Abstract
Objectives
Immune checkpoint inhibitors are associated with adverse cardiovascular events. However, there are no data characterizing cardiovascular events among Asians on immune checkpoint inhibitors. We aim to determine the incidence and risk of cardiac events associated with immune checkpoint inhibitors in an Asian population.
Methods
We performed a retrospective, propensity score-matched cohort study at two tertiary referral centers in Taiwan. Immune checkpoint inhibitor users were matched with non-immune checkpoint inhibitor users based on predetermined clinical variables. The primary outcome was major adverse cardiovascular events, defined as a composite of myocardial infarction, ischemic stroke, acute peripheral occlusive disease, pulmonary embolism, deep venous thrombosis, heart failure, pericardial disease, myocarditis, cardiac arrhythmias and conduction block.
Results
Between January 2010 and November 2021, 868 immune checkpoint inhibitor users were matched 1:1 with non-immune checkpoint inhibitor users. Among immune checkpoint inhibitor users, 67 (7.7%) patients developed major adverse cardiovascular events. During a median follow-up period of 188 days, the incidence rate of major adverse cardiovascular events for immune checkpoint inhibitor and non-immune checkpoint inhibitor users was 94.8 and 46.2 per 1000 patient-years, respectively, resulting in an incidence rate ratio of 2.1 [95% confidence interval: 1.5–2.9]. In multivariate Cox proportional hazard models, immune checkpoint inhibitor users had a 60% increased risk for major adverse cardiovascular events [hazard ratio, 1.6 (95% confidence interval: 1.1–2.3)]. Immune checkpoint inhibitors use was independently associated with increased risk of ischemic stroke [hazard ratio, 3.0 (95% confidence interval: 1.0–9.0)] and pulmonary embolism [hazard ratio, 5.5 (95% confidence interval: 1.4–21.3)]. In multivariate logistic regression analysis, age > 65, metastatic disease, hypertension and baseline platelet-to-lymphocyte ratio < 180 were risk factors for major adverse cardiovascular events.
Conclusions
Among Asians, immune checkpoint inhibitors were associated with an increased risk of major adverse cardiovascular events, particularly ischemic stroke and pulmonary embolism.
Keywords: Immune checkpoint inhibitor, cardiovascular event, immune-related adverse event
The use of immune checkpoint inhibitors is associated with an increased risk of major adverse cardiovascular events, in particular ischemic stroke and pulmonary embolism.
Introduction
Immune checkpoint inhibitors (ICIs) constitute a new paradigm in cancer treatment and are increasingly used in a broad range of cancers (1). They work by blocking inhibitory checkpoints and activating T cells to seek and destroy cancerous cells (2). Despite their efficacy, ∼60–80% of ICI-treated patients develop immune-related adverse events (irAEs) with various clinical manifestations (3). Among these irAEs, cardiovascular adverse events, although relatively uncommon, are increasingly reported in the literature (4–7). Based on previous studies, ICI-associated cardiovascular adverse events may include myocarditis, pericardial disease, vascular thrombotic diseases, heart failure and cardiac arrhythmias (8–13). The incidence of ICI-associated cardiac events is estimated to vary from 5 to 15%, depending on the definitions, types of study and the populations (8–15).
Previous randomized controlled trials (RCTs) investigating the safety and efficacy of ICIs were not designed to report and detect cardiovascular events and these events were underreported (16). Most of the current evidence suggesting an increased risk of cardiovascular adverse events associated with ICIs came from observational studies and meta-analyses. In a large observational study, ICI-treated patients had more than three times the risk of atherosclerotic events compared with patients who were not treated with ICI (9). In a meta-analysis of RCTs, ICI users had a more than three times risk of myocarditis and dyslipidemia, and two times higher risk of pericardial disease, heart failure, myocardial infarction and ischemic stroke compared with non-ICI users (17).
Despite reports on cardiovascular adverse events among ICI users, there have been no reports of cardiac risk associated with ICI treatment among Asian populations. A focused study in an Asian population is merited as Asians have a high baseline susceptibility to cardiovascular events, principally atherosclerotic cardiovascular events and a different cardiac risk profile compared with other populations (18–20). For example, the proportion of premature cardiovascular deaths was close to 2-fold higher in Asia compared with the United States and Europe (20). Therefore, there is a need to characterize the incidence and risk of cardiovascular adverse events associated with ICI use in this unique population. In this study, we describe the incidence and risk of cardiac events associated with ICI use in Asia, using data retrieved from two hospitals in Taiwan.
Methods
Study design
This was a retrospective, propensity-score matched cohort study conducted at Chung Shan Medical University Hospital, Taichung, Taiwan, and Taipei Tzu Chi Hospital, New Taipei City, Taiwan. Both hospitals are tertiary referral centers for cancer. This study was approved by the Institutional Review Board at both hospitals (Chung Shan Medical University Hospital, number CS2–21095, approved on July 2021; Taipei Tzu Chi Hospital, number 10-X-157, approved on November 2021).
We included all patients diagnosed with cancer between January 2010 and November 2021, identified using the International Classification of Diseases (ICD)-9 or ICD-10 codes. Patients who had only one hospital visit, or missing data were excluded. We defined cases as patients who received at least one cycle of ICI, and controls as patients who did not receive any ICI. The index date for cases was determined as the date of the first ICI cycle, and the index date for controls was determined as the date of non-ICI treatment. The non-ICI treatment included chemotherapy, targeted therapy, surgery and radiotherapy. We utilized the electronic medical records to collect data such as age, sex, cancer information, underlying comorbidities, previous cardiotoxic cancer therapies, use of cardioprotective medications. For ICI users, we further collected information on ICI type and treatment cycle, occurrences of other irAEs and clinically indicated laboratory data.
