Abstract
Mycobacterium ulcerans is the causative agent of Buruli ulcer, a tropical ulcerative skin disease. One of the most intriguing aspects of this disease is the presence of extensive tissue damage in the absence of an acute inflammatory response. We recently purified and characterized a macrolide toxin, mycolactone, from M. ulcerans. Injection of this molecule into guinea pig skin reproduced cell death and lack of acute inflammatory response similar to that seen following the injection of viable bacteria. We also showed that mycolactone causes a cytopathic effect on mouse fibroblast L929 cells that is characterized by cytoskeletal rearrangements and growth arrest within 48 h. However, these results could not account for the extensive cell death which occurs in Buruli ulcer. The results presented here demonstrate that L929 and J774 mouse macrophage cells die via apoptosis after 3 to 5 days of exposure to mycolactone. Treatment of cells with a pan-caspase inhibitor can inhibit mycolactone-induced apoptosis. We demonstrate that injection of mycolactone into guinea pig skin results in cell death via apoptosis and that the extent of apoptosis increases as the lesion progresses. These results may help to explain why tissue damage in Buruli ulcer is not accompanied by an acute inflammatory response.
Mycobacterium ulcerans is the causative agent of a tropical skin disease called Buruli ulcer (18), which has recently been recognized as an emerging infection in West Africa (12). Buruli ulcer patients present with indolent, necrotizing ulcerative lesions. The ulcers are characterized by extensive necrosis of the skin and underlying fat. Erythema and focal necrosis, including vascular erosion, are also present. In contrast to other pathogenic mycobacterial diseases such as tuberculosis, there is little evidence of an early acute inflammatory response to the infection, and the bacteria are primarily extracellular. Although the lesions may be quite extensive, covering up to 15% of a patient's skin surface, they are relatively painless.
We have recently purified and characterized a polyketide toxin from M. ulcerans and termed this mycolactone (10). When added to the mouse fibroblast cell line L929, the toxin causes 90 to 100% of the adherent cells to undergo cytoskeletal rearrangement, subsequently rounding up and detaching from the tissue culture plate within 24 to 36 h of treatment. This has been termed the cytopathic effect (CPE) (9, 15, 25). The toxin also causes an arrest in the G0/G1 phase of the cell cycle within 48 h (9, 10). More importantly, mycolactone, when injected intradermally into guinea pigs, is capable of causing a lesion similar to that produced by whole organisms (10). An isogenic mutant which does not produce the toxin is not virulent in the guinea pig model. Taken together, these data provide strong evidence that mycolactone plays a pivotal role in Buruli ulcer pathogenesis.
Necrotic areas in infected guinea pig skin contain many pyknotic nuclei, a finding suggestive of apoptosis. It is impossible to determine by hematoxylin-eosin staining whether these cells are resident or infiltrated immune cells killed at the site of the lesion. In addition, the mechanism of cell death is unknown. To begin to identify the mechanism underlying the histopathology observed in the guinea pig lesions, we decided to look closer at mechanisms of cell death, such as apoptosis.
The cell death apparent in mycolactone-induced guinea pig lesions is at odds with the observation that mycolactone causes growth arrest, with little reduction in viability, in L929 cells. To address the possibility that mycolactone can induce cell death in tissue culture cells with delayed kinetics, we extended the length of time of the cytopathology assay. We also included a macrophage-like cell line, J774, in our experiments to study the possible immune regulatory functions of the toxin. J774 cells exhibit a similar cytopathology to that of L929 cells. A total of 90 to 100% of cells exhibit cytoskeletal rearrangements within 24 to 36 h of treatment and undergo growth arrest. In addition, 24-h pretreatment of J774 cells with toxin inhibits induction of the proinflammatory cytokines, tumor necrosis factor alpha (TNF-α), and interleukin-1 (IL-1) in response to lipopolysaccharide stimulation (L. Pascopella, unpublished results).
We have used here both in vivo and in vitro studies to determine the mechanism of cell death mediated by mycolactone. We demonstrate that apoptotic cells reside in the area of necrosis of the guinea pig lesions infected with either M. ulcerans organisms or the toxin alone. This area of necrosis with apoptosis increases with time, indicating that apoptosis may play a role in Buruli ulcer pathology and, in particular, the lack of inflammation. We also show that the toxin does cause cell death in L929 and J774 cells, with delayed kinetics. Using the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) reaction and genomic DNA analysis, we show that the toxin can cause apoptosis to occur in the two cell lines. Treating the L929 cells with a caspase inhibitor prevents apoptosis, but not cytopathology, suggesting that apoptosis may be a secondary, rather than primary, effect of the toxin on tissue culture cells.
MATERIALS AND METHODS
Eukaryotic cell culture.
L929 mouse fibroblast cells (ATCC CCL1) and mouse macrophage cells J774A.1 (ATCC TIB 67) were purchased from the American Type Culture Collection and passaged in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum (Gibco BRL, Grand Island, N.Y.).
Preparation of mycolactone.
