Abstract
Listeria monocytogenes mutants defective in the actA gene, the plcB gene, and the inlA and inlB genes were less virulent when injected intravenously into BALB/c mice. The growth of these strains as well as of the virulent wild-type strains was increased by treating mice with a neutrophil-specific depleting monoclonal antibody, RB6-8C5. Histologic examination of the livers of the treated animals showed intrahepatocytic proliferation of the listeriae in all cases. Our data show that more than one pathway exists that allows L. monocytogenes to invade parenchymal cells. One pathway most likely involves the actA and plcB gene products, and a second one probably involves the internalins.
Listeria monocytogenes is a bacterial pathogen that most commonly infects immunodeficient individuals, causing infections of the central nervous system (2). It is also a cause of infection of the fetus in pregnant women (2). It is one of the most well-studied bacterial pathogens since it is very useful as a model of an intracellular infectious agent. Despite having been used for a long time as models of macrophage parasites, listeriae can invade nonphagocytic cells such as hepatocytes and endothelial cells (5, 8). Probably because of these properties, neutrophils play a prominent role in the host defense mechanisms against infection by this microbe (1, 5, 7, 10, 18). There is a fairly good idea of the pathways followed by listeria that lead to infection of the different cell types. Molecular and cell biology studies have defined a series of virulence factors that are involved in the pathogenesis of the infection. Several strains with mutations in these factors have been obtained and are being studied in in vitro and in vivo models of infection. Some of the mutants have shown decreased ability in spreading between cells in in vitro assays using different types of cell cultures. Among the most prominent virulence factors are those encoded by the hly, actA, plcB, and inlA and inlB genes (6, 12, 17). The hly gene encodes listeriolysin O, a thiol-activated, oxygen-labile cytolysin that allows the bacteria to lyse the phagosomal membrane and thus escape into the cytosol (3, 6, 12, 17). The ActA protein is then used for the polymerization of actin, leading to extrusion of the bacteria from the infected cell into a neighboring new target cell (6, 12, 13, 17, 19). The lecithinase encoded by plcB is thought to be required for the lysis of the double membrane-bound vacuole that contains the bacteria after the former process has taken place (6, 12, 17, 20). These different steps allow the invasion of nonphagocytic cells residing in the neighborhood of phagocytes that have ingested the bacteria (6, 12, 17). However, an alternative pathway may allow the direct invasion of nonphagocytic cells by listeriae. This pathway uses internalin (InlA) and the product of inlB, which are involved in the induction of endocytosis of the extracellular bacteria by the target cells mediated by E cadherin (9, 15, 16). The knowledge about the activities of these factors stems mostly from in vitro studies. In vivo, it is difficult to study the invasion of nonphagocytic cells, although some authors have reported data obtained after the separation of different cell types by gradient isolation procedures. Also, histological analyses may in theory be used, but they have very low sensitivities.
We sought to test whether the products of listeriae shown in vitro to be important for cell invasion and cell-to-cell spreading are required for the invasion of hepatocytes, as the liver is the main target organ for the in vivo replication of these bacteria. To make such an analysis, we used mice depleted of neutrophils, which are well known to lack the defenses required for the control of parenchymal cell infection by listeriae and therefore allow the detection of intrahepatocytic bacterial growth (1, 5, 7, 10, 18).
Bacterial inocula were prepared from wild-type strains of L. monocytogenes (strains EGD and 10403S) as well as from deficient strains 1942 (with a deleted actA gene, supplied by D. Portnoy, Berkeley, Calif.), 1935 (with a deleted plcB gene, from D. Portnoy), and BUG949 (with a defective inlAB operon, supplied by P. Cossart, Paris, France). Bacteria were cultivated in Antibiotic 3 broth (Difco, Detroit, Mich.) until mid-log phase and kept frozen until use at −70°C. Six- to eight-week-old female BALB/c mice were infected intravenously with different doses of the different strains of listeriae, and bacterial growth was monitored at several time points of infection by performing viability counts on liver and spleen homogenates after serially diluting them and plating them onto Antibiotic 3 agar plates. Mice were either nontreated or treated intravenously with 200 μg of the neutrophil-specific RB6-8C5 monoclonal antibody (prepared from the ascites induced in nude mice by the hybridoma, using a protein G-agarose affinity column [Gibco, Paisley, United Kingdom]) 2 h before infection and at days 2 and 4 of infection.
