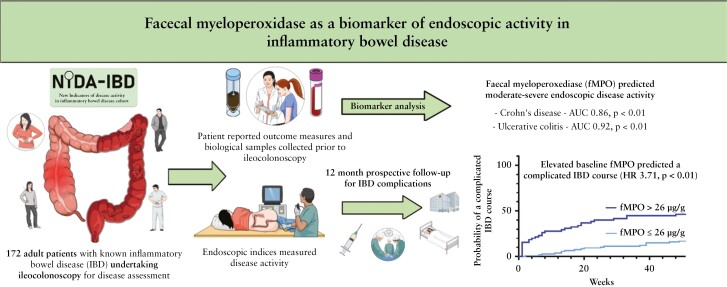
Abstract
Background and Aims
Inflammatory bowel disease [IBD], consisting of Crohn’s disease [CD] and ulcerative colitis [UC], is a relapsing-remitting illness. Treat-to-target IBD management strategies require monitoring of gastrointestinal inflammation. This study aimed to investigate faecal myeloperoxidase [fMPO], a neutrophil granule enzyme, as a biomarker of IBD activity.
Methods
Prospectively recruited participants with IBD, undergoing ileocolonoscopy for disease assessment, provided biological samples and completed symptom questionnaires prior to endoscopy. fMPO, C-reactive protein [CRP], and faecal calprotectin [fCal] were compared with validated endoscopic indices [simple endoscopic score for CD and UC endoscopic index of severity]. Receiver operating characteristic [ROC] curves assessed the performance of fMPO, CRP, and fCal in predicting endoscopic disease activity. Baseline biomarkers were used to predict a composite endpoint of complicated disease at 12 months [need for escalation of biologic/immunomodulator due to relapse, steroid use, IBD-related hospitalisation, and surgery].
Results
A total of 172 participants were recruited [91 female, 100 with CD]. fMPO was significantly correlated with endoscopic activity in both CD [r = 0.53, p < 0.01] and UC [r = 0.63, p < 0.01], and with fCal in all patients with IBD [r = 0.82, p < 0.01]. fMPO was effective in predicting moderate-to-severely active CD [AUROC 0.86, p < 0.01] and UC [AUROC 0.92, p < 0.01]. Individuals with a baseline fMPO > 26 µg/g were significantly more likely to reach the composite outcome at 12 months (hazard ratio [HR] 3.71, 95% confidence interval [CI] 2.07–6.64, p < 0.01).
Conclusions
Faecal myeloperoxidase is an accurate biomarker of endoscopic activity in IBD and predicted a more complicated IBD course during follow-up.
Keywords: Biomarkers, myeloperoxidase, prognosis
Graphical Abstract
1. Introduction
Inflammatory bowel disease [IBD], which encompasses Crohn’s disease [CD] and ulcerative colitis [UC], is characterised by chronic inflammation of the gastrointestinal tract and is estimated to affect over 6.8 million individuals worldwide.1 IBD is associated with periods of relapse and remission through a patient’s lifetime and requires regular surveillance for disease activity.2
Mucosal healing of the gut is the current goal of therapy in IBD and a ‘treat to target’ approach has been shown to maximise benefit.3 This approach is best supported by the use of endoscopic assessment of the gastrointestinal tract to decide on the need for escalation of therapeutic agents.4 However, ileocolonoscopy is invasive for patients who may need serial assessments, expensive in resource-constrained health care jurisdictions, and not without risks to the patient.5,6 Due to these limitations, international guidelines support the use of biomarkers to assess disease activity and treatment response in patients with IBD.2
IBD is characterised by an influx of neutrophils into the gut.7 The major proteins released by neutrophils have considerable potential as accurate biomarkers of inflammation in IBD. Faecal calprotectin [fCal], a neutrophil cytosolic protein, has been extensively studied as a biomarker in IBD.8,9 Extracellular levels of calprotectin increase during periods of inflammation, but oxidative modification and subsequent proteolysis may have an impact on calprotectin structure and function as well as its utility as a biomarker.10,11 The enzyme myeloperoxidase [MPO] is an abundant neutrophil granule enzyme. Through its production of the potent oxidant hypochlorous acid, MPO plays a vital role in killing bacteria and promoting inflammatory tissue damage.12 Faecal myeloperoxidase [fMPO] has previously been measured in small numbers of patients with active IBD, who mainly suffered from UC. However, these studies demonstrated considerable variability in fMPO extraction and measurement and the indices used to measure IBD activity.13–18 Thus, the accuracy and potential for fMPO as a biomarker in IBD remains unclear.
The primary aim of this study was to investigate whether fMPO correlated with endoscopic disease activity in patients with IBD. Additional aims included assessment of the performance of fMPO in comparison with C-reactive protein [CRP] and fCal in predicting endoscopic disease activity in IBD, and the utility of fMPO in predicting a more complicated IBD course over 1 year.
2. Materials and Methods
2.1. Patient selection
Patients with IBD residing within the Canterbury or the West Coast regions of New Zealand, who were undergoing endoscopic disease assessment with ileocolonoscopy, were prospectively recruited into the new indicators of disease activity in inflammatory bowel disease [NIDA-IBD] study between April 2019 and September 2020. Eligible study participants were aged 16 years or older, had a previously established diagnosis of CD or UC, and were identified through endoscopy lists.
2.2. Sample collection and storage
Recruited participants completed stool samples and questionnaires immediately prior to commencing bowel preparation for their ileocolonoscopy. Samples were stored in insulated bags with freezer packs overnight at 4°C before being delivered to a local laboratory and aliquoted and frozen at -80°C for subsequent analysis. Participants were interviewed by study investigators on the day of their ileocolonoscopy to complete measures of demographics, medication and medical history, IBD phenotype according to Montreal Classification, and disease activity measures.19 Venous blood sampling was also performed during this interview.
Study participants were followed up at 6 weeks and 6 months after their ileocolonoscopy for repeat collections of biological samples and study questionnaires. All de-identified data were stored in a secure database [Research Electronic Data Capture, REDCap, Vanderbilt, USA]. A composite outcome of a complicated disease course [need for escalation of biologic agent/immunomodulator due to clinical disease relapse, recurrent corticosteroid use, IBD-related hospitalisation, and surgery] was assessed at 12 months using regional hospital-based patient management software that records all prescriptions, admissions, and surgeries undertaken in the region. This study was approved by the New Zealand Health and Disability Ethics Committee [18/NTA/197] and was conducted in accordance with the World Medical Assembly Declaration of Helsinki. Stool samples collected from the COMFORT cohort, which assessed biomarkers in healthy participants, were used as controls for fCal and fMPO analysis.20
2.3. Disease activity assessment
IBD activity assessment was completed using validated patient questionnaires, study investigator clinical assessment, and validated endoscopic indices of activity. All participants also completed the Inflammatory Bowel Disease Questionnaire [IBDQ-32, McMaster, Canada]21 at each study time point [index, 6 weeks, 6 months]. For CD, the Harvey–Bradshaw Index [HBI], and Crohn’s Disease Activity Index [CDAI] were used to assess patient symptoms.22,23 An HBI > 4 and CDAI > 150 signified clinical activity.24 For UC, the Simple Clinical Colitis Activity Index [SCCAI] was used, with a score > 5 signifying clinical activity.25,26
Endoscopic assessment was completed by the gastroenterologist performing the patients’ ileocolonoscopy. The Simple Endoscopic Score for Crohn’s Disease [SES-CD] was used for endoscopic assessment in CD, with scores 0–2 suggestive of inactive, 3–6 mildly active, 7–15 moderately active, and ≥ 16 severely active disease.27,28 The Ulcerative Colitis Endoscopic Index of Severity [UCEIS] was used for participants with UC, with scores 0–1 suggestive of inactive, 2–4 mildly active, 5–6 moderately active, and ≥ 7 severely active disease.29,30 Scores of ≥ 3 on SES-CD signified active CD, and ≥ 2 on UCEIS signified active UC.
