Abstract
The optimal strategy for imaging after focal therapy for prostate cancer is evolving. This series is an initial report on the use of contrast-enhanced transrectal ultrasound (TRUS) in follow-up of patients after high-intensity focused ultrasound (HIFU) hemiablation for prostate cancer. In 7 patients who underwent HIFU hemiablation, contrast-enhanced TRUS findings were as follows: (1) contrast-enhanced TRUS clearly showed the HIFU ablation defect as a sharply marginated nonenhancing zone in all patients; (2) contrast-enhanced TRUS identified suspicious foci of recurrent enhancement within the ablation zone in 2 patients, facilitating image-guided prostate biopsy, which showed prostate cancer; and (3) contrast-enhanced TRUS findings correlated with multiparametric magnetic resonance imaging and biopsy histologic findings.
Keywords: contrast-enhanced ultrasound, focal therapy, high-intensity focused ultrasound, prostate cancer, transrectal ultrasound
High-intensity focused ultrasound (HIFU) has been used for focal therapy in patients with localized prostate cancer.1 One of the challenges of focal therapy is surveillance during follow-up, since there is no consensus on imaging modalities or valid markers to detect or predict postablation recurrence. Contrast-enhanced transrectal ultrasound (TRUS) uses intravenous microbubble ultrasound (US) contrast agents to assess benign and cancerous prostate tissue by evaluating prostatic perfusion.2–4 Earlier preclinical studies demonstrated that contrast-enhanced TRUS could be used to guide and monitor ablation in a dog model.5,6 Herein, we report an initial clinical experience of using contrast-enhanced TRUS for follow-up after focal HIFU for prostate cancer.
Methods
Patient Selection for HIFU and HIFU Procedure
We identified 7 consecutive patients who underwent HIFU hemiablation, follow-up contrast-enhanced TRUS evaluations, and a 12-month prostate biopsy from our Institutional Review Board–approved database. High-intensity focused US hemiablation was defined as HIFU ablation of the prostate lobe harboring prostate cancer and was performed with either Ablatherm (EDAP TMS, Vaulx-en-Velin, France) or SonaCare (SonaCare Medical, Charlotte, NC) technology. Multiparametric magnetic resonance imaging (MRI) examinations were performed on a 3-T MRI system (GE Healthcare, Milwaukee, WI) using a multichannel phased-array coil. The MRI acquisition protocol included high resolution T2-weighted, diffusion-weighted, and T1-weighted dynamic contrast-enhanced sequences. Parametric apparent diffusion coefficient (ADC) maps were calculated from the diffusion-weighted images. Multiparametric MRI was performed as part of our routine MRI/TRUS fusion-guided prostate biopsy program.7 Prostate cancer (prostate cancer) was assessed with the American Joint Committee on Cancer Staging Manual, seventh edition, and International Society of Urological Pathology standards. Clinically significant prostate cancer was defined as Gleason grade group 2 or higher (Gleason score ≥ 3 + 4). Clinically insignificant prostate cancer was defined as Gleason grade group 1 (Gleason score 6).8
Post-HIFU Follow-up
A digital rectal examination and prostate-specific antigen (PSA) testing were scheduled at 3, 6, and 12 months after hemigland HIFU ablation. Follow-up contrast-enhanced TRUS was offered within 6 and 12 months of follow-up and at the time of prostate biopsy. Multiparametric MRI and prostate biopsy were offered at 12 months or earlier for cause, such as PSA elevation.
Contrast-Enhanced TRUS
After the TRUS transducer was inserted in the patients’ rectum, image settings were optimized. The contrast-enhanced TRUS examination started with conventional TRUS B-mode imaging followed by color or power Doppler imaging. A dual-scan, split-screen mode (simultaneous contrast-enhanced TRUS and B-mode imaging) using low mechanical index settings was used for contrast. Prior to the procedure, the off-label use of microbubble contrast agent for evaluation of the prostate was discussed with the patients. Lumason (Bracco Diagnostic, Inc, Monroe Township, NJ), a sulfur hexafluoride microsphere US contrast agent, was manually agitated for 20 seconds. A 2.5-mL bolus dose of the contrast agent was injected intravenously, followed immediately by a 10-mL normal saline flush. Complete, real-time, 2-minute continuous video clips of the contrast-enhanced TRUS examination, starting from the saline flush, were stored. The contrast-enhanced TRUS video clips were acquired at the base, mid, and apex of the prostate on an axial view. Depending on availability, the US units used were GE LOGIQ E9(GE Healthcare, Buckinghamshire, England) or Philips EPIQ 7 (Philips Healthcare, Bothell, WA). For the GE LOGIQ E9 unit, a GE iC5–9-D transducer was used. For the Philips EPIQ 7 unit, a C10–3v transducer was used. For both systems, a low– mechanical index mode with a frequency of 8 MHz was used.