M. ulcerans 1615 (ATCC 35840) cells were passaged as described elsewhere (9). Mycolactone was prepared as described previously (10). Briefly, late-exponential-phase M. ulcerans bacteria were harvested and extracted with chloroform-methanol (2:1 [vol/vol]) for 2 h at room temperature. The cell debris was separated from the organic phase by centrifugation, and a Folch extraction was performed by the addition of 0.2 volumes of water. The organic phase was dried down and resuspended in a small volume of ice-cold acetone. Mycolactone was purified from the acetone-soluble fraction on a preparative thin-layer chromatography plate (Whatman Pk6F Silica Gel 60; Alltech, Deerfield, Ill.) developed in chloroform-methanol-water (90:10:1 [vol/vol]). Mycolactone runs at an Rf of 0.23 in this solvent system. Mycolactone was eluted from the silica plate in running buffer and weighed, and the effective dose was determined (see below) (9).
Guinea pig skin model of infection.
Hartley female guinea pigs were prepared for intradermal injection by shaving the backs of the animals and anesthetizing them. M. ulcerans bacteria was used at an inoculum of 107 (confirmed by viable plate counting). Bacteria were passed five times through a 25-gauge needle to approximate a uniform suspension. Mycolactone was air dried, resolubilized in a small amount of ethanol, and diluted in mycobacterial medium (Middlebrook 7H9 with OADC supplement). As a negative control, mycobacterial medium was injected. Injections were done with final volumes of 100 μl. All animal experiments were conducted according to the guidelines of the Animal Care and Use Committee at Rocky Mountain Laboratories. Guinea pigs were observed daily for signs of gross pathology and were sacrificed at days 2, 8, and 22 postinjection. Lesions were excised and fixed for 24 h in 3.7% formaldehyde. Samples were embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin-eosin.
TUNEL reaction on guinea pig sections.
Paraffin-embedded guinea pig sections were dewaxed with xylenes and rehydrated through an ethanol series. TUNEL reactions were performed with the In Situ Cell Death Detection POD Kit (Boehringer Mannheim, Indianapolis, Ind.). The sections were treated with 10 μg of proteinase K (Sigma) per ml (15 min at room temperature); endogenous peroxidase was then blocked with 0.3% hydrogen peroxide-phosphate-buffered saline (PBS) (30 min at room temperature) and permeabilized with 0.1% Triton X-100 (2 min on ice). The TUNEL reaction proceeded for 60 min at 37°C with CO2. After a washing with PBS, the slides were incubated with the Converter-POD at a 1:2 dilution in a Tris-NaCl buffer (30 min at 37°C with 5% CO2). For diaminobenzidene (DAB) detection, the slides were incubated with DAB substrate (10 min at room temperature). After staining with hematoxylin, the slides were dehydrated and mounted. For negative controls, the TUNEL reaction was performed on mycobacterial-medium-injected guinea pig skin sections and on M. ulcerans- and mycolactone-treated sections without terminal transferase enzyme.
Cytotoxicity assay.
L929 and J774 cells were plated at 2 × 104 in 24-well tissue culture plates and allowed to adhere overnight at 37°C with CO2. The next day, mycolactone was dissolved in ethanol and diluted in tissue culture media to the appropriate concentration and added to wells. As a control, ethanol was diluted similarly in tissue culture medium and added to the wells. At the indicated times, triplicate wells of each concentration of mycolactone or control cells were harvested by removing the supernatant, washing with PBS, and removing the adherent cells with trypsin followed by a PBS wash. Adherent and nonadherent cells were pooled and tested for presence of dead cells by ethidium homodimer uptake with the Live/Dead Viability/Cytoxicity Kit (Molecular Probes, Inc., Eugene, Oreg.). Five fields of stained cells were visualized on a Bio-Rad MRC 1000 laser confocal microscope. The percent dead and standard error of the mean values were calculated by using StatView 4.51 software (Abacus Concepts).
TUNEL reaction on L929 and J774 cells.
Cells were plated and allowed to adhere overnight. The next day, 300 ng of mycolactone (or an equivalent amount of ethanol diluted in tissue culture media for the negative control) was added, and both adherent and nonadherent cells were harvested and pooled together at the indicated times. The TUNEL reaction was performed by using the Promega Cell Death kit (Promega, Madison, Wis.). Briefly, the cells were fixed with 4% paraformaldehyde-PBS (20 min at 4°C). After a PBS wash, the cells were permeabilized with 0.2% Triton X-100–PBS, and the TUNEL reaction proceeded at 37°C with 5% CO2. The reaction was stopped by washing the cells in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The percentage of TUNEL-positive cells was determined by counting fluorescent cells in at least five fields. Fluorescence and phase pictures were taken on a Nikon Eclipse TE3000 inverted microscope with an Epi-fluorescence attachment (Nikon, Inc., Melville, N.Y.).
DNA fragmentation assay.