2.4. Measurement of full blood count, CRP, and fCal
Venous blood samples were analysed on automated analysers for full blood count, CRP, and albumin at Canterbury Health Laboratories. For fCal analyses, stool samples were extracted using the Calpro Easy Extract device [Calpro AS, Lysaker, Norway] and stored in a commercial Calpro extraction buffer at -80°C. Faecal calprotectin was later measured using a batched enzyme-linked immunosorbent assay [ELISA] where faecal extracts were thawed to room temperature and analysed using the manufacturer’s instructions [CALPRO ELISA, Calpro AS].
2.5. Myeloperoxidase assay
Whole stool samples were stored at -80°C for MPO analyses. Faecal myeloperoxidase protein and activity concentration were measured using a sandwich ELISA previously developed by the study investigators.31 Due to a supplier change, the primary capture antibody for some analyses was changed to anti-myeloperoxidase [4A4] MA1-20074 [Invitrogen, Waltham, MA],32 which behaved comparably to the previous capture antibody [Supplementary Figure 1].
Whole-stool samples were thawed at room temperature, homogenised with a wooden stick, and extracted by collecting 10 mg of stool using an RIDA stool collection tube [R-Biopharm AG, Germany] and diluting it into 1 ml of extraction buffer containing 0.2% [w/v] cetrimonium bromide [CTAB]. As a cationic surfactant, CTAB improves the release of MPO from faecal samples while maintaining the inherent enzymatic properties of MPO.33,34 A standard curve of human purified MPO [Planta Natural Products, Austria] was used on each plate [Supplementary Figure 1A and B]. Samples with high fMPO were diluted in assay buffer until they fell within the linear segment of the standard curves for protein concentration and activity.
Following incubation with the detection antibodies, the concentration of fMPO protein was measured by interpolation using the standard curve of purified human MPO to give a measurement of fMPO protein in ng/ml. The final concentration of fMPO [µg/g] was calculated by accounting for the stool extraction and ELISA plate dilution factors. After antibody capture, fMPO activity was also measured in selected samples before proceeding with the myeloperoxidase protein measurement.31 fMPO activity was measured in 30 randomly selected samples for three separate stratified groups of fMPO protein concentrations that encompassed the range of 0–49 μg/g, 50–199 μg/g, and > 200 μg/g. Specific activity of these samples was calculated as fMPO activity concentration divided by fMPO protein concentration. Myeloperoxidase ELISA techniques are described in more detail in Supplementary Appendix 1. The recovery of fMPO protein in stool samples with this study’s extraction buffer was assessed using spiked stool samples. Ten whole-stool samples from patients with inactive disease were mixed with either buffer alone or human purified myeloperoxidase that was equivalent to an additional 10 ng/ml per well in the ELISA plate. The spiked stool sample was added to extraction buffer, and fMPO protein concentration was measured using the MPO ELISA. fMPO protein was also assessed for its stability in whole stool at either 4°C, 25°C, or 37.5°C for up to 7 days, as well as its freeze-thaw stability, to confirm previously published results.14
2.6. Statistical analysis
2.6.1. Assessment of associations between biomarkers and disease activity measures
Statistical analyses were performed on this dataset using the SPSS 27 statistical package [IBM Corp., Armonk, NY]. Graphs from this data were created using the GraphPad Prism 9 package [GraphPad Software Inc., San Diego, CA]. Biomarker data were analysed for normality using the D’Agostino’s K2test, and fCal and fMPO protein concentrations were subsequently log transformed for parametric analyses. Data were analysed in subgroups of participants with CD and UC. CRP results were dichotomised to values > or < 3 mg/L and chi square testing assessed for differences between individuals with active and inactive IBD. Correlation between biomarkers and endoscopic scores, and between fCal and fMPO protein, were performed using Spearman rank correlations. Student’s t test assessed for differences in fCal and fMPO protein between individuals with endoscopically inactive and active IBD. Analysis of variance [ANOVA] assessed for differences in fCal and fMPO protein between individuals with inactive, mild, moderate, and severely active CD and UC. Receiver operator characteristic curves [ROC] assessed for the precision of biomarkers and symptoms scores in predicting endoscopically active and moderate-to-severely active IBD.
2.6.2. Assessment of biomarkers in response to IBD-related treatments and in predicting a more complicated IBD course
Student’s t tests were used to compare fCal and fMPO protein concentrations at baseline and at 6 months in participants commencing anti-TNFα therapies after recruitment. Six months was the final sample collection point used for this study. ROC analysis assessed for optimal cut-off points of baseline biomarkers in predicting for the composite endpoint at 12 months of follow-up. Log-rank testing was used to compare differences in distributions between individuals reaching and not reaching the composite endpoint at these biomarker set points and Cox regression was used to predict the risk of reaching this outcome at these thresholds.
All de-identified study data are available upon request by contacting the corresponding author of this study.
3. Results
3.1. Description of study participants
A total of 254 patients were approached for this study, with 192 participants recruited [29 individuals declined to participant and a further 33 were unable to be contacted]. Collection of study questionnaires and biological samples was completed in 172 participants in total [CD, n = 100; UC, n = 72]. Further details regarding the characteristics of study participants are included in Table 1 and Supplementary Table 1. The indication for ileocolonoscopy was recorded as either surveillance [CD, n = 57; UC, n = 41] or to assess current disease activity [CD, n = 43; UC, n = 31]; 62 of the individuals with CD and 35 of the individuals with UC had endoscopically active disease.
Table 1.
Description of demographics, clinical phenotype, symptoms, and endoscopic activity in patients in the NIDA-IBD cohort.
| CD [n = 100] | UC [n = 72] | |
|---|---|---|
| Mean age [SD, years] | 45.2 [14] | 49.5 [14] |
| Female participants [%] | 53 [53.0] | 38 [52.8] |
| Median time since IBD diagnosis [range, years] | 13.00 [0–43] | 11.50 [0–54] |
| NZ European ethnicity [%] | 100 [100] | 69 [95.8] |
| Montreal classification of CD [%] | ||
| A1 [%] | 7 [7] | |
| A2 [%] | 75 [75] | |
| A3 [%] | 18 [18] | |
| L1 [%] | 18 [18] | |
| L2 [%] | 21 [21] | |
| L3 [%] | 61 [61] | |
| B1 [%] | 60 [60] | |
| B2 [%] | 20 [20] | |
| B3 [%] | 20 [20] | |
| Perianal disease [%] | 17 [17] | |
| Montreal classification of UC | ||
| E1 [%] | 6 [8.3] | |
| E2 [%] | 33 [45.8] | |
| E3 [%] | 33 [45.8] | |
| Endoscopic remission [%] SES-CD ≤ 2, UCEIS < 2 |
38 [38] | 37 [51.4] |
| Endoscopically active IBD [%] SES-CD > 2, UCEIS ≥ 2 |
62 [62] | 35 [48.6] |
| Mild endoscopic activity [%] | 34 [34] | 22 [30.6] |
| Moderate endoscopic activity [%] | 19 [19] | 7 [9.7] |
| Severe endoscopic activity [%] | 9 [9] | 6 [8.3] |
| Median C-reactive protein [mg/L, IQR] | 3.0 [0–6.8] | 3.0 [0–6.0] |
| Medianfaecal calprotectin[μg/g, IQR] | 115.6 [45.8–350.3] | 146.1 [45.2–853.1] |
| Median faecal myeloperoxidase [μg/g, IQR] | 10.1 [3.0–44.4] | 15.6 [1.7–107.5] |
| Median SES-CD [IQR] | 3.0 [0.0–8.5] | |
| Median UCEIS [IQR] | 1.0 [0.0–4.0] | |
| Median HBI [IQR] | 6.0 [3.0–10.8] | |
| Median CDAI [IQR] | 119.5 [54.3–213.3] | |
| Median SCCAI [IQR] | 4.5 [2.0–8.0] | |
| Mean IBDQ-32 [SD] | 161.37 [37, 32] | 166.13 [40, 29] |
CD , Crohn’s disease; UC , ulcerative colitis; IBD , inflammatory bowel disease; SES-CD , Simple Endoscopic Score for CD; UCEIS , UC Endoscopic Index of Severity; HBI , Harvey–Bradshaw Index; SCCAI , Simple Clinical Colitis Activity Index; IBDQ-32 , Inflammatory Bowel Diseases Questionnaire; IQR, interquartile range; NZ, New Zealand; SD, standard deviation.