Time-Intensity Curve Analysis
An independent radiologist with 6 years of experience performing and interpreting contrast-enhanced US examinations analyzed the stored contrast-enhanced TRUS images. A semiquantitative subjective scale of enhancement was applied to each prostate lobe as well as any enhancing lesions within the lobe as follows, based on the study by Rouvière et al9: E0, no enhancement; E1, mild or heterogeneous enhancement; and E2, marked enhancement.9
Quantitative Analysis
Time-intensity curves were created as follows: QLAB quantification software (Philips Healthcare) was used to postprocess raw data for those contrast-enhanced TRUS examinations done on a Philips EPIQ 7 unit. GE software was used to postprocess raw data for those contrast-enhanced TRUS examinations done on a GE LOGIQ E9 unit. The processing consisted of the following steps: (1) creation of a region of interest (ROI) within the area suspicious for a recurrent tumor (when present) or in the ablated lobe of the prostate if no suspicious focus was seen on contrast-enhanced TRUS images; (2) creation of a second “internal control” ROI at the same depth in the nontreated contralateral gland; (3) correction for “in-plane” motion by proprietary image registration software present on both scanners; (4) extraction of the echo mean for each ROI to create a time-intensity curve; and (5) curve fitting the echo mean time line to the local density random-walk wash-in/wash-out equation to calculate quantitative values, including peak intensity (decibels) and time to peak (seconds).
Results
Patient Demographics
Patient demographics are summarized in Table 1. Median age was 63 years (range, 41–80 years); baseline PSA level, 5.1 ng/mL (range, 3.1–11.0 ng/mL); prostate volume, 26 cm3 (range, 18–46 cm3); and lesion size on multiparametric MRI, 18 mm (range, 9–18 mm; n = 4). One patient had failed prior treatment for prostate cancer. Two patients could not undergo multiparametric MRI because of the presence of a cardiac defibrillator (n = 1) and claustrophobia (n = 1). In our series, 1 patient had International Society of Urological Pathology grade group 1 (Gleason 6) prostate cancer, and 6 patients had Gleason grade group 2 or higher prostate cancer. Over a post-HIFU median follow-up of 15 months (range, 13–20 months), prostate volume decreased by 32.5% (range, 0%–74%), and the PSA level decreased by 82% (range, 30%–95%; 5 patients had > 70% reduction), with a median time to PSA nadir of 3 months (range, 2–12 months).
Table 1.
Demographics, Baseline Characteristics, and Follow up of Patients Who Underwent Contrast-Enhanced TRUS Imaging After Prostate Hemigland HIFU Ablation
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age, y | 46 | 72 | 63 | 61 | 41 | 66 | 80 |
| PSA, ng/mL | 5.2 | 4.0 | 4.2 | 6.5 | 9.1 | 3.1 | 11.0 |
| Clinical stage (DRE) | cT1c | cT2a | cT2a | cT1c | cT1c | cT1c | cT2b |