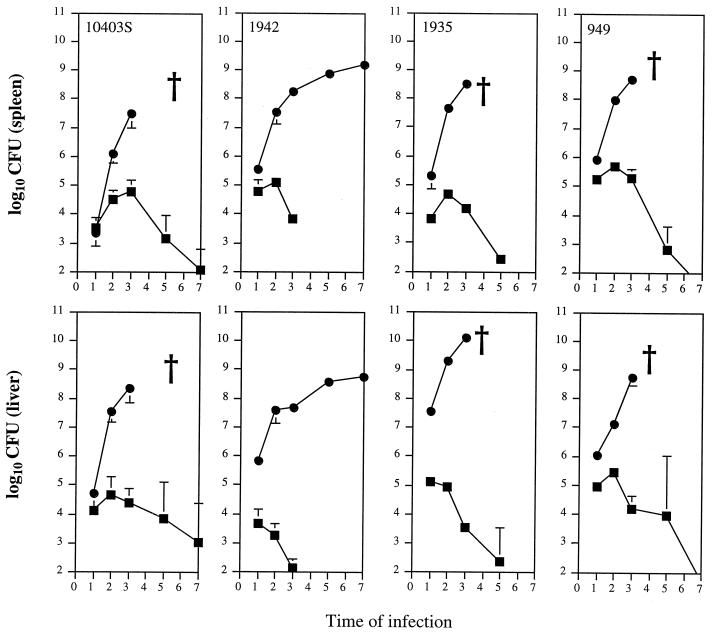
Confirming previous observations, neutrophil depletion caused by the RB6-8C5 monoclonal antibody induced the marked exacerbation of the infection by the wild-type strains 10403S (Fig. 1) and EGD (data not shown; see reference 1). After infection with 1.0 × 103 CFU of strain 10403S, untreated mice controlled the infection. However, neutrophil-depleted animals allowed for the progressive growth of the bacteria, leading to the death of the animals at day 4 of infection. As expected, histological analysis of the livers showed extensive bacterial proliferation inside hepatocytes in neutrophil-deficient animals (data not shown). Mutant strains of listeriae were less virulent, and therefore higher inoculum doses were used. After injection of 1.2 × 105 CFU of the ActA-deficient 1942 strain, bacteria were quickly eliminated in normal BALB/c mice, being undetectable in the organs of those animals at day 4 of infection (Fig. 1). However, neutrophil depletion still exacerbated bacterial proliferation, although the infection followed a nonfatal course until day 7 (Fig. 1). The examination of the histology of the livers of neutrophil-depleted animals infected with strain 1942 revealed foci of infected hepatocytes (Fig. 2). Strains 1935 and BUG949, defective in lecithinase and in the inlAB operon, respectively, were also eliminated from normal mice, although less rapidly than the previous mutant strain (Fig. 1). After infection of neutrophil-depleted animals, both strains caused a progressive infection that caused the death of the hosts at day 4 of infection. Again, listerial proliferation was found to occur in the hepatocytes (data not shown).
FIG. 1.
Growth of L. monocytogenes strains 10403S (wild type), 1942 (ActA deficient), 1935 (PlcB deficient), and BUG949 (InlAB deficient) in the spleens and livers of untreated (squares) or neutrophil-depleted (circles) BALB/c mice. Mice were infected with 1 × 103 CFU of strain 10403, 1.2 × 105 CFU of strain 1942, 3 × 105 CFU of strain 1935, and 6 × 105 CFU of strain 949. Data represent the geometric means of CFU per time point ± 1 standard deviation. Crosses show when animals died from the infection. Times of infection are in days.
FIG. 2.
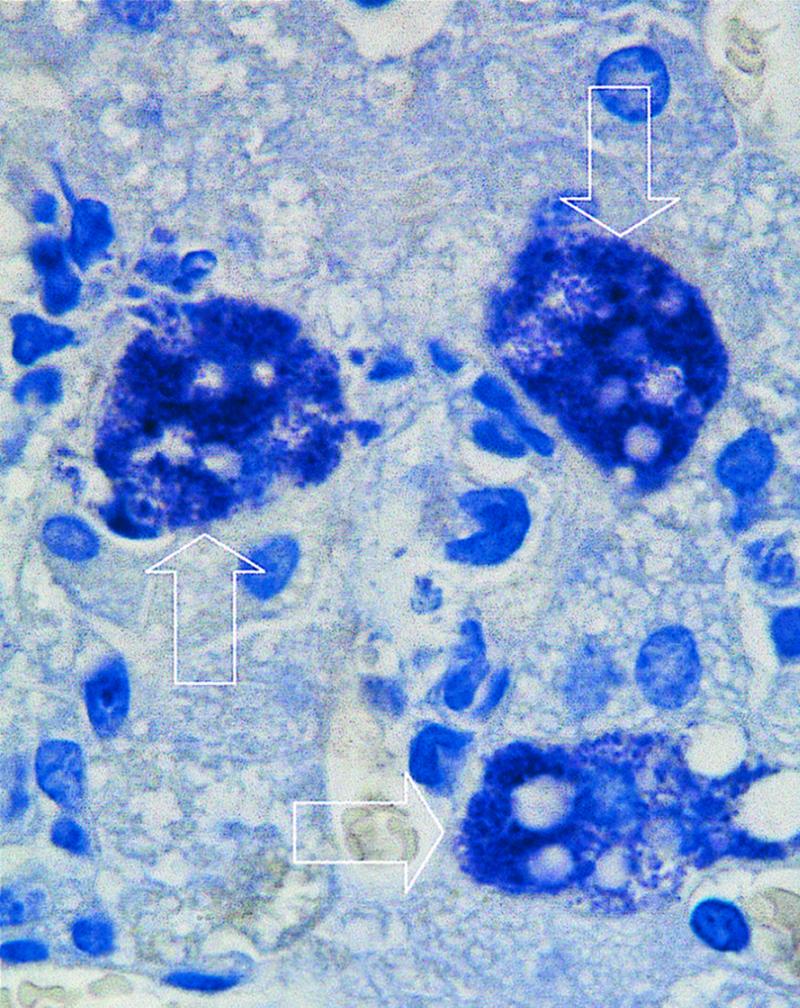
Histology of a liver section from a neutropenic mouse infected for 3 days with L. monocytogenes strain 1942 shows evidence of intrahepatocytic growth of this bacterial mutant. The arrows indicate three hepatocytes heavily infected with bacteria. Magnification, ×2,500.
