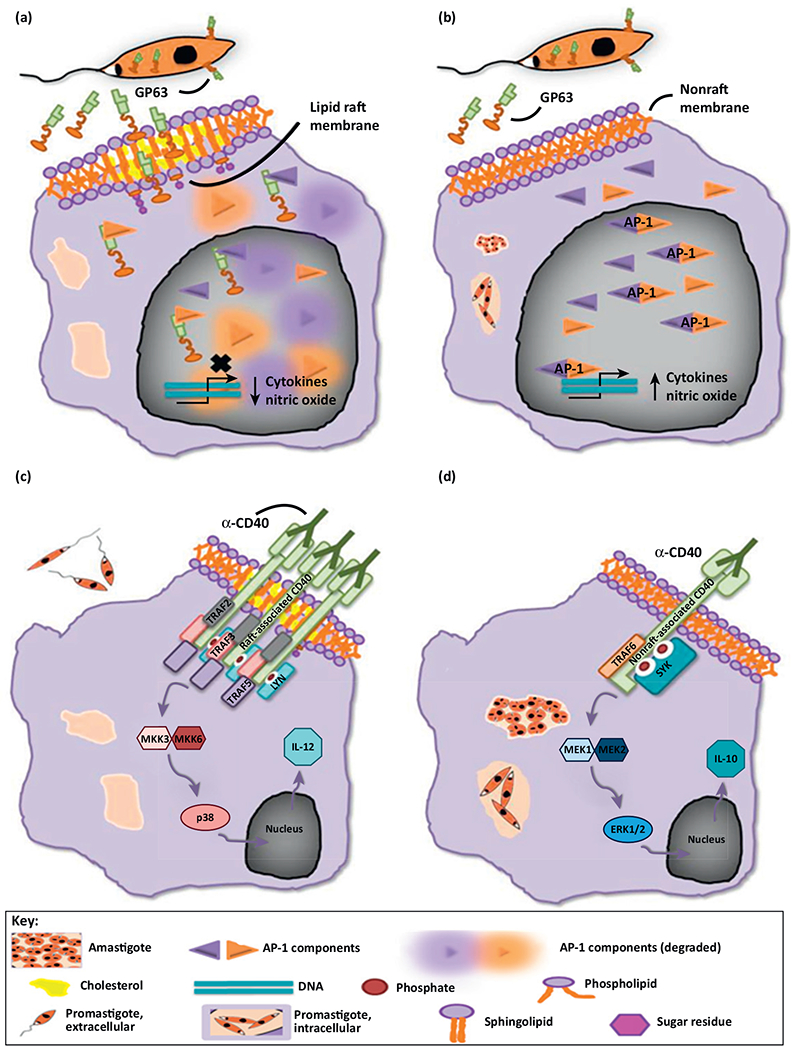
Leishmania infections modulate the host immune response. (a) Early in infection, Leishmania promastigotes release GP63, a prominent surface metalloprotease. GP63 consists of an N-terminal catalytic domain, a central domain, and a C-terminal glycophosphatidylinositol (GPI) anchor, and it is localized inside parasites, at the parasite surface, and/or in a soluble, secreted form. GP63 enters macrophages via an undefined pathway that is phagocytosis-independent and lipid raft-dependent, and can be found inside the cell cytoplasm and in the nucleus. The GPI anchor may be involved in cellular entry via lipid raft membrane domains, because a GPI-less form of GP63 is minimally internalized in macrophages. GP63 actively degrades five of the seven subunits associated with the activator protein 1 (AP-1) transcription factor, essentially abolishing AP-1 activity (degradation is represented by halos surrounding the AP-1 subunits). Without AP-1, the expression of cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1B, and IL-12, and the precursor of nitric oxide (NO), inducible NO synthase (iNOS), is severely reduced or abolished [52]. (b) However, cholesterol chelation (and raft disruption) by methyl-β-cyclodextrin (MβCD) or by intracellular Leishmania parasites results in partial inhibition of c-Jun degradation by GP63 [52]. (c) In uninfected macrophages, stimulated raft-associated CD40 receptor triggers a signaling cascade that results in the production of the proinflammatory cytokine IL-12. Upon binding to CD40 ligand (or in this case, α-CD40), TNF receptor-associated factor 2 (TRAF2), TRAF3, and TRAF5 are recruited to CD40, along with the Src family tyrosine protein kinase, Lyn, within lipid rafts. Lyn activates mitogen-activated protein kinase kinase-3 (MKK-3) and/or MKK-6, which, in turn, results in the phosphorylation and activation of p38. Changes in gene expression stimulated by p38 result in the production of IL-12, a cytokine that promotes infection suppression [67]. (d) In macrophages infected with Leishmania major, a different CD40-mediated signaling cascade is stimulated. Non-raft-associated CD40 is complexed with TRAF6, along with the Syk family kinase, spleen tyrosine kinase (SYK). SYK activates MAPK/ERK kinase-1 (MEK-1) and/or MEK-2, which phosphorylates and activates extracellular signal-regulated protein kinases 1 and 2 (ERK1/2). The gene expression alterations attributed to ERK1/2 activation result in upregulation of the anti-inflammatory cytokine, IL-10. Leishmania infections are promoted by IL-10 release. Interestingly, L. major chelates