In the DNA fragmentation assay, 106 L929 or J774 cells were plated and allowed to adhere overnight at 37°C with 5% CO2. The next day, 300 ng of mycolactone (or an equivalent amount of ethanol diluted in tissue culture medium for control cells) per ml was added. At the indicated times, adherent and nonadherent cells were harvested, combined, washed with PBS, and lysed in 0.4 ml of lysis buffer (20 mM Tris, pH 8.0; 4 mM EDTA; 0.4% Triton X-100; 20 μg of proteinase K per ml; for 20 min at room temperature). Cell debris was separated by centrifugation, and the lysate was extracted with phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol]) twice and ethanol precipitated. The DNA pellet was resuspended in TE plus 10 μg RNase A (30 min at 37°C) per ml. DNA was run on a 1.5% agarose gel, stained with ethidium bromide, and photographed. Control cells were harvested at day 5.
Caspase inhibitor assay.
Mycolactone or (ethanol diluted in tissue culture medium for control cells) was added to L929 cells at 300 ng/ml. Boc-Asp-(Ome)-fluoromethylketone (B-D-FMK) (Enzyme Systems Products, Livermore, Calif.), a general inhibitor of caspases, was resuspended in dimethyl sulfoxide (DMSO) at 100 mM. At the same time as the addition of mycolactone, B-D-FMK was added at 1 μM. Control cells were treated with the same percentage of DMSO (0.001%). Due to the length of the assay and the short half-life of B-D-FMK, the caspase inhibitor was re-added every day. Adherent and nonadherent cells were harvested at each time point, and the TUNEL reaction was performed as described above. Cells were observed microscopically each day and scored as CPE positive when 90 to 100% of cells were rounded and detached. In preliminary experiments, concentrations of B-D-FMK were titrated; 1 μM was the most effective concentration for inhibiting apoptosis without causing toxicity to L929 cells (data not shown).
RESULTS
M. ulcerans organisms and mycolactone cause apoptosis in guinea pig skin.
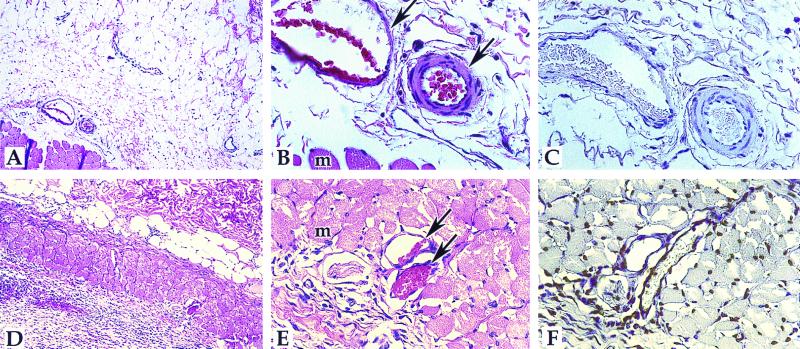
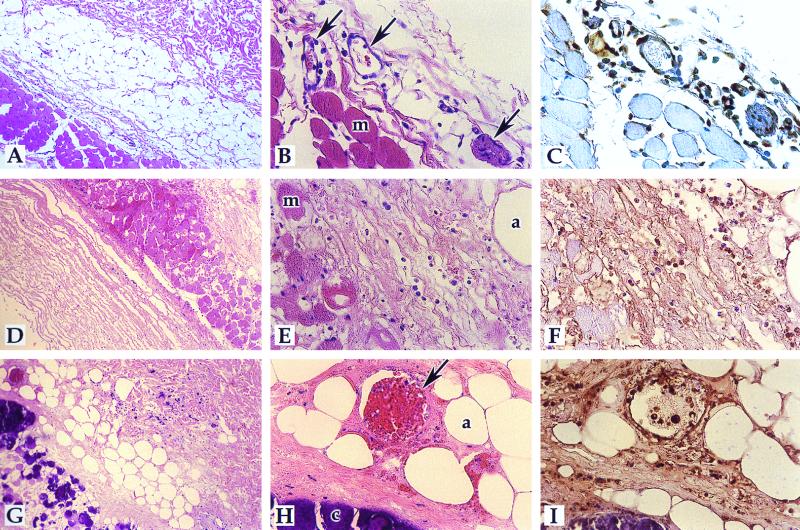
The extensive tissue destruction and presence of cells with pyknotic nuclei present in M. ulcerans-infected lesions suggests that apoptosis may be relevant to the pathogenesis of M. ulcerans. We performed the TUNEL assay on M. ulcerans-infected guinea pig sections 8 days postinjection and found TUNEL-positive nuclei throughout the section (Fig. 1F). An area of early adipocyte destruction, edema, and some vascular disruption could be seen (Fig. 1D to F). As a negative control, mycobacterial medium-treated guinea pig skin was assayed by the TUNEL reaction and very few, if any, TUNEL-positive cells were detected (Fig. 1C). As a second negative control, the TUNEL reaction was also performed on M. ulcerans- and mycolactone-induced lesions in the absence of terminal transferase enzyme, and no positive nuclei were detected (data not shown), indicating that the TUNEL-positive reactions were not due to endogenous peroxidase activity. Next, we performed TUNEL assays on tissue sections from mycolactone-treated guinea pig lesions at different time points postinoculation. Controls were as shown and described in Fig. 1. Guinea pig lesions induced by 100 μg of mycolactone at 2, 8, or 22 days postinoculation (p.i.) were analyzed (10). Apoptotic cells were present in the necrotic areas at all timepoints (Fig. 2), and the area of apoptosis increased over time as the size of the lesion and extent of destruction grew. At all time points, the region of apoptosis was centered around the point of injection, indicating that the toxin was acting locally to mediate the destruction and apoptosis. At 2 days p.i., tissue destruction was in its early stages (Fig. 2A to C). Apoptotic endothelial cells were present around apparently intact blood vessels (Fig. 2C). Infiltrated cells, possibly monocytes, were also apoptotic (Fig. 2C). At 8 days p.i., the tissue destruction was more apparent. Necrosis was apparent in the adipose, muscle, and underlying areas. Blood vessels show leakage into the muscle layer, presumably from microhemorrhages (Fig. 2D to F). Apoptosis was present throughout the necrotic area. The area of edema between adipocytes and muscle with primarily apoptotic cells can be seen (Fig. 2F). At 22 days p.i., tissue destruction was massive (Fig. 2G to I). Edema and calcification were apparent, as well as disrupted blood vessels. All nuclei surrounding the blood vessel were apoptotic (Fig. 2I). The toxin-induced lesions progressed much more rapidly than the bacterium-induced lesions and were more localized around the point of inoculation. There also appeared to be more TUNEL-positive cells in the mycolactone-treated lesions than in the M. ulcerans-infected lesions. These observations can be explained by a more dramatic, although physiological, response to the purified toxin.