Outcome definition
We defined ICI-associated cardiovascular adverse events as incident cardiovascular events occurring after the initiation of ICI. Only cardiac events that occurred for the first time after the initiation of ICI were considered. The primary outcome was major adverse cardiovascular events (MACE), defined as a composite of myocardial infarction, ischemic stroke, acute peripheral occlusive disease, pulmonary embolism, deep venous thrombosis, heart failure requiring hospitalization, pericardial disease, myocarditis, cardiac arrhythmias and conduction block. These were defined as events as each has been reported, in predominately non-Asian populations, to be increased with the use of ICIs (5,9,13–15,21). The secondary outcomes were composites of arterial thrombotic events (composite of myocardial infarction, ischemic stroke and peripheral arterial disease), venous thrombotic events (composite of deep venous thrombosis and pulmonary embolism) and other individual cardiovascular outcomes. Two investigators (Cho-Han Chiang and Cho-Hung Chiang) adjudicated the cardiovascular events independently using a combination of ICD codes, medication use and clinical/imaging findings (Supplementary Table 1) and blinded to group status.
Statistical analysis
To minimize the baseline differences between ICI and non-ICI users, we conducted propensity score matching to match ICI users with non-ICI users based on the following predetermined variables: age, sex, cancer type, presence of metastasis, history of cardiovascular diseases, underlying comorbidities: hypertension (HTN), diabetes mellitus, chronic kidney disease (CKD), hyperlipidemia, chronic obstructive pulmonary disease (COPD) and autoimmune diseases, prior use of cardiotoxic agents: tyrosine kinase inhibitor (TKI), 5-fluorouracil, angiogenesis inhibitor, radiotherapy and anthracyclines, and use of cardioprotective agents: angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), beta-blockers, calcium-channel blockers (CCBs), statins and aspirin. Differences in baseline characteristics between ICI and non-ICI users were compared using standardized mean differences (SMDs) after propensity score matching. An SMD greater than 10% implied an imbalanced distribution of covariates between ICI and non-ICI users.
We calculated the incidence rates of MACE and individual cardiovascular outcomes, and incidence rate ratios using Poisson regression. We performed Kaplan–Meier survival analysis to compare the cumulative incidence curves between ICI and non-ICI users, and conducted Cox proportional hazard model analysis to assess the association between ICI use and the risk of various cardiovascular events. The proportional hazard ratio (HR) assumption was tested based on Schoenfeld residuals. We also performed a subgroup analysis to assess the cardiovascular risk of different classes of ICI: programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). To identify potential risk factors predictive of ICI-associated cardiac events, we used logistic regression model analysis using known cardiovascular risk factors and a stepwise selection approach. A P value <0.05 indicates statistical significance. All analyses were conducted using Stata version 16.0 (StataCorp LLC, College Station, TX).
Results
Patient demographics
Eligible cancer patients treated at Chung Shan Medical University and Taipei Tzu Chi Hospital between 2010 and 2021 were identified using ICD codes. After excluding patients with incomplete data, there remained 868 ICI users (cases) and 56 897 non-ICI users (controls) eligible for analysis (Fig. 1). As expected, ICI users tended to have a higher rate of metastatic disease as compared with non-ICI users (81 vs. 25%, SMD = 133.8) (Supplementary Table 2). Patients who were treated with an ICI also received more types of cardiotoxic therapies in the past as compared with patients who were not treated with ICI. After propensity score matching, all covariates including underlying comorbidities, previous cardiotoxic therapies and previous cardioprotective agents were balanced between the two groups (Table 1 and Supplementary Fig. 1). The median ages for ICI and non-ICI users were 69 (59–77) and 68 (59–78), respectively (Table 1). Lung and hepatobiliary cancers were the most common types of cancer in both ICI and non-ICI users.
Figure 1.
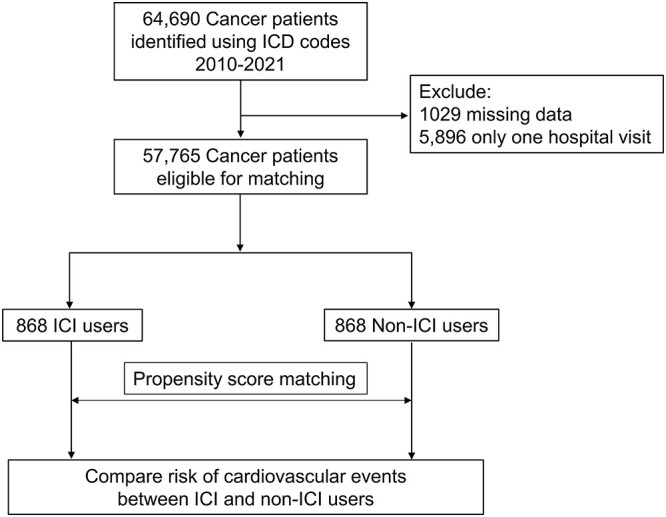
Flowchart of patient enrollment. Abbreviations: ICI, immune checkpoint inhibitor; ICD, International Classification of Diseases.
Table 1.