host cholesterol in a manner equivalent to treatment with MβCD, theoretically disrupting lipid raft domains. Because localization within rafts probably results in receptor clustering and conformational changes, it is possible that this explains the apparent differences in ‘signalosomes’ utilized by uninfected and Leishmania-infected macrophages [67]. In both (b) and (d), the precise parasite stage that causes cholesterol chelation was not clearly identified, and therefore, is represented by intracellular promastigotes and amastigotes.
L. donovani infection of macrophages causes disruption of membrane lipid rafts and changes in membrane fluidity [54]. In antigen presenting cells such as macrophages, CD1d glycoproteins are responsible for signaling that leads to antigen presentation and the activation of T cells and natural killer cells [55]. In uninfected macrophages, CD1d is present in lipid rafts [54]. In L. donovani-infected macrophages, CD1d becomes non-raft-associated [54]. Since L. donovani-infected cells do not express altered levels of CD1d, it is possible that the non-raft localization of CD1d was due to cholesterol chelation by parasites. Likewise, in L. major-infected macrophages, disruption of lipid rafts causes CD40 to localize to non-raft membrane (Figure 2) [56]. When CD40 is raft-associated, it promotes the assembly of an interleukin-12 (IL-12)-promoting CD40 signalosome, which suppresses Leishmania infection [56]. Mislocalization of CD40 to non-raft membrane promotes the assembly of an IL-10-inducing CD40 signalosome, which enhances L. major infection [56]. IL-12 activates natural killer cells and induces T cell differentiation, which, in turn, promotes proinflammatory pathways leading to suppression of infection. IL-10 production, by contrast, promotes an anti-inflammatory response, which supports Leishmania infection.
Targeting lipid rafts for disease management: all hands on deck
Lipid rafts have been identified as putative antiparasite drug targets. Interestingly, targeting chemotherapeutic agents to rafts may increase their effectiveness [57]. Additionally, key lipids in parasite rafts have subtle compositional differences compared with mammalian lipids, making them excellent drug targets [58]. Specifically in mammalian cells, sphingolipids are important for membrane structure and cell signaling [58]. However, many parasites utilize unique inositol-based sphingolipids, including inositol phosphorylceramide (IPC); therefore, the enzymes, such as IPC synthase, which are required for biosynthesis of these unique lipids, could be targeted by novel chemotherapeutics.