As shown above, the lack of neutrophils allowed the unrestrained growth of listeriae in the hepatocytes of infected mice. This approach allowed us, therefore, to assess the ability of the different strains of listeriae to invade those cells. Strains with mutations in the lecithinase gene or in the internalins were better able to proliferate in the organs of neutropenic animals than was the ActA mutant. The latter strain is unable to spread between cells in in vitro cultures (13). It is therefore very interesting to find that this strain still retains some capacity to invade hepatocytes in vivo. Although it is not possible to exclude the possibility that cell-to-cell spread is occurring, we favor the hypothesis that such an invasion of hepatocytes by the ActA mutant is mediated by the InlAB-induced internalization of the bacteria directly by the hepatocytes. This process would occur not only early after infection (as already suggested by Gregory et al. [10]) but also throughout the course of infection in neutropenic animals following the disruption of highly infected hepatocytes and discharge of free bacteria into the tissues. In this regard, it will be interesting to analyze the characteristics of double mutants defective in both the ActA and the internalin pathways. Whereas the mutants analyzed here are able to proliferate in neutropenic mice, it has been reported that listeriolysin mutants fail to do so (4). This may suggest that, once inside the parenchymal cells, this enzyme is needed for further proliferation whatever the pathway of entrance of the bacteria. In addition, there may be other gene products involved in the internalization by nonphagocytic cells such as the hepatocyte. In that respect, it has been shown elsewhere that a murein hydrolase, the p60 protein, may be synergizing with the internalin to mediate cell entrance of listeriae (11, 14).
In conclusion, our data suggest that L. monocytogenes may use distinct pathways to access the cytoplasm of parenchymal cells, namely, in the liver. Among these, the classical listeriolysin O-ActA spreading is most likely the most important, but the alternative induced ingestion mediated by the products of the inl locus plus accessory proteins may represent a secondary mechanism of such invasion.
Acknowledgments
We are indebted to Pascale Cossart and Daniel Portnoy for their gifts of the mutant listeriae and to Regina Silva and Helena Carvalho for support.
I. S. Leal is a fellow of the PRAXIS XXI Programme (Lisbon).
REFERENCES
- 1.Appelberg R, Castro A G, Silva M T. Neutrophils as effector cells of T cell-mediated, acquired immunity in murine listeriosis. Immunology. 1994;83:302–307. [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong D. Listeria monocytogenes. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 1880–1885. [Google Scholar]
- 3.Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature (London) 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 4.Conlan J W, North R J. Role of Listeria monocytogenes virulence factors in survival: virulence factors distinct from listeriolysin are needed for the organism to survive an early neutrophil-mediated host defense mechanism. Infect Immun. 1992;60:951–957. doi: 10.1128/iai.60.3.951-957.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cossart P, Lecuit M. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signalling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czuprynski C J, Brown J F, Maroushek N, Wagner R D, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 8.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaillard J-L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from Gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 10.Gregory S H, Sagnimeni A J, Wing E J. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol. 1996;157:2514–2520. [PubMed] [Google Scholar]
- 11.Hess J, Gentschev I, Szalay G, Ladel C, Bubert A, Goebel W, Kaufmann S H E. Listeria monocytogenes p60 supports host cell invasion by and in vivo survival of attenuated Salmonella typhimurium. Infect Immun. 1995;63:2047–2053. doi: 10.1128/iai.63.5.2047-2053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireton K, Cossart P. Host-pathogen interactions during entry and actin-based movement of Listeria monocytogenes. Annu Rev Genet. 1997;31:113–138. doi: 10.1146/annurev.genet.31.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn M, Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989;57:55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecuit M, Ohayon H, Braun L, Mengaud J, Cossart P. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect Immun. 1997;65:5309–5319. doi: 10.1128/iai.65.12.5309-5319.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mengaud J, Ohayon H, Gounon P, Mege R M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of Listeria monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 17.Portnoy D, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers H W, Unanue E R. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasquez-Boland J-A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]