FIG. 1.
Detection of apoptosis in M. ulcerans-infected guinea pig lesions and media controls. The TUNEL reaction was performed, detected by use of DAB, and counterstained with hematoxylin as described in Materials and Methods. A to C, negative control section injected with mycobacterial medium; D to F, M. ulcerans-injected section (8 days postinjection). A and D, ×83; B, C, E, and F, ×332. Panels A, B, D, and E are hematoxylin-eosin-stained sections; panels C and F are TUNEL sections. Arrows point to the blood vessels; muscle cells (m) are also indicated.
FIG. 2.
Detection of apoptosis in mycolactone-induced guinea pig lesions. The TUNEL reaction was performed, detected by use of DAB, and counterstained with hematoxylin-eosin as described in Materials and Methods. The mycobacterial-medium control is shown in panels A to C of Fig. 1. A to C, 2 days p.i.; D to F, 8 days p.i; G to I, 22 days p.i. Magnifications: A, D, and G, ×83; B, C, E, F, H, and I, ×332. Panels A, B, D, E, G, and H are hematoxylin-eosin-stained sections; panels C, F, and I are TUNEL sections. Arrows point to blood vessels; muscle (m), adipocytes (a), and calcification (c) are also indicated.
Mycolactone-induced cell death of L929 and J774 cells.
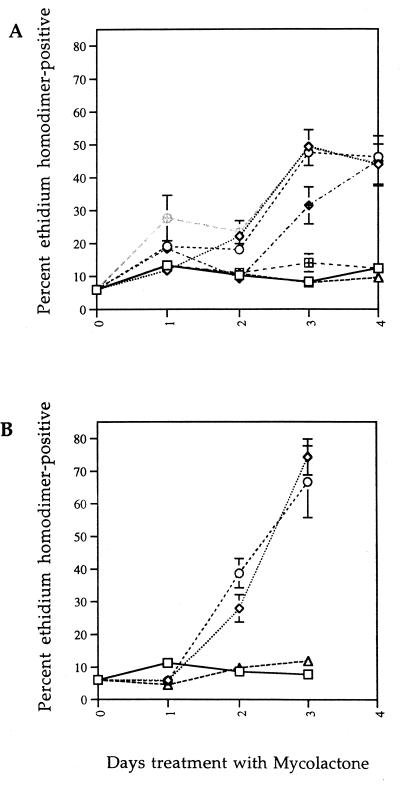
Several characterized bacterial toxins are capable of causing cell cycle arrest followed by delayed cell death (6, 31), and we hypothesized that mycolactone may also cause a delayed cell death on L929 cells. We treated L929 cells with increasing concentrations of mycolactone and assayed for cell death by ethidium homodimer uptake. A wide range of concentrations of mycolactone (3 ng/ml to 3 μg/ml, which corresponds to approximately 4 nM to 4 μM) caused significant cell death of L929 cells after 3 days of treatment (Fig. 3A). Concentrations at or below 300 pg/ml had no effect on cell viability. It is important to note that all concentrations of mycolactone which caused cell death also caused an identical CPE (90 to 100% of cells rounding and growth arrest within 48 h).
FIG. 3.
Mycolactone-mediated cell death of L929 and J774 cells. (A) Cytoxicity on L929 cells. L929 cells were treated with mycolactone at 30 pg/ml (hatched squares), 300 pg/ml (open triangles), 3 ng/ml (hatched diamonds), 30 ng/ml (open diamonds), 300 ng/ml (hatched circles), 3 μg/ml (open circles), or ethanol alone (open squares). (B) Cytoxicity on J774 cells. J774 cells were treated with mycolactone at 300 pg/ml (open triangles), 30 ng/ml (open diamonds), 3 μg/ml (open circles), or ethanol alone (open squares). Cell death was measured by ethidium homodimer uptake as described in Materials and Methods.
