Abstract
Objective
To assess the diagnostic yield of targeted prostate biopsy in African-American (A-A) men using image fusion of multi-parametric magnetic resonance imaging (mpMRI) with real-time transrectal ultrasonography (US).
Patients and Methods
We retrospectively analysed 661 patients (117 A-A and 544 Caucasian) who had mpMRI before biopsy and then underwent MRI/US image-fusion targeted biopsy (FTB) between October 2012 and August 2015. The mpMRIs were reported on a 5-point Likert scale of suspicion. Clinically significant prostate cancer (CSPC) was defined as biopsy Gleason score ≥7.
Results
After controlling for age, prostate-specific antigen level and prostate volume, there were no significant differences between A-A and Caucasian men in the detection rate of overall cancer (35.0% vs 34.2%, P = 0.9) and CSPC (18.8% vs 21.7%, P = 0.3) with MRI/US FTB. There were no significant differences between the races in the location of dominant lesions on mpMRI, and in the proportion of 5-point Likert scoring. In A-A men, MRI/US FTB from the grade 4–5 lesions outperformed random biopsy in the detection rate of overall cancer (70.6% vs 37.2%, P = 0.003) and CSPC (52.9% vs 12.4%, P < 0.001). MRI/US FTB outperformed random biopsy in cancer core length (5.0 vs 2.4 mm, P = 0.001), in cancer rate per core (24.9% vs 6.8%, P < 0.001), and in efficiency for detecting one patient with CSPC (mean number of cores needed 13.3 vs 81.9, P < 0.001), respectively.
Conclusions
Our key finding confirms a lack of racial difference in the detection rate of overall prostate cancers and CSPC with MRI/US FTB between A-A and Caucasian men. MRI/US FTB detected more CSPC using fewer cores compared with random biopsy.
Keywords: prostate cancer, MRI, Likert scoring system, MRI/US image-fusion targeted biopsy, African-American
Introduction
In the USA, African-American (A-A) men have the highest incidence of prostate cancer compared to any other racial or ethnic group [1]. It is also well-documented that A-A men with prostate cancer more frequently present with higher grade tumours. However, it is unclear whether this difference is due to lower access to screening or other socioeconomic and education variables that prevent early diagnosis, or if tumours within the A-A population tend to be more biologically aggressive [2].
Recently, increasing evidence supports the use of MRI with ultrasonography (US) image-fusion targeted prostate biopsy to improve the detection of aggressive prostate cancers, while limiting detection of indolent cancers compared to conventional systematic random biopsy [3–6].
In the present study, we compared outcomes of multi-parametric (mp)MRI and real-time TRUS image-fusion targeted biopsy (FTB) in A-A men with Caucasian men.
To our best of knowledge, the present study is the first to focus on the diagnostic yield of targeted prostate biopsy using image fusion of mpMRI with real-time TRUS in A-A men.
Patients and Methods
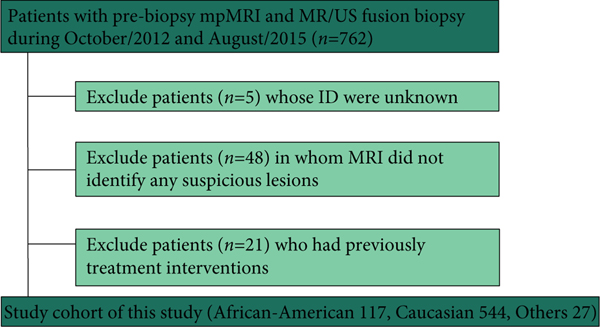
After verification from the Institutional Review Board, we retrospectively reviewed 762 patients who underwent pre-biopsy 3-T mpMRI and subsequent MRI/US FTB between October 2012 and August 2015. A flow diagram of the number of patients who were suitable for study inclusion is presented in Figure 1. All patients underwent pre-biopsy mpMRI and MRI/US FTB sampling was performed if the mpMRI results were abnormal. After excluding patients in whom the mpMRI did not identify any lesions or those who had previous interventions, 117 A-A men were included and compared with 544 Caucasian men.
Fig. 1.
Flow chart of the number of men who were suitable for study inclusion.