| Previous treatment for prostate cancer | No | No | No | No | No | No | Cryo, sEBRT |
| Pre-HIFU multiparametric MRI PI-RADS score |
3 | NAa | 5 | No lesion | 5 | NAb | No lesion |
| Size of lesion, mm | 9 | 18 | 18 | 12 | |||
| Pre-HIFU TRUS and biopsy Prostate volume, cm3 |
27 | 26 | 26 | 46 | 36 | 23 | 18 |
| No. of positive cores | 6 | 6 | 2 | 1 | 2 | 2 | 5 |
| Gleason grade group | 2 | 3c | 3c | 4c | 2 | 1 | 5c |
| Maximum cancer core, % | 40 | 80 | 60 | 5 | 50 | 10 | 50 |
| Follow-up time, mo | 20 | 20 | 16 | 15 | 14 | 13 | 13 |
| Time to PSA nadir, mo | 3 | 6 | 12 | 3 | 2 | 3 | 3 |
| PSA nadir, ng/mL | 0.83 | 0.2 | 0.77 | 3.4 | 2.3 | 2.2 | 0.68 |
| Reduction in PSA at nadir, % | 84 | 95 | 82 | 48 | 75 | 30 | 94 |
| Follow up contrast-enhanced TRUS TRUS prostate volume, cm3 |
15 | 13 | 17 | 12 | 18 | 16 | 18 |
| Reduction in prostate volume, % | 44 | 50 | 35 | 74 | 50 | 30 | 0 |
| Ablation zone visualized | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Time-intensity curve enhancement patterns Enhancement in the treated lobe |
E1 | E0 | E0 | E1 | E0 | E2 | E0 |
| Discrete lesion in the treated lobe | E0 | E0 | E0 | E0 | E2 | E0 | E2 |
| Enhancement in the untreated lobe | E2 | E2 | E1 | E2 | E1 | E2 | E1 |
| Follow-up multiparametric MRI PI-RADS score |
No lesion | NAa | No lesion | No lesion | 4 | NAb | 4 |
| Follow-up prostate biopsy | |||||||
| Follow-up biopsy performed | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Positive biopsy results in treated lobe | No | No | No | No | Yes | Yes | Yes |
| Gleason grade group | 2 | 1 | 4 | ||||
| Maximum cancer core, % | 50 | 20 | 30 | ||||
Cryo indicates cryoablation; DRE, digital rectal examination; E0, no enhancement; E1, mild or patchy enhancement; E2, marked enhancement; NA, not applicable; PI-RADS, Prostate Imaging Reporting and Data System; and sEBRT, salvage external beam radiation therapy.
Patient could not have MRI because of the presence of a defibrillator.
Patient could not have MRI because of claustrophobia.
These 4 patients with high-risk or recurrent prostate cancer specifically requested HIFU hemiablation; additional imaging was performed to rule out metastases.
Contrast-Enhanced TRUS Findings
Follow-up contrast-enhanced TRUS clearly showed the post-HIFU ablation zone in all 7 patients (Figures 1 and 2). Follow-up biopsy at 12 months showed recurrent clinically significant prostate cancer in the treated lobe in 2 patients (Figure 3). Contrast-enhanced TRUS identified and localized the recurrent clinically significant prostate cancer in both patients as a discrete lesion with marked enhancement (E2) within a background of nonenhancement (E0) in the ablated lobe. Contrast-enhanced TRUS findings enabled targeted biopsies of the suspicious lesion in both patients. In both patients with recurrent clinically significant prostate cancer, contrast-enhanced TRUS findings correlated with