Demographics of cases and controls after propensity score matching.
| Demographics | Total | Non-ICI user | ICI user | SMD (%) |
|---|---|---|---|---|
| N = 1736 | N = 868 | N = 868 | ||
| Age | 68 (59–78) | 68 (59–78) | 69 (59–77) | −1.8 |
| Male | 1092 (63%) | 536 (62%) | 556 (64%) | 4.7 |
| History of cardiovascular event | 398 (23%) | 186 (21%) | 212 (24%) | 7.6 |
| Presence of metastasis | 1424 (82%) | 724 (83%) | 700 (81%) | −6.7 |
| Cancer types | ||||
| Head and neck cancer | 176 (10%) | 84 (10%) | 92 (11%) | 3.8 |
| GI cancer | 137 (8%) | 67 (8%) | 70 (8%) | |
| Hepatobiliary cancer | 301 (17%) | 150 (17%) | 151 (17%) | |
| Pancreatic cancer | 23 (1%) | 13 (1%) | 10 (1%) | |
| Lung cancer | 789 (45%) | 397 (46%) | 392 (45%) | |
| Skin cancer | 38 (2%) | 16 (2%) | 22 (3%) | |
| Breast cancer | 48 (3%) | 28 (3%) | 20 (2%) | |
| Gynecologic cancer | 161 (9%) | 80 (9%) | 81 (9%) | |
| Renal and genitourinary | 28 (2%) | 14 (2%) | 14 (2%) | |
| Bone and connective tissue | 10 (1%) | 6 (1%) | 4 (0%) | |
| Others | 25 (1%) | 13 (1%) | 12 (1%) | |
| Common comorbidities | 765 (44%) | 390 (45%) | 375 (43%) | −3.7 |
| Hypertension | 380 (22%) | 188 (22%) | 192 (22%) | 1.2 |
| Diabetes Mellitus | 321 (18%) | 154 (18%) | 167 (19%) | 4.2 |
| Hyperlipidemia | 208 (12%) | 98 (11%) | 110 (13%) | 4.8 |
| CKD | 327 (19%) | 159 (18%) | 168 (19%) | 3.1 |
| COPD | 74 (4%) | 34 (4%) | 40 (5%) | 3.6 |
| History of autoimmune disease | 765 (44%) | 390 (45%) | 375 (43%) | −3.7 |
| Use of cardiotoxic agents | ||||
| Anthracyclines | 683 (39%) | 338 (39%) | 345 (40%) | −1.1 |
| Angiogenesis inhibitor | 683 (39%) | 338 (39%) | 345 (40%) | 2.2 |
| TKI | 588 (34%) | 296 (34%) | 292 (34%) | −1.2 |
| 5-FU | 344 (20%) | 174 (20%) | 170 (20%) | −1.3 |
| Radiation | 192 (11%) | 100 (12%) | 92 (11%) | −3.7 |
| Use of cardiovascular medications | ||||
| ACEI-ARB | 198 (11%) | 91 (10%) | 107 (12%) | 5.8 |
| B-blocker | 175 (10%) | 86 (10%) | 89 (10%) | 1.1 |
| CCB | 147 (8%) | 66 (8%) | 81 (9%) | 5.9 |
| Diuretics | 125 (7%) | 63 (7%) | 62 (7%) | −0.4 |
| Statin | 139 (8%) | 73 (8%) | 66 (8%) | −3.1 |
| Aspirin | 109 (6%) | 55 (6%) | 54 (6%) | −0.5 |
ACEI, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin receptor blockers; CCB, calcium channel blocker; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; TKI, tyrosine kinase inhibitors; SMD, standardized mean differences.
Primary outcome
Among 868 ICI users, 67 patients (74 cases) developed MACE, resulting in an incidence proportion of 7.7% (Supplementary Fig. 2). The most common type of ICI-associated MACE was cardiac arrhythmias (27 cases), followed by pericardial disease (9 cases), ischemic stroke (9 cases) and pulmonary embolism (9 cases). There were three identified cases of ICI-associated myocarditis. Only 1% of the patients received combination therapy (nivolumab plus ipilimumab). The most commonly used regimen was single-agent nivolumab (52%), followed by single-agent pembrolizumab (38%) (Supplementary Table 3). There were more patients receiving a PD-L1 inhibitor and fewer patients receiving a PD-1 inhibitor among cases with MACE. Patients with MACE tended to more commonly develop any other irAE than non-MACE patients (25 vs. 17%, P value = 0.09) (Supplementary Table 4). The baseline white blood count, absolute neutrophil count , neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were all lower in patients who had MACE (Supplementary Table 5).
During a median follow-up period of 188 days, the incidence rate of MACE for ICI and non-ICI users was 94.8 and 46.2 per 1000 patient-years, resulting in an incidence rate ratio of 2.1 [95%confidence interval (CI): 1.5–2.9] (Table 2). In univariate and multivariate Cox proportional hazard models, ICI users had a 60% increase in the risk for MACE [univariate HR, 1.6 (95% CI: 1.1–2.2); multivariate HR, 1.6 (95% CI: 1.1–2.3)] (Table 3). Kaplan–Meier analysis showed that ICI users had a higher cumulative incidence of MACE compared with non-ICI users (Log-rank, P value = 0.01) (Fig. 2). The median overall survival for patients who developed MACE was shorter than that for patients who did not develop MACE [12.3 (interquartile range, IQR: 4.6–25.5) vs. 16.2 (IQR: 4.08–58.4) months; log-rank, P = 0.14] (Supplementary Fig. 3).
Table 2.