The mpMRIs were performed using 3-T clinical MRI instruments and reported on a 5-point Likert scale of suspicion [7] (Table 1). Three-dimensional volume data from mpMRI and real-time TRUS images were visualised on the screen of a computer workstation, the UroStation (Koelis, Grenoble, France) [6,8]. All of the MRI/US FTBs and simultaneous conventional random biopsies were taken at Chesapeake Urology Associates (Owings Mills, MD, USA) and data were analysed at the USC Institute of Urology, Keck School of Medicine, University of Southern California (Los Angeles, CA, USA). A standard random biopsy was taken with conventional systematic 10–12 cores per patient and MRI/US FTB was performed with ≥1 core/lesion.
Table 1.
Likert 5-grade scoring system.
| Likert score | Designation |
|---|---|
|
| |
| 1 | CSPC is highly unlikely to be present |
| 2 | CSPC is unlikely to be present |
| 3 | CSPC is equivocal |
| 4 | CSPC is likely to be present |
| 5 | CSPC is highly likely to be present |
The definition for clinically significant prostate cancer (CSPC) was set at a Gleason score of ≥3+4 in this study.
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS® version 23; IBM Corporation, Armonk, NY, USA) software. Statistically significant differences were assessed using the Mann-Whitney U-test for continuous data and the chi-square test for categorical data. After controlling for age, pre-biopsy PSA level and estimated prostate volume using multivariable logistic regression modelling, we statistically compared overall cancer and CSPC detection rates between A-A and Caucasian men. The results were considered statistically significant at P < 0.05.
Results
The characteristics of the 661 patients, including 117 A-A and 544 Caucasian men, are shown in Table 2. The median age of A-A men was 1.5 years younger than the Caucasian men (63.0 vs 64.5 years, P = 0.003). There were no significant differences between the two cohorts for median pre-biopsy PSA level (7.1 vs 6.9 ng/ml, P = 0.274), estimated prostate volume (52.9 vs 51.9 mL, P = 0.585) and number of lesions on MRI per patient (2.0 vs 2.0, P = 0.878). The study population included the spectrum of men offered prostate biopsy, including i) biopsy naïve men with a clinical suspicion of prostate cancer based on increased PSA level and/or abnormal DRE, ii) men with previous negative biopsy with persistent clinical suspicion of prostate cancer based on increased PSA level and/or abnormal DRE, and iii) men with histologically confirmed cancer on an active surveillance protocol or previous atypical cells. However, there was no statistically difference in the proportion of reasons for biopsy between the two races (P = 0.134).
Table 2.
The patients’ characteristics.
| Characteristic | A-A | Caucasian | P |
|---|---|---|---|
|
| |||
| Number of patients | 117 | 544 | |
| Median (range) | |||
| Age, years | 63.0 (44–81) | 64.5 (30–87) | 0.003 |
| Pre-biopsy PSA level, ng/mL | 7.1 (0.3–61.6) | 6.9 (0.2–51.2) | 0.274 |
| Estimated prostate volume, mL | 52.9 (17.6–178.3) | 51.9 (14.5–247.6) | 0.585 |
| Number of lesions detected on mpMRI/patient | 2.0 (1–5) | 2.0 (1–5) | 0.878 |
| N (%) | |||
| Clinical T stage, cT1/cT2 | 106 (90.6)/8 (6.8) | 492 (90.4)/36 (6.6) | 0.973 |
| Reason for biopsy | |||
| Rising PSA – previous negative biopsy | 49 (41.9) | 238 (43.8) | 0.134 |
| Active surveillance – prostate cancer | 30 (25.6) | 155 (28.5) | |
| Elevated PSA – biopsy naїve | 13 (11.1) | 78 (14.3) | |
| Atypical cells on previous biopsy | 24 (20.5) | 64 (11.8) | |
| Other | 1 (0.9) | 9 (1.7) | |
For conventional random biopsy, there were no significant differences between the A-A and Caucasian men in the detection rates of overall cancer (37.2% vs 42.9%, P = 0.294) and CSPC (12.4% vs 15.9%, P = 0.728) (Table 3).