multiparametric MRI and prostate biopsy findings (Figure 3). One additional patient had recurrent non–clinically significant prostate cancer (Gleason grade group 1) bilaterally on follow-up prostate biopsy, matching bilateral enhancement (E2) seen on contrast-enhanced TRUS imaging. The other 4 patients had no enhancing lesions in the treated lobe on contrastenhanced TRUS imaging, which correlated to no cancer on prostate biopsy (ie, all lobes with negative contrast-enhanced TRUS findings also had negative prostate biopsy findings).
Figure 1.
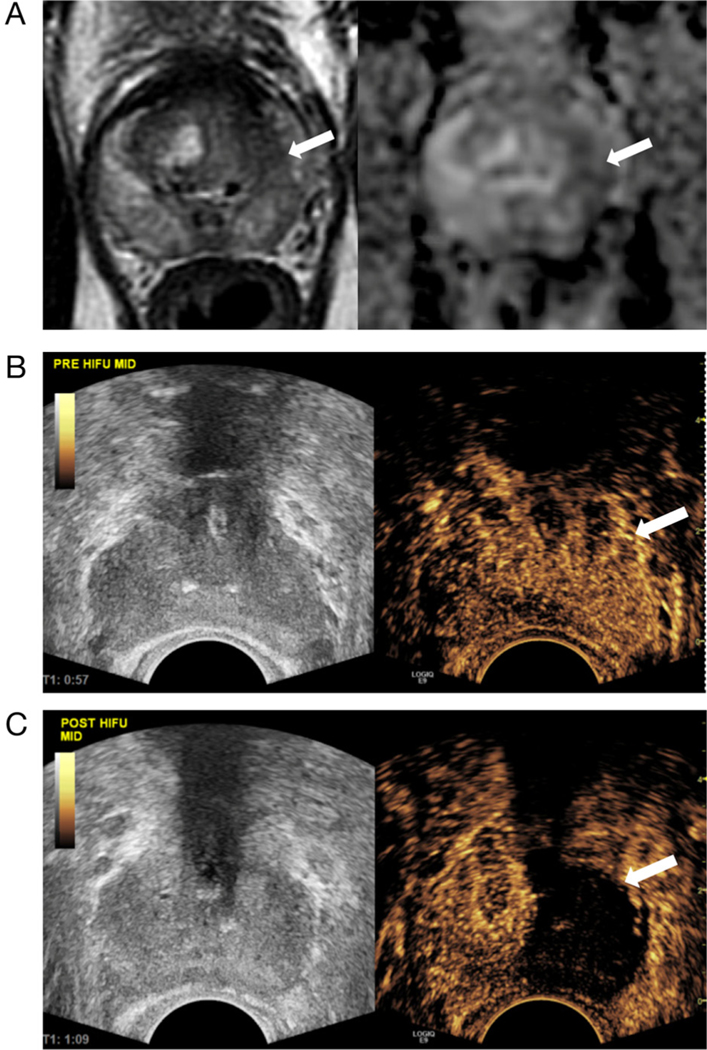
Example of prostate MRI before HIFU and contrastenhanced TRUS appearance of prostate hemiablation with HIFU. A, T2-weighted (left) and ADC (right) MR images show a 1.2-cm left peripheral zone lesion in the 1- to 3-o’clock position, with low T2 and ADC signals (arrows), Prostate Imaging Reporting and Data System category 4. This appearance corresponded to Gleason 3 + 4 prostate cancer on biopsy. B, Split-screen grayscale (left) and contrast-enhanced TRUS (right) images before HIFU ablation. The left prostate lobe shows diffuse slightly increased enhancement (arrow) relative to the right. C, Split-screen grayscale (left) and contrast-enhanced TRUS (right) images immediately after HIFU ablation. The left prostate lobe shows complete absence of enhancement (arrow). The right prostate lobe enhances normally.
Figure 2.
Example of the post-HIFU appearance of the prostate on contrast-enhanced TRUS, with time-intensity curve and histologic correlates: 72-year-old man with prostate cancer (case 2 from Table 1). A, Pretreatment TRUS image shows a 1 × 0.8-cm hypoechoic lesion in the peripheral right mid lobe in the 7-o’clock position (arrow), which corresponded to Gleason 4 + 3 prostate cancer on biopsy. B, Transrectal US image 13 months after right hemigland HIFU ablation shows shrinkage of the ablated right lobe (arrow) versus the left lobe. The ablated right lobe is mildly hypoechoic diffusely relative to untreated left lobe. C, Contrast-enhanced TRUS image shows a clear and well-defined nonenhancing ablated right lobe (arrow) and a normally enhancing left lobe. Yellow and blue circles are manually selected ROIs placed for generation of time-intensity curves. D, Time-intensity curves generated from the ROIs placed in C. Time is on the x-axis (seconds), and peak intensity is on the y-axis (decibels). The untreated left lobe (blue ROI) shows gradual wash-in of contrast, peak enhancement at approximately 50 seconds, followed by gradual wash-out (blue curve). The treated right lobe (yellow ROI) shows a flat waveform (yellow curve), with no quantifiable enhancement. E, Histologic specimen (hematoxylin-eosin, original magnification × 10). Follow-up biopsy at 13 months confirmed necrosis in the ablated right lobe. F, Simultaneous biopsy of the untreated left lobe showed a normal prostate gland.
Figure 3.