Several studies have investigated the influence of existing drugs on parasite rafts. When Giardia was treated with β-lapachone, lipid raft staining was altered, suggesting that raft domains were disrupted [59]. Two drugs, sitamaquine and miltefosine, were tested for interaction with Leishmania rafts [60,61]. The presence of parasite rafts was essential for miltefosine activity. Wild type and miltefosine-resistant Leishmania parasites were stripped of sterols by incubation with cholesterol oxidase or MβCD [61]. In both cases, drug susceptibility in wild type and mutant cells was reduced, and membrane sterol repletion restored drug sensitivity [61]. A biomimetic membrane model was used to demonstrate that condensed domains (rafts) incorporated more miltefosine than fluid phase membrane domains, and this so-called ‘membrane reservoir’ was probably essential for appropriate miltefosine internalization [61]. However, another anti-Leishmania drug, sitamaquine, did not interact with sterols, and sterol depletion by cholesterol oxidase treatment did not significantly affect parasite drug susceptibility [60]. It remains to be seen whether sitamaquine interacts with other Leishmania raft components, such as IPC.
The effects of chemotherapeutics on host cell rafts have also been investigated. For example, amphotericin B sequesters cholesterol and prevents host cell binding by Leishmania [62]. Although the precise mechanism of this inhibition is not yet understood, the researchers proposed that disrupted host cell receptor signaling and function, attributed to raft perturbation, was responsible for their observations [62]. Furthermore, cholesterol-rich domains are required endocytic entry points for some pathogens; Amphotericin B-mediated cholesterol sequestration may reduce Leishmania invasion by eliminating these domains [62].
In some cases, host raft disruption by drugs helped researchers understand host–parasite interactions. For example, lidocaine, a local anesthetic, reversibly disrupted erythrocyte lipid rafts without affecting membrane cholesterol content [63]. Invasion of lidocaine-treated erythrocytes by P. falciparum parasites was inhibited in a dose-dependent manner [63]. The specific mechanism of action of lidocaine allowed researchers to discern that disruption of raft-specific signaling pathways, rather than membrane cholesterol content per se, was likely to be important in host cell invasion [63].
Concluding remarks
It is clear that both parasite and host lipid rafts participate in the virulence programs of eukaryotic pathogens. Parasites ensure their transmission and survival as stowaways by entering host cells through host rafts and/or by altering the architecture and function of host lipid microdomains. Disruption of parasite rafts inhibits adhesion, invasion, motility, and secretion. These parasite functions are all essential for infection. Although much has been learned about the importance of lipid rafts in parasite biology and virulence, several questions remain to be answered (Box 2). Furthermore, there is evidence that chemotherapeutic raft disruption can alter parasite infectivity and/or drug susceptibility. However, an in-depth understanding of drug interaction with parasite and/or host cell lipid rafts will be necessary for novel antiparasitic drug design. Overall, our understanding of infection and immunity has undoubtedly been improved by new insights into lipid rafts – the ‘life rafts’ of parasites.
Box 2. Outstanding questions: key unresolved questions about the role of lipid rafts in parasite biology and virulence.
What is the exact composition of parasite lipid rafts and how does it differ from the composition of host lipid rafts? Because parasite lipid rafts contain unique lipids, for example, inositol phosphorylceramide (IPC) [58] and ergosterol [5], which are not found in host lipid rafts, a more detailed analysis of the lipoid building blocks of parasite rafts is needed. Such information may be used to design new drugs that target the biosynthetic pathways of unique parasite lipids.
Parasites depend on host lipid rafts for adhesion and invasion, but this dependence seems to be parasite stage-specific [7,51]. What regulates this stage reliance on host lipid rafts and how can this be exploited for disease management?
Do multiple subtypes of lipid rafts exist in parasite membranes? There is evidence suggesting that diverse lipid raft domains exist in individual parasites. This prediction is based on the nonoverlapping localizations of DRM proteins (presumably lipid raft proteins) in whole cells [5] or sucrose gradients [6,10,66]. Identification of distinct lipid rafts and discerning their unique functions will provide significant insight into protein trafficking in parasites.
To what extent does manipulation of host lipid rafts by parasites contribute to parasite survival? The ability of parasites to remodel host lipid microdomains may represent an interesting strategy for enhancing parasite survival. Parasites may remodel host membrane through secreted factors (e.g., GP63 [51,52]) or by surface bound factors (e.g., LPG [48–50]). Alterations to host lipid rafts by parasites may disrupt PIP2-based signaling [35], interrupt the function of caveolae [42], inhibit antigen presentation [54], and promote specific cytokine signaling pathways that are beneficial to the parasite [56]. However, it remains to be seen if other functions, such as host cell apoptosis or reactive oxygen synthesis, are also inhibited when host lipid rafts are remodeled by parasites.