Incidence rate ratio of cardiac outcomes between ICI and non-ICI users
| Outcomes | Exposure | Incidence rate, per 1000 patient-years | Incidence rate ratio (95% CI) |
|---|---|---|---|
| MACE | ICI | 94.8 | 2.1 (1.5–2.9) |
| Non-ICI | 46.2 | Reference | |
| Arterial thromboembolism (MI, stroke) | ICI | 20.8 | 3.0 (1.3–6.8) |
| Non-ICI | 7.0 | Reference | |
| Venous thromboembolism (PE, DVT) | ICI | 19.5 | 2.6 (1.1–5.8) |
| Non-ICI | 7.6 | Reference | |
| Myocardial infarction | ICI | 8.3 | 3.9 (0.9–18.7) |
| Non-ICI | 2.1 | Reference | |
| Ischemic stroke | ICI | 12.4 | 3.3 (1.1–10.5) |
| Non-ICI | 3.8 | Reference | |
| Pulmonary embolism | ICI | 12.4 | 7.7 (1.9–44.5) |
| Non-ICI | 1.6 | Reference | |
| Deep venous thrombosis | ICI | 9.7 | 1.5 (0.5–4.2) |
| Non-ICI | 6.5 | Reference | |
| Heart failure hospitalization | ICI | 5.5 | 1.7 (0.4–7.3) |
| Non-ICI | 3.2 | Reference | |
| Pericardial disease | ICI | 12.5 | 2.1 (0.8–5.6) |
| Non-ICI | 5.9 | Reference | |
| Myocarditis | ICI | 4.3 | 7.6 (0.6–402) |
| Non-ICI | 0.6 | Reference | |
| Arrhythmia | ICI | 36.5 | 1.4 (0.8–2.3) |
| Non-ICI | 25.9 | Reference |
MI, myocardial infarction, PAOD, peripheral arterial occlusive disease; PE, pulmonary embolism; DVT, deep venous thrombosis
Note: No PAOD and heart block cases were reported
Table 3.
Univariate and multivariate Cox proportional hazard analysis for various cardiovascular events
| Outcomes | Univariate HR(95% CI) | Multivariate HRa (95% CI) |
|---|---|---|
| MACE | 1.6 (1.1–2.2) | 1.6 (1.1–2.3) |
| Arterial thromboembolism (MI, stroke) | 2.4 (1.1–5.4) | 2.6 (1.1–5.8) |
| Venous thromboembolism (PE, DVT) | 1.9 (0.9–4.1) | 2.0 (0.9–4.3) |
| Myocardial infarction | 2.4 (0.7–8.5) | 2.5 (0.7–9.0) |
| Ischemic stroke | 2.9 (0.9–8.7) | 3.0 (1.0–9.0) |
| Pulmonary embolism | 5.2 (1.3–20.2) | 5.5 (1.4–21.3) |
| Deep venous thrombosis | 1.1 (0.4–2.9) | 1.2 (0.4–3.0) |
| Heart failure hospitalization | 2.0 (0.5–8.0) | 2.3 (0.6–9.4) |
| Pericardial disease | 1.9 (0.8–5.0) | 2.0 (0.8–5.1) |
| Myocarditis | 3.0 (0.3–28.8) | 2.9 (0.3–28.9) |
| Arrhythmia | 1.0 (0.6–1.7) | 1.1 (0.6–1.8) |
MI, myocardial infarction, PAOD, peripheral arterial occlusive disease; PE, pulmonary embolism; DVT, deep venous thrombosis
Note: No PAOD and heart block cases were reported
aAdjusted for age, gender, metastatic disease, cancer type and previous history of cardiovascular disease
Figure 2.
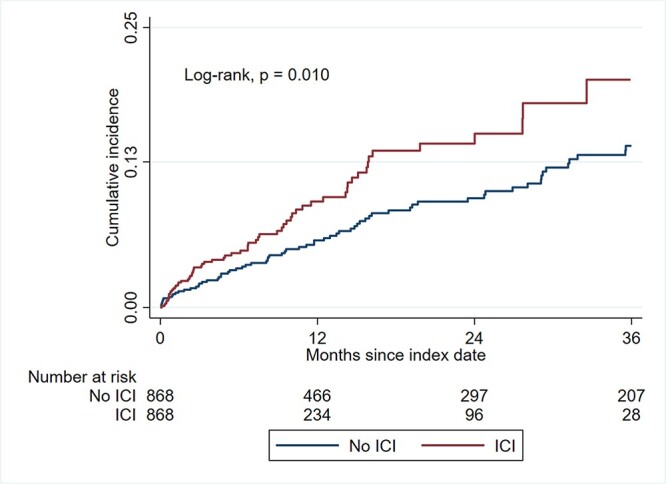
Cumulative incidence of major adverse cardiovascular events. Abbreviations: ICI, immune checkpoint inhibitor.
Secondary outcomes
During the follow-up period, the incidence rate of arterial thromboembolism (ATE) for ICI and non-ICI users was 20.8 and 7.0 per 1000 patient-years, resulting in an incidence rate ratio of 3.0 [95% CI: 1.3–6.8] (Table 2). The incidence rate of venous thromboembolism (VTE) for ICI and non-ICI users was 19.5 and 7.6 per 1000 patient-years, resulting in an incidence rate ratio of 2.6 [95% CI: 1.1–5.8] (Table 2). In multivariate Cox proportional hazard models, ICI users had a 2-fold or higher risk of ATE [multivariate hazard ratio (HR), 2.6 (95% CI: 1.1–5.8)] and VTE [multivariate HR, 2.0 (95% CI: 0.9–4.3)] than non-ICI users (Table 3).
With the individual cardiovascular outcomes, ICI users had a significantly higher risk of ischemic stroke [incidence rate, 12.4 vs. 3.8 per 1000 patient-years; incidence rate ratio 3.3 (95% CI: 1.1–10.5)] and pulmonary embolism [incidence rate, 12.4 vs. 1.6 per 1000 patient-years; incidence rate ratio 7.7 (95% CI: 1.9–44.5)] than non-ICI users (Table 2). In multivariate Cox proportional hazard models, ICI users had a 3-fold or higher risk of ischemic stroke [multivariate HR, 3.0 (95% CI: 1.0–9.0)] and pulmonary embolism [multivariate HR, 5.5 (95% CI: 1.4–21.3)] than non-ICI users (Table 3). The risk of other individual cardiovascular events was numerically but not significantly higher in ICI users.