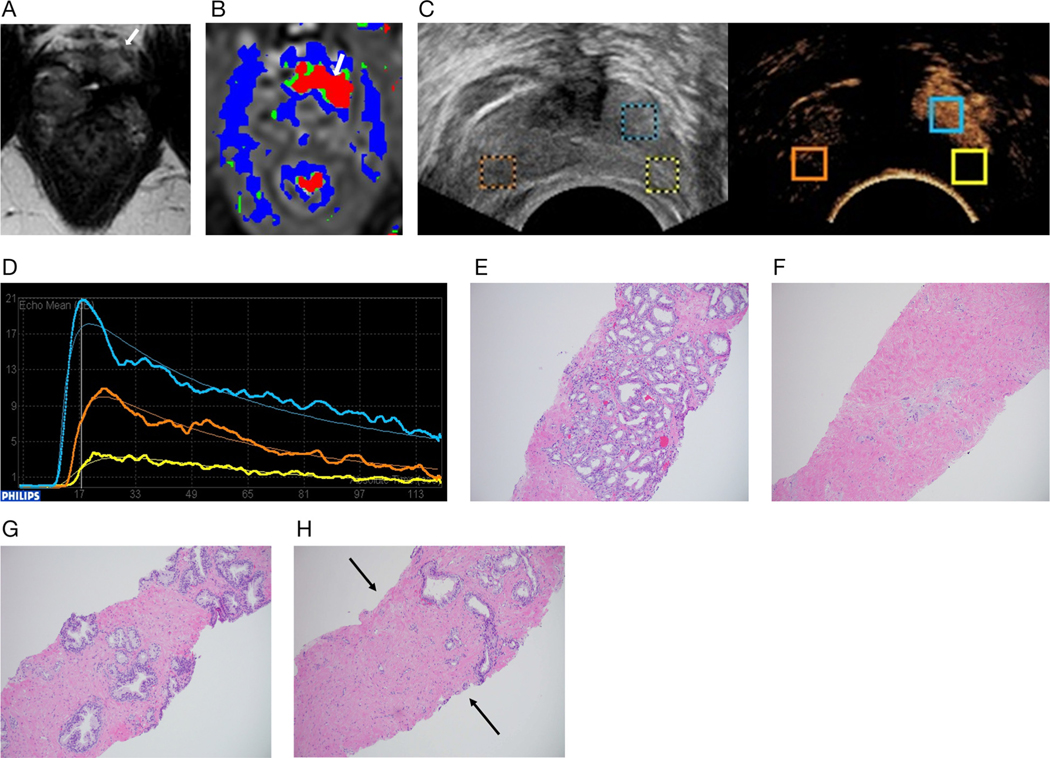
Example of the post-HIFU appearance of recurrent prostate cancer on MRI and contrast-enhanced TRUS, with time-intensity curve and histologic correlates: 41-year-old man with prostate cancer who presented for follow-up with a rising PSA level 1 year after left hemigland HIFU ablation of the prostate for Gleason 3 + 4 prostate cancer in the left apex anterior (case 5 from Table 1). A, Axial T2-weighted MR image at the level of the apex shows atrophy of the treated left versus the right prostate lobe. A linear band of low T2 signal intensity (arrow) at the left anterior apex is nonspecific given the history of HIFU. B, Color map from dynamic contrast-enhanced MR images at the same level as A. There is abnormal rapid enhancement and wash-out (arrow) at the left anterior apex, in the area of low T2 intensity on A, suspicious for a recurrent tumor. C, Split-screen grayscale and contrast-enhanced TRUS transverse images at the level of the prostate apex. Contrast-enhanced TRUS matches MRI findings showing a discrete enhancing suspicious lesion at the left anterior apex, in the otherwise ablated left lobe. Time-intensity curves were created with ROIs placed on the untreated right lobe (orange ROI), ablated left lobe (yellow ROI), and discrete enhancing left anterior apex lesion (blue ROI). D, Time-intensity curves generated from the ROIs placed in C. Time is on the x-axis (seconds), and peak intensity is on the y-axis (decibels). The suspicious lesion in the left apex anterior (blue curve) shows an earlier time to peak and higher peak intensity compared with the untreated right lobe (orange curve). The ablated left lobe (yellow curve) shows a relatively flat waveform, with less enhancement than the untreated right lobe. E, One-year follow up biopsy. Histologic examination of the suspicious enhancing lesion at the left anterior apex (blue ROI) confirmed Gleason 3 + 4 prostate cancer. F, Biopsy of the ablated left lobe (yellow ROI) showed hyalinized prostatic stroma devoid of prostatic glands. G, Biopsy of the normal prostate gland in the untreated right lobe (orange ROI) showed normal prostate glandular tissue. H, Left lobe biopsy including both ablated hyalinized prostatic stoma devoid of prostatic glands (corresponding to yellow ROI) and prostate cancer (corresponding to the blue ROI) that matches the contrastenhanced TRUS findings. Note the sharp delineation (arrows) between hyalinized prostatic stoma devoid of prostatic glands (below and to the left) and prostate cancer (above and to the right).
In both patients with recurrent clinically significant prostate cancer, contrast-enhanced TRUS of the prostate cancer lesions had a shorter time to peak and higher peak intensity than contralateral control ROIs. Time-intensity curves derived from contrast-enhanced TRUS examinations thus objectively differentiated enhancement of treated prostates, untreated prostate lobes, and recurrent cancer, decreasing operator-dependent subjectivity.