In addition to the general questions outlined above, there are also specific outstanding research questions for individual parasites. For example, in E. histolytica, do lipid rafts regulate the assembly of the Gal/GalNAc lectin trimer? Do lipid rafts regulate cellular functions, other than glideosome-based motility in T. gondii? What specific adhesins are affected in Giardia after treatment with the raft-disrupting agent, MβCD?
Acknowledgments
This material is based upon work supported by the National Institute of Food and Agriculture/United States Department of Agriculture (NIFA/USDA) under project number SC-1700312. This article is technical contribution no. 6047 of the Clemson University Experiment Station. The authors would like to thank Mr Thomas Larrew for help with figure illustrations. The authors would also like to thank Drs James Morris, Meredith Morris, and Kimberly Paul (Department of Genetics and Biochemistry, Clemson University), as well as Ms Brenda Welter (Department of Biological Sciences, Clemson University), for critical reading of the manuscript.
Glossary
- Activator protein 1 (AP-1)
transcription factor composed of subunits belonging c-Fos, c-Jun, ATF, and JDP families; AP-1 regulates cellular responses to stress, including infections.
- Amphotericin B
an antifungal antibiotic which binds membrane sterols, causing changes in membrane permeability and leakage of intracellular components.
- Apicomplexa
group of protists that contain an apicoplast, a non-photosynthetic plastid that may play a role in the synthesis of fatty acids, isoprenoids, amino acids, heme, and iron–sulfur clusters.
- β-lapachone
topoisomerase I inhibitor which has been shown to be an effective treatment for infections with viruses or microorganisms.
- Caco-2/TC7
epithelial cell line derived from colorectal adenocarcinoma.
- Caveolae
specific type of lipid rafts containing caveolin proteins, often involved in endocytosis.
- Caveolin proteins
family of integral membrane proteins which play a role in receptor-independent endocytosis and may also act as scaffolding proteins.
- CD1d
an immunoglobulin family protein with a specific role in lipid antigen presentation.
- CD40
a member of the tumor necrosis factor (TNF) receptor protein family expressed by a variety of cells including B cells, macrophages, dendritic cells, and basal epithelial cells; when bound to CD40L, CD40-mediated signaling induces macrophages and dendritic cells to produce particular cytokines.
- Chinese hamster ovary (CHO) cells
epithelial-like cell line
- Cholera toxin B
secreted toxin that binds to GM1 gangliosides; fluorescently labeled form used to identify lipid rafts, which contain GM1 gangliosides.
- Cys6 proteins
Cys6 proteins possess characteristic domains with six conserved cysteines; Cys6 domains are unique to Plasmodium species.
- Cytokine-inducible nitric oxide synthase (iNOS)
cytokine-inducible enzymes that catalyze the production of nitric oxide (NO).
- Detergent-resistant membrane (DRM)
liquid ordered cholesterol- and sphingolipid-rich membrane which is insoluble in non-ionic detergents such as Triton X-100.
- Dual acylation
addition of both palmitoyl and myristoyl groups to a protein; often this modification is required for lipid raft association (see also palmitoylation and myristoylation).
- Filipin
cyclic compound derived from Streptomyces filipinensis that binds cholesterol and disrupts lipid raft function.
- Glycosylphosphatidylinositol (GPI) anchor
a post-translational modification on proteins consisting of phosphatidylinositol, phosphoethanolamine, and a variety of sugar residues.
- HEp2 cells
epithelial cell line that originates from a human laryngeal carcinoma.
- Interferon (IFN)-γ
type II cytokine that is critical for innate and adaptive immunity.
- Interleukin (IL)-1β
an interleukin produced by macrophages and epithelial cells which induces fever and the activation of macrophages and T cells.
- IL-10
an interleukin produced by monocytes which suppresses the function of macrophages, promoting anti-inflammatory pathways.