3.2. Extraction and validation of MPO
The extraction buffer used in this study resulted in mean fMPO protein extraction of 117.5% (standard deviation [SD] 33.12%, n = 10) in spiked stool samples compared with unspiked samples. [Supplementary Figure 2A and B]. This value was similar to the median 107% recovery seen by other groups using a similar extraction buffer.14,31 The variability of fMPO protein recovery in the current system may have been due to sampling variability of fMPO protein throughout solid stool [Supplementary Figure 2C]. fMPO protein was found to be stable for up to 7 days at 4°C, with < 5% reduction at Day 3 and < 20% reduction in fMPO protein concentration at Day 7 compared with Day zero [Supplementary Figure 2D]. There was a significant decrease in fMPO protein concentrations at 3 or more days when samples were stored at 25°C, or after 1 day at 37°C [Supplementary Figure 2D]. Mean time between faecal sampling and ileocolonoscopy was 1 day ± 3 for CD and 1 day ± 2 for UC. Mean time between faecal sampling and processing [storage at -80°C] was 1 day ± 1 for CD and for UC. Freeze-thaw of extraction buffer showed that fMPO protein was stable for up to three freeze-thaw cycles [Supplementary Figure 2E].
3.3. Associations of fMPO protein with fMPO activity
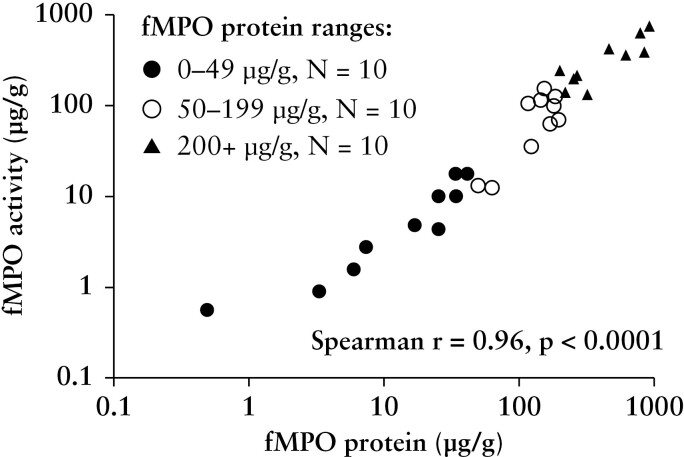
To show that the fMPO protein concentration measured in this study largely reflected an intact form of myeloperoxidase, fMPO activity was measured. In samples randomly selected from ranges of fMPO protein of 0–49 μg/g, 50–199 μg/g, and > 200 μg/g, fMPO activity and fMPO protein concentrations were significantly correlated [r = 0.96, p < 0.001; Figure 1]. The average specific activity of fMPO [[fMPO activity]/[fMPO protein]] in these samples was 57% [± 30 SD], indicating that measured fMPO is mostly active.
Figure 1.
Correlation between faecal myeloperoxidase [fMPO] protein concentration with fMPO activity using randomly selected stool samples to encompass a range of fMPO protein concentrations; 0-–9 μg/g, 50–199 μg/g, and > 200 μg/g. The correlation was performed on the linear data.
3.4. Correlations of fMPO protein with endoscopic disease activity, patient-reported symptoms, and faecal calprotectin
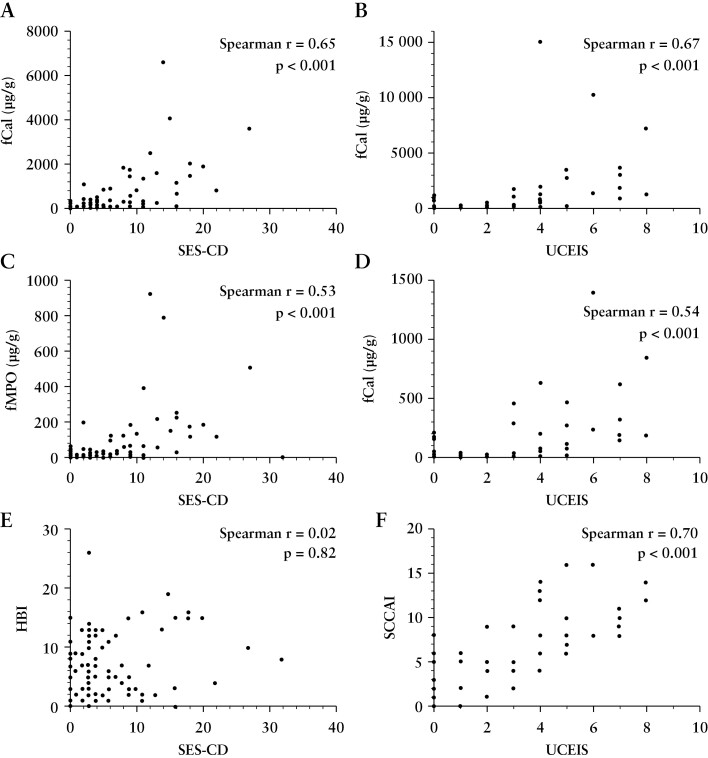
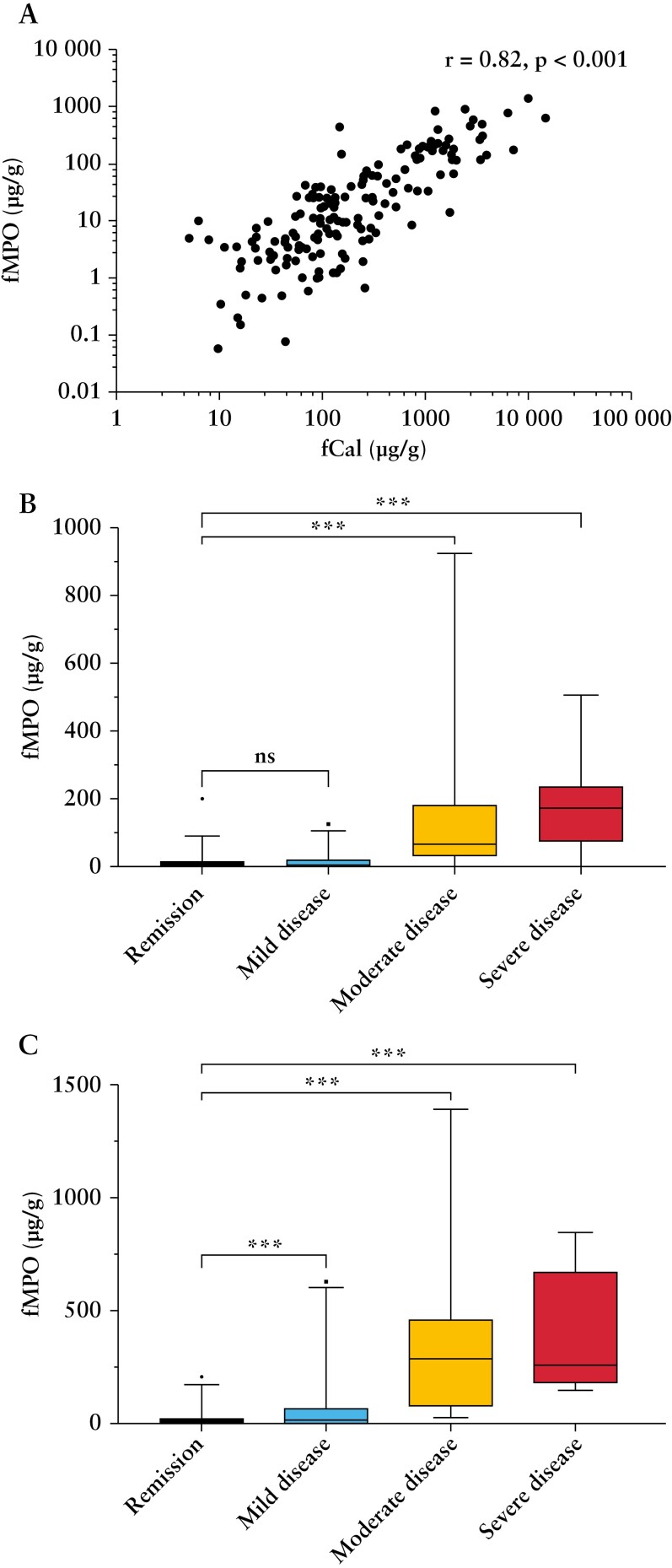
Clinical disease activity was significantly correlated with endoscopic activity in UC [SCCAI, r = 0.70, p < 0.01], but not in CD [CDAI, r = 0.10, p < 0.05; HBI, r = 0.02, p = 0.82]. Patient-reported symptoms in participants with CD [HBI] was not associated with faecal biomarkers [fCal, r = 0.03, p = 0.75; fMPO, r = 0.14, p = 0.16]. Patient-reported symptoms in those with UC [SCCAI] was associated with faecal biomarkers [fCal, r = 0.62, p < 0.001; fMPO, r = 0.54, p < 0.001]. There were correlations between endoscopic disease severity and fCal [CD, r = 0.65, p < 0.01; UC, r = 0.67, p < 0.01], and fMPO [CD, r = 0.53, p < 0.01; UC, r = 0.54, p < 0.01] [Figure 2 and Supplementary Table 2]. There was a significant correlation between fCal and fMPO [r = 0.82, p < 0.001] among all study participants [Figure 3].