The lack of an acute inflammatory response and the paucity of peripheral mononuclear cells in M. ulcerans- and mycolactone-induced lesions raises several interesting hypotheses. The toxin may directly kill macrophages, or it may inhibit their chemotaxis toward the lesion. To address the former possibility, we tested a mouse macrophage cell line J774 to determine whether mycolactone mediates cell death in a manner similar to fibroblasts. J774 cells, like L929 cells, displayed similar morphological changes and dose dependence, although the kinetics of cell death were accelerated in comparison (Fig. 3B). Like L929 cells, the J774 line was affected at concentrations above 300 pg/ml. All concentrations of mycolactone which caused cell death in J774 cells also caused identical CPE, as in the L929 cells.
TUNEL assay and genomic DNA analysis.
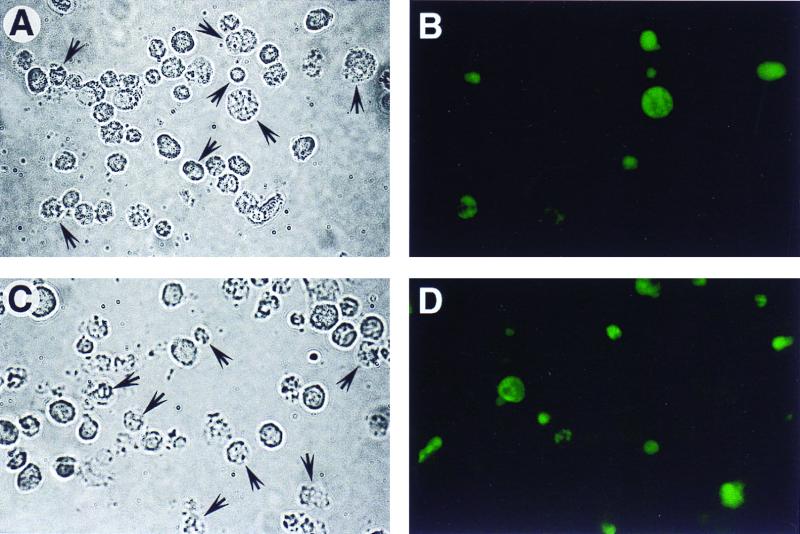
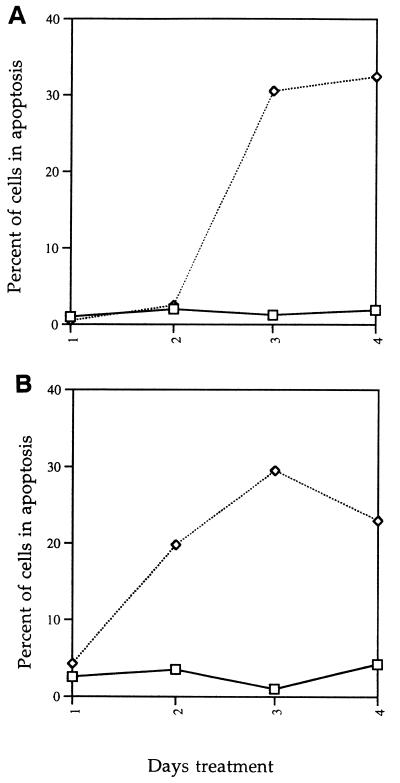
To determine whether mycolactone causes death by apoptosis, we assayed mycolactone-treated L929 and J774 cells for DNA fragmentation by TUNEL and gel electrophoresis assays. At 3 days after treatment, L929 cells reacted positively in TUNEL assays (Fig. 4B). J774 cells reacted positively after 2 days treatment with mycolactone (Fig. 4D). Control cells were negative by the TUNEL assay. A kinetic analysis of the percentage of cells positive by this assay indicated that apoptosis occurred with slightly different kinetics in the two cell lines, occurring after 2 days of treatment in the macrophage cell line as opposed to after 3 days of treatment for the fibroblasts (Fig. 5).
FIG. 4.
TUNEL reaction of L929 and J774 cells. (A and B) The TUNEL reaction was performed on L929 cells treated with mycolactone at 300 ng/ml for 3 days. (C and D) TUNEL reaction was performed on J774 cells treated with mycolactone at 300 ng/ml for 3 days. Panels A and C are phase-contrast micrographs, and panels B and D are fluorescence micrographs. Arrows in panels A and C identify the TUNEL-positive cells.
FIG. 5.
Quantitation and kinetic analysis of apoptotic L929 and J774 cells. The TUNEL reaction was performed on L929 (A) and J774 (B) cells and quantitated as described in Materials and Methods. Symbols: □, control (ethanol-treated) cells; ◊, mycolactone-treated cells.