Subgroup analyses
We conducted a subgroup analysis to investigate if a particular ICI drug class is associated with an increased risk of MACE. We did not analyze patients treated with a CTLA-4 inhibitor (ipilimumab) as only 15 patients received a CTLA-4 inhibitor. We excluded patients from this subgroup analysis if they have received both PD-1 and PD-L1 inhibitors. Among 671 PD-1 inhibitor users, the incidence rate of MACE was 87.5 per 1000 patient-years, resulting in an incidence rate ratio of 1.9 (95% CI: 1.3–2.8) when compared with non-ICI users (Supplementary Table 6). Among 145 PD-L1 inhibitor users, the incidence rate of MACE was 172.2 per 1000 patient-years, resulting in an incidence rate ratio of 3.7 (95% CI: 2.1–6.2) when compared with non-ICI users. In univariate and multivariate Cox proportional hazard models, PD-L1 inhibitor users had a nearly 2-fold higher risk of MACE compared with PD-1 inhibitor users [multivariate HR, 2.8 (95% CI: 1.7–4.8) vs. 1.5 (95% CI: 1.0–2.2)].
Predictive variables for ICI-associated cardiovascular events
In model 1, incorporating prespecified clinical variables, among ICI users, age and presence of metastatic disease were independently associated with an increased risk of MACE (Table 4). In model 2, using a stepwise selection approach, age ≥ 65, presence of metastatic disease, HTN at baseline and development of any irAE were independently associated with an increased risk of MACE. By contrast, use of a PD-1 inhibitor and use of beta-blockers were associated with a lower risk of MACE. In model 3, using a stepwise selection that includes peripheral blood markers, a baseline PLR < 180 was independently associated with a 2-fold increased risk for MACE [odds ratio, 2.00 (95% CI: 1.15–3.50)], P value = 0.02).
Table 4.
Multivariate logistic regression analysis for major adverse cardiac events
| Covariates | Odds ratio (95% CI) | P value |
|---|---|---|
| Multivariate model 1 | ||
| Age | 1.03 (1.01–1.05) | 0.02 |
| Metastasis | 3.47 (1.35–8.88) | 0.01 |
| Cardiovascular disease | 0.84 (0.45–1.58) | 0.60 |
| Hypertension | 1.50 (0.85–2.68) | 0.17 |
| Diabetes mellitus | 1.14 (0.62–2.11) | 0.67 |
| Hyperlipidemia | 0.98 (0.50–1.92) | 0.96 |
| Chronic kidney disease | 0.95 (0.44–2.03) | 0.89 |
| Any other irAE | 1.66 (0.92–2.99) | 0.09 |
| Multivariate model 2 | ||
| Age ≥ 65 | 2.15 (1.25–3.71) | 0.006 |
| Metastasis | 3.48 (1.36–8.87) | 0.009 |
| PD-1 | 0.51 (0.29–0.92) | 0.02 |
| Hypertension | 1.75 (1.02–2.98) | 0.04 |
| Beta-blocker | 0.26 (0.08–0.86) | 0.03 |
| Any other irAE | 1.73 (0.95–3.14) | 0.07 |
| Multivariate model 3 a | ||
| Age ≥ 65 | 2.08 (1.19–3.57) | 0.01 |
| Metastasis | 3.57 (1.40–9.11) | 0.01 |
| PD-1 | 0.58 (0.32–1.05) | 0.07 |
| Hypertension | 1.72 (1.00–2.94) | 0.05 |
| Beta-blocker | 0.25 (0.07–0.83) | 0.02 |
| Any other irAE | 1.70 (0.93–3.10) | 0.08 |
| Baseline PLR < 180 | 2.00 (1.15–3.50) | 0.02 |
irAE, immune-related adverse events; PD-1, programmed cell death protein 1; PLR, platelet-lymphocyte ratio
aOnly 727 patients have baseline lab data for analysis
Discussion
Evidence from RCTs and real-world observational studies have reported severe and fatal irAEs related to ICIs (11,22). In this propensity-score matched cohort study among Asian patients, ICI treatment was associated with a 1.6-fold increased risk of cardiovascular events compared with non-ICI treatment. Patients treated with ICIs had an increased risk of arterial and venous thrombotic events, in particular ischemic stroke and pulmonary embolism. Notably, PD-L1 inhibitors were associated with a ∼2-fold higher risk of cardiovascular events compared with PD-1 inhibitors. Our findings have important implications for Asian patients who are currently treated with ICI and potential candidates for ICI treatment.
RCTs have not consistently reported an association between ICI use and cardiovascular outcomes for several reasons: conventional cancer trials were not designed to report cardiovascular outcomes (16), patients recruited in these trials tended to have better performance status (23) and patients with a history of cardiovascular diseases were often excluded from these trials (24). Nevertheless, some studies have investigated the association between ICI and the risk of cardiovascular events. In a population-based study conducted in Denmark, ICI was associated with a 2– 5-fold increased risk of composite cardiac events in lung cancer and melanoma patients (13). Similarly, in a large single-center study, ICI was associated with a 3-fold increased risk of atherosclerotic cardiovascular events (9). Recently, a safety meta-analysis of RCTs showed that ICI was associated with a 1.5–4-fold increased risk of cardiac events, including dyslipidemia, myocarditis, pericardial diseases, heart failure, ischemic stroke and myocardial infarction (17).