Figure 2.
Correlations between faecal biomarkers and clinical symptoms with endoscopic activity in inflammatory bowel disease.[A] Faecal calprotectin [fCal] and Simple Endoscopic Score for Crohn’s Disease [SES-CD]. [B] fCal and Ulcerative Colitis Endoscopic Index of Severity [UCEIS]; [C] Faecal myeloperoxidase protein [fMPO] and SES-CD. [D] fMPO and UCEIS. [E] SES-CD and Harvey–Bradshaw index [HBI]. [F] UCEIS and Simple Clinical Colitis Activity Index [SCCAI].
Figure 3.
Faecal myeloperoxidase [fMPO] protein was significantly correlated with faecal calprotectin [fCal] in patients with inflammatory bowel disease [A]. fMPO protein differed significantly between individuals in endoscopic remission [SES-CD ≤ 2, UCEIS ≤ 1] and those with moderate [SES-CD 7–15, UCEIS 5–6] or severely active [SES-CD > 15, UCEIS ≥ 7] Crohn’s disease [B] and ulcerative colitis [C]. SES-CD, Simple Endoscopic Score for Crohn’s Disease; UCEIS, Ulcerative Colitis Endoscopic Index of Severity. Mild disease = SES-CD 3–6, UCEIS 2–4. ns = p > 0.05; ***p < 0.001. Correlations for panel A performed on linear biomarker data.
Subgroup analysis of only individuals with endoscopically active disease [SES-CD > 2, UCEIS ≥ 2] showed that both faecal biomarkers remained significantly correlated with endoscopic disease activity [CD fCal, r = 0.63, p < 0.001; CD fMPO, r = 0.61, p < 0.001; UC fCal, r = 0.70, p < 0.001; UC fMPO, r = 0.70, p < 0.001] and each other [r = 0.82, p < 0.001]. In individuals with endoscopically active disease, faecal biomarkers were correlated with patient-reported symptoms in UC [SCCAI vs fCal, r = 0.69, p < 0.001; SCCAI vs fMPO, r = 0.60, p < 0.001] but not in CD [HBI vs fCal, r = 0.13, p = 0.30; HBI vs fMPO, r = 0.15, p = 0.25]. Patient symptoms were associated with endoscopic indices in individuals with endoscopically active UC [r = 0.65, p < 0.001] but not in CD [r = 0.04, p = 0.75].
The associations between faecal biomarkers and endoscopic disease activity were similar between males [CDfCal 0.62, p < 0.001, CDfMPO 0.50, p < 0.001, UCfCal r = 0.69, p < 0.001, UCfMPO r = 0.60, p < 0.001] and females [CDfCal 0.68, p < 0.001, CDfMPO 0.56, p < 0.001, UCfCal r = 0.69, p < 0.001, UCfMPO r = 0.69, p < 0.001]. The correlation between fCal and fMPO was also similar between males [r = 0.82, p < 0.001] and females [r = 0.80, p < 0.001].
3.5. Biomarker concentrations based on endoscopic activity in the NIDA-IBD cohort
In individuals with CD, CRP was ≥ 3 mg/L in a greater proportion of patients with endoscopically active vs inactive disease (active 53.2%; inactive 25.7%; X2[1, N = 100] = 6.90, p < 0.01). The same relationship was also seen in CRP analysis in individuals with UC (active 62.9%; inactive 21.6%; X2[1, N = 72] = 12.58, p < 0.01).
There were no differences in fCal or fMPO protein concentrations (geometric mean, 95% confidence interval [CI]) between individuals with CD and UC (fCal-CD, 140.58 µg/g; UC, 184.51 µg/g; 95% confidence interval [CI] 0.45–1.28; p = 0.31; fMPO- CD, 17.14 µg/g; UC, 1.60 µg/g; 95% CI 0.46–1.46; p = 0.50).
Faecal calprotectin and myeloperoxidase were significantly lower in individuals with endoscopically inactive versus active CD and UC [p < 0.01] [Table 2]. There was a significant difference in fCal concentration between individuals with endoscopic remission and those with mild [p = 0.02 CD; p < 0.01 UC], moderate [p < 0.01 CD; p < 0.01 UC] and severely active [p < 0.01 CD, p < 0.01 UC] IBD [Table 2]. Faecal MPO protein also differed significantly between individuals with endoscopic remission and those with moderate [p < 0.01 CD; p < 0.01 UC] and severely active [p < 0.01 CD; p < 0.01 UC] IBD [Table 3 and Figure 3].
Table 2.
Comparison of concentrations of C-reactive protein [CRP], faecal calprotectin [fCal], and faecal myeloperoxidase [fMPO] protein between individuals in endoscopic remission and those with active, mild, moderate, and severe disease activity.
| Crohn’s disease | |||||
|---|---|---|---|---|---|
| Biomarker concentration [geometric mean, 95% CI] | Remission [SES-CD ≤ 2] | Endoscopically active [SES-CD > 2] | Mild Activity [SES-CD 3–6] | Moderate Activity [SES–CD 7-15] | Severe Activity [SES-CD > 15] |
| CRP [mg/L] | 2.1 [1.46–3.03] |
4.26a [3.1–5.9] |
3.0a [2.0–4.7] |
5.10a [2.72–9.58] |
10.2a [4.35–24.29] |
| fCal [µg/g] | 54.60 [35.16–84.77] |
244.69a [169.02–357.81] |
109.95a [75.94–159.17] |
601.85a [317.35–1141.39] |
749.95a [249.64–2252.96] |
| fMPO [µg/g] | 6.69 [4.22–10.49] |
22.20a [14.01–35.16] |
8.41 [5.47–13.07] |
64.72a [29.67–139.77] |
89.12a [21.33–376.15] |
| Ulcerative colitis | |||||
|---|---|---|---|---|---|
| Biomarker concentration [geometric mean, 95% CI] | Remission [UCEIS ≤ 1] | Endoscopically active [UCEIS ≥ 2] | Mild activity [UCEIS 2–4] | Moderate activity [UCEIS 5–6] | Severe Activity [UCEIS ≥ 7] |
| CRP [mg/L] | 1.80 [1.35–2.44] |
6.96a [4.01–12.18] |
1.80a [1.35–2.43] |
9.87a [1.72–56.26] |
33.11a [13.74–79.84] |
| fCal [µg/g] | 56.83 [36.23–88.23] |
645.48a [376.15–1096.63] |
333.62a [175.91–632.70] |
1685.81a [492.75–5825.50] |
2344.90a [1053.63–5218.68] |
| fMPO [µg/g] | 5.21 [3.03–8.93] |
60.34a [33.12–103.95] |
26.84a [13.33–54.05] |
194.42a [59.74–632.70] |
307.97a [144.03–651.97] |
SES-CD, Simple Endoscopic Score for Crohn’s Disease; UCEIS, Ulcerative Colitis Endoscopic Index of Severity; CI, confidence interval.