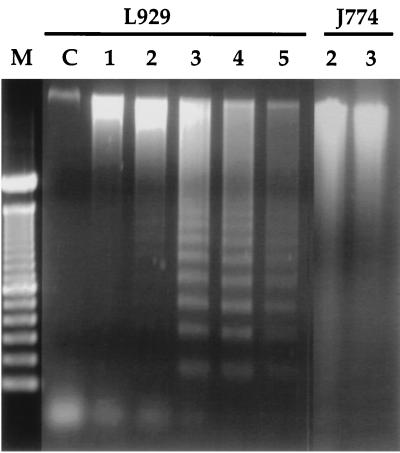
The ability of mycolactone to induce apoptotic-like DNA fragmentation was confirmed by extraction of whole genomic DNA from treated cells and electrophoresis on agarose gels. L929 cells showed the characteristic DNA laddering of apoptotic cells after 3 days of treatment (Fig. 6). J774 DNA was analyzed at day 2 and day 3 posttreatment with mycolactone, corresponding to the days with the highest percentage of TUNEL-positive cells. We were unable to detect laddering of J774 DNA with this assay, even though the experiment was repeated several times. Instead, the DNA appeared degraded compared to the control (ethanol-treated) cells (Fig. 6).
FIG. 6.
Electrophoretic analysis of DNA from L929 and J774 cells exposed to mycolactone. Genomic DNA was extracted from cells treated with 300 ng of mycolactone per ml and electrophoresed on a 1.5% agarose gel. Lane M, molecular weight markers; lane C, control L929 cells (treated with ethanol and harvested at day 5). Numbers above the lanes indicate days of treatment with mycolactone.
Inhibition of apoptosis in L929 cells.
The lag time between the CPE (cytoskeletal rearrangement) and apoptosis led us to ask whether the two events are interdependent. We hypothesized that, by inhibiting apoptosis, we may alter either the kinetics or the severity of the CPE.
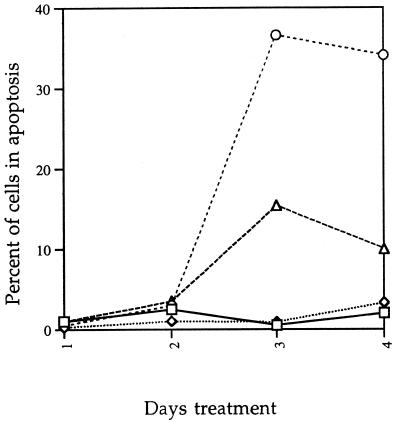
Inhibitors of the IL-1β-converting enzyme family of caspases have been shown to inhibit apoptosis (20, 27, 32). A pan-caspase inhibitor, B-D-FMK, was used to inhibit apoptosis of mycolactone-treated L929 cells. B-D-FMK was added to L929 cells at the same time as mycolactone and re-added daily (due to inactivation by endogenous cysteine proteases). Cells were analyzed microscopically for CPE as defined in Materials and Methods and then harvested and processed for TUNEL reaction. L929 cells treated with mycolactone and B-D-FMK show normal CPE, but B-D-FMK inhibits progression to apoptosis compared to the mycolactone-treated L929 cells. B-D-FMK treatment alone caused no CPE and did not induce apoptosis (Fig. 7). We then asked whether inhibition of apoptosis by B-D-FMK would change the cell death phenotype by assaying for ethidium homodimer uptake. There was no difference in either the kinetics of cell death or the percentage of dead cells regardless of whether or not B-D-FMK was added to the mycolactone-treated cells (data not shown). This finding indicates that if apoptosis is inhibited, the mycolactone-treated cells die by necrosis.
FIG. 7.
B-D-FMK inhibits mycolactone-induced apoptosis. L929 cells were treated with ethanol only (□), 1 μM B-D-FMK only (◊), mycolactone only (○), or mycolactone plus B-D-FMK (▵). Cells were harvested at each day and processed for the TUNEL reaction.
DISCUSSION
Previous work from our laboratory demonstrated that the M. ulcerans polyketide toxin, mycolactone, causes Buruli ulcer-like lesions in a guinea pig model of infection (10, 25). These lesions are characterized by extensive cell damage with many cells containing pyknotic nuclei. Mycolactone also causes a CPE on L929 cells within 48 h of treatment (defined as 90 to 100% of cells exhibiting cytoskeleton rearrangements and growth arrest), with minimal loss of viability after 48 h. To resolve the apparent paradox of the two phenotypes and to begin to define the mechanism underlying the histopathology, we looked at cell death in both systems.
To determine the relevance of apoptosis to pathology, we performed TUNEL assays on M. ulcerans- and mycolactone-induced guinea pig lesions and found that apoptotic cells were present in the lesions. By studying mycolactone-induced lesions over time postinjection, it was apparent that the area of apoptosis increased over time as the size of the lesion increased. Not all pyknotic nuclei were TUNEL positive. One explanation for this finding is that not all cell death is apoptosis mediated. An alternative hypothesis is that apoptosis is the primary cause of cell death but that at any given time point not all cells in the lesion are TUNEL positive. Due to the dearth of guinea pig cell-specific markers, we have not definitely identified affected cells in the lesions.