Discussion
High-intensity focused US uses focused US waves to generate thermal energy and destroy tissue. It is an alternative minimally invasive treatment for localized (stage T1–T2) prostate cancer in patients not undergoing prostatectomy.10,11 Conventional preprocedure imaging before HIFU can include a combination of MRI and TRUS, as well as staging studies including computed tomography and bone scans.
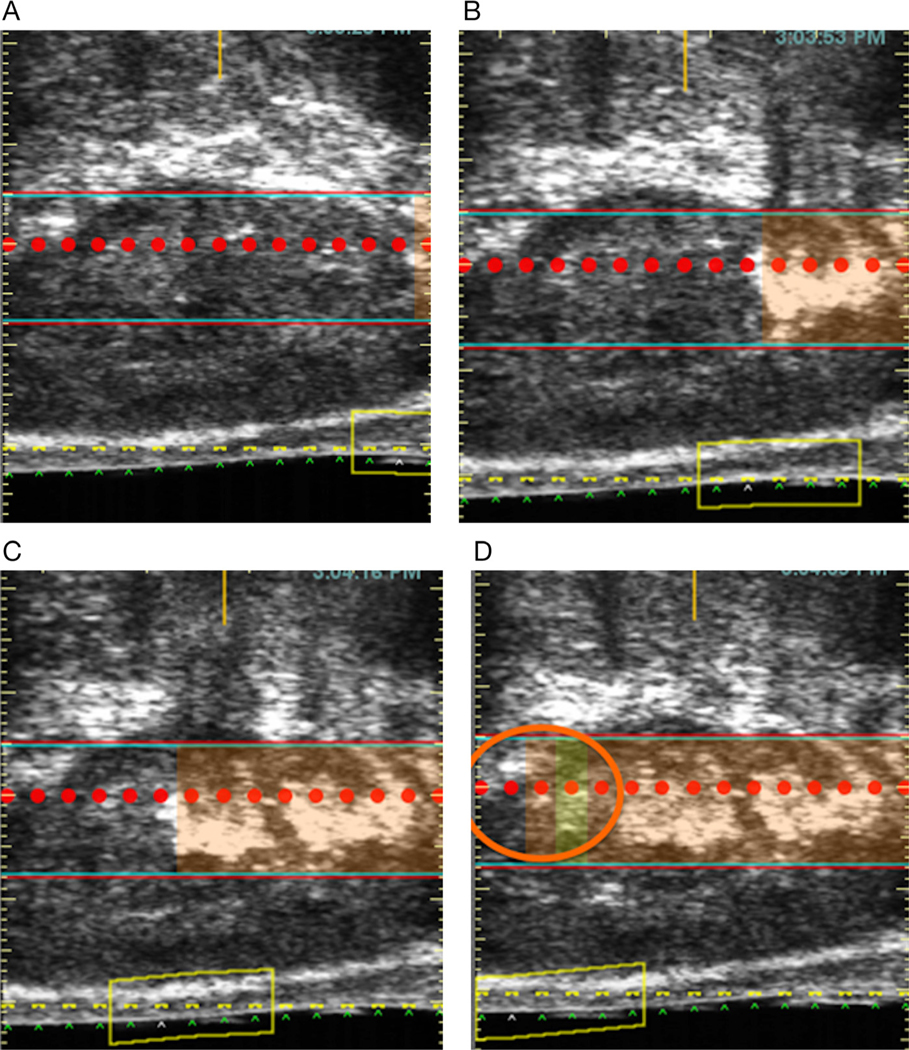
Intraprocedural imaging during HIFU has been limited to visual assessment of cavitation (echogenic bands developing during HIFU), a transient phenomenon that provides poor anatomic resolution (Figure 4). Conventional grayscale TRUS is widely used to guide prostate biopsy; however, it cannot diagnose prostate cancer or distinguish ablated prostate from normal prostate with high accuracy. Color Doppler US can accurately assess macrovessels, but not vessels less than 200 μm in diameter.12
Figure 4.
Example of echogenic cavitation during HIFU. TRUS images (A–D) show the development of cavitation, manifesting as echogenicity at the level of the red band and corresponding to the region of ablation. The cavitation, in addition to being transient and having poor anatomic resolution, is variably seen. In the final frame (D), no echogenic cavitation is seen (orange circle) to correspond to the region being treated.