Our study adds value to the current literature by extending these findings to an Asian population. In addition, we note several important differences between our study and previous reports. First, we found an increased risk of VTEs, specifically pulmonary embolism, associated with ICI. Although VTEs were frequently reported among ICI users (10,21,25), a recent meta-analysis of RCTs did not find an increased risk of VTEs associated with ICI use (17). Metastatic disease is a risk factor for VTEs (26), and the higher rates of metastatic disease among ICI users might be a confounder that increases the risk of VTEs in the ICI-treated group. Nevertheless, we included metastatic disease in the propensity score model where we matched ICI and non-ICI users, and we adjusted for metastatic disease in the Cox Proportional Hazard model analysis, minimizing the concern for metastatic disease as a confounding variable. Further studies are required to ascertain if ICIs are associated with VTEs. We detected three cases of myocarditis (incidence of 0.35%). This is a rate lower than reported by previous studies (5,11,17). This lower rate of myocarditis is likely due to misclassification of myocarditis in large cohort studies as other cardiovascular diseases such as heart failure, pericarditis or cardiac arrhythmia. Cardiac magnetic resonance imaging, one of the diagnostic modalities for myocarditis (7,14,27), is not routinely available and applied in our cohorts presenting with other potential manifestations of myocarditis, likely leading to an underdiagnosis of this condition in our study. In addition, only 15 (1.7%) of our patients received ipilimumab mono or combination therapy, both of which are risk factors for myocarditis (5). We did not detect a statistically significantly higher risk of myocardial infarction, also likely because of the short follow-up time in the ICI cohort. The median follow-up time in this study was only 188 days since the initiation of ICI, and ICI-related atherosclerotic changes might not have developed within this short timeframe (9). We did observe an increased risk of ischemic stroke and composite ATE in our analysis, which is consistent with the theory that ICI might lead to atherosclerotic changes and progression of atherosclerotic plaques (9). We did not detect an increased risk of heart failure among ICI users, which differed from a recent meta-analysis showing that ICI use was associated with a 2-fold increased risk of heart failure (17). This discrepancy might be because we only included incident (first-time) heart failure hospitalizations and the meta-analysis included recurrent heart failure hospitalizations (17). We also observed a shorter median overall survival for patients who developed ICI-associated MACE, though the association was not statistically significant. This was contrary to the observations that the development of ICI-associated irAEs is associated with an improved survival outcome (28–30). This is likely because immune-related cardiovascular events can be of a greater severity. For example, the mortality for myocarditis is estimated to be >25% (5,31,32). In a systematic review of 125 trials, deaths due to cardiovascular irAEs comprise about 10% of all ICI-related deaths (33). In line with these findings, the development of serious non-cardiovascular irAEs such as hepatitis and pneumonitis is associated with a poor prognosis (22,34,35).
In this study, we observed an incidence proportion of 7.7% per 1000 patient-years for a composite cardiovascular outcome, which was lower than that reported by previous observational studies. In a single-center study, conducted by Chitturi and colleagues, the incidence proportion for MACE comprising cardiovascular death, nonfatal myocardial infarction, nonfatal stroke and hospitalization for heart failure was 13.3% (36). In a population-based study performed by D’Souza and colleagues, the absolute 1-year risk for a composite cardiovascular outcome comprising arrhythmia, cardiac death/arrest, chest pain, heart failure, myocarditis/pericarditis and stroke was 9.7% (13). This lower rate in our study could be due to several reasons. First, in these observational studies, only patients with lung cancer or melanoma were included. Lung cancer patients have one of the highest risks of cardiovascular diseases among all types of cancer (37), so the incidence of cardiac events would likely be higher than that in a general cancer population. Second, the study performed by D’Souza and colleagues had a longer follow-up time than our study, thereby allowing for the development of more cardiovascular events. Third, these studies were conducted in Caucasian populations, which had different baseline risk of cardiovascular diseases compared with Asian populations (19). For example, East Asians were found to have higher stroke rates and lower coronary heart disease rates compared with the United States and Scandinavian populations (18). Consistent with the literature, our study found a higher ICI-associated stroke incidence proportion (9/868 vs. 0/135) and lower ICI-associated MI incidence rate (6/868 vs. 1/135) compared with the single-center study by Chitturi and colleagues (36).
The optimal approach to detection and management of ICI-associated adverse cardiovascular events remains unclear. We report several clinical variables that may aid clinicians to identify patients at an increased risk of developing adverse cardiac events from ICI treatment. Age, metastatic disease and HTN are well-recognized risk factors for cardiovascular diseases (26,38–40). A lower baseline NLR and PLR have been reported to predict the occurrences of irAEs (41,42). Both NLR and PLR appear to represent the balance between immunoreaction and non-specific inflammation, potentially influencing the response to ICIs (41). In a secondary analysis of five RCTs in cardiovascular diseases, baseline NLR correlated positively with risk of cardiac events (43); in this study, a higher NLR seemed to reflect a higher degree of inflammation and myocardial damage. By contrast, in a case–control study of ICI-treated patients, a higher NLR was noted on admission with myocarditis and was associated with worse outcomes (44). Even though we did not find an association between baseline NLR and risk of ICI-associated cardiovascular event in our multivariate logistic regression analysis, we did observe a lower NLR in patients who developed MACE. In contrast to NLR, baseline PLR was independently associated with risk of MACE after adjusting for other clinical variables. Similar to a previous study investigating irAEs (41), 70% of patients who developed MACE had a PLR lower than 180. Thus, baseline PLR may be used to predict irAEs that include cardiovascular adverse events, in combination with other clinical variables. Of note, we found a lower risk of cardiac events among ICI patients treated with beta-blockers, consistent with a cardioprotective role of beta-blockers in cancer therapy (45). Whether beta-blockers can be utilized to reduce the risk of ICI-associated cardiovascular events remains to be determined by future studies.