Biomarker significantly different from endoscopic remission [p < 0;05].
Table 3.
Area under the receiver operating characteristic curve [AUROC] and optimal cut-off points for biomarkers (C-reactive protein [CRP], faecal calprotectin [fCal], and faecal myeloperoxidase [fMPO] protein) to maximise sensitivity and specificity in predicting for endoscopic disease activity and moderate-severe endoscopic activity in IBD.
| Predicting endoscopic activity [SES-CD > 2, UCEIS > 1] | |||
|---|---|---|---|
| Crohn’s disease | AUROC | Optimal cut-off points | Sensitivity/specificity [%] |
| CRP | 0.66a | 3.5 mg/L | 53/74 |
| fCal | 0.77a | 58.24 µg/g | 87/60 |
| fMPO | 0.69a | 10.25 µg/g | 63/69 |
| Ulcerative colitis | AUROC | Optimal cut-off points | Sensitivity/specificity [%] |
|---|---|---|---|
| CRP | 0.73a | 3.5 mg/L | 63/78 |
| fCal | 0.89a | 137.50 µg/g | 89/78 |
| fMPO | 0.85a | 4.64 | 94/62 |
| IBD combined | Optimal cut-off points | Sensitivity/specificity [%] | |
|---|---|---|---|
| CRP | 0.69a | 3.5 mg/L | 56/76 |
| fCal | 0.81a | 147.61 µg/g | 65/82 |
| fMPO | 0.76a | 7.39 µg/g | 75/67 |
| Predicting moderate-severe endoscopic activity [SES-CD > 6, UCEIS > 4] | |||
|---|---|---|---|
| Crohn’s disease | AUROC | Optimal cut-off points | Sensitivity/specificity [%] |
| CRP | 0.70a | 3.5 mg/L | 68/67 |
| fCal | 0.87a | 252.14 µg/g | 79/84 |
| fMPO | 0.86a | 23.07 µg/g | 82/81 |
| Ulcerative colitis | AUROC | Optimal cut-off points | Sensitivity/specificity [%] |
|---|---|---|---|
| CRP | 0.81a | 10.5 mg/L | 62/93 |
| fCal | 0.94a | 270.00 µg/g | 100/76 |
| fMPO | 0.92a | 75.60 µg/g | 92/88 |
| IBD combined | AUROC | Optimal cut-off points | Sensitivity/specificity [%] |
|---|---|---|---|
| CRP | 0.74a | 3.5 mg/L | 73/67 |
| fCal | 0.88a | 252.14 µg/g | 83/79 |
| fMPO | 0.87a | 58.83 µg/g | 76/90 |
SES-CD , Simple Endoscopic Score for Crohn’s Disease; UCEIS , Ulcerative Colitis Endoscopic Index of Severity; IBD , inflammatory bowel disease.
Area under the receiver operating characteristic curve [AUROC] statistically significant [p < 0.05].
Subgroup analyses showed that fCal was significantly elevated in individuals who had isolated ileal activity [geometric fCal mean inactive disease vs active disease, 3.85 µg/g vs 5.17 µg/g; 95% CI 1.82–7.54; p < 0.05]. This was not observed with fMPO protein [geometric fMPO mean inactive disease vs active disease, 5.52 µg/g vs 10.78 µg/g; 95% CI 0.90–4.22; p = 0.09].
3.6. Receiver operative characteristic analysis of biomarkers and symptom scores in IBD activity assessment
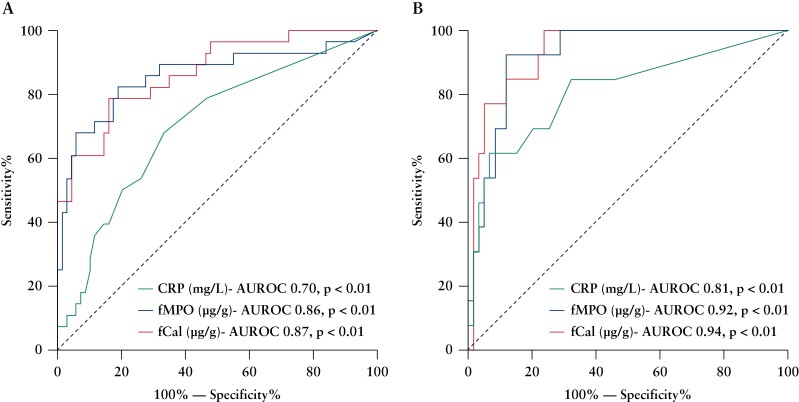
ROC analysis showed that fCal and fMPO protein were effective in predicting endoscopic disease activity in patients with CD and UC [p < 0.01 for both] [Table 3]. These biomarkers were also strong predictors for the presence of moderate to severely active CD and UC on endoscopy [Table 3 and Figure 4]. CRP was less effective than the faecal biomarkers in predicting moderate to severely active IBD [p < 0.05] [Table 3 and Figure 4]. Clinical symptoms performed similarly to fCal and fMPO protein in predicting for the presence of moderate-to-severe endoscopically active disease in UC [SCCAI, AUROC 0.92, p < 0.01] but not CD [HBI, AUROC 0.53, p = 0.66; CDAI, AUROC 0.64, p < 0.05].
Figure 4.
Faecal calprotectin [fCal] and faecal myeloperoxidase [fMPO] protein both predicted moderat- to-severe endoscopic activity in CD [A] and UC [B], in comparison with C-reactive protein [CRP]. CD, Crohn’s disease; UC, ulcerative colitis; AUROC, area under the receiver operating characteristic curve.
3.7. Optimal cut-off points of CRP, fCal, and fMPO protein in assessing disease activity
Coordinate points on ROC analyses were used to determine optimal cut-off points of CRP, fCal, and fMPO protein to maximise sensitivity and specificity in predicting endoscopic disease activity and moderate-severe endoscopic activity in CD and UC [Table 3].
3.8. Responsiveness of fMPO protein to IBD related treatment
There were significant limitations with the collection and analysis of NIDA-IBD related biological samples at the 6-week and 6-month follow up intervals due to the impact of the COVID-19 pandemic. Of the 172 patients initially recruited, 92 participants had complete study data at 6 weeks and 46 participants at 6 months. Subgroup analyses were performed to assess for changes in fCal and fMPO protein concentrations at 6 months in patients commencing a biologic therapy after their index ileocolonoscopy [n = 5 patients]. Paired t tests showed only significant differences in fMPO protein [p = 0.01] but not fCal [p = 0.19] between baseline and at 6 months in these individuals.