- IL-12
an interleukin produced by macrophages and dendritic cells which activates natural killer cells and induces T cell differentiation, promoting proinflammatory pathways.
- Inner membrane complex (IMC)
double membrane aleveolin sacs (vesicles) which play a role in parasite replication and gliding motility.
- Kinetoplastid
single cell flagellate protozoans which contain a kinetoplast which is a network of concatenated circular DNAs.
- Lidocaine
drug that can be used to disrupt lipid rafts without affecting cholesterol content in red blood cells.
- Lipid rafts
tightly packed, cholesterol- and sphingolipid-rich membrane microdomains; lipid rafts are involved in organizing trafficking and signaling pathways in a variety of cell types.
- Lipophosphoglycan (LPG)
eukaryotic surface molecule made up of lipid and polysaccharide (glycan) connected via a phosphodiester bond; LPG is commonly found in protozoan parasites such as Leishmania spp. where it plays a role in modulating host immune response.
- Methyl-β-cyclodextrin (MβCD)
chemical commonly used to chelate cholesterol from membranes, thereby disrupting lipid rafts.
- Miltefosine
a phospholipid drug with antineoplastic and antifungal properties also used to treat cutaneous and visceral leishmaniasis.
- Myristoylation
the addition of the saturated 14 carbon fatty acid, myristic acid, to a glycine residue via amide linkages.
- Palmitoylation
the addition of palmitic acid, a 16 carbon saturated fatty acid to cysteine residues by thioester linkages.
- Parasitophorous vacuole membrane (PVM)
vacuolar membrane that surrounds intracellular parasites during invasion and intracellular replication; vacuole lipids originally derive from host cell lipids and are expanded and modified by the parasite.
- Phosphatidylinositol bisphosphate (PIP2)
phospholipid component of membranes; PIP2 serves as a substrate for hydrolysis by phospholipase C.
- Phosphatidylinositol-specific phospholipase C
class of enzymes that cleave PIP2 into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3).
- Protein tyrosine phosphatases
group of enzymes that remove phosphate groups from tyrosine residues and participate in regulation of signal transduction.
- Raft patching
antibody crosslinking of raft-associated proteins leading to aggregation of rafts; facilitates the visualization of rafts by microscopy.
- Sitamaquine
oral drug which is an 8-aminoquinoline analog; proposed as possible treatment for visceral leishmaniasis.
- Sucrose gradient fractionation
separation of molecules (in this case protein) by buoyancy in a sucrose gradient; often used in fractionation of raft components, which are more buoyant than surrounding membrane.
- Tumor necrosis factor (TNF)-α
cytokine produced by macrophages, natural killer cells, and T cells which promotes inflammatory pathways.
- Transferrin
iron-binding glycoprotein that is taken up by cells through receptor-mediated endocytosis.
References
- 1.Simons K and Sampaio JL (2011) Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol 3, a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humen MA et al. (2011) Lipid raft-dependent adhesion of Giardia intestinalis trophozoites to a cultured human enterocyte-like Caco-2/TC7 cell monolayer leads to cytoskeleton-dependent functional injuries. Cell. Microbiol 13, 1683–1702 [DOI] [PubMed] [Google Scholar]
- 3.Mittal K. et al. (2008) Entamoeba histolytica: lipid rafts are involved in adhesion of trophozoites to host extracellular matrix components. Exp. Parasitol 120, 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welter BH et al. (2011) Localization to lipid rafts correlates with increased function of the Gal/GalNAc lectin in the human protozoan parasite, Entamoeba histolytica. Int. J. Parasitol 41, 1409–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denny PW and Smith DF (2004) Rafts and sphingolipid biosynthesis in the kinetoplastid parasitic protozoa. Mol. Microbiol 53, 725–733 [DOI] [PubMed] [Google Scholar]