On gadolinium-enhanced T1-weighted MRI done during the first week after HIFU focal therapy of the prostate, the ablation zone appears as a nonenhancing region. The size of this nonenhancing ablation zone decreases by approximately 60% by 1 month, as the treatment zone contracts. In addition to atrophy of the treated area, diffusely decreased T2 signal intensity and a variable ADC signal are common.13
Unlike MRI, contrast-enhanced TRUS shows contrast uptake in even the smallest intratumoral vessels, such as the neovascularity commonly seen in prostate cancer.2 Contrast-enhanced TRUS uses contrast agents consisting of microbubbles with an average diameter of 2 to 6 μm.14 Identification of microvasculature in contrast-enhanced TRUS-guided biopsy may minimize false-negative results due to a sampling error.15
Contrast-enhanced TRUS has been used for diagnosis of prostate cancer in treatment-naïve prostate glands with encouraging early outcomes.4 However, reports of contrast-enhanced TRUS for evaluation of the prostate after HIFU are limited. One study evaluated contrast-enhanced TRUS after whole-gland HIFU ablation during a short follow-up period of 1.5 months.9 The authors found that prostate devascularization after focal therapy manifested as a lack of contrast uptake on contrast-enhanced TRUS, shown to correlate with nonviable tissue on biopsy. In that study, foci of residual enhancement in ablated prostate had a high probability of harboring viable tissue. Unlike MRI, changes shown on contrast-enhanced TRUS were stable from day 1 to day 45 after treatment.9
We hypothesized that contrast-enhanced TRUS can evaluate focal HIFU treatment efficacy postoperatively for a longer follow-up. Follow-up contrast-enhanced TRUS examinations done between 6 and 12 months after HIFU clearly showed the ablation defect in all 7 patients. Contrast-enhanced TRUS also showed recurrent clinically significant prostate cancer as a focal enhancing lesion in 2 patients, correlating with multiparametric MRI and prostate biopsy findings. To our knowledge, this pilot study was the first to do the following: (1) explore the utility of contrast-enhanced TRUS in the clinical setting of focal therapy (hemigland HIFU ablation) for prostate cancer; and b) perform quantitative analysis, including time-intensity curves and classification of enhancement patterns (E0, E1, E2) after focal HIFU ablation c) provide correlation with both mpMRI and PBx histology, and d) provide longer-term follow-up after HIFU ablation (median of 15 months).
A major challenge of focal therapy for prostate cancer is the uncertainty of the optimal method for following patients. After focal therapy, PSA testing is not accurate for predicting or identifying prostate cancer recurrence.1,16 Multiparametric MRI outperforms PSA testing for detecting prostate cancer recurrence after focal therapy.16 However, using multiparametric MRI for follow-up assessment of focal therapy has numerous limitations, including its high costs, limited availability, recognized list of contraindications, the need for expertise in interpretation of post–focal therapy MRI, and the lack of standardized reporting, as the Prostate Imaging Reporting and Data System system does not apply to posttreatment evaluation.7 One important multiparametric MRI parameter suggesting postablation recurrence of prostate cancer is avid dynamic contrast enhancement in a recurrent tumor (Figure 3).17 In a similar fashion, the recurrent prostate cancer showed more rapid and intense enhancement (shorter time to peak and higher peak intensity) on contrast-enhanced TRUS time-intensity curves than the normal contralateral control tissue.
Contrast-enhanced TRUS has potential to be a cost-effective and noninvasive procedure that can be performed quickly by either urologists or radiologists. It is widely available, as most US machines have contrast-enhanced US capability. The contrast agents used in contrast-enhanced TRUS have minimal side effects and can be used in patients with impaired renal function, hip prostheses, cardiac devices (pacemakers and defibrillators), and claustrophobia, all potential contraindications to multiparametric MRI.18 Although not detailed in this series, contrast-enhanced TRUS examinations can be performed intraoperatively during HIFU treatment, which is a crucial advantage over MRI.
This case series was a pilot exploration of the clinical utility of contrast-enhanced TRUS in the follow-up of patients after HIFU focal therapy, with encouraging early outcomes. Limitations of this series included the small patient population, which precluded a detailed statistical analysis. Nevertheless, individual “per-patient” detailed outcomes were promising. Further evaluations with longer followups, multiple readers, and larger patient cohorts allowing for statistical analyses are needed.