Table 3.
Overall cancer detection rate and CSPC detection rate with random biopsy.
| A-A, n/N (%) | Caucasian, n/N (%) | P | |
|---|---|---|---|
|
| |||
| Overall cancer detection rate | 42/113 (37.2) | 226/527 (42.9) | 0.294 |
| CSPC detection rate | 14/113 (12.4) | 84/527 (15.9) | 0.728 |
On the pre-biopsy mpMRI, the proportion of the dominant lesion on the 5-point Likert scale scoring was not statistically different between the two races (Table 4).
Table 4.
5-point Likert scale scoring on mpMRI in A-A and Caucasian men (P = 0.425).
| Likert score | A-A, n (%) | Caucasian, n (%) |
|---|---|---|
|
| ||
| 1 | 15 (12.8) | 64 (11.8) |
| 2 | 42 (35.9) | 163 (30.0) |
| 3 | 42 (35.9) | 190 (34.9) |
| 4 | 8 (6.8) | 61 (11.2) |
| 5 | 9 (7.7) | 64 (11.8) |
| Unknown | 1 (0.9) | 2 (0.4) |
| Total | 117 (100) | 544 (100) |
Table 5 (A) shows the overall cancer detection rates in the dominant lesions with MRI/US FTB. There were no significant differences in the overall cancer detection rates for the grade 1–2 (lower suspicion) lesions (14.0% vs 18.5%, P = 0.429) and the grade 4–5 (higher suspicion) lesions (70.6% vs 69.6%, P = 0.934) between A-A and Caucasian men, respectively. However, the cancer detection rate for the grade 3 (equivocal suspicion) lesions in A-A men was significantly higher than that in Caucasian men (47.6% vs 28.9%, P = 0.019).
Table 5.
Overall cancer (A) and CSPC (B) detection rates with MRI/US FTB.
| Likert score | A. Overall cancer detection rate | B. CSPC detection rate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| A-A, n/N (%) | Caucasian, n/N (%) | P | A-A, n/N (%) | Caucasian, n/N (%) | P | |
|
| ||||||
| 1–2 | 8/57 (14.0) | 42/227 (18.5) | 0.429 | 1/57 (1.8) | 16/227 (7.0) | 0.109 |
| 3 | 20/42 (47.6) | 55/190 (28.9) | 0.019 | 11/42 (26.2) | 34/190 (17.9) | 0.219 |
| 4–5 | 12/17 (70.6) | 87/125 (69.6) | 0.934 | 9/17 (52.9) | 66/125 (52.8) | 0.991 |
| Total | 41/117 (35.0) | 186/544 (34.2) | 0.915 | 22/117 (18.8) | 118/544 (21.7) | 0.288 |
Table 5 (B) shows the CSPC detection rates in the dominant lesions with MRI/US FTB. There were no differences in any of the scoring groups between A-A and Caucasian men.
After controlling for age, pre-biopsy PSA level and prostate volume, there were no significant differences between A-A and Caucasian men in the detection rate of overall cancer (35.0% vs 34.2%, P = 0.915) and CSPC (18.8% vs 21.7%, P = 0.288) for all of the suspicious lesions (grade 1–5).
In A-A men, MRI/US FTB from the grade 4–5 lesions outperformed random biopsy and MRI/US FTB from the grade 1–3 lesions in the detection rate of overall cancer (70.6% vs 37.2% vs 28.3%, P = 0.003) and CSPC (52.9% vs 12.4% vs 12.1%, P < 0.001), respectively.
For the location of dominant lesions on mpMRI, there were no significant differences between A-A and Caucasian men [Table 6 (A)].
Table 6.