The mechanisms underlying cardiovascular adverse events resulting from ICI treatment have not been fully elucidated but are based on solid scientific plausibility. These same immune checkpoints being targeted for cancer are also critical regulators of atherosclerosis. For example, inhibition of PD-1 and PD-L1 is associated with an increase in atherosclerotic plaque in animal models, through a mechanism of increased vascular adhesion and a marked infiltration of CD4- and CD8-positive T-cells (46,47). Possible other explanations include increased T-cell activity against antigens in cardiomyocytes, elevated concentrations of inflammatory mediators and autoantibodies, and accelerated atherosclerotic progression (9,48). For example, in myeloid progenitor cells, PD-1 knockout induced cholesterol synthesis and suppressed cholesterol metabolism, leading to increased levels of cholesterol levels intracellularly (49).
Previous studies investigating the relative toxicity profile of PD-1 and PD-L1 treatment have produced conflicting results. In a systematic review conducted by Pillai et al., PD-1 and PD-L1 treatment exhibited similar incidences of irAEs in Non-Small Cell Lung Cancer patients (50). However, two meta-analyses of RCTs reported that PD-1 inhibitors were associated with higher rates of grade 3 or higher adverse events than PD-L1 inhibitors (33,51). To date, there were scarce clinical reports on the effects of different ICI drug classes on cardiac risks. In a case series of two ICI-associated myocarditis, patients had an increased expression of PD-L1 in their injured myocardium (31). In preclinical studies, depletion of PD-L1 by genetic deletion or inhibiting antibody worsened transient myocarditis to fatal disease, suggesting a cardioprotective role of PD-L1 (52). Consistent with these findings, we found that PD-L1 inhibitors were associated with a higher risk of cardiac events than PD-1 inhibitors. A plausible explanation is that although blockade of PD-1 promotes the recruitment and activation of T cells, blockade of PD-L1 additionally promotes the infiltration of polymorphonuclear leukocytes, which together produce exaggerated acute inflammatory response damaging the myocardium (52). More studies are required to determine if a particular class or type of ICI is associated with a higher risk of cardiac events.
There were several limitations to this study. First, this was a retrospective study and there were some missing data. Furthermore, cardiac outcomes could only be adjudicated based on a review of medical records and might not be accurate. Nevertheless, the adjudication should have influenced both the ICI and non-ICI groups similarly and therefore should not have affected the risk estimation. Second, there could be residual confounders that were not included in the propensity score matching model used to adjust for the baselines between ICI and non-ICI users. However, we included most of the known covariates that were deemed to have influences on cardiovascular risks in cancer patients in our model. Third, it was difficult to determine if a previous cardiovascular event might modify the exposure to ICI treatment. However, a history of cardiac disease is not an exclusion from most ICI efficacy trials and is not considered a contraindication to ICI use (53–55). Finally, the onset of ICI-associated cardiovascular adverse events can occur after the end of ICI treatment and our short follow-up duration for ICI users might underestimate the incidence and risk of cardiac events associated with ICI use.
Conclusion
In conclusion, ICIs are associated with an increased risk of cardiovascular events, in particular ischemic stroke and pulmonary embolism, among Asian populations. There were differences in the incidence and risk of ICI-associated cardiovascular incidence events between our study and those conducted in the United States and Europe due to differences in study design and ethnicity. Age, metastatic disease, HTN and baseline PLR may be used in combination to identify patients who might be at risk of developing ICI-associated cardiovascular events.
Disclosures
Dr Neilan has been a consultant to and received fees from Parexel Imaging, Intrinsic Imaging, H3-Biomedicine, AbbVie, C4-Therapeutics, Roche and Genentech, outside of the current work. Dr Neilan also reports consultant fees from Bristol Myers Squibb for a Scientific Advisory Board and consultancy focused on myocarditis related to immune checkpoint inhibitors. Dr Neilan has received grant funding from Astra Zeneca and BMS.
Supplementary Material
Contributor Information
Cho-Han Chiang, Department of Medicine, Mount Auburn Hospital, Harvard Medical School, Boston, MA, USA.
Cho-Hung Chiang, Department of General Division, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan; Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan.
Kevin Sheng-Kai Ma, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Center for Global Health, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; Graduate Institute of Biomedical Electronics and Bioinformatics, College of Electrical Engineering and Computer Science, National Taiwan University, Taipei, Taiwan.
Yuan Ping Hsia, Department of Family Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Foundation, New Taipei City, Taiwan.
Yu-wen Lee, Department of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Han-Ru Wu, Department of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Cho-Hsien Chiang, Department of Medical Education, Kuang Tien General Hospital, Taichung, Taiwan; London School of Hygiene & Tropical Medicine, London, UK.
Chun-Yu Peng, Department of Medicine, Danbury Hospital, Danbury, Connecticut, USA; Department of Medicine, National Taiwan University College of Medicine, Taipei, Taiwan.
James Cheng-Chung Wei, Division of Allergy, Immunology and Rheumatology, Chung Shan Medical University Hospital, Taichung, Taiwan.
Her-Shyong Shiah, Department of Hematology and Oncology, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan; Graduate Institute of Cancer Biology and Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan.
Cheng-Ming Peng, Department of Family Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Foundation, New Taipei City, Taiwan; Da Vinci Minimally Invasive Surgery Center, Chung Shan Medical University Hospital, Taichung, Taiwan.
Tomas G Neilan, Cardio-Oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA; Cardiovascular Imaging Research Center (CIRC), Division of Cardiology and Department of Radiology, Massachusetts General Hospital, Boston, MA, USA.