In summary, ablated prostate tissue showed a clear signal void (“black hole” or E0 enhancement pattern) on contrast-enhanced TRUS images. This signal void contrasted sharply with the marked enhancement (E2 enhancement pattern) in patients with recurrent tumors. The quantitative time-intensity curves confirmed the semiquantitative scale and visual observations by showing rapid and intense enhancement (short time to peak and high peak intensity) in recurrent cancer. In our initial pilot series, these findings were robust and unambiguous when compared with multiparametric MRI and confirmed on prostate biopsy. Contrast-enhanced TRUS provides initial encouraging outcomes; therefore, it has potential to add to focal therapy for prostate cancer follow-up as a valuable imaging modality to assess treatment effects and prostate cancer recurrence.
Acknowledgments
This trial is supported in part by National Institutes of Health/National Cancer Institute Grant R01 CA205058–01 (AA, MCS, ISG).
Abbreviations
- ADC
apparent diffusion coefficient
- HIFU
high-intensity focused ultrasound
- MRI
magnetic resonance imaging
- PSA
prostate-specific antigen
- ROI
region of interest
- TRUS
transrectal ultrasound
- US
ultrasound
References
- 1.Rischmann P, Gelet A, Riche B, et al. Focal high intensity focused ultrasound of unilateral localized prostate cancer: a prospective multicentric hemiablation study of 111 patients. Eur Urol 2017; 71:267–273. [DOI] [PubMed] [Google Scholar]
- 2.Wink M, Frauscher F, Cosgrove D, et al. Contrast-enhanced ultrasound and prostate cancer: a multicentre European research coordination project. Eur Urol 2008; 54:982–992. [DOI] [PubMed] [Google Scholar]
- 3.Xu G, Wu J, Yao MH, et al. Parameters of prostate cancer at contrast-enhanced ultrasound: correlation with prostate cancer risk. Int J Clin Exp Med 2015; 8:2562–2569. [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, Zhu ZQ, Zhou ZG, et al. Contrast-enhanced transrectal ultrasound for prediction of prostate cancer aggressiveness: the role of normal peripheral zone time-intensity curves. Sci Rep 2016; 6:38643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JB, Merton DA, Wansaicheong G, et al. Contrast enhanced ultrasound for radio frequency ablation of canine prostates: initial results. J Urol 2006; 176:1654–1660. [DOI] [PubMed] [Google Scholar]
- 6.Liu JB, Wansaicheong G, Merton DA, et al. Canine prostate: contrast-enhanced US-guided radiofrequency ablation with urethral and neurovascular cooling—initial experience. Radiology 2008; 247:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, version 2. Eur Urol 2016; 69:16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: the seventh edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 9.Rouvière O, Glas L, Girouin N, et al. Prostate cancer ablation with transrectal high-intensity focused ultrasound: assessment of tissue destruction with contrast-enhanced US. Radiology 2011; 259: 583–591. [DOI] [PubMed] [Google Scholar]
- 10.Crouzet S, Chapelon JY, Rouvière O, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur Urol 2014; 65:907–914. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed HU, Hindley RG, Dickinson L, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 2012; 13:622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halpern EJ, Ramey JR, Strup SE, Frauscher F, McCue P, Gomella LG. Detection of prostate carcinoma with contrast-enhanced sonography using intermittent harmonic imaging. Cancer 2005; 104:2373–2383. [DOI] [PubMed] [Google Scholar]
- 13.Rouvière O, Lyonnet D, Raudrant A, et al. MRI appearance of prostate following transrectal HIFU ablation of localized cancer. Eur Urol 2001; 40:265–274. [DOI] [PubMed] [Google Scholar]
- 14.Quaia E Microbubble ultrasound contrast agents: an update. Eur Radiol 2007; 17:1995–2008. [DOI] [PubMed] [Google Scholar]
- 15.Xie SW, Li HL, Du J, et al. Contrast-enhanced ultrasonography with contrast-tuned imaging technology for the detection of prostate cancer: comparison with conventional ultrasonography. BJU Int 2012; 109:1620–1626. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson L, Ahmed HU, Hindley RG, et al. Prostate-specific antigen vs magnetic resonance imaging parameters for assessing oncological outcomes after high intensity-focused ultrasound focal therapy for localized prostate cancer. Urol Oncol 2017; 35:30.e9–30.e15. [DOI] [PubMed] [Google Scholar]
- 17.De Visschere PJ, De Meerleer GO, Futterer JJ, Villeirs GM. Role of MRI in follow-up after focal therapy for prostate carcinoma. AJR Am J Roentgenol 2010; 194:1427–1433. [DOI] [PubMed] [Google Scholar]
- 18.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013; 39:187–210. [DOI] [PubMed] [Google Scholar]