- 6.Goldston AM et al. (2012) Exposure to host ligands correlates with co-localization of Gal/GalNAc lectin subunits in lipid rafts and PIP2 signaling in Entamoeba histolytica. Eukaryot. Cell 11, 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes MC et al. (2007) Novel strategy in Trypanosoma cruzi cell invasion: implication of cholesterol and host cell microdomains. Int. J. Parasitol 37, 1431–1441 [DOI] [PubMed] [Google Scholar]
- 8.Yoneyama KAG et al. (2006) Characterization of Leishmania (Viannia) braziliensis membrane microdomains, and their role in macrophage infectivity. J. Lipid Res 47, 2171–2178 [DOI] [PubMed] [Google Scholar]
- 9.Sanders PR et al. (2005) Distinct protein classes including novel merozoite surface antigens in raft-like membranes of Plasmodium falciparum. J. Biol. Chem 280, 40169–40176 [DOI] [PubMed] [Google Scholar]
- 10.Proellocks NI et al. (2007) Plasmodium falciparum Pf34, a novel GPI-anchored rhoptry protein found in detergent-resistant microdomains. Int. J. Parasitol 37, 1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richard D. et al. (2009) Identification of rhoptry trafficking determinants and evidence for a novel sorting mechanism in the malaria parasite Plasmodium falciparum. PLoS Pathog. 5, e1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arévalo-Pinzón G. et al. (2010) Conserved high activity binding peptides from the Plasmodium falciparum Pf34 rhoptry protein inhibit merozoites in vitro invasion of red blood cells. Peptides 31, 1987–1994 [DOI] [PubMed] [Google Scholar]
- 13.Mongui A. et al. (2010) Identification and characterization of the Plasmodium vivax thrombospondin-related apical merozoite protein. Malar. J 9, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Perez DA et al. (2011) Identification, characterization and antigenicity of the Plasmodium vivax rhoptry neck protein 1 (PvRON1). Malar. J 10, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mongui A. et al. (2008) Characterisation of the Plasmodium vivax Pv38 antigen. Biochem. Biophys. Res. Commun 376, 326–330 [DOI] [PubMed] [Google Scholar]
- 16.Angel DI et al. (2008) The Plasmodium vivax Pv41 surface protein: identification and characterization. Biochem. Biophys. Res. Commun 377, 1113–1117 [DOI] [PubMed] [Google Scholar]
- 17.Li J. et al. (2012) Pv12, a 6-Cys antigen of Plasmodium vivax, is localized to the merozoite rhoptry. Parasitol. Int 61, 443–449 [DOI] [PubMed] [Google Scholar]
- 18.García J. et al. (2009) Identification of conserved erythrocyte binding regions in members of the Plasmodium falciparum Cys6 lipid raft-associated protein family. Vaccine 27, 3953–3962 [DOI] [PubMed] [Google Scholar]
- 19.Laughlin RC et al. (2004) Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Infect. Immun 72, 5349–5357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrêa JR et al. (2007) Transferrin uptake may occur through detergent-resistant membrane domains at the cytopharynx of Trypanosoma cruzi epimastigote forms. Mem. Inst. Oswaldo Cruz 102, 871–876 [DOI] [PubMed] [Google Scholar]
- 21.Corrêa JÈR et al. (2008) Transferrin uptake in Trypanosoma cruzi is impaired by interference on cytostome-associated cytoskeleton elements and stability of membrane cholesterol, but not by obstruction of clathrin-dependent endocytosis. Exp. Parasitol 119, 58–66 [DOI] [PubMed] [Google Scholar]
- 22.Daher W and Soldati-Favre D (2009) Mechanisms controlling glideosome function in apicomplexans. Curr. Opin. Microbiol 12, 408–414 [DOI] [PubMed] [Google Scholar]
- 23.Johnson TM et al. (2007) Immobilization of the type XIV myosin complex in Toxoplasma gondii. Mol. Biol. Cell 18, 3039–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders PR et al. (2007) Identification of protein complexes in detergent-resistant membranes of Plasmodium falciparum schizonts. Mol. Biochem. Parasitol 154, 148–157 [DOI] [PubMed] [Google Scholar]
- 25.Tyler KM et al. (2009) Flagellar membrane localization via association with lipid rafts. J. Cell Sci 122, 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godsel LM and Engman DM (1999) Flagellar protein localization mediated by a calcium–myristoyl/palmitoyl switch mechanism. EMBO J. 18, 2057–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tull D. et al. (2010) Membrane protein SMP-1 is required for normal flagellum function in Leishmania. J. Cell Sci 123, 544–554 [DOI] [PubMed] [Google Scholar]