3.9. Associations of biomarkers with a more complicated disease course
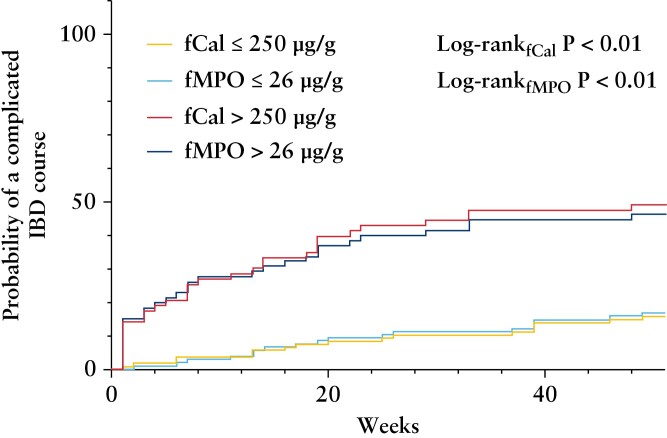
All 172 patients were followed for 12 months after recruitment, and 49 participants reached the composite endpoint of complicated disease. Of these individuals, 36 had escalation of biologic/immunomodulatory agents due to disease flare, 10 had corticosteroid use, 16 required an IBD-related hospitalisation, and 10 underwent IBD-related surgery. Individuals reaching the composite endpoint had a significantly higher fCal [geometric mean 487.85 µg/g vs 100.48 µg/g, p < 0.01] and fMPO protein [geometric mean 45.60 µg/g vs 9.87 µg/g, p < 0.01] at baseline than those not reaching this endpoint. Nine of the 49 participants reaching the composite endpoint had inactive symptoms at baseline [HBI < 5, SCCAI < 5]. ROC analyses showed that both baseline fCal [AUROC 0.75, p < 0.01] and fMPO protein [AUROC 0.72, p < 0.01] predicted for this composite outcome. Concentrations of fCal of 250 µg/g [sensitivity 65%, specificity 74%] and fMPO protein of 26 µg/g [sensitivity 63%, specificity 74%] were the most accurate predictors of a complicated disease course. Kaplan–Meier analyses [Figure 5] showed significant differences in reaching the composite endpoint at 12 months of follow-up when baseline fCal and fMPO protein concentrations were dichotomised above/below threshold values identified on ROC analyses [fCal log-rank p < 0.01, fMPO log-rank p < 0.01]. Cox-regression analyses showed that participants with baseline biomarker concentrations above these thresholds were at a significantly increased risk of reaching the composite endpoint (fCal, hazard ratio [HR] 4.35, 95% CI 2.41–7.85, p = 0.01; fMPO, HR 3.71, 95% CI 2.07–6.64, p < 0.01).
Figure 5.
Kaplan–Meier Curves showing differences in probability in reaching a composite outcome of escalation of immunomodulator/biologic therapy, corticosteroid use, IBD related hospitalisation, and surgery during 12 months of follow-up based on threshold values of baseline biomarkers [faecal calprotectin, fCal, >250 µg/g<; faecal myeloperoxidase, fMPO, fMPO > 26 µg/g<]. IBD, inflammatory bowel disease.
4. Discussion
Neutrophil activation is a key driver in the pathophysiology of IBD,7 a chronic gastrointestinal condition associated with long-term complications such as stricture formation, fistula, need for surgery, and colorectal malignancy.35–37 The current study has shown that the neutrophil-derived protein MPO can be readily extracted from faecal samples and was an effective biomarker of endoscopic activity in IBD. Faecal myeloperoxidase and faecal calprotectin had similar performance characteristics in predicting moderate-to-severely active IBD, which represents a cohort of patients that may specially benefit from treatment modifications. Elevated levels of faecal myeloperoxidase at baseline also predicted a more complicated disease course, such as the need for escalation of biologic/immunomodulator therapies, corticosteroid use, IBD related hospitalisation, and surgery at 12 months of follow-up.
Treatment of IBD aims to reduce gut inflammation to prevent disease progression and complications. Assessment for gastrointestinal mucosal inflammation has traditionally relied upon ileocolonoscopy, which is expensive for the health system and invasive for patients.5 The effect of tight disease control in IBD through the use of non-invasive biomarkers has been associated with superior outcomes and cost-effectiveness compared with symptom-based therapeutic escalation alone.3,38,39 Thus, the use of a specific biomarker such as faecal calprotectin is now considered part of routine best practice.2 The current study is aligned with previous observations showing that symptom severity does not always reflect intestinal inflammation, specially in CD, and can be unreliable to guide treatment modifications if used alone.40–42 Thus, non-invasive biomarkers in IBD activity assessment are likely to play a pivotal role in guiding treatment decisions.
Faecal calprotectin is an effective biomarker of IBD activity and has also previously been shown to be a predictor of disease relapse and response to treatment.43–46 Despite these benefits, fCal is associated with significant cost in resource-constrained environments due to the requirement of specific capture antibodies for measurements via ELISA, and prolonged turnaround times when batched analyses are performed. Point-of-care fCal tests have been developed in recent years, but inter-test concordance is often poor, and the cost of individual tests or specialised readers for interpreting the results is often higher than ELISA methods.47–49 Furthermore, calprotectin subunits are susceptible to hypochlorous acid-mediated oxidation and modification due to the activity of neutrophil-derived myeloperoxidase at sites of infection and inflammation.10 This oxidation makes calprotectin more susceptible to proteolysis. Thus measures of unmodified calprotectin may underestimate the true inflammatory burden.50
MPO has previously been measured in the stool of patients with IBD but with mixed results. This may be due in part to difficulties in extracting myeloperoxidase, which is tightly bound to the solid fraction of stool, and may account for the significant variability in extraction of MPO from faeces ranging from 20% to 100%.13,14,31,51 Furthermore, the methods used to measure fMPO varied and ranged from radio-immunoassays to ELISAs.14,52 The MPO extraction buffer used in the current study contained 0.2% cetrimonium bromide and resulted in the extraction of 117.5% +/- 33.1% of MPO in spiked stool samples [Supplementary Figure 2]. The stability of fMPO protein in different storage conditions was also investigated in this study and showed that fMPO protein concentrations remained stable for up to 7 days when samples were stored at 4°C, which is comparable to fCal and an important consideration when assessing the utility and practical application of biomarker assessment for real-world use.53,54 Previous studies investigating fMPO as a biomarker in IBD have also generally been limited to 10–75 study participants, and predominantly included those with UC.13,15,16,51,55 These studies investigated the utility of fMPO to predict disease activity but used a variety of measures to assess this, including symptoms/clinical assessment, alternative biomarkers, endoscopic activity, and histological assessment.13,16,51,55 Until now, there has been no definitive study that links fMPO to endoscopic findings in both CD and UC using validated endoscopy scoring systems and laboratory methodology in a large IBD cohort and control group.
The current study highlights that fMPO protein is an accurate biomarker of gut inflammation in IBD. The sensitivity and specificity of this biomarker improved when predicting for moderate-to-severely active IBD. Thus, fMPO protein may be able to identify individuals with significant IBD activity who may benefit most from treatment modification. Both fCal and fMPO protein had lower diagnostic accuracy at identifying individuals with CD who had mild endoscopic activity.
Previous studies have identified higher neutrophil activity in colonic than in ileal CD.56 Consequently, the level of mucosal inflammation seen in individuals with isolated ileal activity may be below the sensitivity threshold of biomarker detection. This remains an ongoing issue for the use of biomarkers in guiding the management of small bowel CD.56 Being able to identify individuals with a high inflammatory burden is a key priority, given the potential to initiate disease-modifying therapies which target these inflammatory pathways in a timely fashion.57
International guidelines promote the use of biomarkers to monitor for treatment response and IBD relapse.2,57 Although not specifically powered to investigate the response of biomarkers to IBD treatment, the current study has shown that fMPO protein levels reduced at 6 months following the introduction of biologic therapies for IBD. This expands on previous observations of fMPO protein normalising in patients with UC in response to treatment with 5-aminosalicylates.58,59 The current study also showed that a baseline fMPO protein reading above 26 µg/g was predictive of a more complicated IBD course at 12 months of follow-up. This novel observation is in line with previous reports of baseline fCal predicting disease relapse during follow-up.43,60 These findings add further utility for the use of non-invasive biomarkers in order to stratify patients who may have a more complicated disease course, who may have a more complicated disease course and may benefit from closer disease monitoring and tailoring of IBD-specific therapies.