We have demonstrated here that longer exposure to the toxin causes cell death of L929 cells within 72 h across a wide range of mycolactone concentrations. J774 cells show a similar dose dependence of mycolactone and a cell death by 48 h, which is slightly earlier than for the L929 cells. Using TUNEL and DNA fragmentation assays, we showed that mycolactone induced apoptosis coincident with cell death. It is important to note that both the J774 and L929 cultures show 90 to 100% cytopathicity but that the percentage of TUNEL-positive cells over a period of days showed a maximum of 20 to 35% undergoing apoptosis. Cytopathicity and apoptosis may be two independent primary events, with apoptosis affecting only a subset of the population. Another hypothesis is that apoptosis is a secondary result of the CPE. Inhibition of anchorage-dependent cell spreading has been shown to trigger apoptosis (8, 24, 30), as have fungal metabolites, such as cytochalasins, which effect actin polymerization (28, 30). By using a pan-caspase inhibitor, we showed that mycolactone is capable of causing a CPE in L929 cells without progression to apoptosis, demonstrating that the CPE is not dependent on apoptosis.
Although the mycolactone-treated L929 cells reproducibly showed a strong DNA fragmentation pattern, we were unable to demonstrate DNA fragmentation with J774 cells despite repeated attempts. There is much information in the literature describing apoptosis occurring without the presence of DNA ladder formation (5, 21, 22, 29). Perhaps the window of time that the DNA ladder would appear in J774 cells was too narrow for us to detect and, therefore, the DNA appeared intact, as in control cells, or degraded, as in mycolactone-treated cells.
Mycolactone is a complex polyketide-derived macrolide. Macrolides are broadly characterized into groups based on the number of carbons present in the lactone ring (4). Mycolactone is a 12-membered ring macrolide which is the group of macrolides containing the smallest lactone ring. Other 12-membered ring macrolides include methymycin, litorin, patulolide, cladospolide, and recefeiolide (4). Although they share a similar lactone ring size, the rest of their chemical structures bear little resemblance to mycolactone, and no biological activity has been assigned for them approximating the activity we have observed here with mycolactone. Other macrolides that induce apoptosis in tissue culture cells include erythromycin, bafilomycin A1, antimycin A, and ionomycin (1–3, 11, 13, 17). The concentrations of these macrolides used to induce apoptosis are between 60 ng/ml and 6 μg/ml, which is the mycolactone concentration range in which we observe similar effects. These examples may or may not be relevant to mycolactone-mediated apoptosis. Whether mycolactone exerts its effects by any of these mechanisms is currently under investigation.
Pathogenic mycobacteria, such as M. tuberculosis, have been shown to induce apoptosis in cultured monocytes (14, 19). Further analysis of the mycobacterial products which may regulate apoptosis demonstrated that culture filtrate of M. tuberculosis, supernatant from heat-treated organisms, and purified protein derivative induce apoptosis (16, 26). Whether these extracts contain any mycolactone-like molecules is possible but as yet unknown.
The most distinctive and unique feature of M. ulcerans pathology is the presence of cell damage in the absence of an acute inflammatory response. Why does this cell death not lead to an acute inflammatory response? The data presented here suggest a hypothesis for how mycolactone as a single molecule might lead to this property. Recently, it has been shown that mycolactone suppressed the production of inflammatory cytokines (23). These data could explain why neutrophils are absent at early time points following infection. The results presented here from both in vitro and in vivo experiments suggest that cell death results from apoptosis. Apoptosis as a phenomenon is associated with a lack of inflammatory response (7). If apoptosis is a major cause of pathology in Buruli ulcer, this could explain why an acute inflammatory reaction does not develop in these lesions despite extensive cell damage.
ACKNOWLEDGMENTS
We thank R. Wells and C. Favara for excellent technical assistance. We also thank L. Barker, T. Hackstadt, L. Perry, J. Portis, and J. Van Putten for critical reading of the manuscript.
REFERENCES
- 1.Aagaard-Tillery K M, Jelinek D F. Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell Immunol. 1994;156:493–507. doi: 10.1006/cimm.1994.1193. [DOI] [PubMed] [Google Scholar]
- 2.Aagaard-Tillery K M, Jelinek D F. Differential activation of a calcium-dependent endonuclease in human B lymphocytes. Role in ionomycin-induced apoptosis. J Immunol. 1995;155:3297–3307. [PubMed] [Google Scholar]
- 3.Aoshiba K, Nagai A, Konno K. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob Agents Chemother. 1995;39:872–877. doi: 10.1128/aac.39.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryskier A, Agouridas C, Gasc J-C. Classification of macrolide antibiotics. In: Bryskier A J, Butzler J-P, Neu H C, Tulkens P M, editors. Macrolides: chemistry, pharmacology and clinical uses. Paris, France: Arnette S.A.; 1993. pp. 5–66. [Google Scholar]