- 28.Tull D. et al. (2004) SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellum membrane in Leishmania. Mol. Biol. Cell 15, 4775–4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Paulo Martins V. et al. (2010) Acylation-dependent export of Trypanosoma cruzi phosphoinositide-specific phospholipase C to the outer surface of amastigotes. J. Biol. Chem 285, 30906–30917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno S. et al. (1994) Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J. Exp. Med 180, 1535–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maric D. et al. (2011) Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J. Biol. Chem 286, 33109–33117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emmer BT et al. (2010) Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot. Cell 9, 934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobo K. et al. (2007) Diversity of raft-like domains in late endosomes. PLoS ONE 2, e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tull D. et al. (2012) Acylation-dependent and independent membrane targeting and distinct functions of small myristoylated proteins (SMPs) in Leishmania major. Int. J. Parasitol 42, 239–247 [DOI] [PubMed] [Google Scholar]
- 35.Murphy SC et al. (2007) Cytoplasmic remodeling of erythrocyte raft lipids during infection by the human malaria parasite Plasmodium falciparum. Blood 110, 2132–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrias E. et al. (2007) Participation of macrophage membrane rafts in Trypanosoma cruzi invasion process. Biochem. Biophys. Res. Commun 363, 828–834 [DOI] [PubMed] [Google Scholar]
- 37.Priotto S. et al. (2009) Trypanosoma cruzi: participation of cholesterol and placental alkaline phosphatase in the host cell invasion. Exp. Parasitol 122, 70–73 [DOI] [PubMed] [Google Scholar]
- 38.Sartori M. et al. (2003) Role of placental alkaline phosphatase in the internalization of trypomatigotes of Trypanosoma cruzi into HEp2 cells. Trop. Med. Int. Health 8, 832–839 [DOI] [PubMed] [Google Scholar]
- 39.Saslowsky DE et al. (2002) Placental alkaline phosphatase is efficiently targeted to rafts in supported lipid bilayers. J. Biol. Chem 277, 26966–26970 [DOI] [PubMed] [Google Scholar]
- 40.Medina FA et al. (2007) Immune dysfunction in caveolin-1 null mice following infection with Trypanosoma cruzi (Tulahuen strain). Microb. Infect 9, 325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueno N. et al. (2009) Differences in human macrophage receptor usage, lysosomal fusion kinetics and survival between logarithmic and metacyclic Leishmania infantum chagasi promastigotes. Cell. Microbiol 11, 1827–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adesse D. et al. (2010) Trypanosoma cruzi infection results in the reduced expression of caveolin-3 in the heart. Cell Cycle 9, 1639–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park DS et al. (2002) Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am. J. Pathol 160, 2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiss AL and Botos E (2009) Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell. Mol. Med 13, 1228–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinet AF et al. (2011) Exclusion of synaptotagmin V at the phagocytic cup by Leishmania donovani lipophosphoglycan results in decreased promastigote internalization. Microbiology 157, 2619–2628 [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez NE et al. (2011) Stage-specific pathways of Leishmania infantum chagasi entry and phagosome maturation in macrophages. PLoS ONE 6, e19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez NE et al. (2006) Role of caveolae in Leishmania chagasi phagocytosis and intracellular survival in macrophages. Cell. Microbiol 8, 1106–1120 [DOI] [PubMed] [Google Scholar]