The current study shows that fMPO protein concentration and fMPO activity are significantly correlated in the faeces of patients with IBD. This shows that fMPO is predominantly active and largely intact, indicating that most of the available MPO is being captured. This highlights the possibility that MPO may be contributing to bowel damage in IBD through the generation of hypochlorous acid or free radical species. Future studies are planned to investigate whether MPO-mediated oxidative damage is associated with the mucosal inflammation and bowel injury seen over the longitudinal course of this disease.
A limitation of this study was the variability in endoscopic scoring due to the lack of central reading of ileocolonoscopic findings, which may have also influenced the thresholds of disease activity to which individuals were stratified. A significant proportion of included patients were having ileocolonoscopy for surveillance of IBD-associated dysplasia, resulting in many study participants having inactive IBD. Having a larger proportion of participants with moderate-to-severe disease activity is likely to have strengthened the current study by further defining optimal biomarker thresholds to identify such individuals and improve the overall generalisability of the study findings. Although endoscopic disease assessment is considered the gold standard measure of disease activity in IBD, the role of histological healing as a treatment target has become more prominent in recent years.61 Due to resource constraints and the variability of indices of histological activity in IBD, histological assessments were not undertaken in this study as it is outside usual clinical management. These data may provide additional insights into the mechanistic implications of MPO activity promoting intestinal damage in IBD and would also validate the findings on endoscopic indices. Furthermore, participants with CD did not undertake cross-sectional imaging of their small bowel and thus disease activity may be underestimated in these individuals. Additional assessment of the performance of fMPO protein in individuals with isolated small bowel disease activity would help further evaluate the utility of this biomarker in this cohort in comparison with fCal.
In this study, faecal sample collection and handling were carried out to reflect real-world clinical practice and required temporary storage at a participant’s home in an insulated cool-pack prior to delivery to a local laboratory and storage at -80°C. Although the insulated pack aimed to reflect 4°C and participants were advised to store samples in a refrigerator or freezer prior to delivery to a laboratory, this was a potential source of reduced detection of fMPO protein and activity at the time of processing due to the imperfect nature of these storage conditions. However, the reduction in fMPO protein concentrations were only seen after 3 days of storage at room temperature, and most samples in this cohort were frozen to -80°C by this time. The stool extraction techniques in this study used commercially available devices that are commonly used and extract an approximate weight and volume of stool; however, exact quantification of stool wet weight may have improved the accuracy of faecal biomarker assessment.
In the largest cohort of patients with IBD investigating fMPO to date, the current study has shown that fMPO protein is an effective biomarker of disease activity when compared with ileocolonoscopy. Faecal myeloperoxidase has similar precision in predicting moderate-to-severely active IBD as faecal calprotectin, declines following the initiation of biologic therapies, and predicts a more complicated IBD course.
Supplementary Material
Contributor Information
Akhilesh Swaminathan, Department of Medicine, University of Otago, Christchurch, New Zealand; Department of Gastroenterology, Christchurch Hospital, Christchurch, New Zealand.
Grace M Borichevsky, Centre for Free Radical Research, Department of Pathology and Biomedical Science, University of Otago, Christchurch, New Zealand.
Teagan S Edwards, Centre for Free Radical Research, Department of Pathology and Biomedical Science, University of Otago, Christchurch, New Zealand.
Esther Hirschfeld, Department of Medicine, University of Otago, Christchurch, New Zealand.
Thomas C Mules, Department of Gastroenterology, Christchurch Hospital, Christchurch, New Zealand.
Chris M A Frampton, Department of Medicine, University of Otago, Christchurch, New Zealand.
Andrew S Day, Department of Paediatrics, University of Otago, Christchurch, New Zealand.
Mark B Hampton, Centre for Free Radical Research, Department of Pathology and Biomedical Science, University of Otago, Christchurch, New Zealand.
Anthony J Kettle, Centre for Free Radical Research, Department of Pathology and Biomedical Science, University of Otago, Christchurch, New Zealand.
Richard B Gearry, Department of Medicine, University of Otago, Christchurch, New Zealand; Department of Gastroenterology, Christchurch Hospital, Christchurch, New Zealand.
Funding
This work was supported by the New Zealand Society of Gastroenterology Janssen Research Grant and the Royal Australasian College of Physicians Research Entry Grant.
Conflict of Interest
AS has received honoraria for educational activities for Janssen [unrelated to this manuscript]. ASD has served on advisory boards for Janssen, Abbvie, and Nestle [all unrelated to this manuscript]. RBG has received research grants, served on advisory boards, and received honoraria for educational activities for Janssen, AbbVie, and Zespri [unrelated to this manuscript].
Author Contribution
AS, GMB, TSE, ASD, MBH, AJK, and RGB conceived the study design. AS, GMB, EH, and TCM acquired the study data and AS, GMB, and EH performed the laboratory analyses. AS, GMB, and CMF performed the statistical analyses and ASD, MBH, AJK, RBG assisted in the interpretation of the study results. AS, GMB, and TCM prepared the initial article draft. All authors contributed to the critical revision of this manuscript and approved the final submitted version.
Conference presentations: the findings from this study were previously presented at the New Zealand Society of Gastroenterology Annual Scientific Meeting, Christchurch, New Zealand, 17–19 November 2021.
References
- 1. Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE] Initiative of the International Organization for the Study of IBD [IOIBD]: determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. [DOI] [PubMed] [Google Scholar]
- 3. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease [CALM]: a multicentre, randomised, controlled phase 3 trial. Lancet 2017;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 4. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 5. Annese V, Daperno M, Rutter MD, et al. European evidence-based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013;7:982–1018. [DOI] [PubMed] [Google Scholar]
- 6. Ho SSC, Keenan JI, Day AS.. Patient perceptions of current and potential inflammatory bowel disease diagnostic and monitoring tests. Intern Med J 2021. Doi: 10.1111/imj.15298. [DOI] [PubMed] [Google Scholar]
- 7. Fournier BM, Parkos CA.. The role of neutrophils during intestinal inflammation. Mucosal Immunol 2012;5:354–66. [DOI] [PubMed] [Google Scholar]
- 8. RØseth AG, Fagerhol MK, Aadland E, Schjønsby H.. Assessment of the neutrophil dominating protein calprotectin in feces: a methodologic study. Scand J Gastroenterol 1992;27:793–8. [DOI] [PubMed] [Google Scholar]
- 9. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- 10. Hoskin TS, Crowther JM, Cheung J, et al. . Oxidative cross-linking of calprotectin occurs in vivo, altering its structure and susceptibility to proteolysis. Redox Biol 2019;24:101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edwards TS, Dickerhof N, Magon NJ, Paton LN, Sly PD, Kettle AJ.. Formation of calprotectin-derived peptides in the airways of children with cystic fibrosis. J Immunol 2022;208:979–90. [DOI] [PubMed] [Google Scholar]
- 12. Winterbourn CC, Kettle AJ, Hampton MB.. Reactive oxygen species and neutrophil function. Annu Rev Biochem 2016;85:765–92. [DOI] [PubMed] [Google Scholar]
- 13. Masoodi I, Kochhar R, Dutta U, et al. Evaluation of fecal myeloperoxidase as a biomarker of disease activity and severity in ulcerative colitis. Dig Dis Sci 2012;57:1336–40. [DOI] [PubMed] [Google Scholar]