Funding
This work was supported by National Institutes of Health/National Heart, Lung, Blood Institute (R01HL137562, R01HL130539, K24HL150238 to Dr Neilan). Dr T. Neilan holds the Michael and Kathryn Park Chair in Cardiology and was also supported, in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg, Christina and Paul Kazilionis and a Hassenfeld Scholar Award.
References
- 1. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018;118:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vuong JT, Stein-Merlob AF, Nayeri A, Sallam T, Neilan TG, Yang EH. Immune checkpoint therapies and atherosclerosis: mechanisms and clinical implications: JACC state-of-the-art review. J Am Coll Cardiol 2022;79:577–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity: JACC: CardioOncology primer. JACC CardioOncol 2021;3:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ball S, Ghosh RK, Wongsaengsak S, et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol 2019;74:1714–27. [DOI] [PubMed] [Google Scholar]
- 9. Drobni ZD, Alvi RM, Taron J, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 2020;142:2299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moik F, Chan WE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood 2021;137:1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gong J, Drobni ZD, Zafar A, et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J Immunother Cancer 2021;9:e002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Souza M, Nielsen D, Svane IM, et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J 2021;42:1621–31. [DOI] [PubMed] [Google Scholar]
- 14. Thavendiranathan P, Zhang L, et al. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol 2021;77:1503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Awadalla M, Mahmood SS, Groarke JD, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol 2020;75:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemporary clinical trials communications 2018;11:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dolladille C, Akroun J, Morice PM, et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J 2021;42:4964–77. [DOI] [PubMed] [Google Scholar]
- 18. Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation 2008;118:2702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC: Asia 2021;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong J, Drobni ZD, Alvi RM, et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur J Cancer 2021;158:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonsu JM, Guha A, Charles L, et al. Reporting of cardiovascular events in clinical trials supporting FDA approval of contemporary cancer therapies. J Am Coll Cardiol 2020;75:620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonsu J, Charles L, Guha A, et al. Representation of patients with cardiovascular disease in pivotal cancer clinical trials. Circulation 2019;139:2594–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deschênes-Simard X, Richard C, Galland L, et al. Venous thrombotic events in patients treated with immune checkpoint inhibitors for non-small cell lung cancer: a retrospective multicentric cohort study. Thromb Res 2021;205:29–39. [DOI] [PubMed] [Google Scholar]
- 26. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715–22. [DOI] [PubMed] [Google Scholar]
- 27. Bonaca MP, Olenchock BA, Salem JE, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive Pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol 2020;6:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrelli F, Grizzi G, Ghidini M, et al. Immune-related adverse events and survival in solid Tumors treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother 2020;43:1–7. [DOI] [PubMed] [Google Scholar]
- 30. Foster CC, Couey MA, Kochanny SE, et al. Immune-related adverse events are associated with improved response, progression-free survival, and overall survival for patients with head and neck cancer receiving immune checkpoint inhibitors. Cancer 2021;127:4565–73. [DOI] [PubMed] [Google Scholar]
- 31. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol 2019;5:1008–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Zhang Y, Jia X, et al. Effect of immune-related adverse events and pneumonitis on prognosis in advanced non-small cell lung cancer: a comprehensive systematic review and meta-analysis. Clin Lung Cancer 2021;22:e889–900. [DOI] [PubMed] [Google Scholar]
- 35. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 2020;18:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chitturi KR, Xu J, Araujo-Gutierrez R, et al. Immune checkpoint inhibitor-related adverse cardiovascular events in patients with lung cancer. JACC CardioOncol 2019;1:182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoon DW, Shin DW, Cho JH, et al. Increased risk of coronary heart disease and stroke in lung cancer survivors: a Korean nationwide study of 20,458 patients. Lung Cancer 2019;136:115–21. [DOI] [PubMed] [Google Scholar]
- 38. Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis 2019;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zöller B, Ji J, Sundquist J, Sundquist K. Risk of coronary heart disease in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer 2012;48:121–8. [DOI] [PubMed] [Google Scholar]
- 40. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension 2020;75:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pavan A, Calvetti L, Dal Maso A, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist 2019;24:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee PY, Oen KQX, Lim GRS, et al. Neutrophil-to-lymphocyte ratio predicts development of immune-related adverse events and outcomes from immune checkpoint blockade: a case-control study. Cancers (Basel) 2021;13:1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J 2021;42:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Drobni ZD, Zafar A, Zubiri L, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc 2020;9:e018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol 2018;71:2281–90. [DOI] [PubMed] [Google Scholar]
- 46. Fernandez DM, Rahman AH, Fernandez NF, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med 2019;25:1576–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poels K, van Leent MMT, Boutros C, et al. Immune checkpoint inhibitor therapy aggravates T cell-driven plaque inflammation in atherosclerosis. JACC CardioOncol 2020;2:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 49. Strauss L, Mahmoud MAA, Weaver JD, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol 2020;5:eaay1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer 2018;124:271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sonpavde GP, Grivas P, Lin Y, Hennessy D, Hunt JD. Immune-related adverse events with PD-1 versus PD-L1 inhibitors: a meta-analysis of 8730 patients from clinical trials. Future Oncol 2021;17:2545–58. [DOI] [PubMed] [Google Scholar]
- 52. Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed Death-1 ligand 1 (PD-L1) regulates CD8+ T-cell–mediated injury in the heart. Circulation 2007;116:2062–71. [DOI] [PubMed] [Google Scholar]
- 53. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017;123:1904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in Resectable lung cancer. N Engl J Med 2018;378:1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