- 48.Winberg ME et al. (2009) Leishmania donovani lipophosphoglycan inhibits phagosomal maturation via action on membrane rafts. Microb. Infect 11, 215–222 [DOI] [PubMed] [Google Scholar]
- 49.Dermine JF et al. (2005) Leishmania donovani lipophosphoglycan disrupts phagosome microdomains in J774 macrophages. Cell. Microbiol 7, 1263–1270 [DOI] [PubMed] [Google Scholar]
- 50.Vinet AF et al. (2009) The Leishmania donovani lipophosphoglycan excludes the vesicular proton-ATPase from phagosomes by impairing the recruitment of synaptotagmin V. PLoS Pathog. 5, e1000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez MA et al. (2009) Leishmania GP63 alters host signaling through cleavage-activated protein tyrosine phosphatases. Sci. Signal 2, ra58. [DOI] [PubMed] [Google Scholar]
- 52.Contreras I. et al. (2010) Leishmania-induced inactivation of the macrophage transcription factor AP-1 is mediated by the parasite metalloprotease GP63. PLoS Pathog. 6, e1001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinonen KM et al. (2009) Protein tyrosine phosphatases PTP-1B and TC-PTP play nonredundant roles in macrophage development and IFN-γ signaling. Proc. Natl. Acad. Sci. U.S.A 106, 9368–9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakraborty D. et al. (2005) Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J. Immunol 175, 3214–3224 [DOI] [PubMed] [Google Scholar]
- 55.Brigl M and Brenner MB (2004) CD1: antigen presentation and T cell function. Annu. Rev. Immunol 22, 817–890 [DOI] [PubMed] [Google Scholar]
- 56.Rub A. et al. (2009) Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nat. Immunol 10, 273–280 [DOI] [PubMed] [Google Scholar]
- 57.Rajendran L. et al. (2010) Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov 9, 29–42 [DOI] [PubMed] [Google Scholar]
- 58.Young SA et al. (2012) Sphingolipid and ceramide homeostasis: potential therapeutic targets. Biochem. Res. Int 2012, Article ID 248135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Correa G. et al. (2009) Cell death induction in Giardia lamblia: effect of β-lapachone and starvation. Parasitol. Int 58, 424–437 [DOI] [PubMed] [Google Scholar]
- 60.Coimbra E. et al. (2010) Mechanism of interaction of sitamaquine with Leishmania donovani. J. Antimicrob. Chemother 65, 2548–2555 [DOI] [PubMed] [Google Scholar]
- 61.Saint-Pierre-Chazalet M. et al. (2009) Membrane sterol depletion impairs miltefosine action in wild-type and miltefosine-resistant Leishmania donovani promastigotes. J. Antimicrob. Chemother 64, 993–1001 [DOI] [PubMed] [Google Scholar]
- 62.Paila YD et al. (2010) Amphotericin B inhibits entry of Leishmania donovani into primary macrophages. Biochem. Biophys. Res. Commun 399, 429–433 [DOI] [PubMed] [Google Scholar]
- 63.Koshino I and Takakuwa Y (2009) Disruption of lipid rafts by lidocaine inhibits erythrocyte invasion by Plasmodium falciparum. Exp. Parasitol 123, 381–383 [DOI] [PubMed] [Google Scholar]
- 64.Emmer BT et al. (2009) Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J. Cell Sci 122, 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fridberg A et al. (2008) Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J. Cell Sci 121, 522–535 [DOI] [PubMed] [Google Scholar]
- 66.Tull D. et al. (2012) Acylation-dependent and independent membrane targeting and distinct functions of small myristoylated proteins (SMPs) in Leishmania major. Int. J. Parasitol 42, 239–247 [DOI] [PubMed] [Google Scholar]
- 67.Garg R. et al. (2008) Leishmania infantum promastigotes reduce entry of HIV-1 into macrophages through a lipophosphoglycan-mediated disruption of lipid rafts. J. Infect. Dis 197, 1701–1708 [DOI] [PubMed] [Google Scholar]
- 68.Parish L. et al. (2011) Ookinete-interacting proteins on the microvillar surface are partitioned into detergent resistant membranes of Anopheles gambiae midguts. J. Proteome Res. 10, 5150–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Girolamo F. et al. (2008) Plasmodium lipid rafts contain proteins implicated in vesicular trafficking and signalling as well as members of the PIR superfamily, potentially implicated in host immune system interactions. Proteomics 8, 2500–2513 [DOI] [PubMed] [Google Scholar]