- 14. Peterson CGB, Eklund E, Taha Y, Raab Y, Carlson M.. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol 2002;97:1755–62. [DOI] [PubMed] [Google Scholar]
- 15. Saiki T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med J 1998;45:69–73. [DOI] [PubMed] [Google Scholar]
- 16. Sugi K, Saitoh O, Hirata I, Katsu K.. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol 1996;91:927–34. [PubMed] [Google Scholar]
- 17. Peterson CGB, Lampinen M, Hansson T, Lidén M, Hällgren R, Carlson M.. Evaluation of biomarkers for ulcerative colitis comparing two sampling methods: fecal markers reflect colorectal inflammation both macroscopically and on a cellular level. Scand J Clin Lab Invest 2016;76:393–401. [DOI] [PubMed] [Google Scholar]
- 18. Silberer H, Küppers B, Mickisch O, et al. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab 2005;51:117–26. [PubMed] [Google Scholar]
- 19. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol J 2005;19:5A–36A. [DOI] [PubMed] [Google Scholar]
- 20. Heenan PE, Gearry RB.. The Christchurch cOhort to investigate Mechanisms FOr gut Relief and improved Transit [COMFORT]. Intest Inflamm Dis 2020; 5:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96:804–10. [PubMed] [Google Scholar]
- 22. Harvey RF, Bradshaw JM.. A simple index of Crohn’s-disease activity. Lancet 1980;315:514. [DOI] [PubMed] [Google Scholar]
- 23. Best WR, Becktel JM, Singleton JW, Kern F.. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 24. Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P.. Correlation between the Crohn’s disease activity and Harvey–Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol 2010;8:357–63. [DOI] [PubMed] [Google Scholar]
- 25. Walmsley R, Ayres R, Pounder R, Allan R.. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jowett SL, Seal CJ, Barton JR, Welfare MR.. Use of the simple clinical colitis activity index [SCCAI] to define relapse of ulcerative colitis [UC]. Gut 2001;48:A1–5.11286195 [Google Scholar]
- 27. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 28. Sipponen T, Nuutinen H, Turunen U, Färkkilä M.. Endoscopic evaluation of Crohn’s disease activity: comparison of the CDEIS and the SES-CD. Inflamm Bowel Dis 2010;16:2131–6. [DOI] [PubMed] [Google Scholar]
- 29. Travis SPL, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity [UCEIS]. Gut 2012;61:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie T, Zhang T, Ding C, et al. Ulcerative Colitis Endoscopic Index of Severity [UCEIS] versus Mayo Endoscopic Score [MES] in guiding the need for colectomy in patients with acute severe colitis. Gastroenterol Rep 2018;6:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoskin TS. Neutrophil Activation in Inflammatory Bowel Disease. [Thesis.] Department of Medicine, University of Otago, Christchurch; 2011. [Google Scholar]
- 32. Thermofisher. Myeloperoxidase Antibody [MA1-20074].https://www.thermofisher.com/antibody/product/Myeloperoxidase-Antibody-clone-4A4-Monoclonal/MA1-20074 Accessed October 26, 2021.
- 33. Pulli B, Ali M, Forghani R, et al. . Measuring myeloperoxidase activity in biological samples. PLoS One; 8:e67976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shabani M, Ani M, Movahedian A, Samsam Shariat SZA.. Kinetic investigation of myeloperoxidase upon interaction with copper, cadmium, and lead ions. Iran Biomed J 2011;15:107–12. [PMC free article] [PubMed] [Google Scholar]
- 35. Tarrant KM, Barclay ML, Frampton CMA, Gearry RB.. Perianal disease predicts changes in Crohn’s disease phenotype: results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol 2008;103:3082–93. [DOI] [PubMed] [Google Scholar]
- 36. Eglinton T, Reilly M, Chang C, Barclay M, Frizelle F, Gearry R.. Ileal disease is associated with surgery for perianal disease in a population-based Crohn’s disease cohort. Br J Surg 2010;97:1103–9. [DOI] [PubMed] [Google Scholar]
- 37. Beaugerie L, Itzkowitz SH.. Cancers complicating inflammatory bowel disease. N Engl J Med 2015;372:1441–52. [DOI] [PubMed] [Google Scholar]
- 38. Colombel JF, D’haens G, Lee WJ, Petersson J, Panaccione R.. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis 2020;14:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ungaro R, Colombel JF, Lissoos T, Peyrin-Biroulet L.. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol 2019;114:874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Jong MJ, Huibregtse R, Masclee AAM, Jonkers DMAE, Pierik MJ.. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol 2018;16:648–63.e3. [DOI] [PubMed] [Google Scholar]
- 41. Mules TC, Swaminathan A, Hirschfeld E, et al. . The impact of disease activity on psychological symptoms and quality of life in patients with inflammatory bowel disease: results from the Stress, Anxiety and Depression with Disease Activity [SADD] Study. Aliment Pharmacol Ther 2021;55:201–11. [DOI] [PubMed] [Google Scholar]
- 42. Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis 2015;21:824–31. [DOI] [PubMed] [Google Scholar]
- 43. Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005;54:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology 2015;148:938–47.e1. [DOI] [PubMed] [Google Scholar]
- 45. Heida A, Park K, van Rheenen PF.. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis 2017;23:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kostas A, Siakavellas SI, Kosmidis C, et al. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J Gastroenterol 2017;23:7387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. National Institute for Health and Care Excellence. Point-of-Care and Home Faecal Calprotectin Tests for Monitoring Treatment Response in Inflammatory Bowel Disease. Medtech innovation briefing [MIB132].2017. https://www.nice.org.uk/advice/mib132. Accessed March 4, 2022.
- 48. Vinding KK, Elsberg H, Thorkilgaard T, et al. Fecal calprotectin measured by patients at home using smartphones—a new clinical tool in monitoring patients with inflammatory bowel disease. Inflamm Bowel Dis 2016;22:336–44. [DOI] [PubMed] [Google Scholar]
- 49. Labaere D, Smismans A, Van Olmen A, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United Eur Gastroenterol J 2014;2:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 2005;77:598–625. [DOI] [PubMed] [Google Scholar]
- 51. Soomro S, Venkateswaran S, Vanarsa K, et al. Predicting disease course in ulcerative colitis using stool proteins identified through an aptamer-based screen. Nat Commun 2021;12:3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bengtsson J, Adlerberth I, Östblom A, Saksena P, Öresland T, Börjesson L.. Effect of probiotics [Lactobacillus plantarum 299 plus Bifidobacterium Cure21] in patients with poor ileal pouch function: a randomised controlled trial. Scand J Gastroenterol 2016;51:1087–92. [DOI] [PubMed] [Google Scholar]
- 53. Lasson A, Stotzer PO, Öhman L, et al. . The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis 2015;9:26–32. [DOI] [PubMed] [Google Scholar]
- 54. Whitehead S, Brookes M, French J, Ford C, Gama R.. PTH-075 The effect of storage conditions on the stability of faecal calprotectin. Gut 2015;64:A439. [Google Scholar]
- 55. Peterson CGB, Sangfelt P, Wagner M, Hansson T, Lettesjö H, Carlson M.. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scand J Clin Lab Invest 2007;67:810–20. [DOI] [PubMed] [Google Scholar]
- 56. Pierre N, Salée C, Vieujean S, et al. Review article: distinctions between ileal and colonic Crohn’s disease: from physiology to pathology. Aliment Pharmacol Ther 2021;54:779–91. [DOI] [PubMed] [Google Scholar]
- 57. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020;14:4–22. [DOI] [PubMed] [Google Scholar]
- 58. Jiang XE, Yang SM, Zhou XJ, Zhang Y.. Effects of mesalazine combined with bifid triple viable on intestinal flora, immunoglobulin and levels of cal, MMP-9, and MPO in feces of patients with ulcerative colitis. Eur Rev Med Pharmacol Sci 2020;24:935–42. [DOI] [PubMed] [Google Scholar]
- 59. Wagner M, Peterson CG, Ridefelt P, Sangfelt P, Carlson M.. Fecal markers of inflammation used as surrogate markers for treatment outcome in relapsing inflammatory bowel disease. World J Gastroenterol 2008;14:5584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mao R, Xiao Y lian, Gao X, et al. . Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis 2012;18:1894–9. [DOI] [PubMed] [Google Scholar]
- 61. Gupta A, Yu A, Peyrin-Biroulet L, Ananthakrishnan AN.. Treat to target: the role of histologic healing in inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021;19:1800–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.