- 5.Cohen G M, Sun X-M, Snowden R T, Dinsdale D, Skilleter D N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992;286:331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Rycke J, Mazars P, Nougayrede J-P, Tasca C, Boury M, Herault F, Valette A, Oswald E. Mitotic block and delayed lethality in HeLa epithelia cells exposed to Escherichia coli BM2-1 producing cytotoxic necrotizing factor type 1. Infect Immun. 1996;64:1694–1705. doi: 10.1128/iai.64.5.1694-1705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dini L, Ruzittu M T, Falasca L. Recognition and phagocytosis of apoptotic cells. Scanning Microsc. 1996;10:239–252. [PubMed] [Google Scholar]
- 8.Fukai F, Mashimo M, Akiyama K, Goto T, Tanuma S, Katayama T. Modulation of apoptotic cell death by extracellular matrix proteins and a fibronectin-derived antiadhesive protein. Exp Cell Res. 1998;242:92–99. doi: 10.1006/excr.1998.4076. [DOI] [PubMed] [Google Scholar]
- 9.George K M, Barker L P, Welty D M, Small P L C. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect Immun. 1998;66:587–593. doi: 10.1128/iai.66.2.587-593.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George K M, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small P L C. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 11.Grand R J A, Milner A E, Mustoe T, Johnson G D, Owen D, Grant M L, Gregory C D. A novel protein expressed in mammalian cells undergoing apoptosis. Exp Cell Res. 1995;218:439–451. doi: 10.1006/excr.1995.1177. [DOI] [PubMed] [Google Scholar]
- 12.Horsburgh C R, Jr, Meyers W M. Buruli ulcer. In: Horsburgh C R Jr, Nelson A M, editors. Pathology of emerging infections. Washington, D.C.: ASM Press; 1997. pp. 119–134. [Google Scholar]
- 13.Kaushal G P, Ueda N, Shah S V. Role of caspases (ICE/CED3 proteases) in DNA damage and cell death in response to a mitochondrial inhibitor, antimycin A. Kidney Int. 1997;52:438–445. doi: 10.1038/ki.1997.350. [DOI] [PubMed] [Google Scholar]
- 14.Klinger K, Tchou-Wong K M, Brandli O, Aston C, Kim R, Chi C, Rom W N. Effects of mycobacteria on regulation of apoptosis in mononuclear phagocytes. Infect Immun. 1997;65:5272–5278. doi: 10.1128/iai.65.12.5272-5278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieg R E, Hockmeyer W T, Connor D H. Toxin of Mycobacterium ulcerans. Arch Dermatol. 1974;110:783–788. doi: 10.1001/archderm.110.5.783. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Bassiri H, Rossman M D, Kramer P, Eyuboglu A F, Torres M, Sada E, Imir T, Carding S R. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human γδ T cells: a mechanism for the loss of γδ T cells in patients with pulmonary tuberculosis. J Immunol. 1998;161:1558–1567. [PubMed] [Google Scholar]
- 17.Long X, Crow M T, Sollott S J, O'Neil L, Menees D S, Hipolito M D, Boluyt M O, Asai T, Lakatta E G. Enhanced expression of p53 and apoptosis induced by blockade of the vacuolar proton ATPase in cardiomyocytes. J Clin Investig. 1998;101:1453–1461. doi: 10.1172/JCI345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCallum P, Tolhurst J C, Buckle G, Sissons H A, Buckle G, Tolhurst J C. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93–122. [PubMed] [Google Scholar]
- 19.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Geurin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulvey M A, Lopez-Boado Y S, Wilson C L, Roth R, Parks W C, Heuser J, Hultgren S J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 21.Oberhammer F, Wilson J W, Dive C, Morris I D, Hickman J A, Wakeling A E, Walker P R, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ormerod M G, O'Neill C F, Robertson D, Harrap K R. Cisplatin induces apoptosis in a human ovarian carcinoma cell line without concomitant internucleosomal degradation of DNA. Exp Cell Res. 1994;211:231–237. doi: 10.1006/excr.1994.1082. [DOI] [PubMed] [Google Scholar]
- 23.Pahlevan A A, Wright D J, George K M, Small P L, Foxwell B M. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell functions and expression of NFκB. J Immunol. 1999;163:3928–3935. [PubMed] [Google Scholar]
- 24.Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in culture human endothelial cells. J Cell Biol. 1994;127:537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read J K, Heggie C M, Meyers W M, Connor D H. Cytotoxic activity of Mycobacterium ulcerans. Infect Immun. 1974;9:1114–1122. doi: 10.1128/iai.9.6.1114-1122.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas M, Barrera L F, Puzo G, Garcia L F. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J Immunol. 1997;159:1352–1361. [PubMed] [Google Scholar]
- 27.Sarin A, Wu M-L, Henkart P A. Different interleukin-1β-converting enzyme (ICE) family protease requirements for the apoptotic death of T lymphocytes triggered by diverse stimuli. J Exp Med. 1996;184:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauman I, Berry S J. Cytochalasin-D treatment triggers premature apoptosis of insect ovarian follicle and nurse cells. Int J Dev Biol. 1993;37:441–450. [PubMed] [Google Scholar]
- 29.Schulz-Osthoff K, Walczak H, Droge W, Krammer P H. Cell nucleus and DNA fragmentation are not required for apoptosis. J Cell Biol. 1994;127:15–20. doi: 10.1083/jcb.127.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott G, Cassidy L, Busacco A. Fibronectin supresses apoptosis in normal human melanocytes through an integrin-dependent mechanism. J Investig Dermatol. 1997;108:147–153. doi: 10.1111/1523-1747.ep12332650. [DOI] [PubMed] [Google Scholar]
- 31.Sugai M, Kawamoto T, Peres S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. Dual signaling of the fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]