Location of dominant lesions on mpMRI (A), and overall cancer (B) and CSPC (C) detection rates in the PZ and TZ with MRI/US FTB.
| Location | A. Dominant lesions on MRI | B. Overall cancer detection rate | C. CSPC detection rate | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| A-A, n/N (%) | Caucasian, A-A, n/N (%) | A-A, n/N (%) | Caucasian, A-A, n/N (%) | P | A-A, n/N (%) | Caucasian, A-A, n/N (%) | P | |
|
| ||||||||
| PZ | 83/117 (70.9) | 348/544 (64.0) | 26/83 (31.3) | 118/348 (33.9) | 0.654 | 14/83 (16.9) | 82/348 (23.6) | 0.188 |
| TZ | 18/117 (15.4) | 110/544 (20.2) | 8/18 (44.4) | 38/110 (34.5) | 0.417 | 5/18 (27.8) | 17/110 (15.5) | 0.169 |
| P | 0.339 | 0.286 | 0.902 | 0.223 | 0.072 | |||
Table 6 (B and C) shows the detection rates of overall cancer and CSPC in the peripheral zone (PZ) and transition zone (TZ) with MRI/US FTB. There were no significant differences between A-A and Caucasian men, as well as between the PZ and TZ.
Table 7 shows the comparison between conventional random biopsy and MRI/US FTB for the dominant lesions in A-A men. A higher proportion of cores were positive for any cancers using MRI/US FTB than random biopsy (24.9% vs 6.8%, P < 0.001). The median cancer core length with MRI/US FTB was significantly greater than that of random biopsy (4.97 vs 2.39 mm, P < 0.001). In addition, the mean number of cores needed to detect one patient with any cancer was 27.3 and 7.15 for random biopsy and MRI/US FTB, respectively (P < 0.001). The mean number of cores needed to detect one patient with CSPC was 81.9 and 13.3 for random biopsy and MRI/US FTB, respectively (P < 0.001).
Table 7.
Comparison between random biopsy and MRI/US FTB in A-A men.
| Variable | Random biopsy | MRI/US FTB | P |
|---|---|---|---|
|
| |||
| Total cores, n | 1147 | 293 | |
| Cancer positive cores, n | 78 | 73 | |
| Positive for any cancer per core, n/N cores (%) | 78/1147 (6.8) | 73/293 (24.9) | <0.001 |
| Maximum cancer core length, mm, mean | 2.39 | 4.97 | 0.001 |
| Efficiency in detecting one patient with overall cancer, mean number of cores needed | 27.3 | 7.15 | <0.001 |
| Efficiency in detecting one patient with CSPC, mean number of cores needed | 81.9 | 13.3 | <0.001 |
Discussion
To our knowledge, the present study is the first to focus on the diagnostic yield of targeted prostate biopsy in A-A men using image fusion of mpMRI with real-time TRUS.
Our key finding is that MRI/US FTB detected more CSPC in A-A men using fewer cores compared with conventional random biopsy; and also, at the time of diagnosis of prostate cancer using MRI/US FTB, there were statistically no differences in the detection rates of prostate cancer between A-A and Caucasian men.
After controlling for age, pre-biopsy PSA level and estimated prostate volume, there were no statistically significant differences in the overall cancer and CSPC detection rates between the races. Recently, mpMRI has been increasingly used in patients with a high suspicion of prostate cancer and MRI/US FTB may replace the more traditional TRUS-guided biopsy in the future [9].
In the present study, MRI/US FTB detected more CSPC using fewer cores compared with conventional random biopsy. These results confirm that MRI/US FTB is a useful tool for detecting CSPC independently of race. Moreover, MRI/US FTB findings strongly correlated with the level of suspicion on mpMRI in A-A men, as well as Caucasian men.
Higher scores (grade 4–5) on mpMRI correlated strongly with a higher likelihood of any cancer (70.6%) and CSPC (52.9%) in A-A men. Thus, MRI/US FTB should be considered for grade 4–5 lesions. In contrast, lower scores (grade 1–2) on mpMRI may be useful in predicting a low likelihood of high-grade cancer, so MRI/US FTB could potentially be avoided. Grade 3 is equivocal and biopsy indication for grade 3 needs to be evaluated in further investigations.
Interestingly, however, our present study showed that the overall cancer detection rate only for the grade 3 (equivocal suspicion) lesions in A-A men was significantly higher than in Caucasian men (47.6% vs 28.9%, P = 0.019). However, there were no differences in the CSPC detection rate for the grade 3 lesions between the two races. Taken together, this may imply that the grade 3 lesions in A-A men have a tendency to contain more clinically insignificant prostate cancer compared to Caucasian men. Further investigations will be needed to clarify this issue.
In general, prostate cancer remains the most common malignancy and the second leading cause of cancer death among men in the USA [10]. In addition, it has previously been reported that prostate cancer exhibits the most striking racial difference, as A-A men are at a 1.4-times higher risk of being diagnosed and at 2–3-times higher risk of dying from prostate cancer, compared to Caucasian men [2,11,12]. Furthermore, A-A men present at a more advanced disease stage, are administered different treatment regimens, and have shorter progression-free survival after treatment [2,13–15]. It is known that prostate cancer racial difference exists at stages of presentation, diagnosis, treatment regimens and subsequent survival, and quality of life. The difference is complex involving biological, socioeconomic and sociocultural determinants [2,16–19].
The biological contribution to prostate cancer difference in A-A vs Caucasian men is an area of great research interest. Devaney et al. [20] recently reported that genome-wide methylation patterns differed by ethnic/racial groups, which suggests distinct differences in the aetiology of prostate cancer in A-A vs Caucasian men.
It has also been reported that A-A men more often estimated their likelihood of prostate cancer as 0% at biopsy, despite higher risk. Reasons for these low estimates and their potential contribution to poor treatment outcomes of A-A patients require further investigation [21].
On the other hand, in a large population-based study of patients with low-risk low-volume prostate cancer, it was reported that A-A men were no more likely to have pathologically aggressive features than Caucasian men [22]. In recent years, A-A men have experienced greater improvement in prostate cancer survival, and the racial difference has decreased [23].
Taken together, it is still controversial as to whether A-A race is an independent predictor of adverse oncological outcomes in patients with prostate cancer. Therefore, future follow-up data of the present study population may help in identifying answers to this question.
There are several limitations to the present study. First, the present study is retrospective and the study population heterogeneous, comprising of biopsy naїve men, men with prior negative biopsy, and men with prior positive biopsy. Second, the MRIs were reviewed independently by fellowship-trained abdominal radiologists with potentially varying levels of experience in prostate MRI interpretation because of the practical clinical setting of the present study. Third, we used a Likert scale as a reporting system of prostate MRI. Although 5-point Likert scale scoring and Prostate Imaging-Reporting and Data System version 2 (PI-RADS v2) are well-known reporting systems of prostate MRI, controversy still exists on the best way to report [24–26]. Additionally, our reference standard is based on prostate biopsy rather than the final prostatectomy specimen. Also our present analysis excluded patients without visible lesions on MRI and subsequently the rate of CSPC in such patients has not been assessed. Furthermore, we classified all Gleason ≥7 as CSPC; however, the definition of CSPC is controversial and debatable with no universally accepted definition. Lastly, the extra costs and efforts associated with mpMRI and MRI/US FTB should be further investigated with consideration of possible cost savings due to reductions in repeat biopsies.
Conclusions
The present study is the first to focus on the diagnostic yield of targeted prostate biopsy in A-A men using image fusion of mpMRI with real-time TRUS. Our present findings indicate a lack of different racial outcomes for CSPC detected with MRI/US FTB. Furthermore, we also found that MRI/US FTB detected more CSPC using fewer cores compared with conventional random biopsy, with histological findings correlating with the level of suspicion on mpMRI.
Acknowledgements
We thank Jie Cai for his valuable help. This study was supported in part by the National Institutes of Health (NIH) / National Cancer Institute (NCI) grant -R01CA205058 (PI: Gill IS).
Abbreviations:
- A-A
African-American
- CSPC
Clinically significant prostate cancer
- FTB
image-fusion targeted biopsy
- mpMRI
multi-parametric MRI
- PZ
peripheral zone
- TZ
transition zone
- US
ultrasonography
Footnotes
Conflicts of Interest
None declared.
References
- 1.Barrington WE, Schenk JM, Etzioni R et al. Difference in association of obesity with prostate cancer risk between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol 2015; 1: 342–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate 2011; 71: 985–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqui MM, Rais-Bahrami S, Turkbey B et al. Comparison of MR/ ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng X, Rosenkrantz AB, Mendhiratta N et al. Relationship between prebiopsy multiparametric magnetic resonance imaging (MRI), biopsy indication, and MRI-ultrasound fusion-targeted prostate biopsy outcomes. Eur Urol 2016; 69: 512–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ukimura O, Marien A, Palmer S et al. Trans-rectal ultrasound visibility of prostate lesions identified by magnetic resonance imaging increases accuracy of image-fusion targeted biopsies. World J Urol 2015; 33: 1669–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baco E, Rud E, Eri LM et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol 2015; 69: 149–56 [DOI] [PubMed] [Google Scholar]
- 7.Dickinson L, Ahmed HU, Allen C et al. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imaging 2013; 37: 48–58 [DOI] [PubMed] [Google Scholar]
- 8.Ukimura O, Desai MM, Palmer S et al. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J Urol 2012; 187: 1080–6 [DOI] [PubMed] [Google Scholar]
- 9.Alberts AR, Schoots IG, Bokhorst LP, van Leenders GJ, Bangma CH, Roobol MJ. Risk-based patient selection for magnetic resonance imaging-targeted prostate biopsy after negative transrectal ultrasound-guided random biopsy avoids unnecessary magnetic resonance imaging scans. Eur Urol 2016; 69: 1129–34 [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30 [DOI] [PubMed] [Google Scholar]
- 11.Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol 2007; 177: 444–9 [DOI] [PubMed] [Google Scholar]
- 12.Gaines AR, Turner EL, Moorman PG et al. The association between race and prostate cancer risk on initial biopsy in an equal access, multiethnic cohort. Cancer Causes Control 2014; 25: 1029–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imperato PJ, Nenner RP, Will TO. Radical prostatectomy: lower rates among African-American men. J Natl Med Assoc 1996; 88: 589–94 [PMC free article] [PubMed] [Google Scholar]
- 14.Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol 2010; 28: 1069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritch CR, Morrison BF, Hruby G et al. Pathological outcome and biochemical recurrence-free survival after radical prostatectomy in African-American, Afro-Caribbean (Jamaican) and Caucasian-American men: an international comparison. BJU Int 2013; 111: E186–90 [DOI] [PubMed] [Google Scholar]
- 16.Bostwick DG, Burke HB, Djakiew D et al. Human prostate cancer risk factors. Cancer 2004;101(Suppl.):2371–490. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz K, Powell IJ, Underwood W 3rd, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 2009; 74: 1296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moul JW, Douglas TH, McCarthy WF, McLeod DG. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol 1996; 155: 1667–73 [PubMed] [Google Scholar]
- 19.Freedland SJ, Jalkut M, Dorey F, Sutter ME, Aronson WJ. Race is not an independent predictor of biochemical recurrence after radical prostatectomy in an equal access medical center. Urology 2000; 56: 87–91 [DOI] [PubMed] [Google Scholar]
- 20.Devaney JM, Wang S, Furbert-Harris P et al. Genome-wide differentially methylated genes in prostate cancer tissues from African-American and Caucasian men. Epigenetics 2015; 10: 319–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmerich JA, Ahmad FS, Meltzer DO, Dale W. African American men significantly underestimate their risk of having prostate cancer at the time of biopsy. Psychooncology 2013; 22: 338–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber D, Chhabra A, Rineer J, Weedon J, Schwartz D. A Population-based study of men with low-volume low-risk prostate cancer: does African-American race predict for more aggressive disease? Clin Genitourin Cancer 2015; 13: 259–64 [DOI] [PubMed] [Google Scholar]
- 23.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers: results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol 2015; 1: 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinreb JC, Barentsz JO, Choyke PL et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur Urol 2016; 69: 16–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenkrantz AB, Kim S, Lim RP et al. Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology 2013; 269: 482–92 [DOI] [PubMed] [Google Scholar]
- 26.Roethke MC, Kuru TH, Schultze S et al. Evaluation of the ESUR PI-RADS scoring system for multiparametric MRI of the prostate with targeted MR/TRUS fusion-guided biopsy at 3.0 Tesla. Eur Radiol 2014; 24: 344–52 [DOI] [PubMed] [Google Scholar]